Abstract
There have been few studies examining the short-term effect of high-impact activities on bone metabolism measured by bone serum marker concentrations. The purpose of this study was to examine the effect of short-term high-impact jump activity on bone turnover in female college-aged non-athletes. Twenty six healthy females were randomly assigned to a control or jump group. The subjects jumped 5 days per week for 2 weeks. The participants completed 10 jumps per session. A general health questionnaire and a bone-specific physical activity assessment instrument (BPAQ) were completed. BPAQ scores were calculated based on the past history of exercise. Blood draws were taken in both groups before and after the two-week experimental period. The vertical ground reaction force (VGRF) of all jumps and jump height were measured for each subject daily and the osteogenic index (OI) was measured. Concentrations of serum osteocalcin (OC), Bone Specific Alkaline Phosphatase (BAP), C-Terminal Telopeptides of Type I Collagen (CTX) and plasma Tartrate-Resistant Acid Phosphatase (TRAP5b) were assessed pre and post jump protocol to measure bone formation and resoprtion respectively. A significant interaction (time x group) was found in TRAP5b, and BAP values (p < 0.05). There was a significant decrease in CTX and BAP values in the jump group (p < 0.05) after the two week jump protocol. No significant interactions or changes were observed in OC values for either the jump or the control group. Two weeks of jump activity consisting of 10 jumps/day for 5 days/week with a weekly osteogenic index of 52.6 significantly decreased markers of bone resorption (TRAP5b and CTX) and bone formation (BAP) in young female non-athletes.
Key points
Please provide 3-5 bullet points of the study.
Inductive qualitative methodologies can encourage the much-needed voice of female youth in sport and physical activity research.
Vignettes serve, not only as a method to illustrate data, but also as a medium to teach contextually relevant information to participants and sport science service providers.
The barriers and solutions to female youth engagement in physical activity are best understood through the perspectives of the intended participant.
Female youth can serve as central informants in the development and analysis of research projects relating to female youth physical activity.
Key words: Jump exercise, vertical ground reaction force, bone serum markers, randomized controlled study
Introduction
The benefit of mechanical loading or exercise on bone mass and strength has been identified in both human (Kohrt et al., 2004) and animals models (Iwamoto et al., 1999; Umemura et al., 2008). Jumping activities are particularly osteogenic to bone since they result in high magnitude loads and high loading rates (Lanyon and Rubin, 1984). Jumping protocols are especially effective in children prior to the onset of puberty (Hind and Burrows, 2007) as well as post puberty (Eliakim et al., 1997; Johannsen et al., 2003; Kannus et al., 1995). In addition, low-repetition, high-impact jumps enhanced bone mineral density (BMD) by 2.6% at the femoral neck in female college students following a 6 month exercise program (Kato et al., 2006). Significant increases in femoral neck BMD after 5 months of jump training have even been reported in premenopausal women (Bassey et al., 1998; Burshell et al., 2010).
Bone serum markers are a measurement that may quantify the effectiveness of a jump protocol for bone adaptation at early time points. Previous studies have measured changes in bone mass, strength, or size by dissection (for animal), magnetic resonance imaging (MRI), peripheral quantitative computed tomography (pQCT), and dual energy X-ray absorptiometry (DEXA) (Bassey et al. , 1998; Fuchs and Snow, 2002; Fuchs et al., 2001; Johannsen et al., 2003; Umemura et al., 1995; 2002b; 2008). However, the limitation of these methods is the length of time needed between measurements to accurately quantify any changes in bone mass. On the other hand, bone serum markers can measure the dynamic status of the bone and examine systematic changes rather than changes of a specific bone site (Syed and Khan, 2002).
Studies using endurance training consisting of walking, running, aerobic dance and stair climbing have measured bone serum markers in both males and females (Adami et al., 2008; Eliakim et al., 1997). An increase in bone formation markers and a decrease in bone resorption markers were found in adolescent males (Eliakim et al., 1997). In pre-menopausal women following 4 weeks of exercise, an increase in bone formation and no change in bone resorption markers were reported (Adami et al. 2008). Bone formation increased significantly accompanied by an increase in resorption after the first 8 weeks in male and female army recruits (Evans et al., 2008). After 16 weeks of training (marching, running, jumping), no additional increases in bone formation were reported and bone resorption returned to baseline levels. Previous studies utilizing bone serum markers to determine the effectiveness of jump or resistance exercise protocols have reported increased bone formation markers following a 12 week protocol (resistance training) (Lester et al., 2009) and an 8 week jump protocol (Erickson and Vukovich, 2010) with no changes in bone resorption. Short term exercise to exhaustion in males (high impact and endurance protocols) resulted in increased bone resorption markers (Rantalainen et al., 2009; Scott et al., 2010; 2011).
The development of exercise protocols focused on bone mass improvement necessitates a measurement to determine the effectiveness of these protocols. The osteogenix index (OI) (Turner and Robling 2003) incorporates the magnitude of loading and frequency of loading into an equation yielding a quantity to compare osteogenic effectiveness between protocols. Three studies to date reported the osteogenic index (OI) of their protocols (Erickson and Vukovich, 2010; Lester et al., 2009; Rantalainen et al., 2011) with two studies of young males and females reporting increased bone formation markers but no changes in markers of bone resorption after 8 weeks of training. These studies did not find a correlation between the magnitude of OI and the changes in bone formation markers (Erickson and Vukovich, 2010; Lester et al., 2009). However, acute exhaustive exercise protocols report increased bone resorption markers ( Rantalainen et al., 2009; Scott et al., 2010; 2011) and a correlation between bone formation and OI was reported following an acute (1 session) exhaustive jumping protocol in young males (Rantalainen et al., 2009). However, we are unaware of any jump studies looking at a short-term protocol (2 weeks) on bone serum markers. Therefore, the purpose of this study was to examine the effect of short-term (2 weeks) high-impact jump activity on bone metabolism in female college-aged non-athletes. Determining the effectiveness of dynamic loading protocols early may help to efficiently design exercise programs to improve bone strength.
Methods
Participants
Twenty six non-athletic females (19-24 years of age) participated in the study. Participants were randomly assigned to either the control group (n = 13) or the jump group (n = 13). Exclusion criteria included: currently exercising more than 3 days a week, a history of bone disorders (e.g., osteoporosis), abnormal menstruation, eating disorders, pregnancy, smoking, or a lower extremity injury within six months of the study. Participants who were taking oral contraceptives were included in the study. The distribution of OC users among the participants was 3 out of 13 in the control group and 5 out of 13 in the jump group. All participants completed a health history questionnaire and gave written consent prior to participation. The Human Subjects Institutional Review Board at Temple University approved the protocol prior to enrollment.
Experimental design
The research design consisted of a pre-test post-test randomized group design. The independent variables were group (jumping versus control) and time (pre-test, post-test). The dependent variables were measures of bone formation including serum levels of osteocalcin (OC) and bone specific alkaline phosphatase (BAP) and measures of bone resorption including tartrate-resistant acid phosphatase (TRAP5b) and C-terminal telopeptides of type I collagen (CTX). Vertical ground reaction force (VGRF) was measured and defined as the maximum Z-axis load on the force plate during a 5 second reading of the jump activity.
Procedures: Anthropometric assessment
Participant height (centimeters) was attained from the questionnaire and weight was recorded (kilograms) by using a Kistler Force Plate sampled at 1000 Hz (Kistler Instrumentation Corp., Winterhur, Switzerland).
Questionnaire
The General Health questionnaire was used to check if the participant met the criteria to participate in the study. The BPAQ, a bone-specific physical activity assessment instrument (Weeks and Beck, 2008), was used. The participants were asked to fill out the chart with their physical activity history from birth to present time. This chart was also used to ensure the participants did not meet the exclusion criteria.
Training protocol
After random assignment to groups, the jump group participated in one jumping session five days per week for two weeks. The jumping session consisted of ten two-legged drop jumps (DJ) (Noyes et al., 2005)(Figure 1). A drop jump from a box followed by a maximal effort vertical jump has been reported to have higher VGRF values than other styles (McKay et al., 2005). A 3.0 times body weight in the VGRF at landing was reported to be a sufficient bone stimulus for young adults (Bassey et al., 1998; Fuchs et al., 2001) and in the current protocol, jumps with VGRF values below 3.0 were excluded and repeated to maximize the mechanical stimuli. The ground reaction forces were measured for all jumps. The Vertec, a device to measure vertical jump height, was used to maximize voluntary effort by instructing the jumper to touch the highest marker possible on each jump. A recent study has shown that focusing on the rungs of a Vertec as an external focus enhanced jump height (Wulf and Dufek, 2009). In this study, each jump was performed with a 30-second rest interval between jumps based on rat studies that reported that intervals between loading cycles could enhance the anabolic effects on bone (Robling et al., 2001) and specifically a 30-second interval between individual loading cycles had more anabolic effects on bone in rats than a 3-second interval (Umemura et al., 2002b). Participants in the control group did not perform the jumping protocol.
Figure 1.
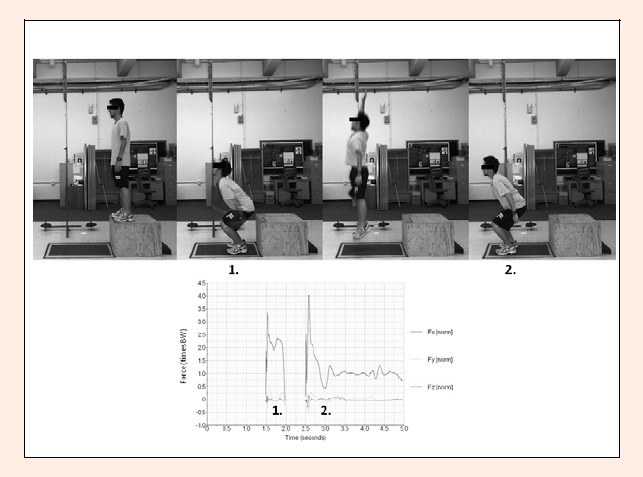
Images of the plyometric jump used in the training protocol. Participants began on a 40cm box, dropped to the forceplate and jumped as high as possible (using the Vertec measuring device) and landing on the forceplate. Representative force plate tracing illustrating the magnitudes of the two landings.
Jump intensity measurements
VGRF of each jump was recorded using a Kistler Force Plate (Kistler Instrumentation Corp., Winterhur, Switzerland) at a sampling rate of 1000 Hz. The participant was instructed to land on the force plate when performing jump activities. The recorded VGRF was used to exclude jumps with values lower than 3.0 times body weight. The coefficient of variation (CV) was calculated for repeatability. The jumps were very repeatable between days with a between-day CV (variability between days for each subject) of 3.8% for the 1st landing and 5.9% for the 2nd landing. The osteogenic index (OI) was used to evaluate the osteogenic potential of the jumping protocol (Turner and Robling, 2003). The average weekly OI for each participant was calculated using their average maximum peak vertical ground reaction (10 jumps/session; 5 days/week). The weekly osteogenic index (OI) was calculated based on the equation from Turner and Robling (2003).
OI (1 session/day) = [(peak vertical ground reaction force) x ln (jumps +1)] x 5[1]
Bone serum markers
Antecubital vein blood draws of 7 ml were performed pre-test and post-test to determine bone formation markers (osteocalcin (OC) and bone specific alkaline phosphatase (BAP)) and bone resorption markers (tartrate-resistant acid phosphatase (TRAP5b) and C-terminal telopeptides of type I collagen (CTX)). The blood collected in a vacutainer tube was kept on an ice bath immediately after the blood draw, and the whole blood was centrifuged at 1,600 revolutions per minutes for 20 min at 4°C within 2 hours of the blood draw. The plasma was then divided and transferred into five 2 mL cryovials (Fisher Scientific International, Inc., Hampton, NH) and stored at -80°C. Post-test blood samples were taken 48 hours after the last jumping session to avoid any effect of the last jump session. All samples were taken at the same time of day for each participant (pre-post) but all participants did not have blood drawn at the same time of day as a group (0800 h - 1400 h). The participants were not instructed to fast prior to the blood draw.
Tartrate-resistant acid phosphatase (TRAP5b): Serum concentrations of TRAP5b (U/I) were assessed in duplicate using the MicroVue TRAP5b enzyme immunoassay (QUIDEL Corporation, San Diego, CA), according to manufacturer guidelines. The intra-assay CV was 2.2 % and the inter-assay CV was 3.0 %.
C-Terminal Telopeptides of Type I Collagen (CTX): Serum concentrations of c-terminal telopeptides of type I collagen (ng/mL), a bone serum marker used to determine bone resorption, were measured using the Serum CrossLaps ELISA (Immunodiagnostic Systems, Fountain Hills, AZ), according to manufacturer guidelines. The intra-assay CV was 1-8% and the inter-assay CV was 3-6%.
Osteocalcin (OC): Plasma concentrations of osteocalcin (ng/mL) were determined using the MicroVue Osteocalcin enzyme immunoassay (QUIDEL Corporation, San Diego, CA), according to manufacturer guidelines. The intra- assay CV was 4.8-10 % and the inter-assay CV was 4.8-9.8%.
Bone Specific Alkaline Phosphatase (BAP): Serum concentrations of bone specific alkaline phosphatase (U/L), a bone serum marker used to determine bone formation, were measured using the MicroVue BAP immunoenzymetric assay (Quidel Corporation, San Diego, CA), according to manufacturer guidelines. The intra-assay CV was 4-6% and the inter-assay CV was 5-8%.
Data analysis
All statistical procedures were completed using the statistical package for Social Sciences (SPSS, version 18.0, SPSS Inc, Chicago, IL.). Descriptive statistics were calculated for group characteristics and reported as means ± standard deviations (SD) and differences between the groups were determined using an unpaired t-test. Two-way repeated measures ANOVA [2 groups (jump and control) X 2 times (pre-test and post-test)] was computed to determine interactions between the jump and control groups for the dependent variables (osteocalcin, BAP, Trap5b, CTX,). An unpaired t-test was used to determine in the changed (pre to post) for each group differed (an analysis that supports the interaction). An unpaired T-test was also used to determine any differences at baseline. To determine time changes (pre to post) within groups, paired t-tests were performed. A level of p ≤ 0. 05 was considered significant. Sample size estimation was calculated using G-Power 3.1.0; based on the mean differences and standard deviation of a paired t-test for CTX and TRAP5b, a power of .8 would be obtained with a sample size ranging from 7-20.
Results
Anthropometric measurements
Descriptive statistics indicated that the variables of age, height, weight, exercise per week, and BPAQ score had no significant difference (p = 0.2463-0.8920) between groups (Table 1).
Table 1.
Anthropometric data and BPAC for participants in jump group and control group. No significant differences were found between the Jump and Control group for any measure based on t-test. Data are means (SD).
| Jump (n=13) | Control (n=13) | |
|---|---|---|
| Age (yr) | 20.8 (1.1) | 20.4 (1.4) |
| Height (m) | 1.65 (.06) | 1.63 (.07) |
| Weight (kg) | 63.9 (8.1) | 65.3 (12.8) |
| Exercise/Week | 2.3 (1.3) | 2.7 (1.4) |
| Oral contraceptive use (n) | 5 | 3 |
| BPAQ (Pre) | 34.9 (32.9) | 21.2 (22.9) |
Bone serum markers
Serum concentrations of TRAP5b at baseline were (mean ± SD: control, 2.64 ± 0.74 U·I-1; jump, 2.69 ± 0.76 U·I-1) and post training protocol (control, 2.92 ± 0.97 U/I; jump, 2.55 ± 0.73 U·I-1). There was no significant difference detected in the baseline values between the jump and control group using a t-test (p = 0.9086). A two-way repeated measures ANOVA revealed significant interactions (time x group) in TRAP5b values (p = 0.0202) (Figure 2A). A t-test of the differences (post-pre) between groups was also significant (p = 0.0101). A paired t-test indicated that the decrease in TRAP5b in the jump group did not reach significance (p = 0.0551) and no significant change in the control group (p = 0.1239) (Figure 3A).
Figure 2.
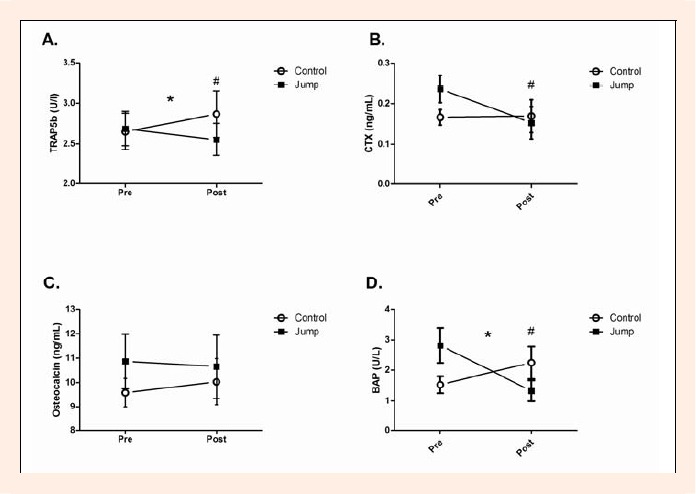
Pre-test and Post-test bone serum marker concentration in jump group and control group. Two-way repeated measures ANOVA was computed to determine a (time x group) interaction for each marker. Significance does not indicate an increase or decrease in the magnitude of the markers just that control and jump groups responded differently. Unpaired t-tests were used to detect differences in the change between the differences post-pre between the control and jump groups a similar analysis to the interaction term. A. Serum Trap5b levels (U/I) B. Serum CTX levels (ng·mL-1) C. Serum Osteocalcin levels (ng·mL-1) D. Serum BAP levels (U/L) Means ± SEM * Significant interaction (time x group) p < 0.05 # Significant difference (post - pre) between groups.
Figure 3.
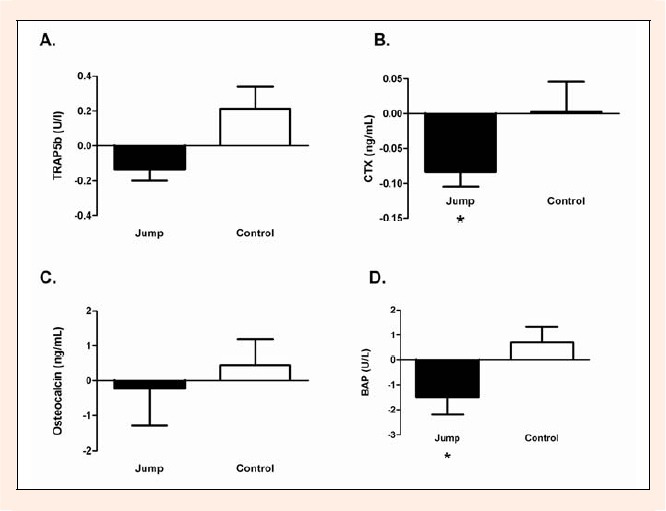
Differences (Post-Pre) in Bone Serum Marker Concentrations within the jump group and control group. Paired t-test were used to determine changes from pre to post within groups. A. Serum Trap5b levels (U/I) B. Serum CTX levels (ng·mL-1) C. Serum Osteocalcin levels (ng·mL-1) D. Serum BAP levels (U/L) Means ± SEM * Significant difference (p < 0.05).
Serum concentrations of CTX at baseline were (mean ± SD: control, 0.17 ± 0.06 ng·mL-1; jump, 0.24 ± 0.11 ng·mL-1) and post training protocol (control, .17 ± 0.13 ng·mL-1; jump, .15 ± 0.12 ng·mL-1). There was no significant difference detected in the baseline values between the jump and control group using a t-test (p = 0.0870). A two-way repeated measure ANOVA revealed no interactions (time x group) with a significance level of (p= 0.0885) (Figure 2B). However, a t-test of the differences (post-pre) between groups was significant (p = 0.0442). A paired t-test indicated a significant decrease in CTX in the jump group (p = 0.0032) and no significant change in the control group (p = 0.9495) (Figure 3B).
Serum concentrations of OC at baseline were (mean ± SD: control, 10.9 ± 2.9 ng·mL-1; jump, 9.6 ± 1. 6 ng·mL-1) and post training protocol (control, 10.0 ± 2.5 ng·mL-1; jump, 10.7 ± 3.4 ng·mL-1). There was no significant difference detected in the baseline values between the jump and control group using a t-test (p = 0.3299). A two-way repeated measure ANOVA revealed no interactions (time x group) with a significance level of (p = 0.6221) (Figure 2C). A t-test of the differences (post-pre) between groups was not significant (p = 0.3110). A paired t- test indicated no significant changes in the jump group (p = 0.4246) or the control group (p = 0. 5682) (Figure 3C).
Serum concentrations of BAP at baseline were (mean ± SD: control, 1.53 ± 0.87 U·I-1; jump, 2.82 ± 1.73 U·I-1) and post training protocol (control, 2.25 ± 1.67 U·I-1; jump, 1.33 ± 1.02 U·I-1). The difference detected in the baseline values between the jump and control group using a t-test did not reach significance (p = 0.0526). A two-way repeated measures ANOVA revealed significant interactions (time x group) in BAP values (p =0.0286) (Figure 2D). A t-test of the differences (post-pre) between groups was also significant (p = 0.0286). A paired t-test indicated a significant decrease in BAP in the jump group (p = 0.0333) and no significant change in the control group (p = 0.2665) (Figure 3D).
Vertical ground reaction forces and osteogenic index
The average magnitude of the vertical ground reaction force (VGRF) for the 1st landing was 4.13 ± 0.72 times BW and for the 2nd landing was 3.88 ± 0.079 times BW. The average weekly osteogenic index (OI) for the jump group was 52.6 ± 8.7.
Discussion
An intensity of 3 times body weight for all jumps was maintained for the 2 week, 10 jumps/day (50 jumps/week) protocol. Bone resorption measured by TRAP5b and CTX resulted in variable outcomes. The change in TRAP5b was significantly different from the change in control but was not statistically significantly lower post jump intervention than pre (p = 0.0551). Whereas the CTX values were significantly lower post jumping compared to pre jumping however the change was not significantly different from the changes in control (interaction term). There was no significant change in bone formation measured by serum osteocalcin levels but a significant decrease in BAP levels. Bone structure benefits both from an increase in bone formation (osteoblast activity) and a decrease in bone resorption (osteoclast activity) following loading (Lee et al., 2003).
The magnitude and frequency of the jump training in this study was well controlled. Loading magnitude has been identified as an indicator of the osteogenic potential of an exercise protocol compared to the time or duration spent in an exercise activity (Turner and Robling, 2003). A 3.0 times body weight in the vertical ground reaction force (VGRF) at landing was reported to be a sufficient bone stimulus to induce the improvement in bone mineral density in young adults (Bassey et al., 1998, Fuchs et al., 2001). McKay et al., 2005 reported that a drop jump (DJ) and a counter movement jump (CMJ) following a drop jump (DJ) from a box elicited higher VGRF magnitudes than other jump styles, such as jumping jacks, alternating feet, and side to side jumps. All jumps during the protocol were greater than 3.0 times BW on either the first or second landing.
Evidence supports the efficacy of low-repetition high-impact jump exercises in enhancing bone mineral density (BMD) at the femoral neck in young women (Bassey et al., 1998; Kato et al., 2006) and also in improving the mechanical strength of bone in rats ( Honda et al., 2003; Umemura et al., 1997). Studies suggest that 10 jumps/day with maximal effort is an effective protocol to stimulate a positive response in bone and there was minimal additional effect with increased number of repetitions (> 40) since the mechanosensitivity of the bone to loading tends to fade as loading repetition is increased (Turner and Robling, 2003; Nagasawa et al., 2008). The attenuated sensitivity, however, can recover after 30 seconds of rest between jumps (Umemura et al., 2002b). The frequency of exercises per week is another important factor that can maximize the effectiveness of jump exercises on bone adaptation. Umemura et al., 2008 performed a study comparing the following exercise frequencies; one session per week (W1), three sessions per week (W3), five sessions per week (W5), and seven sessions per week (W7). They reported that the effect was significantly higher in the W7 group than the W1 and W3 groups, but the difference was not significantly different between the W5 and W7 groups. Five sessions per week and 30 second rest periods between jumps were used in the current study based on these results.
Any loading stimuli needs to exceed a certain threshold to induce an adaptive response to the bone (Schriefer et al., 2005a; 2005b). To exceed the threshold, the participants' maximum voluntary effort for the jump protocol was required throughout the 2-week protocol. The osteogenic index (OI), a measurement incorporating the magnitude and frequency the jumps, attempts to quantify the osteogenic effects of an exercise program. The average weekly OI in the current study of 52.6 was higher than other exercise studies that had maximum weekly OI's of 36.9 (Lester et al., 2009) and 34.0 (Erickson and Vukovich, 2010) but lower than the OI of a protocol to exhaustion (OI - 110) (if calculated using the Turner and Robling formula an OI of 43 results) (Rantalainen et al., 2009). However, all the studies did not calculate the osteogenic index (OI) similar to the formulae in Turner and Robling (2003) and therefore comparisons are difficult.
In the present study, bone resorption, measured by TRAP5b and CTX, was affected by the protocol. In the jump group, TRAP5b values were decreased by 5.0% (p = 0.0551) (did not reach significance) and CTX by 35% (p < 0.05). The decreased resorption may indicate a response prior to an increase in bone formation markers that has been reported following longer training protocols (8 weeks) (Erickson and Vukovich, 2010; Lester et al., 2009). TRAP5b is one of the first enzymes to be increased during osteoclast activity and has been associated with the number of osteoclasts (Rissanen et al., 2009). Whereas CTX, a measure of cross-link fragments in the serum, occurs later in the resorption cycle and may better represent osteoclast activity compared to TRAP5b (Rissanen et al., 2009). This larger decrease in CTX compared to TRAP5b may suggest there is a decrease in osteoclast activity rather than apoptosis of osteoclasts following the 2 week protocol. However, increased bone resorption markers, specifically CTX resulted from exhaustive jump (Rantalainen et al., 2009) and treadmill (Scott et al., 2011) protocols. One limitation of using CTX as an outcome measure for this study was the lack of fasting prior to blood draws and the variability in time of day for the blood draws. CTX values may decrease up to 50% following feeding and our change in CTX was 35%, in addition, the interaction term from the two-way ANOVA was not significant potentially in part due to the lack of control of nutritional status pre blood draw. This limitation may have also affected the other bone markers however; the impact would be less than that on the CTX levels. Future studies need to confirm if increased bone resorption follows most loading protocols or is associated only with exhaustive protocols.
The decrease in bone formation markers was not consistent following the jump protocol; a decrease in OC (2.2%) was not significant but the decrease in BAP (53%) was significant. Bone formation seems to be suppressed similar to bone resorption potentially indicative of a reversal phase in preparation for an increase in bone formation. Longer durations of training may result in suppressed bone resorption and increased bone formation that has been reported in animal exercise models (Nagasawa et al., 2008; Umemura et al., 2002a).
These data give an insight into the bone marker response to very short training protocols, the data suggest at early time points in a training protocol bone formation and bone resorption are both suppressed. However, more time intervals are needed to determine the time course of the bone serum markers, the increase in bone resorption markers following acute exercise needs to be integrated with the decreases in bone resorption markers following non-exhaustive training protocols. The time course for any increases in bone formation markers also needs to be established.
There were no significant changes in bone formation markers as indicated by OC value. Recent studies suggest that serum OC was affected not only by osteoblast activity, but also by the energy status of the body (Wolf, 2008) specifically OC is affected by glucose metabolism (Motyl et al., 2010). Even though the jump protocol was well controlled, participants' eating habits were not controlled nor observed, and there is a possibility that participants' diet before and during the intervention period affected the OC values.
The association between oral contraceptive use and bone health is still controversial. Oral contraceptives may have positive effects such as increased bone mass in young premenopausal women (Massaro et al., 2010; Wei et al., 2011), negative effects such as increased condylar resorption (Gunson et al., 2009) or no effect on bone mass (Vestergaard et al., 2008). There were 5 subjects (38.5%) in the jump group and 3 subjects (23.1%) in the control group who were using oral contraceptives and the effect of oral contraceptive on bone metabolism after high- impact exercise needs further investigation.
Conclusion
In conclusion, the results of the present study indicate that 2 weeks of jump activity consisting of 10 jumps/day for 5 days/week significantly decreased bone resorption measured by TRAP5b and CTX bone serum markers in young female non-athletes. On the other hand, bone formation measured by serum osteocalcin levels did not change significantly from the jumps but significant decreases were found in BAP levels.
Acknowledgments
Funding support for this project was provided by the Temple University's Office of the Senior Vice Provost for Undergraduate Studies through the The Creative Arts, Research and Scholarship Program (CARAS).
Biographies
Kohei Kishimoto
Employment
Temple University.
Degree
MSc, ATC.
Research interests
Bone metabolism.
Ryan P. Lynch
Employment
Temple University.
Degree
BSc, ATC.
Research interests
Bone metabolism.

Jamie Reiger
Employment
Temple University.
Degree
BSc, ATC.
Research interests
Bone metabolism.

Vanessa R. Yingling
Employment
Temple University.
Degree
PhD.
Research interests
Bone metabolism, biomechanics.
E-mail: yingling@temple.edu
References
- Adami S., Gatti D., Viapiana O., Fiore C.E., Nuti R., Luisetto G., Ponte M., Rossini M.(BONTURNO Study Group)(2008)Physical activity and bone turnover markers: a cross-sectional and a longitudinal study.Calcified Tissue International 883(6), 388-392 [DOI] [PubMed] [Google Scholar]
- Bassey E.J., Rothwell M.C., Littlewood J.J., Pye D.W.(1998)Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise.Journal of Bone and Mineral Research: The Official Journal of The American Society for Bone and Mineral Research 113(12), 1805-1813 [DOI] [PubMed] [Google Scholar]
- Burshell A.L., Moricke R., Correa-Rotter R., Chen P., Warner M.R., Dalsky G.P., Taylor K.A., Krege J.H.(2010)Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate.Bone 446(4), 935-939 [DOI] [PubMed] [Google Scholar]
- Eliakim A., Raisz L.G., Brasel J.A., Cooper D.M.(1997)Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males.Journal of Bone and Mineral Research: The Official Journal of The American Society for Bone and Mineral Research 112(10), 1708-1713 [DOI] [PubMed] [Google Scholar]
- Erickson C.R., Vukovich M.D.(2010)The osteogenic index and changes in bone markers during a jump-training program: A pilot study. Medicine and Science in Sports and Exercise 42(8): 1485-92 [DOI] [PubMed] [Google Scholar]
- Evans R.K., Antczak A.J., Lester M., Yanovich R., Israeli E., Moran D.S.(2008)Effects of a 4-month recruit training program on markers of bone metabolism.Medicine and Science in Sports and Exercise 440(11 Suppl), S660-670 [DOI] [PubMed] [Google Scholar]
- Fuchs R.K., Bauer J.J., Snow C.M.(2001)Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial.Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research 116(1), 148-156 [DOI] [PubMed] [Google Scholar]
- Fuchs R.K., Snow C.M.(2002)Gains in hip bone mass from high-impact training are maintained: a randomized controlled trial in children.The Journal of Pediatrics 1141(3), 357-362 [DOI] [PubMed] [Google Scholar]
- Gunson M.J., Arnett G.W., Formby B., Falzone C., Mathur R., Alexander C.(2009)Oral contraceptive pill use and abnormal menstrual cycles in women with severe condylar resorption: a case for low serum 17beta-estradiol as a major factor in progressive condylar resorption.American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, its Constituent Societies, and the American Board of Orthodontics 1136(6), 772-779 [DOI] [PubMed] [Google Scholar]
- Hind K., Burrows M.(2007)Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials.Bone 440(1), 14-27 [DOI] [PubMed] [Google Scholar]
- Honda A., Sogo N., Nagasawa S., Shimizu T., Umemura Y.(2003)High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as Sham rats.Journal of Applied Physiology 995(3), 1032-1037 [DOI] [PubMed] [Google Scholar]
- Iwamoto J., Yeh J.K., Aloia J.F.(1999)Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat.Bone 224(3), 163-169 [DOI] [PubMed] [Google Scholar]
- Johannsen N., Binkley T., Englert V., Neiderauer G., Specker B.(2003)Bone response to jumping is site-specific in children: a randomized trial.Bone 333(4), 533-539 [DOI] [PubMed] [Google Scholar]
- Kannus P., Haapasalo H., Sankelo M., Sievanen H., Pasanen M., Heinonen A., Oja P., Vuori I.(1995)Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players.Annals of Internal Medicine 1123(1), 27-31 [DOI] [PubMed] [Google Scholar]
- Kato T., Terashima T., Yamashita T., Hatanaka Y., Honda A., Umemura Y.(2006)Effect of low-repetition jump training on bone mineral density in young women.Journal of Applied Physiology 1100(3), 839-843 [DOI] [PubMed] [Google Scholar]
- Kohrt W.M., Bloomfield S.A., Little K.D., Nelson M.E., Yingling V.R.(2004)American College of Sports Medicine Position Stand: physical activity and bone health.Medicine and Science in Sports and Exercise 336(11), 1985-1996 [DOI] [PubMed] [Google Scholar]
- Lanyon L.E., Rubin C.T.(1984)Static vs dynamic loads as an influence on bone remodelling.Journal of Biomechanics 117(12), 897-905 [DOI] [PubMed] [Google Scholar]
- Lee K., Jessop H., Suswillo R., Zaman G., Lanyon L.(2003).Endocrinology: bone adaptation requires oestrogen receptor-alpha.Nature 4424(6947), 389. [DOI] [PubMed] [Google Scholar]
- Lester M.E., Urso M.L., Evans R.K., Pierce J.R., Spiering B.A., Maresh C.M., Hatfield D.L., Kraemer W.J., Nindl B.C.(2009)Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training.Bone 445(4), 768-776 [DOI] [PubMed] [Google Scholar]
- Massaro M., Di Carlo C., Gargano V., Formisano C., Bifulco G., Nappi C.(2010)Effects of the contraceptive patch and the vaginal ring on bone metabolism and bone mineral density: a prospective, controlled, randomized study.Contraception 881(3), 209-214 [DOI] [PubMed] [Google Scholar]
- McKay H., Tsang G., Heinonen A., Mac Kelvie K., Sanderson D., Khan K.M.(2005).Ground reaction forces associated with an effective elementary school based jumping intervention.British Journal of Sports Medicine 339(1), 10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K.J., McCabe L.R., Schwartz A.V.(2010)Bone and glucose metabolism: a two-way street.Archives of Biochemistry and Biophysics 5503(1), 2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Honda A., Sogo N., Umemura Y.(2008)Effects of low-repetition jump exercise on osteogenic response in rats.Journal of Bone and Mineral Metabolism 226(3), 226-230 [DOI] [PubMed] [Google Scholar]
- Noyes F.R., Barber-Westin S.D., Fleckenstein C., Walsh C., West J.(2005)The drop-jump screening test: difference in lower limb control by gender and effect of neuromuscular training in female athletes.The American Journal of Sports Medicine 333(2), 197-207 [DOI] [PubMed] [Google Scholar]
- Rantalainen T., Heinonen A., Linnamo V., Komi P., Takala T., Kainulainen H.(2009)Short-term bone biochemical response to a single bout of high-impact exercise.Journal of Sports Science & Medicine 88(4), 553-559 [PMC free article] [PubMed] [Google Scholar]
- Rantalainen T., Hoffren M., Linnamo V., Heinonen A., Komi P.V., Avela J., Nindl B.C.(2011)Three-month bilateral hopping intervention is ineffective in initiating bone biomarker response in healthy elderly men.European Journal of Applied Physiology 1111(9), 2155-2162 [DOI] [PubMed] [Google Scholar]
- Rissanen J.P., Ylipahkala H., Fagerlund K.M., Long C., Vaananen H.K., Halleen J.M.(2009)Improved methods for testing antiresorptive compounds in human osteoclast cultures.Journal of Bone and Mineral Metabolism 227(1), 105-109 [DOI] [PubMed] [Google Scholar]
- Robling A.G., Burr D.B., Turner C.H.(2001)Recovery periods restore mechanosensitivity to dynamically loaded bone. The Journal of Experimental Biology 2204(Pt 19), 3389-3399 [DOI] [PubMed] [Google Scholar]
- Schriefer J.L., Robling A.G., Warden S.J., Fournier A.J., Mason J.J., Turner C.H.(2005a)A comparison of mechanical properties derived from multiple skeletal sites in mice.Journal of Biomechanics 338(3), 467-475 [DOI] [PubMed] [Google Scholar]
- Schriefer J.L., Warden S.J., Saxon L.K., Robling A.G., Turner C.H.(2005b)Cellular accommodation and the response of bone to mechanical loading.Journal of Biomechanics 338(9), 1838-1845 [DOI] [PubMed] [Google Scholar]
- Scott J.P., Sale C., Greeves J.P., Casey A., Dutton J., Fraser W.D.(2011)The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise.Journal of Applied Physiology 1110(2), 423-432 [DOI] [PubMed] [Google Scholar]
- Scott J.P., Sale C., Greeves J.P., Casey A., Dutton J., Fraser W.D.(2010)The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running.The Journal of Clinical Endocrinology and Metabolism 995(8), 3918-3925 [DOI] [PubMed] [Google Scholar]
- Syed Z., Khan A.(2002)Bone densitometry: applications and limitations.Journal of Obstetrics and Gynaecology Canada 224(6), 476-484 [DOI] [PubMed] [Google Scholar]
- Turner C.H., Robling A.G.(2003)Designing exercise regimens to increase bone strength.Exercise and Sport Sciences Reviews 331(1), 45-50 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Baylink D.J., Wergedal J.E., Mohan S., Srivastava A.K.(2002a)A time course of bone response to jump exercise in C57BL/6J mice.Journal of Bone and Mineral Metabolism 220(4), 209-215 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Ishiko T., Tsujimoto H., Miura H., Mokushi N., Suzuki H.(1995)Effects of jump training on bone hypertrophy in young and old rats.International Journal of Sports Medicine 116(6), 364-367 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Ishiko T., Yamauchi T., Kurono M., Mashiko S.(1997)Five jumps per day increase bone mass and breaking force in rats.Journal of Bone and Mineral Research 112(9), 1480-1485 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Nagasawa S., Honda A., Singh R.(2008)High-impact exercise frequency per week or day for osteogenic response in rats.Journal of Bone and Mineral Metabolism 226(5), 456-460 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Sogo N., Honda A.(2002b)Effects of intervals between jumps or bouts on osteogenic response to loading.Journal of .Applied.Physiology 993(4), 1345-1348 [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L.(2008)Fracture risk in very young women using combined oral contraceptives.Contraception 778(5), 358-364 [DOI] [PubMed] [Google Scholar]
- Weeks B.K., Beck B.R.(2008)The BPAQ: a bone-specific physical activity assessment instrument.Osteoporosis International 119(11), 1567-1577 [DOI] [PubMed] [Google Scholar]
- Wei S., Jones G., Thomson R., Dwyer T., Venn A.(2011)Oral contraceptive use and bone mass in women aged 26-36 years. Osteoporosis International 22(1): 351-355 [DOI] [PubMed] [Google Scholar]
- Wolf G.(2008).Energy regulation by the skeleton. Nutrition Reviews666(4), 229-233 [DOI] [PubMed] [Google Scholar]
- Wulf G., Dufek J.S.(2009)Increased jump height with an external focus due to enhanced lower extremity joint kinetics.Journal of Motor Behavior 441(5), 401-409 [DOI] [PubMed] [Google Scholar]


