Abstract
The purpose of this investigation was to determine if creatine supplementation assisted with reducing the amount of exercise induced muscle damage and if creatine supplementation aided in recovery from exercise induced muscle damage. Two groups of subjects (group 1 = creatine; group 2 = placebo) participated in an eccentric exercise protocol following 7 and 30 days of creatine or placebo supplementation (20 g.d-1 for 7 d followed by 6g.d-1 for 23 d = 30 d). Prior to the supplementation period, measurements were obtained for maximal dynamic strength, maximal isometric force, knee range of motion, muscle soreness, and serum levels of creatine kinase (CK) and lactate dehydrogenase (LDH). Following 7 days of creatine supplementation, on day 8, subjects began consuming 6 g.d-1 of creatine for 23 days. Additionally on days 8 and 31, subjects performed an eccentric exercise protocol using the knee extensors to induce muscle damage. Indirect markers of muscle damage, including maximal isometric force, knee range of motion, muscle soreness, and serum levels of CK and LDH, were collected at 12, 24, and 48 hours following each exercise bout. The results indicated that acute bouts of creatine have no effect on indirect markers of muscle damage for the acute (7 days) bout. However, maximal isometric force was greater for the creatine group versus placebo for the chronic (30 days) bout. This suggests that the ergogenic effect of creatine following 30 days of supplementation may have a positive impact on exercise induced muscle damage.
Key points.
Eccentric muscle actions highly associated with exercise induced muscle damage.
Creatine supplementation has ergogenic effect to increase protein synthesis.
Creatine supplementation does not attenuate exercise induced muscle damage with short term supplementation (7 days).
Increased maximal isometric force seen with creatine supplementation after 30 days following exercise induced muscle damage.
Ergogenic effect of creatine supplementation may contribute to reduced exercise induced muscle damage.
Key words: Soreness, isometric force, eccentric.
Introduction
Creatine supplementation has been widely studied as an ergogenic aid relative to performance in high-intensity activities (Burke et al., 1996; Casey et al., 1996; Greenhaff et al., 1993; Harris et al., 1992; Kreider, et al., 1998; Odland et al., 1997; Terjung, et al., 2000; Volek, et al., 1999; Willoughby and Rosene, 2001). Others have examined clinical aspects of creatine supplementation such as potential adverse effects in muscle injury (Krieder, et al., 1998), thermoregulation (Kern et al., 2001; Rosene et al., 2004; Volek, et al., 2001), and renal complications (Boswell et al., 2003). These studies have led investigators to examine potential clinical benefits of creatine supplementation such as with recovery from exercise induced muscle damage (Rawson et al., 2001).
Exercise-induced muscle damage has been shown to result from strenuous, unaccustomed exercise. The damage that occurs is primarily due to eccentric muscle actions and affects the structural composition of muscle leading to impairments in performance. These structural changes in muscle include mechanical factors, alterations in calcium homeostasis due to sarcoplasmic reticulum disruption, and the inflammatory response (Clarkson and Sayers, 1999).
As part of the repair process, protein synthesis is enhanced via several mechanisms including the stress proteins which have been found to be up-regulated following a bout of eccentric exercise (Willoughby et al., 2003). Creatine supplementation has also been shown to impact protein synthesis resulting in alterations in skeletal muscle composition. Twelve weeks of creatine supplementation (25g.d-1 for 1 wk; followed by 5g.d-1 for 12 wks) resulted in increased muscle fiber cross sectional area when compared to placebo for type I (35% vs 11%), type IIa (36% vs 15%), and type IIb fibers (35% vs 6%) (Volek et al., 1999). Additionally, greater increases were found for type I, type IIa, and type IIx MHC mRNA expression for creatine supplemented individuals compared to placebo. For these changes in MHC mRNA expression, subjects were supplemented with 6g.d-1 for 90 days with no loading phase (Willoughby and Rosene, 2001).
Rawson et al., 2001 reported that 5 days of creatine supplementation (20g.d-1) did not reduce indirect markers of muscle damage or reduce recovery time following eccentric exercise. Increased muscular strain as a result of the eccentric exercise was believed to have caused structural damage within the muscle thereby limiting creatine’s effects on cellular membrane stability. As such, sarcolemmal and sarcoplasmic reticulum damage may have been too extensive for a 5-day supplementation protocol to have any impact (Rawson et al., 2001).
The symptoms of delayed onset muscle soreness which include strength loss, pain, muscle tenderness, stiffness, and swelling, have been reported to occur within 48 hours of damage and last beyond 5 days. Degradation of contractile proteins appears to contribute to decreases in muscular force 5-28 days post eccentric exercise (Ingalls et al., 1998). Therefore, reductions in force output immediately following a bout of eccentric exercise and up to 5 days may be related to the inflammatory response associated with cellular membrane damage (Connelly, Sayers et al., 2003). Any effects of creatine supplementation impacting myofibrillar protein content would not be evident until between the 5 and 28 day time period.
Athletes have also anecdotally reported decreased fatigue, decreased muscle soreness, and decreased recovery time while supplementing with creatine. With evidence to support increased myofibrillar protein synthesis, muscular hypertrophy, and muscular strength with creatine supplementation it is possible that creatine supplementation will have positive effects on indirect markers of exercise-induced muscle damage (Willoughby and Rosene, 2001; Volek, et al., 1999). Based on previous reports of enhanced creatine uptake with exercise and positive effects on skeletal muscle composition, (Burke et al., 1996; Casey, et al., 1996; Greenhaff, et al., 1993; Harris et al., 1993; Kreider, et al., 1998; Odland, et al., 1997; Terjung, et al., 2000; Volek, et al., 1999; Willoughby and Rosene, 2001) longer supplementation protocols may also be helpful in decreasing exercise-induced muscle damage. Therefore the purpose of this investigation was to determine the effects of 7 and 30 days of creatine versus placebo supplementation on indirect markers of muscle damage following a bout of eccentric exercise.
Methods
Subject
Twenty males, were randomly assigned to a creatine (CR) or placebo (P) group (CR = 10; P = 10). For the CR group subjects were 21.6 ± 1.65 yrs, 1.77 ± 0.07 m, 84.0 ± 8.3 kg, and 12.95 ± 4.76% body fat. For the P group subjects were 21.60 ± 0.70 yrs, 1.753 ± 0.06 m, 84.0 ± 13.4 kg, and 11.75 ± 4.72% body fat. The subjects were physically active (consistent physical activity for 6 months prior to beginning the study) and free of creatine supplementation for at least 60 days prior to beginning the study. All subjects were required to read and complete a medical history form to ensure that eligibility criteria were met. All subjects were informed of the purpose and possible risks involved in the investigation and were required to read and sign an informed consent prior to participation. All procedures were approved by the University Institutional Review Board.
Blood sampling
For serum creatine kinase (CK) and lactate dehydrogenase (LDH), blood was drawn from the antecubital vein into a 10 mL collection tube via a Vacutainer apparatus. The blood samples stood for 10 min, were centrifuged to extract the serum and frozen at -20°C for later analysis. Blood samples were obtained prior to each eccentric exercise bout and also at 12, 24, and 48 hours post-exercise. Serum CK and LDH were analyzed via reflectance spectrophotometry with the dry-chemistry technique utilizing the DT60 II Chemistry System (Orthoclinical Diagnostics, Raritan, NJ) at 680 nm (CK) and 340 nm (LDH) following manufacture’s guidelines.
Muscle strength assessment
Maximal dynamic strength (MDS) of the dominant (acute) and non-dominant (longer-term) thigh was assessed using a Body Masters (Rayne, LA) seated leg extension machine via a standard one repetition maximum (1-RM) test prior to the eccentric exercise protocol. The concentric 1-RM measure was used to determine the eccentric load of 150% of the concentric 1-RM. A maximum of four sets was used to determine the 1-RM in order to counteract muscle fatigue (Willoughby and Pelsue, 1998; Willoughby and Rosene, 2001).
Maximal isometric force (MIF) was determined using the Biodex System 2 (Shirley, NY) isokinetic dynamometer. Subjects were seated with the leg positioned at approximately 45° of knee flexion. The Biodex was set at 0° per second or in isometric mode and the subject performed 3 maximal isometric contractions with 1 minute rest between trials. The average score was used as the criterion.
Knee range of motion and muscle soreness
Knee range of motion (KROM) was assessed using standard goniometric techniques with the subject in the prone position (Norkin and White, 1995). The evaluator passively moved the involved knee into the flexed position (heel moving towards the buttocks) while the hips were maintained in neutral. The position of the knee at which the subject attempted to lift the hips off the table or indicated maximal knee motion was used as the measurement.
Perceived muscle soreness (SOR) was assessed by each subject placing a mark along a 25.4 cm continuum, with 0 indicating no muscle soreness and 25.4 cm indicating very, very sore (Willoughby et al., 2003)
Testing protocol
Two groups of subjects (group 1 = creatine; group 2 = placebo) participated in an eccentric exercise protocol following 7 and 30 days of creatine or placebo supplementation. Prior to the supplementation period, baseline measures were obtained for MDS, MIF, KROM, SOR, and serum levels of CK and LDH.
Following the 7 days of creatine supplementation (20 g.d-1), on day 8 (acute effect), subjects began consuming 6 g.d-1 of creatine for 29 days. Additionally on day 8, subjects performed a knee extension eccentric exercise protocol to induce muscle damage of the knee extensors. The eccentric exercise protocol consisted of a warm- up bout of 1 set of 10 repetitions at 50% of the previously determined concentric 1-RM. Subjects then performed 7 sets of 10 repetitions at 150% of the concentric 1-RM using eccentric contractions, with each repetition lasting 2-3 seconds and 15 seconds rest between each repetition. A 3 min rest was employed between each set (Willoughby et al., 2003). Subjects were required to refrain from strenuous exercise 3 days prior to the exercise bout. Indirect markers of muscle damage including MIF, KROM, SOR were assessed at 12 hours post-eccentric exercise and every 24 hours thereafter for 5 days (Rawson et al., 2001). Blood samples were obtained prior to each eccentric exercise bout and also at 12, 24, and 48 hours post-exercise. After 30 days of supplementation, on day 31 (longer-term effect), subjects repeated the knee extension eccentric exercise protocol on the non-dominant leg to counteract the repeat bout effect. To determine the correct eccentric load, the concentric 1-RM of the non-dominant leg was assessed on day 21 of the supplementation period, in addition to all other measures, following procedures previously described. Measurements for indirect markers of muscle damage on the non- dominant leg were repeated at 12 hours post-eccentric exercise and every 24 hours thereafter for 5 days (Rawson et al., 2001). Blood samples were obtained prior to each eccentric exercise bout and also at 12, 24, and 48 hours post-exercise. Subjects were instructed to consume a normal mixed diet throughout the duration of the study. During the 36 day study period, subjects completed four, 3-day dietary recalls for analysis of nutrient intake. Additionally, subjects were required to refrain from any additional strenuous activity (increasing exercise duration, intensity, beginning a new exercise regimen, etc). Normal daily activities and/or exercise were permitted.
Statistical analysis
Before analyses of the dependent variables, independent group t-tests were performed to ensure that the creatine and placebo groups were similar across diet. Analyses were preformed individually for the acute and chronic conditions. For both conditions, 2 X 7 ANOVAs with time as the repeated factor were computed to examine interactions or differences among the independent variables. The independent variables included the treatment groups (creatine or placebo) and time (pre to day 5). The dependent variables were MIF, KROM, and SOR. In addition, 2 X 4 ANOVAs with time as a repeated factor were computed for LDH and CK for both acute and chronic phases. The levels for time included pretest up to 48 hr. The alpha level was set at 0.05 and when post hoc analyses were performed, the Bonferroni adjustment was used to adjust for multiple analyses.
Results
The independent group t-tests for dietary intake of carbohydrate, protein and fat was not significantly different (p > 0.05) for the two groups. It was therefore determined that the groups were similar for dietary intake.
Acute condition: No significant (p > 0.05) difference or interaction was found for KROM. For MIF and SOR only significant (p < .05) time differences were found. With respect to MIF, 12 hr was significantly less than days 3, 4, and 5 (Figure 1). For SOR, pre test scores were significantly less than hr 12, 24 and 48; 12 hr was significantly greater than days 4 and 5. In addition, 48 hr was significantly greater than days 3, 4, and 5; and lastly, day 3 was significantly greater than days 4 and 5 (Figure 2). LDH and CK were not significantly different across time or group (p > 0.05).
Figure 1.
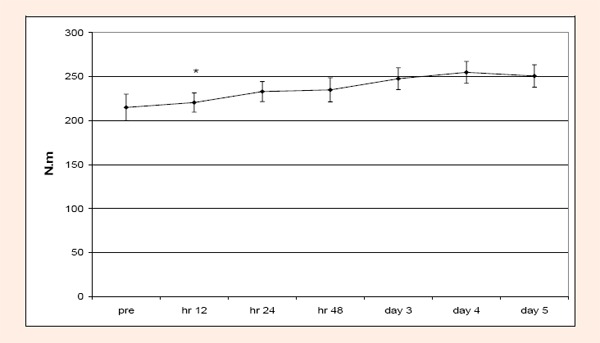
Changes over time for MIF during the acute condition. MIF for hour 12 was significantly less (*) than days 3, 4, and 5.
Figure 2.
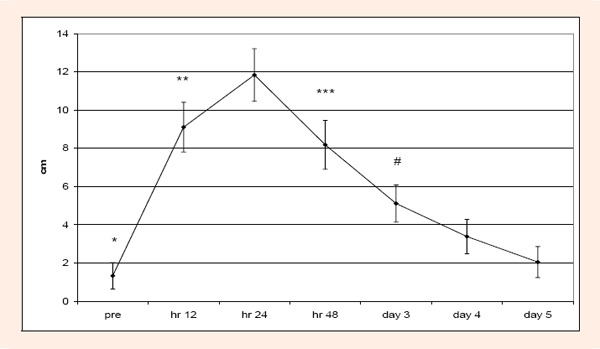
Changes over time for SOR during the acute condition. Pretest SOR scores were significantly less (*) than SOR scores at hours 12, 24, and 48. Hour 12 scores were significantly higher (**) than days 4 and 5. Hour 48 scores were significantly higher (***) than days 3, 4, and 5. Day 3 scores were significantly higher (#) than days 4 and 5.
Chronic condition: KROM, and LDH were not significantly (p > 0.05) different with respect for group or time. SOR was significantly (p < 0.05) different across time. Pre test scores were significantly less than hours 12, 24, and 48. Hour 24 was significantly greater than day 5; and hr 48 and day 3 were significantly greater than days 4 and 5 (Figure 3). CK was significant (p < 0.05) across time. Pre test scores were significantly less than hours 12, 24, and 48 (Figure 4).
Figure 3.
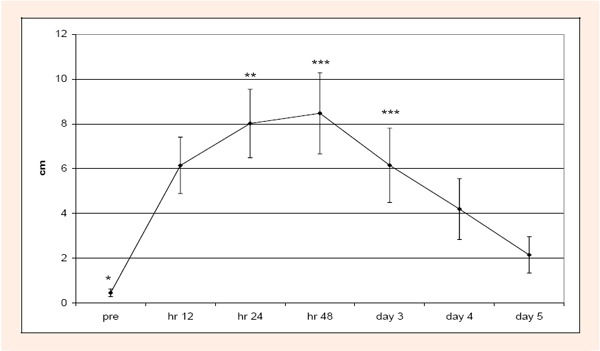
Changes over time for SOR scores during the chronic condition. Pretest scores were significantly less (*) than hours 12, 24, and 48. Hour 24 scores were significantly higher (**) than day 5. Hour 48 and day 3 scores were significantly higher (***) than days 4 and 5.
Figure 4.
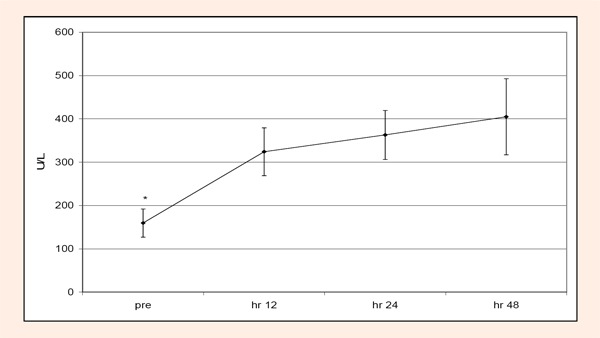
Changes in creatine kinase (CK) over time during the chronic condition. Pretest CK values were significantly less (*) than hours 12, 24, and 48.
For the chronic MIF data, one subject was eliminated for the analyses. Values for the subject were not considered within normal values for MIF. For MIF there was a significant (p < 0.05) time and treatment effect. For the time effect, MIF scores were significantly lower at 12 hours versus hours 24 and 48 and days 3, 4, and 5. Hour 24 MIF scores were significantly lower than days 3, 4, and 5. At 48 hours MIF scores were significantly greater than at 12 hours and days 4 and 5. On day 3 MIF scores were significantly greater than at 12 and 24 hours. On days 4 and 5 MIF scores were significantly greater than 12, 24, and 48 hours (Figure 5). For the treatment effect, MIF scores for the creatine group were significantly greater versus the placebo group (Figure 6).
Figure 5.
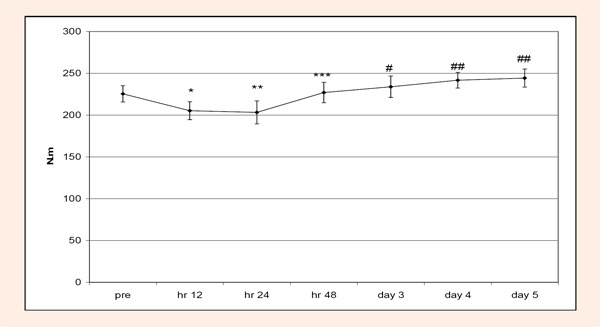
Changes in MIF scores during the chronic condition. Hour 12 scores were significantly lower (*) than hours 24, 48 and days 3, 4, and 5. Hour 24 scores were significantly lower (**) than days 3, 4, and 5. Hour 48 scores were significantly higher (***) than hour 12 and days 4 and 5. Day 3 scores were significantly higher (#) than at hours 12 and 24. Day 4 and 5 scores were significantly higher (##) than hours 12, 24, and 48.
Figure 6.
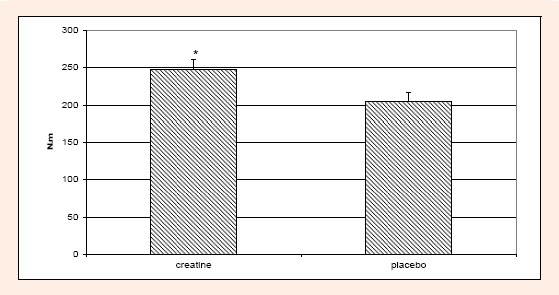
Difference in MIF scores between creatine and placebo groups for the chronic condition. The creatine group had significantly higher MIF scores (*) than the placebo group.
Discussion
This investigation examined the acute and longer-term (chronic) effects of creatine supplementation on exercise-induced muscle damage. Anecdotal reports have indicated that individuals supplementing with creatine have a decreased recovery time during and following exercise, subsequently these individuals report greater/more effective exercise sessions. The consequences of exercise-induced muscle damage particularly that of eccentric exercise, include a myriad of events that lead to reductions in muscle force, increased soreness, and impaired muscle function (Thompson et al., 2001). Therefore strategies utilized to reduce the negative effects of exercise-induced muscle damage would have great benefit to those wishing to maximize performance.
As a result of exercise- induced muscle damage, there is injury to the cell membrane which triggers the inflammatory response leading to the synthesis of prostaglandins and leukotrines (Connolly et al., 2003). Additionally, alterations in sarcolemmal and sarcoplasmic reticulum membranes are evident. This damage may result in increased intracellular calcium levels which may be associated with muscle degradation. As such, ingestion of exogenous creatine may provide protective effects via increased phosphocreatine synthesis which may aid in stabilizing the sarcolemmal membranes and thereby reducing the extent of damage (Rawson et al., 2001).
In the current investigation only MIF following the chronic condition resulted in creatine having greater MIF versus placebo. This suggests that the creatine supplementation may reduce the extent of muscle damage when supplementing for more than 30 days. However, since no other indices of muscle damage resulted in differences between creatine and placebo, it is plausible that since these subjects were active males, that an ergogenic effect of creatine supplementation may have occurred. Previous investigators have reported increases in muscle fiber size and molecular changes with 12 weeks of creatine supplementation (Volek et al., 1999; Willoughby and Rosene, 2001). These investigations incorporated a resistance training program that contributed to the resultant changes. In the present investigation subjects were not required to refrain from training, therefore there was the potential for similar ergogenic effects. The resultant muscle force differences in the chronic condition support an ergogenic benefit, while the results from the acute condition are similar to previous reports with short-term supplementation (Rawson et al., 2001).
Neural factors such as enhanced neural recruitment patterns, enhanced motor unit synchronization and increased excitability of the ?-motor neuron have been attributed to strength gains early in a training program, the first 6-8 weeks. In addition, there appear to be intramuscular structural adaptations, including muscular hypertrophy and fiber type conversion (type IIb converts to type IIa) that occurs during 6 weeks of training (Staron et al., 1994). Creatine supplementation has been found to enhance intramuscular adaptations to strength training both at the fiber and molecular level (Volek et al., 1999; Willoughby and Rosene, 2001).
In the present investigation, it is plausible to expect that increased MIF scores resulted in part due to neural adaptations. However, the creatine group exhibited a greater increase in MIF versus the placebo group in the chronic condition. Therefore an ergogenic benefit of creatine supplementation is the most plausible explanation for differences in MIF. Ingalls, Warren, and Armstrong (1998) reported that decreased muscle force following muscle damage is a result of proteolysis at days 14 and 28 post damage. Early decrements in force (within 5 days of damage) were not attributed to proteolysis. The MIF differences between Cr and P conditions at 30 days post damage may be explained by enhanced protein synthesis versus degradation, most likely within the MHC as previously reported (Ingalls et al., 1998; Willoughby and Rosene, 2001).
Previous research has shown positive effects of creatine supplementation on muscle protein synthesis (Willoughby and Rosene, 2001). In the event that there is increased protein synthesis, and therefore reduced proteolysis, then creatine supplementation may have a positive influence on performance when muscle damage occurs. Traditional supplementation protocols have been utilized to rapidly increase muscle creatine levels (Harris et al., 1992; Hultman et al., 1996) and then maintain these levels (Robinson, 2000). Therefore, the protocols have been divided into a loading and maintenance phase. The loading phase is incorporated to rapidly increase muscle creatine levels by as much as 20%.(Harris et al., 1992). The maintenance phase allows for these increased muscle creatine levels to remain for the duration of supplementation. Any positive effects of creatine on the attenuation of muscle damage may be found under such supplementation conditions.
Indices of muscle damage, other than MIF, did not differ between the creatine and placebo conditions. However, the resultant time effects were consistent with the progression of recovery from muscle damage. It has been shown that muscle creatine levels show increases in just 2 days (Vandenderghe et al. 1999) and that signs and symptoms of muscle damage present within 48 hours of the event (Connolly et al., 2003). In the present investigation under both acute and chronic conditions there was evidence of symptoms of muscle damage within the 48 hour period, particularly SOR. These findings are similar to Rawson et al., 2001 who reported symptoms of muscle damage however, no difference between creatine and placebo groups following 5 days of creatine supplementation at 20 g.d-1.
The elevations of CK during the chronic condition may be indicative of using the non-dominant leg for the subsequent muscle damage bout. Increased CK in blood is a result of eccentric exercise which is unfamiliar to the muscle or muscle group, (Clarkson and Tramblay, 1992; Rawson et al., 2001) therefore CK elevations in the non-dominant compared to the dominant leg would be expected. Additionally, the CK elevations in the chronic condition suggest increased sarcolemmal or sarcoplasmic reticulum membrane damage. Since the non-dominant leg was used to induce muscle damage in the chronic condition, increased CK is most likely due to greater sarcolemmal and sarcoplasmic reticulum membrane instability as a result of mechanical stress from the eccentric exercise (Rawson et al., 2001).
Conclusion
In summary, short-term creatine supplementation did not appear to attenuate the effects of exercise induced muscle damage when compared to placebo treatments. However, the long-term effects appeared to have had an ergogenic effect on muscle when muscles were subjected to isometric force development. Anectdotal reports of attenuation of muscle damage and decreased recovery time may be associated with the increased energy availability of PCr associated with creatine supplementation, as well as the possible molecular changes in muscle. The eccentric protocol utilized in the current investigation and that of Rawson et al., 2001 were designed to create situations of significant muscle damage. Therefore the possibility exists that creatine’s ergogenic effects on muscle may require greater than 7 days to positively impact muscle damage. Future studies may need to focus on lesser amounts of exercise induced muscle damage, such as may occur with regular weight-bearing athletic type activity, for short-term creatine supplementation protocols to see a potential benefit of creatine supplementation in decreasing recovery time and attenuating exercise induced muscle damage. In addition, longer-term supplementation protocols should investigate additional muscle performance measures, such as dynamic strength, to determine if muscle damage is attenuated or is recovery time decreased due to the ergogenic effects of creatine supplementation.
Acknowledgments
The creatine and placebo supplements were provided by AST Sports Science, Golden, CO, USA. All experimental procedures comply with the current laws of the United States.
Biographies
John Rosene
Employment
Associate Professor and Director of the Human Performance Laboratory at Plymouth State University in Plymouth, NH.
Degree
DPE, ATC
E-mail: jmrosene@plymouth.edu
Tracey Matthews
Employment
Associate Professor at Springfield College in Springfield, MA.
Degree
DPE
Christine Ryan
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Keith Belmore
Employment
An assistant athletic trainer at Plymouth State University in Plymouth, NH.
Degree
MSc, ATC
Alisa Bergsten
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Jill Blaisdell
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
James Gaylord
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Rebecca Love
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Michael Marrone
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Kristine Ward
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
Eric Wilson
Employment
Undergraduate exercise physiology student at Plymouth State University in Plymouth, NH.
Degree
BSc
References
- 1.Boswell L., Mistry D., Okusa M., Patrie J., MacKnight J., Frick K., Watson D., Weltman J., Gieck J., Weltman A. (2003) Creatine supplementation does not affect renal function at rest or during exercise. Medicine & Science in Sports & Exercise Suppl 35, S400 [Google Scholar]
- 2.Burke L.M., Pyne D.B., Telford R.D. (1996) Effect of oral creatine supplementation on single-effort sprint performance in elite swimmers. International Journal of Sport Nutrition 6, 222-233 [DOI] [PubMed] [Google Scholar]
- 3.Casey A., Constantin-Teodosiu D., Howell S., Hultman E., Greenhaff P.L. (1996) Creatine ingestion favorably affects performance in muscle metabolism during maximal exercise in humans. American Journal of Physiology 271, E31-E37 [DOI] [PubMed] [Google Scholar]
- 4.Clarkson P.M., Sayers S.P. (1999) Etiology of exercise-induced muscle damage. Canadian Journal of Applied Physiology 24, 234-248 [DOI] [PubMed] [Google Scholar]
- 5.Clarkson P.M., Tramblay I. (1992) Exercise-induced muscle damage, repair, and adaptation in humans. Journal of Applied Physiology 65, 1-6 [DOI] [PubMed] [Google Scholar]
- 6.Connolly A.J., Sayers S.P., McHugh M.P. (2003) Treatment and prevention of delayed onset muscle soreness. Journal of Strength and Conditioning Research 17, 197-208 [DOI] [PubMed] [Google Scholar]
- 7.Greenhaff P.L., Bodin K., Harris R.C., Hultman E., Jones D.A., McIntyre D.B., Soderlund K., Turner D.L. (1993) The influence of oral creatine supplementation on muscle phosphocreatine resynthesis following intense contraction in man. Journal of Physiology 467, 75 [Google Scholar]
- 8.Harris R.C., Soderlund K., Hultman E. (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science 83, 367-374 [DOI] [PubMed] [Google Scholar]
- 9.Hultman E., Soderlund K., Timmons J., Cederblad G., Greenhaff P. (1996) Muscle creatine loading in men. Journal of Applied Physiology 81, 232-237 [DOI] [PubMed] [Google Scholar]
- 10.Ingalls C. P., Warren G.L., Armstrong R.B. (1998) Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. Journal of Muscle Research and Cell Motility 119, 215-224 [DOI] [PubMed] [Google Scholar]
- 11.Kern M., Podewils L.J., Vukovich M., Buono M.J. (2001) Physiological response to exercise in the heat following creatine supplementation. Journal of Exercise Physiology online 4, 18-27 [Google Scholar]
- 12.Kreider R.B., Ferreira M., Wilson M., Grindstaff P., Plisk S., Reinardy J., Cantler E., Almada A.L. (1998) Effects of creatine supplementation on body composition, strength, and sprint performance. Medicine & Science in Sports & Exercise, 30, 73-82 [DOI] [PubMed] [Google Scholar]
- 13.Norkin C., White D. J. (1995) Measurement of joint motion: A guide to goniometry. 2nd edition Philadelphia:F. A. Davis [Google Scholar]
- 14.Odland L.M., MacDougall J.D., Tarnopolsky M.A., Elorriaga A., Borgmann A. (1997) Effect of oral creatine supplementation on muscle [Pcr] and short-term maximum power output. Medicine & Science in Sports & Exercise 29, 216-219 [DOI] [PubMed] [Google Scholar]
- 15.Rawson E.S., Gunn B., Clarkson P.M. (2001) The effects of creatine supplementation on exercise-induced muscle damage. Journal of Strength Conditioning Research 15, 178-184 [PubMed] [Google Scholar]
- 16.Robinson S.J. (2000) Acute quadriceps compartment syndrome and rhabdomyolysis in a weight lifter using high-dose creatine supplementation. Journal of the American Board of Family Practice 13, 134-137 [DOI] [PubMed] [Google Scholar]
- 17.Rosene J.M., Whitman S.A., Fogarty T.D. (2004) A comparison of thermoregulation with short-term creatine supplementation between genders in a thermoneutral environment. Journal of Athletic Training 339, 50-55 [PMC free article] [PubMed] [Google Scholar]
- 18.Sorichter S., Mair J., Koller A., Gebert W., Rama D., Calzolari C., Artner-Dworzak E., Puschendorf B. (1997) Skeletal troponin I as a marker of exercise-induced muscle damage. Journal of Applied Physiology 83, 1076-1082 [DOI] [PubMed] [Google Scholar]
- 19.Staron R.S., Karapondo D.L., Kraemer W.J., Fry A.C., Gordon S.E., Falkel J.E., Hagerman F.C., Hikida R.S. (1994) Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. Journal of Applied Physiology 776, 1247-1255 [DOI] [PubMed] [Google Scholar]
- 20.Terjung R.L., Clarkson P., Eichner E.R., Greenhaff P.L., Hespel P.J., Israel R.G., Kraemer W.J., Meyer R.A., Spriet L.L., Tarnopolsky M.A., Wagenmakers A.J.M., Williams M.H. (2000) The physiological and health effects of oral creatine supplementation. Medicine & Science in Sports & Exercise 332, 706-717 [DOI] [PubMed] [Google Scholar]
- 21.Thompson H.S., Scordilis S.P., Clarkson P.M., Lohrer W.A. (2001) A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiologica Scandinavia 171, 187-193 [DOI] [PubMed] [Google Scholar]
- 22.Vandenberghe K., Van Hecke P., Van Leemputte M., Vanstapel F., Hespel P. (1999) Phosphocreatine resynthesis is not affected by creatine loading. Medicine & Science in Sports & Exercise 31, 236-242 [DOI] [PubMed] [Google Scholar]
- 23.Volek J.S., Duncan N.D., Mazzetti S.A., Staron R.S., Putukian M., Gomez A.L.L., Pearson D.R., Fink W.J., Kraemer W.J. (1999) Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Medicine & Science in Sports & Exercise 31, 1147-1156 [DOI] [PubMed] [Google Scholar]
- 24.Volek J.S., Mazzetti S.A., Farquhar W.B., Barnes B.R., Goomez A.L., Kraemer W.J. (2001) Physiological responses to short-term exercise in the heat after creatine loading. Medicine & Science in Sports & Exercise 33, 1101-1108 [DOI] [PubMed] [Google Scholar]
- 25.Willoughby D.S., Pelsue S. (1998) Muscle strength and qualitative myosin heavy chain isoform mRNA expression after moderate- and high-intensity weight training in the elderly. Journal of Aging and Physical Activity 6, 327-329 [Google Scholar]
- 26.Willoughby D.S., Rosene J.M. (2001) Effects of oral creatine and heavy resistance training on myosin heavy chain expression. Medicine & Science in Sports & Exercise 33, 1674-1681 [DOI] [PubMed] [Google Scholar]
- 27.Willoughby D.S., Rosene J.M., Myers J. (2003) Ubiquitin and HSP-72 expression and apoptosis after an acute bout of eccentric exercise. Journal of Exercise Physiology online 6, 96-104 [Google Scholar]


