Abstract
This study investigated the effect of a high-volume compared to a low-volume resistance training session on maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). Twenty male subjects with resistance training experience (6.2 ± 3.2 y), in a crossover trial, completed two resistance training protocols (high-volume: 5 sets per exercise; low-volume: 2 sets per exercise) and a control session (no exercise) on 3 separate occasions. MIP and MEP decreased by 13.6% (p < 0.01) and 14.7% (p < 0.01) respectively from pre-session MIP and MEP, following the high-volume session. MIP and MEP were unaffected following the low-volume or the control sessions. MIP returned to pre-session values after 40 minutes, whereas MEP remained significantly reduced after 60 minutes post-session by 9.2% compared to pre-session (p < 0.01). The findings suggest that the high-volume session significantly decreased MIP and MEP post-session, implicating a substantially increased demand on the respiratory muscles and that adequate recovery is mandatory following this mode of training.
Key points.
Respiratory muscular strength performance is acutely diminished following a high-volume whole-body resistance training session.
Greater ventilatory requirements and generation of IAP during the high-volume resistance training session may have contributed to the increased demand placed on the respiratory muscles.
Protracted return of respiratory muscular strength performance to baseline levels may have implications for individuals prior to engaging in subsequent exercise bouts.
Key words: Respiratory pressures, core stability, hyperventilation, intra-abdominal pressure, Valsalva maneuver
Introduction
High-volume resistance training sessions with short to moderate recovery between sets (< 3 min) are common practices employed for muscular hypertrophy (Kraemer et al., 2000; Marx et al., 2001; McAardle and Foglia, 1969). These sessions are associated with increased metabolic acidosis and ventilatory demand (Collins et al., 1991; Kang et al., 2005). In addition, Al-Bilbeisi and McCool, 2000 showed that the diaphragm is recruited during resistance training exercises via increases in transdiaphragmatic pressure, and suggested that this type of training may provide a strength training stimulus to the respiratory muscles. This was later confirmed by increased respiratory muscle strength within non-trained individuals following a 16-week resistance training program (DePalo et al., 2004). It is therefore not surprising that greater maximal inspiratory pressure (MIP), a non-invasive measure of inspiratory muscle strength (Green et al., 2002), and diaphragm thickness was found in weight-lifters compared to untrained individuals (McCool et al. , 1997). However, a reduction in MIP and maximal expiratory pressure (MEP) has been reported following resistance exercises (Gomez et al., 2009).
Contraction of the abdominal muscles in conjunction with tensing of the diaphragm during resistance exercises is thought to provide stabilization of the lower back, referred to as “core stability” during a lift (DePalo et al., 2004). Ringquist, 1966 found a significant correlation between MIP and trunk flexor strength, implicating a relationship between increased inspiratory muscle strength with core muscle strength. Core stability is a key determinant to performing exercises such as the squat and deadlift at high relative intensities, e. g., at >80% of one repetition maximum (1RM) (Zatsiorsky, 1995), intensities at which activation of the Valsalva maneuver (VM) is inevitable (MacDougall et al., 1992). VM acts to augment intra-abdominal pressure (IAP) (increased pressurization of the abdominal cavity) (Nachemson et al., 1986; Goldish et al., 1994) which promotes core stability (Hemborg et al., 1985; Hodges et al., 2001; 2003; Shirely et al., 2003). Notably, the expiratory muscles (rectus abdominus, transverse abdominus, and internal/external obliques) are engaged during the VM (Cresswell et al., 1992). Hence, whilst high-volume resistance training facilitates muscular hypertrophy, its demand on the respiratory muscular system may be sufficient to lead to fatigue. This study explored the effect of a high-volume compared to a low-volume resistance training session on MIP and MEP.
Methods
Subjects
Twenty male (age 26.8 ± 5.4 y; body mass 89.1 ± 12.8 kg; height 1.79 ± 0.05 m), non-smoking Caucasians with 6.2 ± 3.2 years of resistance training experience participated in this study. All subjects were experienced with resistance training and followed muscular hypertrophy training practices in accordance with the ACSM position statement (Ratamess et al., 2009). Pulmonary function was normal for all subjects and was defined by forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) above predicted values developed by the third National Health and Nutrition Examination Survey (Hankinson et al., 1999). Prior to the commencement of the study, subjects performed 1RM tests and were familiarized with the respiratory tests (MIP and MEP). The study was approved by the University of Sydney Human Research Ethics Committee.
Study procedure
This is a crossover, repeated measures design with random allocation of subjects into three sequences (high-volume, low-volume, and control sessions). Random sequence allocation was conducted by way of a sealed envelope. Subjects were instructed to refrain from exercise outside of their activities of daily living for 48 hours prior to each session. Respiratory measurements of MIP and MEP were conducted 15 min prior to all sessions (pre-session), and 5 min following the completion of these sessions (post-session). Resistance exercise warm-up sets, performed following the respiratory measurements, consisted of 1- 2 sets of each exercise at perceived light loads. The control session involved sitting quietly for 50 min, while the high- and low-volume resistance training sessions involved performing 5 and 2 sets respectively of 10 repetitions (or to failure if unable to reach 10 repetitions in a set) at 70% 1RM of four exercises (bench press, squat, seated shoulder press, and deadlift) with 90 s recovery between sets. The order in which subjects performed these exercises was randomized, with the experimenter blinded to this randomization. The speed of exercise repetitions during the sessions and 1RM testing was controlled at 2 s concentric phase and 3 s eccentric phase (with no rest in- between repetitions) through the full range of motion available. This involved full extension during the lifting phase for all lifts, while during the lowering phase, bar to chest for the bench press, thighs parallel to the floor for the squat, bar to clavicle for the seated shoulder press, and until the 20kg plates touched the floor for the deadlift. The high- and low-volume resistance training sessions took approximately 50 and 25 min to complete respectively. During the 90 s recovery period between sets for each exercise, subjects breathed through a mouthpiece where VE (minute ventilation), RR (respiratory rate), and PETCO2 (partial pressure of end-tidal carbon dioxide) were measured.
1RM
A 1RM was assessed for each of the four exercises (bench press, squat, seated shoulder press, and deadlift) to determine the loads for the resistance training bouts, according to the ACSM's Guidelines for Exercise Testing and Prescription (ACSM, 2009). Following a warm-up with a light load, and approximately 5 min rest, subjects performed an estimated maximal effort repetition. If this lift was successful, another lift was attempted with a heavier load with 5 min rest between attempts. This cycle was continued until the subject was unable to complete a lift, with the 1RM being the heaviest load that was successfully lifted.
Respiratory muscle strength, lung function and ventilatory measurements
Measurements conducted for pre and post-sessions included MIP and MEP, which were recorded using a differential pressure transducer (model MP-45, Validyne Engineering, Northridge, California). The transducer was calibrated before each session by using a U-tube water manometer. Measurement protocols followed that outlined by ATS/ERS (Green et al., 2002). Both MIP and MEP were performed with a plastic tube 3cm in diameter and 15cm long, with a plastic flanged mouthpiece (MADA, Italy) and a small air leak 7.5 cm from the mouthpiece. The small leak in the tube was necessary to prevent generation of high buccal pressures, and in addition subjects were required to hold their cheeks with one hand during the performances. Verbal encouragement was given to the subjects' during testing to ensure that motivation levels remained high. To help subjects achieve maximal effort, visual feedback from a computer was used to track pressures generated during MIP and MEP. Subjects rested approximately 1 min between efforts. MIP and MEP were defined as the peak pressure generated within three efforts in which the peak pressure differed by < 5%. In most cases, no more than six efforts were required. MIP and/or MEP that showed deterioration post-session were repeated every 20 min for 1 h to follow their recovery to baseline.
Recovery ventilatory responses (VE, RR, and PETCO2) were recorded breath-by-breath for 60 s at the conclusion of each set of exercise (within 5 s of the last repetition), with subjects seated while wearing a nose clip and breathing through a mouthpiece connected to the Ultima CPX pulmonary function testing system (Medgraphics Milano, Italy). Prior to each session, the pneumotach was calibrated with multiple comparisons to a 3-liter syringe while O2 and CO2 analyzers were calibrated automatically by the pulmonary function system software following the presentation of high- and low-span gases. This procedure was in accordance with system requirements (Medgraphics Cardiorespiratory Diagnostics Systems, 2001). Ventilatory measurements were taken during the recovery between sets of exercises because of the greater responses that occur within this period for resistance training exercises compared to during the actual exercise (Scott, 2009; Scott, 2011). Responses were averaged over 60 s for each set due to the non-steady state nature of the ventilatory responses following resistance exercises. The average values for recovery responses for each exercise following 5 and 2 sets were calculated for each subject.
Data analysis
Data for all measurements are presented as means ± standard deviation (SD). Data recorded were analyzed by Statistical Package for Social Science (SPSS) version 17.0. Statistics were calculated using a two factor [time (pre-post) x condition (low-volume vs. high-volume vs. control)] repeated measures analysis of variance (ANOVA) for each measurement (MIP, MEP, VE, RR, and PETCO2). Where significant main effects were found, post hoc analyses were calculated using the Student-Neuman-Keuls method. An alpha level of p < 0.05 indicated statistical significance.
Results
Respiratory muscle strength and ventilatory measures
There were no significant differences between the high-volume, low-volume, and control sessions for the pre-session MIP and MEP indicating high reproducibility (Table 1). Significant differences were found for MIP and MEP between pre- and post-session for the high-volume session. MIP decreased by 13.6% (p < 0.01) from pre-session (Figure 1A); MEP decreased by 14.7% (p < 0.01) from pre-session (Figure 1B). MIP returned to pre-session values after 40 minutes, whereas MEP remained significantly reduced after 60 minutes post-session by 9.2% compared to pre- session (p < 0.01). There were also no significant differences pre- and post-session for MIP and MEP in either the low-volume or control session.
Table 1.
Pre-session MIP and MEP. No significant differences between sessions for MIP and MEP.
| Control | Low-volume | High-volume | |
|---|---|---|---|
| MIP (cmH2O) | 161.9 (23.8) | 162.4 (19.6) | 162.9 (22.2) |
| MEP (cmH2O) | 224.1 (39.2) | 223.5 (40.6) | 224.9 (43.3) |
Figure 1.
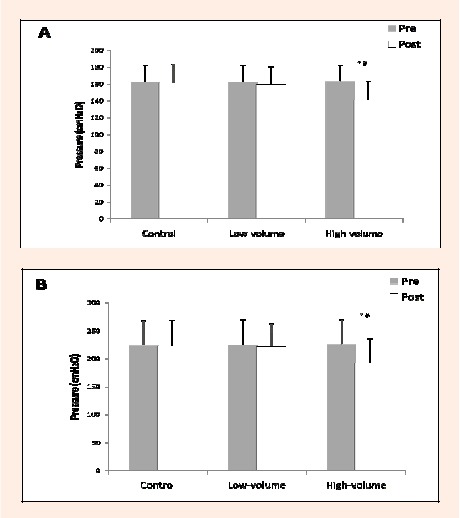
Maximal inspiratory pressure and B. maximal expiratory pressure, pre- and post-exercise and control sessions.* significantly different from Pre- value (p < 0.01); # significantly different from low-volume and control (p < 0.01).
Recovery ventilatory responses
Mean recovery VE and RR were significantly increased for each exercise (p < 0.05), while PETCO2 was significantly lower for each exercise (p < 0.05) within the high-volume session compared to the lower volume session (Figure 2). A trend for recovery VE and RR from greatest to lowest was found for the deadlift, squat, shoulder press, and bench press respectively. Tidal volume during each exercise did not change between the high-volume and low-volume sessions. During the high-volume session the majority (~ 78%) of subjects were unable to complete the full 10 repetitions on the final sets of each exercise and terminated at the highest repetitions possible prior to failure to lift the load.
Figure 2.
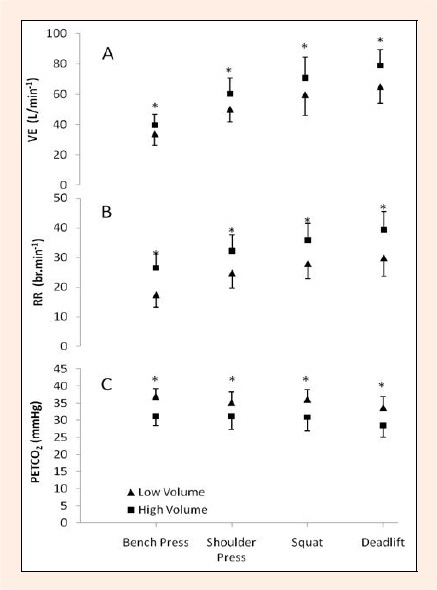
Recovery ventilatory responses. A. minute ventilation (VE), B. respiratory rate (RR), C. partial pressure of end-tidal carbon dioxide (PETCO2).* significant difference between sessions (p < 0.05).
Discussion
In regular weight trainers, we showed a diminished MIP and MEP following the high-volume resistance training session, with no deterioration following the low-volume session. The reduction in MIP and MEP was protracted into the recovery period. In addition, increased VE, RR and decreased PETCO2 were recorded for all exercises in the high-volume compared with the low-volume session.
The significant and protracted reductions in both MIP and MEP following a whole-body resistance exercise session suggests that the respiratory muscles were not only engaged during this mode of exercise, but recovery of the muscles was slow. Similarly Gomez et al., 2009 also found reductions in MIP and MEP following a resistance exercise. However, these reductions were achieved through a bout of exhaustive sit-ups involving only the abdominals. Two potential factors which may contribute to the increased work of the respiratory muscles during high- resistance exercise are the engagement of the diaphragm and abdominal muscles in maintaining trunk stability, and hyperventilation due to increased VE. Some authors have suggested that the work placed on the respiratory muscles is 25-50% higher during hyperventilation compared with exercise at the same VE (Aaron et al., 1992; McGregor et al., 1962; Milic-Emili and Petit, 1960).
Trunk rigidity, and therefore core stability, is provided by an increased IAP (Hemborg et al., 1985; Hodges et al., 2001; Hodges et al., 2003). Core stability is vital during weight-lifting, especially in exercises where support for the lumbar region is needed such as those requiring high axial loading (i.e. squats and deadlifts). The diaphragm and expiratory muscles contribute to increases in IAP via the VM (Cresswell et al., 1992; Goldish et al., 1994; Nachemson et al., 1986). Breath hold (BH) also produces a similar effect (Hagins et al., 2004; Hagins et al., 2006). It was identified that the subjects in this study performed the VM or BH as they approached repetition failure, during the execution of lifts within the later sets of the high volume session. These observations concurred with subject feedback at study termination. The performance of VM or BH was especially evident during the squats and deadlifts, and according to previous research, the IAP was expected to be greater for these exercises compared to the bench press and shoulder press (Harman et al., 1988). The diminished MIP and MEP following the high-volume session may have resulted from fatigue of the diaphragm and abdominal muscles as a result of the volume of work placed upon these muscles to maintain IAP (thus core stability) throughout the high-volume session. However, diaphragmatic fatigue cannot be confirmed unless transdiaphragmatic twitch pressure (Pdi twi) was measured (Johnson et al., 1996). Therefore reductions in MIP and MEP post-exercise cannot be attributed directly to specific muscles, but rather to the global respiratory muscles that contribute to generating these pressures (Green et al., 2002). Alternatively, depletion of immediate energy sources (phosphagens) or glycogen store within the respiratory muscles may have led to the reductions in MIP and MEP (Ianuzzo et al., 1987).
Hyperventilation, demonstrable in mean recovery PETCO2 occurred following each exercise. The recovery PETCO2 was significantly lower for the high-volume compared to the low-volume session. This is not surprising given the increased volume of work (sets x repetitions) and relatively short recoveries between sets from the high-volume session. Whilst all subjects completed 10 repetitions at 70% 1RM for all sets of exercises during the low-volume session, the majority of subjects performed less than 10 repetitions on the last sets for the high- volume session. Reaching repetition failure during the last sets of the high volume-session suggests sub-optimal replenishment of high energy phosphates and possibly elevated blood lactate. The hyperventilatory response following exercise would therefore be part of the compensatory (bicarbonate buffering) system to regulate pH levels within the blood. However, a major limitation of this study was that blood lactate concentration was not measured to confirm increased metabolic responses.
It was noteworthy that the MIP reduction following the high-volume session took 40 minutes to return to pre-session values, whereas MEP reduction was not restored after 60 minutes had elapsed. A slower recovery rate for MEP may be linked to fiber type differences of the muscles involved in MIP and MEP. The expiratory muscles typically contain a higher proportion of fast- twitch muscles compared to the main inspiratory muscle (i.e. diaphragm) (Keens et al., 1978). Therefore, the extended delay for MEP to return to pre-session values is suggestive that these muscles are particularly prone to extended fatigue. Since the monitoring of decreased respiratory measurements lasted for only 60 min, it is unknown how long the MEP would take to return to baseline level. Evidence suggests that expiratory muscle fatigue can impair exercise performance (Verges et al., 2007; Taylor and Romer, 2008). Therefore, this may have implications for individuals prior to engaging in subsequent exercise bouts (resistance or aerobic).
We cannot exclude the possibility that the reduction in MIP and MEP was influenced by motivation. However, all subjects in this study were highly experienced with the concept of performing maximally (regularly undertaken in the context of their weight- training) and well rested in the 48 h preceding testing. Further, subjects were familiar with all tests, received equal encouragement in all tests, and were provided visual bio-feedback which they could use as a target for their effort. Together this supports the contention that the observed decrement in respiratory performance was a direct function of physiological factors affecting the force generation of the global respiratory muscles.
Conclusion
In conclusion, MIP and MEP showed a significant reduction following the high-volume whole-body resistance training session within regular weight trainers. Diminished respiratory muscular strength may be explained by the increased demands placed on the respiratory muscles to maintain core stability in addition to the ventilatory demand of hyperventilation post-session. The protracted return of MIP and MEP to baseline levels suggests that recovery is requisite for subsequent training bouts.
Acknowledgements
No funding was received for the study and there was no conflict of interest from the results of this study among the authors. The results of the present study do not constitute endorsement by ACSM. We are grateful for help with statistics from Dr Rob Heard, Discipline Behavioural and Social Sciences in Health/Discipline
Biographies
Daniel A. Hackett
Employment
University of Sydney (Australia), Associate Lecturer
Degree
BExSc, MHlthSc (Hons)
Research interests
Resistance training.
E-mail: daniel.hackett@sydney.edu.au
Nathan A. Johnson
Employment
University of Sydney (Australia), Academic
Degree
BMedSc, MHlthSc, PhD
Research interests
Exercise, health and disease
E-mail: nathan.johnson@sydney.edu.au
Chin-Moi Chow
Employment
University of Sydney (Australia), Senior Academic
Degree
BSc, PhD
Research interests
Exercise, sleep
E-mail: chin-moi.chow@sydney.edu.au
References
- Aaron E.A., Johnson B.D., Seow C.K., Dempsey J.A.(1992)Oxygen cost of exercise hyperpnea: measurement. Journal of Applied Physiology 72, 1810-1817 [DOI] [PubMed] [Google Scholar]
- ACSM (2009)Guidelines for exercise testing and prescription. 8th edition Lippincott, Williams and Wilkins, Philadelphia: 90 [Google Scholar]
- Al-Bilbeisi F.D., McCool F.D.(2000)Diaphragm recruitment during nonrespiratory activities. American Journal of Respiratory and Critical Care Medicine 162, 456-459 [DOI] [PubMed] [Google Scholar]
- Collins M. A., Cureton K.J., Hill D.W., Ray C.A.(1991)Relationship of heart rate to oxygen uptake during weight lifting exercise. Medicine and Science in Sports and Exercise 23, 636-640 [PubMed] [Google Scholar]
- Cresswell A.G., Grundstrom H., Thorstensson A.(1992)Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta Physiologica Scandinavica 144, 409-418 [DOI] [PubMed] [Google Scholar]
- De Palo V. A., Parker A.L., Al-Bilbeisi F., McCool F.D.(2004)Respiratory muscle strength training with nonrespiratory maneuvers. Journal of Applied Physiology 96, 731-734 [DOI] [PubMed] [Google Scholar]
- Goldish G. D., Quast J.E., Blow J.J., Kuskowski M.A.(1994)Postural effects on intra-abdominal pressure during Valsalva maneuver. Archives of Physical Medicine and Rehabilitation 75, 324-327 [DOI] [PubMed] [Google Scholar]
- Gomez C. L., Strongoli L.M., Coast J.R.(2009)Repeated abdominal exercise induces respiratory muscle fatigue. Journal of Sports Science and Medicine 8, 543-547 [PMC free article] [PubMed] [Google Scholar]
- Green M., Road J., Sieck G.C., Similowski T.(2002)Tests of respiratory muscle strength. In: ATS/ERS statement on respiratory muscle testing. American Journal of Respiratory and Critical Care Medicine 166, 528-54712456380 [Google Scholar]
- Hagins M., Pietrek M., Sheikhzadeh A., Nordin M.(2006)The effects of breath control on maximum force and IAP during a maximum isometric lifting task. Clinical Biomechanics 21, 775-780 [DOI] [PubMed] [Google Scholar]
- Hagins M., Pietrek M., Sheikhzadeh A., Nordin M., Axen K.(2004)The effects of breath control on intra-abdominal pressure during lifting tasks. Spine 29, 464-469 [DOI] [PubMed] [Google Scholar]
- Hankinson J. L., Odencrantz J.R., Fedan K.B.(1999)Spirometric reference values from a sample of the general U.S. population. American Journal of Respirataory and Critical Care Medicine 159, 179-187 [DOI] [PubMed] [Google Scholar]
- Harman E. A., Frykman P.N., Clagett E.R., Kraemer W.J.(1988)Intra-abdominal and intra-thoracic pressures during lifting and jumping. Medicine and Science in Sports and Exercise 20, 195-201 [DOI] [PubMed] [Google Scholar]
- Hemborg B., Moritz U., Lowing H.(1985) Intra-abdominal pressure and trunk muscle activity during lifting IV. The causal factors of the intra-abdominal pressure rise. Scandinavian Journal of Rehabilitation Medicine 17, 25-38 [PubMed] [Google Scholar]
- Hodges P., Kaigle Holm A., Holm S., Ekstrom L., Cresswell A., Hansson T., Thorstensson A.(2003)Intervertebral stiffness of the spine is increased by evoked contraction of transverse abdominis and the diaphragm: in vivo porcine studies. Spine 28, 2594-2601 [DOI] [PubMed] [Google Scholar]
- Hodges P. W., Cresswell A.G., Daggfeldt K., Thorstensson A.(2001)In vivo measurement of the effect of intra-abdominal pressure on the human spine. Journal of Biomechanics 34, 347-353 [DOI] [PubMed] [Google Scholar]
- Ianuzzo C.D., Spalding M.J., Williams H.(1987)Exercise-induced glycogen utilization by the respiratory muscles. Journal of Applied Physiology 62, 1405-1409 [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Aaron E.A., Babcock M.A., Dempsey J.A.(1996)Respiratory muscle fatigue during exercise: implications for performance. Medicine and Science in Sports and Exercise 28, 1129-1137 [DOI] [PubMed] [Google Scholar]
- Kang J., Hoffman J.R., Im J., Spiering B.A., Ratamess N.A., Rundell K.W., Nioka S., Cooper J., Chance B.(2005)Evaluation of physiological responses during recovery following three resistance exercise programs. Journal of Strength and Conditioning Research 19, 305-309 [DOI] [PubMed] [Google Scholar]
- Keens T. G., Bryan A.C., Levision H., Ianuzzo C.D.(1978)Developmental pattern of muscle fiber types in human ventilatory muscles. Journal of Applied Physiology 44, 909-913 [DOI] [PubMed] [Google Scholar]
- Kraemer W.J., Ratamess N., Fry A.C., Triplett-McBride T., Koziris L.P., Bauer J.A., Lynch J.M., Fleck S.J.(2000)Influence of resistance training volume and periodization on physiological and performance adaptations in college women tennis players. American Journal of Sports Medicine 28, 626-633 [DOI] [PubMed] [Google Scholar]
- Mac Dougall J.D., McKelvie R.S., Moroz D.E., Sale D.G., McCartney N., Buick F.(1992)Factors affecting blood pressure during heavy weight lifting and static contractions. Journal of Applied Physiology 73, 1590-1597 [DOI] [PubMed] [Google Scholar]
- Marx J.O., Ratamess N.A., Nindl B.C., Gotshalk L.A., Volek J.S., Doh,i K., Bush J.A., Gómez A.L., Mazzetti S.A., Fleck S.J., Häkkinen K., Newton R.U., Kraemer W.J.(2001)Low-volume circuit versus high-volume periodized resistance training in women. Medicine and Science in Sports and Exercise 333, 635-643 [DOI] [PubMed] [Google Scholar]
- McAardle W.D., Foglia G.F.(1969)Energy cost and cardiorespiratory stress of isometric and weight training exercises. Journal of Sports Medicine 9, 23-29 [PubMed] [Google Scholar]
- McCool F. D., Conomos P., Benditt J.O., Cohn D., Sherman C.B., Hoppin F.G.(1997)Maximal inspiratory pressures and dimensions of the diaphragm. American Journal of Respiratory and Critical Care Medicine 155, 1329-1334 [DOI] [PubMed] [Google Scholar]
- McGregor M., Donevan R.E., Anderson N.M.(1962)Influence of carbon dioxide and hyperventilation on cardiac output in man. Journal of Applied Physiology 17, 933-937 [DOI] [PubMed] [Google Scholar]
- Medgraphics Cardiorespiratory Diagnostics Systems (2001)Breeze suite quick start operator manual: an introduction to pulmonary function testing. Medical Graphics Corporation, St Paul (MN) 2-3 [Google Scholar]
- Milic-Emili G., Petit J.M.(1960)Mechanical efficiency of breathing. Journal of Applied Physiology 15, 359-362 [DOI] [PubMed] [Google Scholar]
- Nachemson A. L., Andersson G.B.J., Schultz A.B.(1986)Valsalva maneuver biomechanics: Effects on lumbar trunk loads of elevated intra-abdominal pressures. Spine 11, 476-479 [PubMed] [Google Scholar]
- Ratamess N., Alvar B., Evetoch T.K., Housh T.J., Kibler W.B., Kraemer W.J., Triplett N.T.(2009)Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise 41, 687-708 [DOI] [PubMed] [Google Scholar]
- Ringquist T.(1966)The ventilatory capacity in healthy subjects. An analysis of casual factors with special reference to the respiratory forces. Scandinavian Journal of Clinical and Laboratory Investigation 18, 1-179 [PubMed] [Google Scholar]
- Scott C.B.(2011)Quantifying the immediate recovery energy expenditure of resistance training. Journal of Strength and Conditioning Research 25, 1159-1163 [DOI] [PubMed] [Google Scholar]
- Scott C.B., Croteau A., Ravlo T.(2009)Energy expenditure before, during, and after the bench press. Journal of Strength and Conditioning Research 23, 611-618 [DOI] [PubMed] [Google Scholar]
- Shirley D., Hodges P.W., Eriksson A.E., Gandevia S.C.(2003)Spinal stiffness changes throughout the respiratory cycle. Journal of Applied Physiology 95, 1467-1475 [DOI] [PubMed] [Google Scholar]
- Taylor B.J., Romer L.M.(2008)Effect of expiratory muscle fatigue on exercise tolerance and locomotor muscle fatigue in healthy humans. Journal of Applied Physiology 104, 1442-1451 [DOI] [PubMed] [Google Scholar]
- Verges S., Sager Y., Erni C., Spengler C.M.(2007)Expiratory muscle fatigue impairs exercise performance. European Journal of Applied Physiology 101, 225-232 [DOI] [PubMed] [Google Scholar]
- Zatsiorsky V.M.(1995) Science and practice of strength training. Human Kinetics, Champiagn (IL) 141-150 [Google Scholar]


