Abstract
Psychostimulant addicts often take high doses of drugs, and high doses of psychostimulants such as methamphetamine (METH) are neurotoxic to striatal dopamine (DA) terminals. Yet, the effects of high doses of METH on drug-seeking and drug-taking behavior have not been examined. In the present study, we found that single high doses of METH in rats (10–20 mg/kg) dose-dependently increased cocaine self-administration under fixed-ratio 2 (FR2) reinforcement conditions, while higher doses (40 mg/kg×1 or 10 mg/kg/2 h×4) caused high mortality among rats maintained on daily cocaine self-administration. The increased cocaine self-administration appeared to be a compensatory response to reduced cocaine reward after METH, because the same doses of METH caused a dose-dependent reduction both in “breakpoint” levels for cocaine self-administration under progressive-ratio reinforcement and in nucleus accumbens DA response to acute cocaine. Further, METH (10–20 mg/kg) produced large DA release (4000%–6000% over baseline), followed by a significant reduction in striatal DA and 3,4-dihydroxyphenylacetic acid (DOPAC) contents, but without significant changes in striatal DA transporter levels. These findings suggest that the present high doses of METH caused striatal DA depletion or hypofunction without severe damage in DA terminals, which may contribute to the increased cocaine-taking behavior observed in the present study. Provided that the present doses of METH may mimic METH overdose incidents in humans, the present findings suggest that METH-induced DA depletion or neurotoxicity may lead to an increase in subsequent drug-taking and drug-seeking behavior.
Keywords: methamphetamine, cocaine, dopamine, self-administration, drug-taking, drug reward
Psychostimulant addiction is often characterized by a pattern of escalating use (Kramer et al., 1967; Cook et al., 1993). The neural mechanisms underlying such escalating use are unknown. Since some psychostimulants such as methamphetamine (METH) are neurotoxic to striatal dopamine (DA) terminals, as exemplified by a significant reduction in striatal DA, dopamine transporter (DAT) or DA receptor levels in both humans and experimental animals (Volkow et al., 2001; Wilson et al., 1996; Cadet et al., 2003, 2007; Riddle et al., 2006), it was hypothesized that METH neurotoxicity in DA may contribute to escalating use or augmented psychostimulant-taking behavior.
Previous studies have shown that multiple high doses of METH significantly attenuate METH’s actions on locomotion (Wallace et al., 1999), conditioned place preference (CPP) (Itzhak et al., 2002) and striatal DA (Cass et al., 1997; Wallace et al., 1999), suggesting that METH-induced DA neurotoxicity may lead to a reduction in psychomotor-stimulating and rewarding responses to METH. However, this view is challenged by other studies demonstrating that high METH doses produce augmented locomotion and stereotypic responses to METH (Itzhak et al., 2002; Kita et al., 1998), augmented CPP (Gehrke et al., 2003) and augmented striatal DA release to METH (Kazahaya et al., 1989). The reasons for these discrepancies are unclear. One possibility is that repeated METH administration may also induce enduring neuroadaptations, such as sensitization of locomotion and striatal DA transmission (White and Kalivas, 1998; Mizoguchi et al., 2008), which may confound METH neurotoxicity-induced changes in behavior and neurochemistry. This suggests that repeated METH treatment may be not an ideal approach to determine whether METH-induced DA neurotoxicity contributes to augmented drug-taking behavior in rats. Therefore, in the present study, we investigated the effects of high doses of METH on cocaine (not METH) self-administration to avoid repeated METH administration. In addition, METH self-administration usually displays rapid dose escalation over time (Kitamura, 2006; Mandyam et al., 2007), making it difficult to distinguish the effects produced by METH pretreatment from effects produced by METH self-administration itself.
Therefore, in the present study, we first investigated whether single (10, 20, 40 mg/kg) or multiple (10 mg/kg/2 h×4) high doses of METH altered cocaine self-administration under low fixed ratio (FR2) and progressive-ratio (PR) reinforcement. To study possible mechanisms underlying changes in cocaine self-administration, we further assessed the effects of METH on striatal DA response to cocaine, and the effects of METH on extracellular DA and striatal DA contents as well as DAT levels. We found that single non-fatal high doses (10–20 mg/kg) of METH produced a dose-dependent increase in cocaine self-administration predominantly by METH-induced striatal DA depletion and hypofunction in rats.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague–Dawley rats (275–300 g) were obtained from Charles River Laboratories (Raleigh, NC, USA). They were housed individually in a climate-controlled animal room with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Research Council, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse. All experiments were designed to minimize number of animals used and any discomfort experienced by the animals.
Cocaine self-administration under FR2 reinforcement
I.v. surgery and cocaine self-administration protocols were as previously described (Xi et al., 2005, 2006). Briefly, i.v. catheterization was performed under sodium pentobarbital (60 mg/kg i.p.) anesthesia with aseptic surgical technique. After 7 days of recovery from surgery, animals were placed into standard operant chambers for cocaine self-administration. Initially, animals pressed the active lever for cocaine (1 mg/kg/infusion) for 3 h per day under fixed-ratio (FR1) reinforcement, under which animals received a cocaine infusion for every lever-press. Animals were then switched to 0.5 mg/kg/infusion under FR2 reinforcement under which animals received a cocaine infusion after every second lever-press. Cocaine infusions were associated with light and sound cues. Inactive lever-presses were counted, but had no consequence.
To determine whether high doses of METH alter cocaine-taking behavior, we first assessed the effects of multiple dosing regimens of METH pretreatment on cocaine self-administration under FR2 reinforcement conditions. Animals were divided into five experimental groups after the following criteria of self-administration stability were met: less than 10% variability in inter-response interval and less than 10% variability in number of presses on the active lever for at least three consecutive days. Then, each group of rats received either vehicle (saline), or one of four METH doses (10, 20, 40 mg/kg, or 10 mg/kg/2 h×4, i.p.) in their home cages. Self-administration testing (under FR2 reinforcement) began again 24 h after the METH treatment. The effects of METH on cocaine self-administration were observed daily for 7 days. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine injections per 3 h session. Since all animals completed the maximal 50 cocaine infusions over different periods of time after vehicle or METH administration, we used cocaine self-administration rate (infusions/h) to evaluate the effects of METH on cocaine self-administration behavior.
Cocaine self-administration under PR reinforcement
To determine whether METH-induced changes in cocaine self-administration under FR2 reinforcement were due to a change in cocaine’s rewarding efficacy, we further assessed the effects of METH pretreatment on break-point for cocaine self-administration under PR reinforcement, a paradigm highly sensitive to changes in drug reward strength (Richardson and Roberts, 1996). Three additional groups of rats were used for the PR self-administration experiment. All animals were initially trained for cocaine self-administration under FR1 and FR2 reinforcement as described above, and then switched to cocaine self-administration under PR reinforcement after stable FR2 self-administration was established. During PR cocaine self-administration the work requirement (i.e. lever presses) to receive a single i.v. cocaine infusion was raised progressively within each test session according to the following PR series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492 and 603 until the break-point was reached (Richardson and Roberts, 1996). The break-point was defined as the maximal work demand (i.e. number of lever presses) completed for the last cocaine infusion prior to a 1-h period during which no infusions were obtained by the animal. Animals were allowed to continue daily sessions of cocaine self-administration under PR reinforcement conditions until day-to-day variability in break-point fell within one to two ratio increments for three consecutive days. Once a stable break-point was established, subjects received either vehicle (saline) or one dose of METH (10, 20 mg/kg i.p.). PR cocaine self-administration testing began again 24 h after the METH treatment. The effects of METH on cocaine self-administration under PR reinforcement were observed daily for 7 days.
In vivo brain microdialysis
To determine whether a DA-related mechanism may underlie METH-induced changes in cocaine self-administration, in vivo microdialysis with high performance liquid chromatography (HPLC) was used to measure nucleus accumbens (NAc) DA response to cocaine after METH and also acute METH-induced changes in extracellular DA in the NAc. Two groups of rats were used in this experiment. In one group of rats (n=30), we first observed the unilateral NAc DA response to systemic administration of cocaine (10 mg/kg i.p.) 7 days after different METH doses (0, 10, 20 mg/kg i.p.) (n=7–8 rats each dose group). We then observed the DA response to local perfusion of gradually escalating concentrations (1, 10, 100, 1000 μM) of cocaine into the contralateral NAc 9 days after METH in the same group of rats. We used two different days (7 versus 9 days after METH) for microdialysis in these experiments because of the danger of cross-contaminating and confounding effects on NAc DA of administering both systemic and intra-NAc cocaine in the same animals on the same day. In another group of rats (n=29), we first observed the effects of acute METH on extracellular DA in the NAc from one NAc on the test day (i.e. METH injection day), and then further observed the NAc DA response to local perfusion of cocaine 3 or 14 days after METH from the contralateral NAc (n=5–8 rats each treatment group).
In vivo microdialysis protocols were as reported previously (Xi et al., 2006). Rats were anesthetized with sodium pentobarbital, and guide cannulae (20 gauge, Plastics One, Roanoke, VA, USA) were surgically implanted into the NAc (AP+1.6 mm, ML±1.4 mm, DV-4.3 mm, 6° from vertical, according to the rat brain atlas of Paxinos and Watson, 1998). The guide cannulae were fixed to the skull with four stainless steel skull screws (Small Parts, Inc., Miami Lakes, FL, USA) and dental acrylic. Microdialysis probes were inserted into the NAc 12 h before the onset of microdialysis to minimize damage-induced neurotransmitter release. Twelve hours after probe insertion, perfusion of microdialysis buffer (5 mM glucose, 2.5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, 0.15% phosphate-buffered saline, pH 7.4) through the probe (2.0 μl/min) began and continued for at least 2 h before sampling started. Samples were collected every 20 min into 10 μl 0.5 M perchloric acid to prevent DA degradation. After sample collection, all samples were frozen at −80 °C until analyzed.
After microdialysis experiments were completed, rats were anesthetized with a high dose of pentobarbital (>100 mg/kg i.p.) and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed and placed in 10% formalin for 1 week. The tissue was blocked around the NAc, sectioned coronally (100 μm thick) by vibratome, and stained with Cresyl Violet. Sections were then examined by standard light microscopy.
Microdialysate DA was measured by HPLC with an ESA Biosciences, Inc. (Chelmsford, MA, USA) electrochemical (EC) detection system as described previously (Xi et al., 2006), upgraded by a Coulochem III EC detector. Areas under curve (AUC) for DA were measured and quantified with external standard curves. The minimum detection limits for DA were 1–10 fmol.
Body temperature and locomotion measurement
METH or vehicle (0.9% NaCl) was given i.p. in home cages, during which time both deep body (rectal) temperature and locomotion were monitored in two separate groups of rats. Before METH administration, animals were placed in locomotor detection chambers (Accuscan Instruments, Inc., Columbus, OH, USA) for 3 days (5 h per day) for environmental habituation. Next, each rat was given either saline or one of two doses (10, 20 mg/kg i.p.) of METH. Immediately after the METH injection, animals were placed into the locomotor chambers to record locomotion for 5 h. Data were collected in 10-min intervals using VersaMax version 3.0 software (Accuscan Instruments, Inc.). Total distance was used to evaluate the effects of different doses of METH on locomotor behavior.
Brain tissue DA and DOPAC measurement
To determine whether the present non-fatal doses of METH produce similar DA neurotoxicity as other higher dosing regimens reported previously (Cadet et al., 2003, 2005), we measured DA and 3,4-dihydroxyphenylacetic acid (DOPAC) contents in rat striatum (n=63) at 1, 3, 7, or 14 days after 10 or 20 mg/kg METH (n=7 in each group). The control rats (n=7) were decapitated 1 day after vehicle (saline, 0.5 ml i.p.) treatment. After decapitation, the rat brains were rapidly removed, and the entire striatum (including dorsal striatum and NAc) in each rat was dissected and stored at −80 °C until assayed. We chose to use the whole striatum, rather than the dorsal striatum (caudate putamen) or the ventral striatum (NAc) because it is very difficult to precisely dissect these two striatal subregions ex vivo. The protocols for measuring brain tissue DA and DOPAC were as reported previously (Krasnova et al., 2000). The striatal tissue samples were weighed, ultrasonicated in 10% perchloric acid containing 10 ng/mg of the internal standard dihydroxybenzylamine and centrifuged at 25,000×g for 12 min. The analytical column was a Symmetry C18 3.5 μm (4.6×150.0 mm) (Waters Corporation, Milford, MA, USA) connected to a Coulochem III detector (ESA Biosciences, Inc.). The mobile phase consisted of 0.01 M sodium dihydrogen phosphate, 0.01 M citric acid, 2 mM sodium EDTA, 1 mM sodium octylsulfate, 10% methanol, pH 3.5 and was used at a flow rate of 0.8 ml/min and a temperature of 4 °C. EZChrome Elite software system from ESA Biosciences, Inc. was used for data collection and analysis. AUCs for DA and DOPAC peaks were measured and quantified with external standard curves.
[125I]RTI-121 autoradiography
Finally, to determine whether the present non-fatal doses of METH produced damage to striatal DA terminals, we used [125I]RTI-121 autoradiography to measure striatal DAT (Boja et al., 1995), a DA marker located predominantly on presynaptic DA terminals (Mengual and Pickel, 2004). The autoradiographic protocol was as previously reported (Hirata et al., 1996). In brief, at 1, 3 or 7 days after METH (10 or 20 mg/kg i.p., n=6 in each group), rat brains were rapidly removed after decapitation, frozen in isopentane on dry ice, and stored at −70 °C. Sections (30 μm) were cut at −20 °C and thaw-mounted on gelatin-coated glass slides. The sections were then incubated with 100,000 cpm/ml of [125I]RTI-121 (specific activity: 2200 Ci/mmol) at room temperature for 60 min in a buffer containing 137.0 mM NaCl, 2.7 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4 and 10.0 mM NaI. Specific DAT binding was determined in the presence of 10 μM GBR-12909. At the end of the incubation, the slides were washed twice with fresh buffer, dipped in ice cold distilled water and dried under a cool air stream. The slides were then apposed to radiosensitive films (Biomax MS, Eastman Kodak Company, New Haven, CT, USA) with a plastic standard (125I microscales, Amersham, Little Chalfont, Buckinghamshire, UK) for 3 days at 4 °C. After film development, images were scanned, and the images were analyzed using NIH Image v1.63.
Drugs
Cocaine HCl and (+)-METH HCl (METH) (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in physiological saline. [125I]RTI-121 (radioiodinated 3β-[4-(trimethylstannyl)phenyl]-tropan-2β-carboxylic acid isopropyl ester) was purchased from PerkinElmer (Boston, MA, USA). GBR-12909 {1-[2-[bis(4-fluorophenyl)me-thoxy]ethyl]-4-(3-phenylprophyl)piperazine dihydrochloride} was purchased from Research Biochemicals International (Natick, MA, USA). Other chemicals were purchased from Sigma-Aldrich.
Data analyses
All data are presented as means (±SEM). One-way analysis of variance (ANOVA) and/or two-way ANOVA with repeated measures over time were used to analyze the effects of METH or cocaine on behavior and neurochemistry. Individual group comparisons were carried out using the Student–Newman–Keuls method. The null hypothesis was rejected at P<0.05.
RESULTS
METH pretreatment potentiates cocaine self-administration under FR2 reinforcement
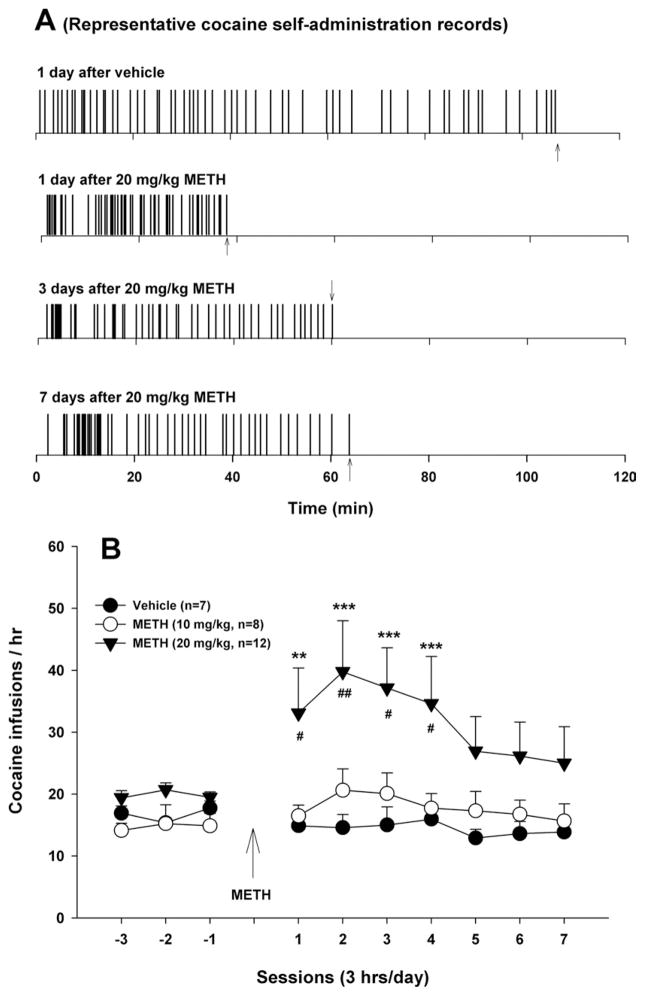
Fig. 1 A shows representative cocaine self-administration records. Fig. 1B shows mean cocaine self-administration rates, illustrating that 20 mg/kg METH produced a significant increase in cocaine self-administration, an effect that lasted as long as 7 days. Two-way ANOVA for repeated measures performed on the data shown in Fig. 1B revealed a significant METH treatment main effect (F2,24= 3.98, P<0.05), a significant time main effect (F9,18=2.86, P<0.005) and a significant treatment×time interaction (F24,216=1.85, P<0.05). Individual group comparisons revealed a significant increase in cocaine self-administration after 20 mg/kg (t=3.83, P<0.01), but not after 10 mg/kg (t=1.26, P>0.05) METH, when compared with the vehicle control group. Higher doses (40×1 or 10 mg/kg/2 h×4, i.p.) caused death of the majority of cocaine self-administration rats within 6–10 h after the METH administration.
Fig. 1.
Effects of METH (10, 20 mg/kg i.p.) on cocaine self-administration under FR2 reinforcement. (A) Representative cocaine self-administration records, illustrating that 20 mg/kg METH produced a significant increase in cocaine self-administration rates at 1, 3, and 7 days after METH. Each vertical line represents a cocaine infusion (0.5 mg/kg/infusion). The arrows (↑) indicate the last injection of the maximal 50 cocaine infusion. (B) Mean cocaine self-administration (infusion) rates after METH or vehicle. ** P<0.01, *** P<0.001, when compared with baselines before METH administration in each dose group. # P<0.05, ## P<0.01, when compared with the same time point in the vehicle control group.
METH pretreatment lowers break-point for cocaine self-administration under PR reinforcement
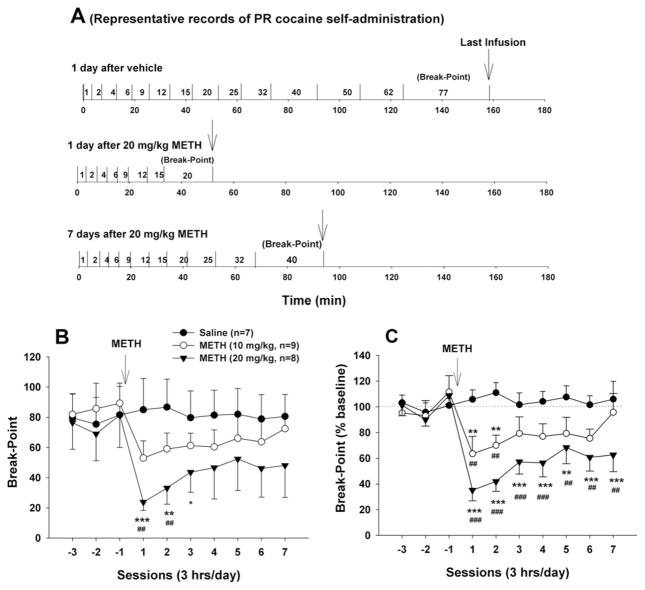
Fig. 2 A shows representative records of cocaine self-administration under PR reinforcement, illustrating that 20 mg/kg METH significantly lowered the break-point from 77 responses after vehicle to 20 responses at 1 day (24 h) post-METH, and then back to 40 responses at day 7 post-METH. Fig. 2B and 2C illustrate mean break-point levels and percent changes in break-point levels, respectively, before and after METH administration. Two-way ANOVA for repeated measures over time (sessions) for the raw break-point data (Fig. 2B) revealed significant main effects of treatment (F2,22=9.88, P<0.001) and time (F9,20=4.35, P<0.001) and a significant treatment×time interaction (F18,220=2.92, P<0.001). Mean individual group comparisons revealed a significant overall reduction in break-point (i.e. AUC) after 20 mg/kg METH (t=4.42, P<0.001), but not after 10 mg/kg (t=1.79, P>0.05) METH, when compared with the vehicle control group. Similarly, two-way ANOVA with repeated measures over time (sessions) for the data shown in Fig. 2C revealed significant treatment (F2,22=7.99, P<0.01) and time main effects (F9,20=4.07, P<0.001) and a significant treatment×time interaction (F18,220=3.45, P<0.001). Mean individual group comparisons revealed a significant overall reduction in break-point level (AUC data) after 10 mg/kg (t=2.94, P<0.05) or 20 mg/kg METH (t=3.96, P<0.001), when compared with the vehicle treatment group. Other individual time-point comparisons with the baseline in each treatment group are labeled directly on Fig. 2B and 2C for simplicity and clarity (* P<0.05, ** P<0.01, *** P<0.001, compared to baseline in each group).
Fig. 2.
Effects of METH (10, 20 mg/kg i.p.) on cocaine self-administration under PR reinforcement. (A) Representative records of an individual animal illustrating a reduction in the PR break-point for cocaine self-administration from 77 after vehicle (upper trace) to 20 at 1 day (24 h) after 20 mg/kg METH (middle trace) and then to 40 at 7 days after 20 mg/kg METH (lower trace). Each vertical line indicates a cocaine infusion (0.5 mg/kg/infusion). The number between the vertical lines indicates the work demand (progressively increased PR ratio, i.e. number of lever presses) for a subsequent cocaine infusion. (B) Time courses of mean break-point levels for cocaine self-administration after 10 or 20 mg/kg METH. (C) Time courses of mean break-points for cocaine self-administration as percent of baseline after 10 or 20 mg/kg METH. ** P<0.01, *** P<0.001, when compared to baselines before METH administration in each dose group. ## P<0.01, ### P<0.001, when compared with the same time point in the vehicle control group.
METH pretreatment decreases NAc DA responses to cocaine
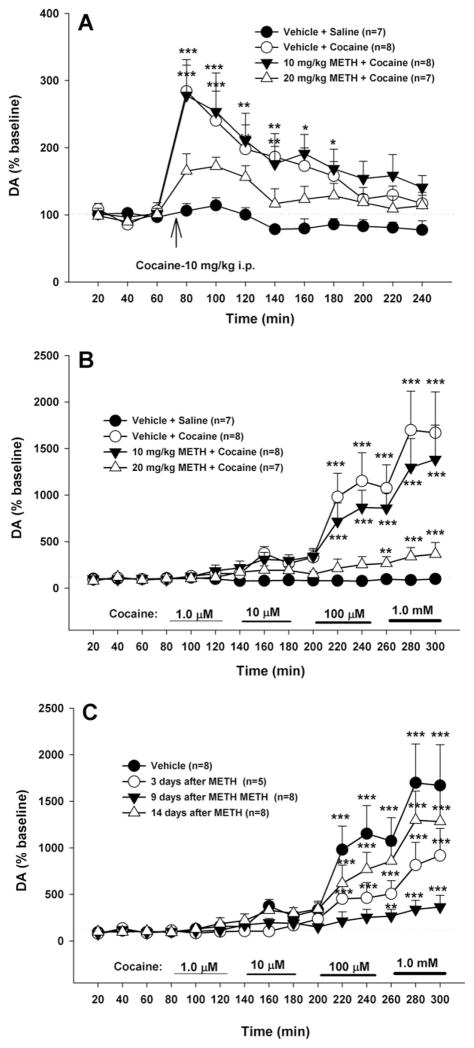
Fig. 3 A shows that METH pretreatment significantly attenuated systemic cocaine-induced increases in extracellular NAc DA. Two-way ANOVA with repeated measures revealed significant treatment (F3,26=12.10, P<0.001) and time main effects (F11,33=13.81, P<0.001), and a significant treatment×time interaction (F33,286=2.33, P<0.001). Post hoc individual group comparisons indicated a significant overall reduction in the DA response to cocaine (AUC data) after 20 mg/kg (t=3.81, P<0.01), but not after 10 mg/kg (t=1.74, P>0.05) METH, when compared with the vehicle pretreatment group. Fig. 3B shows the effects of local perfusion of gradually escalating concentrations of cocaine (1–1000 μM) into the NAc on extracellular DA in the NAc in control and METH-treated rats measured 9 days after METH. Two-way ANOVA with repeated measurements over time (or cocaine dose) revealed a statistically significant treatment (i.e. different doses of METH) main effect (F3,27=7.45, P<0.001), time main effect (F14,378=26.58, P<0.001), and treatment×time interaction (F42,378=6.77, P<0.001). Post hoc individual group comparisons revealed a significant overall reduction in cocaine-enhanced NAc DA (AUC data) 9 days after 20 mg/kg (t=3.23, P<0.05), but not 10 mg/kg (t=0.53, P>0.05) METH pre-treatment. Fig. 3C shows the effects of local perfusion of cocaine into the NAc on extracellular DA in rats 3, 9 and 14 days after 20 mg/kg METH pretreatment. Two-way ANOVA for repeated measurements over time (isomorphic with local cocaine doses) revealed a statistically significant treatment main effect (F3,21=5.56, P<0.001). Individual group comparisons indicated a significant reduction in DA response to cocaine (AUC data) at 3 days (t=2.98, P<0.01), 9 days (t=3.66, P<0.001), but not 14 days (t=0.31, P>0.05) after METH. Other individual time-point comparisons with baseline in each treatment group are labeled directly on Fig. 3 for simplicity.
Fig. 3.
Effects of METH (10, 20 mg/kg i.p.) on cocaine-induced increases in extracellular NAc DA. (A) The effect of METH on systemic cocaine-enhanced extracellular DA measured 7 days after METH; (B) the effect of different doses of METH on intra-NAc cocaine-enhanced extracellular DA 9 days after METH. (C) The effects over time (3, 9, 14 days) of 20 mg/kg METH on intra-NAc cocaine-enhanced DA. * P<0.05, ** P<0.01, *** P<0.001, when compared with baselines before METH administration in each dose group.
Acute METH causes hyperthermia and hypolocomotion
Fig. 4 shows that the present doses of METH caused significant and dose-dependent hyperthermia and significant hypolocomotion measured immediately after METH. Two-way ANOVA with repeated measures over time for the data shown in Fig. 4A reveals a statistically significant METH treatment main effect (F2,19=6.35, P<0.001) and a significant time main effect (F6,114=13.76, P<0.001). Individual group comparisons indicate a statistically significant increase in body temperature over 6 h after 10 or 20 mg/kg METH administration. Similarly, two-way ANOVA with repeated measures over time for the data shown in Fig. 4B reveals a statistically significant METH treatment main effect (F(2,20)=5.73, P<0.001) and a significant time main effect (F(23,460)=12.51, P<0.01). Individual group comparisons indicate a statistically significant decrease in locomotion after 10 or 20 mg/kg METH administration, compared with the vehicle control group.
Fig. 4.
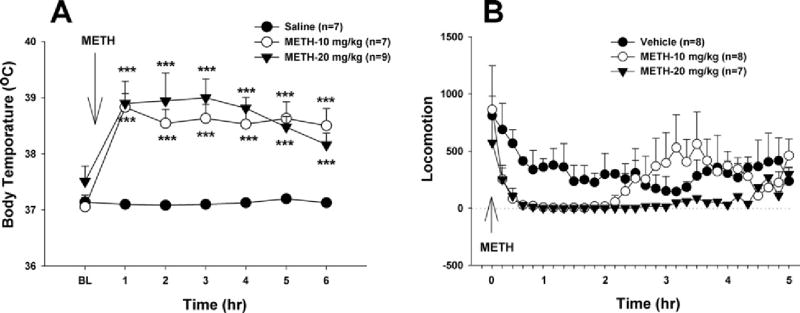
Effects of acute METH (10, 20 mg/kg i.p.) on deep body temperature (A) and locomotion (B). *** P<0.001, when compared with each time point in the vehicle control group.
Acute METH causes robust NAc DA release
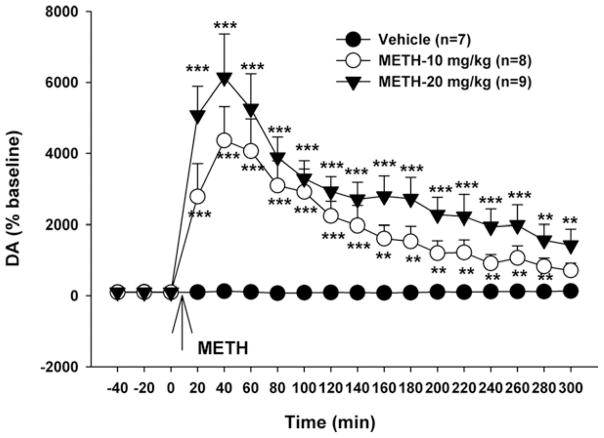
To determine whether the reduction in NAc DA response to cocaine was due to METH-induced DA release with consequent depletion of terminal DA, we measured acute METH-induced changes in extracellular NAc DA and subsequent changes in striatal DA and DOPAC contents. Fig. 5 shows that acute METH caused robust and dose-dependent increases (4000%–6000% of baseline) in extracellular NAc DA. Two-way ANOVA for repeated measures over time revealed significant treatment (F2,21=14.59, P<0.001) and time (F17,34=32.83, P<0.001) main effects. Individual group comparisons revealed a significant overall increase in extracellular DA after 10 mg/kg (t=3.38, P<0.01) or 20 mg/kg (t=5.35, P<0.001) METH, when compared to the vehicle treatment group at all post-METH time points.
Fig. 5.
Effects of METH (10, 20 mg/kg i.p.) on extracellular NAc DA. ** P<0.01, *** P<0.001, when compared to baseline.
Fig. 6 shows the locations of the microdialysis probes used for the data shown in Figs. 3 and 5, demonstrating that the active membrane portions of the probes were located in the NAc of the striatum.
Fig. 6.
Schematic reconstructions of positions of active microdialysis membranes within the NAc. Dialysis membranes tended to span the length of the core and shell compartments of the NAc.
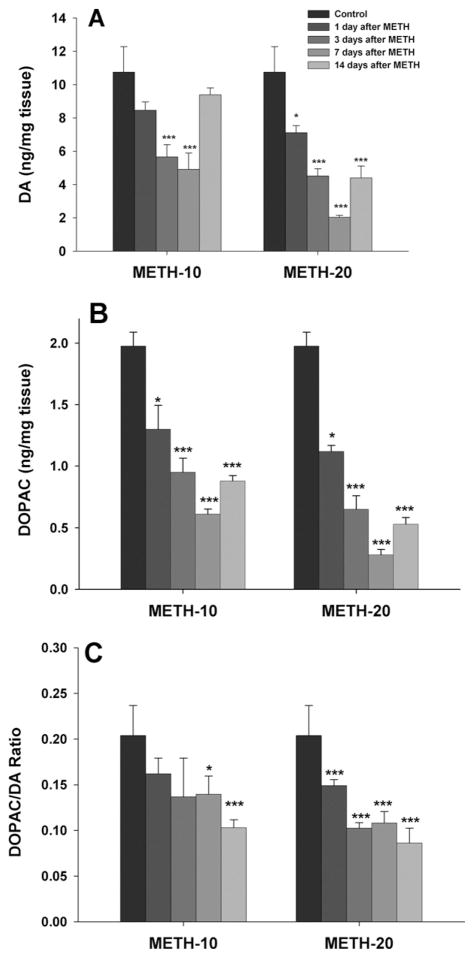
METH decreases striatal DA and DOPAC contents as well as DA turnover
Fig. 7 shows that METH caused time- and dose-dependent decreases in whole striatal DA (Fig. 7A) and DOPAC (Fig. 7B) contents, as well as in the DOPAC/DA ratio (Fig. 7C). The decreases in DA and DOPAC were observed on day 1, reached the lowest point on day 7, and then partially recovered by day 14 after the METH injections. One-way ANOVA for the DA data shown in Fig. 7A revealed a statistically significant reduction in striatal DA after 10 mg/kg (F4,30=9.29, P<0.001) or 20 mg/kg (F4,30=18.72, P<0.001) METH. Post hoc analyses revealed a significant reduction in striatal DA at 3 and 7 days after 10 mg/kg METH, and at all time points after 20 mg/kg METH (Fig. 7A). Similarly, DOPAC contents after 10 mg/kg (F4,30=15.62, P<0.001) or 20 mg/kg (F4,30=29.25, P<0.001) METH were substantially decreased at all post-METH time points (Fig. 7B). In addition, METH produced a significant reduction in DA turnover (DOPAC/DA ratio) at 7 and 14 days after 10 mg/kg METH (F4,30=6.42, P<0.001), and at all time points after 20 mg/kg METH (F4,30=7.26, P<0.001) (Fig. 7C).
Fig. 7.
Effects of METH (10, 20 mg/kg i.p.) on striatal tissue DA (A) and DOPAC (B) contents, as well as DOPAC/DA ratio (C) over time. * P<0.05, ** P<0.01, *** P<0.001, when compared with the vehicle control group.
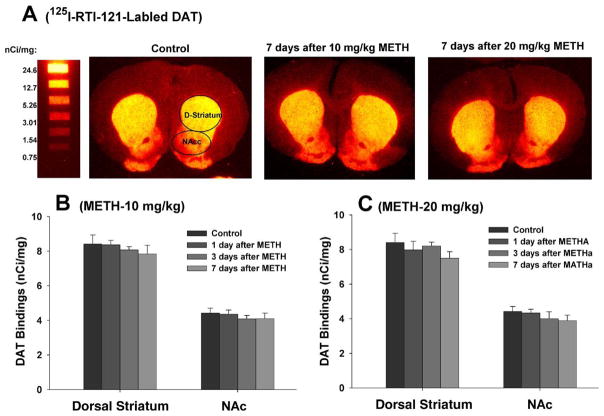
METH fails to alter striatal DAT levels
Fig. 8 shows that METH did not significantly alter DAT bindings in either the dorsal or ventral (NAc) striatum after 10 mg/kg (Fig. 8B: dorsal striatum, F3,14=0.10, P=NS; NAc: F3,14=0.44, P=NS) or 20 mg/kg (Fig. 8C: dorsal striatum, F3,14=0.98, P=NS; NAc, F3,14=1.02, P=NS) METH.
Fig. 8.
Effects of METH (10, 20 mg/kg i.p.) on striatal DAT levels. (A) Representative [125I]RTI-121 autoradiographic images, illustrating striatal DAT density before and 7 days after 10 or 20 mg/kg METH. (B) Averaged effects of 10 mg/kg METH on striatal DAT density over time in the dorsal and ventral (NAc) striatum. (C) Averaged effects of 20 mg/kg METH on striatal DAT density over time in the dorsal striatum and ventral NAc.
DISCUSSION
The major findings of the present study are (1) single high doses of METH (10–20 mg/kg) dose-dependently increased cocaine self-administration under FR2 reinforcement, while higher single or multiple doses caused high mortality among cocaine self-administration rats; (2) the same doses of METH dose-dependently lowered break-point levels for cocaine self-administration under PR reinforcement, suggesting that the increased cocaine self-administration under FR2 reinforcement could be a compensatory response to reduced cocaine reward after METH; (3) the same doses of METH also produced a dose-dependent reduction both in NAc DA response to acute cocaine and in striatal DA/DOPAC contents, suggesting a DA-dependent mechanism involved in METH-enhanced cocaine self-administration; (4) METH did not produce a significant reduction in striatal DAT levels, suggesting that the present doses of METH predominantly cause DA depletion or hypo-function, rather than severe damage to striatal DA terminals; and finally, (5) some differences in time courses (or pattern) between the changes in cocaine self-administration and the changes in striatal DA suggest that a non-DA mechanism may be also involved in post-METH alterations in cocaine self-administration.
Previous studies have shown that repeated high doses of METH significantly alter the rewarding (as assessed by CPP) and psychomotor-stimulating effects of METH as described in the introduction. However, little is known about whether high doses of METH alter drug-taking or drug-seeking behavior. In the present study, we found that 10 mg/kg METH modestly (not statistically significant, when compared to the pre-METH baseline or the vehicle control group) enhanced, 20 mg/kg METH robustly increased FR2 cocaine self-administration, while higher doses (40 mg/kg, or 10 mg/kg/2 h×4) of METH caused a high rate of mortality among cocaine self-administration rats. Since the same single high doses of METH also caused severe hyperthermia, sweating, extreme difficulty in locomotion and breathing, as well as a significant reduction in striatal DA contents and DA response to cocaine (see discussion below), it is suggested that the present doses of METH are neurotoxic in rat brain, and that this caused augmented cocaine-taking behavior. As noted, the present METH doses (10, 20 mg/kg i.p.) are much lower than other METH dose regimens (10 mg/kg/2 h×4 or 20–100 mg/kg×1, s.c.) that are commonly used in studying METH DA neurotoxicity (Cadet et al., 2003, 2007; Riddle et al., 2006). However, these higher METH dosing regimens caused high mortality among cocaine self-administration rats in the present study, and are therefore of no use for studying subsequent drug-seeking or drug-taking behavior. The presently-observed high mortality seen with the very high METH dosing regimens could be related to differences in species (mice versus rats), age or body weight of rats (2 weeks–2 months, 100–250 g in previous studies versus 3–4 months, 400–600 g in the present study), drug use history (naive rats in previous studies versus cocaine self-administration rats), METH delivery route (s.c. versus i.p.), or observation time post-METH (1–24 h in previous studies versus 1–14 days in the present study). This is partly supported by a finding that aging rats display increased susceptibility to METH-induced DA neurotoxicity (Imam and Ali, 2001).
Previous studies have shown that bilateral 6-OHDA lesions of midbrain DA cells result in increased amphetamine self-administration (Deminière et al., 1984), while 6-OHDA lesions of NAc DA terminals produce extinction of cocaine self-administration (Roberts et al., 1980; Caine and Koob, 1994; Pettit et al., 1984). This is congruent with the general finding that effects produced by intracerebral 6-OHDA are heterogeneous and critically influenced by site of infusion (Simola et al., 2007). In addition, the present high doses of METH predominantly caused depletion of striatal DA, rather than severe degeneration of striatal DA terminals (see discussion below). Thus, in the present experiments, cocaine would still be able to bind to DAT in structurally intact terminals to initiate cocaine’s rewarding effects, whereas in 6-OHDA-treated animals suffering from DA terminal destruction, cocaine would not be able to enhance extracellular DA and therefore be unable to produce rewarding effects.
It is well documented that the dose-related effects of cocaine self-administration display an inverted “U” shaped curve (Yokel and Wise, 1975; Belin et al., 2008). That is, cocaine self-administration rate exhibits a dose-dependent increase at low doses (ascending slope), but a dose-dependent reduction at high doses (descending slope). The cocaine self-administration rate on the descending slope has been thought to be a function of cocaine reward strength, i.e. low cocaine doses produce low reward which in turn results in a compensatory increase in cocaine self-administration, with the converse occurring at high cocaine doses (Yokel and Wise, 1975; Xi et al., 2005). Since the present cocaine dose (0.5 mg/kg/infusion) was located on the high dose end of the descending slope (Xi et al., 2005), we interpret the METH-induced increase in cocaine self-administration in the present study to be a compensatory response to a reduction in cocaine reward after METH. This interpretation is further supported by our finding that METH dose-dependently lowered the break-point for PR cocaine self-administration. Since PR break-point is highly dose-dependent, the PR self-administration procedure is considered a sensitive measure of drug reward efficacy (Arnold and Roberts, 1997; Stafford et al., 1998). Together, our cocaine self-administration findings under both FR and PR reinforcement support a conclusion that METH dose-dependently reduces cocaine’s rewarding efficacy. This conclusion is further supported by our findings that lower doses of DA receptor antagonists produce a compensatory increase in cocaine self-administration under low FR reinforcement, but a decrease in break-point levels for cocaine self-administration under PR reinforcement. However, higher doses of DA receptor antagonists produce a robust reduction in both PR break-point and cessation of cocaine self-administration under low FR reinforcement (Yokel and Wise, 1975; Xi et al., 2005, 2007; Peng et al., 2008).
Given the important role of striatal DA in drug reward (Wise, 2005), we hypothesize that attenuated striatal DA response to cocaine may underlie the attenuation of cocaine reward observed in this study. Indeed, the present METH doses caused a dose-dependent reduction in NAc DA response to acute cocaine, an effect that lasted 3–9 days and recovered after 2 weeks. This is contrary to the enhanced striatal DA response to acute cocaine reported after multiple low doses of METH (Kazahaya et al., 1989; Davidson et al., 2005). This could be related to the fact that repeated low METH doses may cause neuroadaptations predominantly, rather than neurotoxicity, as noted above (introduction). In contrast, the present doses of METH produced robust and dose-dependent increases in extracellular DA, and subsequent long-lasting decreases in striatal DA, DOPAC, and DA turnover. These findings suggest that the presently-observed METH-induced reduction in striatal DA content may be the result of excessive DA release and depression of DA synthesis. A similarly robust increase in extracellular DA (Pereira et al., 2002) and subsequent decrease in striatal DA content were reported previously after a single high METH dose (15–40 mg/kg) (Peat et al., 1983; Fukumura et al., 1998; Cappon et al., 2000).
In contrast, we did not see a significant alteration in striatal DAT binding 1–7 days after METH. This is consistent with a recent report that a single injection of 25 mg/kg METH failed to decrease DA fiber density in limbic brain areas of adult gerbils (Brummelte et al., 2006). These data suggest that the present non-fatal METH doses may not produce severe damage to striatal DA terminals. This is consistent with previous reports that much higher single doses (30–100 mg/kg) or repeated high METH doses (10–15 mg/kg/2 h×4) are required to produce significant striatal DA terminal damage, as assessed by reduced DAT binding or DA uptake, DAT internalization and/or high molecular weight DAT complex formation (Fukumura et al., 1998; Cappon et al., 2000; Baucum et al., 2004; Zhu et al., 2006; Cen et al., 2008). Taken together, the present findings, for the first time, demonstrate that non-fatal high doses of METH cause striatal DA depletion or hypofunction, rather than striatal DA terminal damage. This may, in part, underlie the reduction in striatal DA response to cocaine and in cocaine reward, and the compensatory increases in cocaine-taking behavior.
In addition to lesioning striatal DA terminals, high doses of METH are also neurotoxic to brain 5-HT neuronal somatodendritic regions and/or axonal terminals (Cadet et al., 2003, 2007; Quinton and Yamamoto, 2006). Selective destruction of brain 5-HT neurons by 5,7-dihydroxytryptamine or 3,4-methylenedioxymethamphetamine (MDMA) also produces an increase in cocaine self-administration (Fletcher et al., 2001) and cocaine-induced CPP (Fletcher et al., 1999; Horan et al., 2000), suggesting that a serotoninergic mechanism could also be involved in the METH-induced increases in the cocaine self-administration observed in the present study. Clearly, more study is required to clarify this issue.
CONCLUSION
In conclusion, the present study, for the first time, demonstrates that a single high dose of METH causes significant long-lasting increases in cocaine self-administration and also a long lasting reduction in striatal DA response to cocaine, suggesting that a DA-dependent mechanism may underlie the increase in cocaine-taking behavior in rats. Provided that the present doses of METH may mimic METH high dose or overdose incidents in human psycho-stimulant users (Fairbairn et al., 2008) and that other METH dose regimens also produce significant DA neurotoxicity in psychostimulant users (Cadet et al., 2003, 2007), the present finding suggests that METH-induced DA depletion or neurotoxicity may contribute to augmentation of subsequent drug-seeking and drug-taking behavior.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH).
Abbreviations
- ANOVA
analysis of variance
- AUC
area under curve
- CPP
conditioned place preference
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- EC
electrochemical
- FR1
fixed ratio 1
- FR2
fixed ratio 2
- HPLC
high performance liquid chromatography
- METH
methamphetamine
- NAc
nucleus accumbens
- PR
progressive ratio
References
- Arnold JM, Roberts DCS. A critique of fixed ratio and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja JW, Cadet JL, Kopajtic TA, Lever J, Seltzman HH, Wyrick CD, Lewin AH, Abraham P, Carroll FI. Selective labeling of the dopamine transporter by the high affinity ligand 3β-(4-[125I]iodophenyl)tropane-2β-carboxylic acid isopropyl ester. Mol Pharmacol. 1995;47:779–786. [PubMed] [Google Scholar]
- Brummelte S, Grund T, Czok A, Teuchert-Noodt G, Neddens J. Long-term effects of a single adult methamphetamine challenge: minor impact on dopamine fibre density in limbic brain areas of gerbils. Behav Brain Funct. 2006;2:12–22. doi: 10.1186/1744-9081-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotoxicol Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav. 1994;61:213–221. doi: 10.1901/jeab.1994.61-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphet-amine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Cen X, Nitta A, Ibi D, Zhao Y, Niwa M, Taguchi K, Hamada M, Ito Y, Ito Y, Wang L, Nabeshima T. Identification of piccolo as a regulator of behavioral plasticity and dopamine transporter internalization. Mol Psychiatry. 2008;13:451–463. doi: 10.1038/sj.mp.4002132. [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- Davidson C, Lee TH, Ellinwood EH. Acute and chronic continuous methamphetamine have different long-term behavioral and neurochemical consequences. Neurochem Int. 2005;46:189–203. doi: 10.1016/j.neuint.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Deminière JM, Simon H, Herman JP, Le Moal M. 6-Hydroxydo-pamine lesion of the dopamine mesocorticolimbic cell bodies increases (+)-amphetamine self-administration. Psychopharmacology. 1984;83:281–284. doi: 10.1007/BF00464795. [DOI] [PubMed] [Google Scholar]
- Fairbairn N, Wood E, Stoltz JA, Li K, Montaner J, Kerr T. Crystal methamphetamine use associated with non-fatal overdose among a cohort of injection drug users in Vancouver. Public Health. 2008;122:70–78. doi: 10.1016/j.puhe.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Chambers JW. Selective destruction of brain serotonin neurons by 5,7-dihydroxytryptamine increases responding for a conditioned reward. Psychopharmacology. 1999;147:291–299. doi: 10.1007/s002130051170. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Robinson SR, Slippoy DL. Pre-exposure to (±)3,4-methylenedioxy-methamphetamine (MDMA) facilitates acquisition of intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2001;25:195–203. doi: 10.1016/S0893-133X(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998;806:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- Gehrke BJ, Harrod SB, Cass WA, Bardo MT. The effect of neurotoxic doses of methamphetamine on methamphetamine-conditioned place preference in rats. Psychopharmacology. 2003;166:249–257. doi: 10.1007/s00213-002-1318-5. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714:95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Ashby CR., Jr Enhancement of conditioned place preference response to cocaine in rats following sub-chronic administration of 3,4-methylenedioxymethamphetamine (MDMA) Synapse. 2000;35:160–162. doi: 10.1002/(SICI)1098-2396(200002)35:2<160::AID-SYN9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Aging increases the susceptibility to methamphetamine-induced dopaminergic neurotoxicity in rats: correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001;78:952–959. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity in mice: long-lasting sensitization to the locomotor stimulation and desensitization to the rewarding effects of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1177–1183. doi: 10.1016/s0278-5846(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Kazahaya Y, Akimoto K, Otsuki S. Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biol Psychiatry. 1989;25:903–912. doi: 10.1016/0006-3223(89)90270-9. [DOI] [PubMed] [Google Scholar]
- Kita T, Takahashi M, Wagner GC, Kubo K, Nakashima T. Methamphetamine-induced changes in activity and water intake during light and dark cycles in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1185–1196. doi: 10.1016/s0278-5846(98)00069-4. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Bychkov ER, Lioudyno VI, Zubareva OE, Dambinova SA. Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats. Neuroscience. 2000;95:113–117. doi: 10.1016/s0306-4522(99)00400-5. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual E, Pickel VM. Regional and subcellular compartmentation of the dopamine transporter and tyrosine hydroxylase in the rat ventral pallidum. J Comp Neurol. 2004;468:395–409. doi: 10.1002/cne.10979. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peat MA, Warren PF, Gibb JW. Effects of a single dose of methamphetamine and iprindole on the serotonergic and dopaminergic system of the rat brain. J Pharmacol Exp Ther. 1983;225:126–131. [PubMed] [Google Scholar]
- Peng X-Q, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaër E, Muńoz C, Gardner EL, Xi Z-X. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2008;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FC, Imam SZ, Gough B, Newport GD, Ribeiro CF, Slikker W, Jr, Macedo TR, Ali SF. Acute changes in dopamine release and turnover in rat caudate nucleus following a single dose of methamphetamine. J Neural Transm. 2002;109:1151–1158. doi: 10.1007/s00702-002-0754-z. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopa-mine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotoxicol Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- Stafford D, Lesage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost–variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Yang Z, Li S-J, Li X, Dillon C, Peng X-Q, Spiller K, Gardner EL. Levotetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006;27:131–136. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]