Abstract
We investigated the relationship between age and muscle size in both the appendicular and trunk regions of 1507 Japanese men and women aged 20 to 95 years. Seven hundred twenty-two men (young [aged 20-39 years], n = 211; middle-aged [aged 40-59 years], n = 347; and old [aged 6095 years], n = 164) and 785 women (young, n = 207; middle-aged, n = 341; and old, n = 237) were recruited for this cross-sectional study. Muscle thickness (MTH) and subcutaneous fat thickness (FTH) were measured by ultrasound at 8 sites on the anterior and posterior aspects of the body. MTH was expressed in terms relative to limb length (MTH/L) or height (MTH/Ht). Percent body fat was estimated from FTH, and fat-free mass (FFM) was calculated. In men, a graded decrease in FFM was found in all age groups. In women, FFM was similar in the young and middle-aged groups, but was lower in the oldest group. Age was significantly and inversely correlated with FFM in men (r = - 0.358, p < 0.01), but not in women (r = -0.08). On the other hand, age was strongly and inversely correlated with quadriceps MTH/L (men, r = -0.529; women, r = -0.489; both p < 0.001) and abdomen MTH/Ht (men, r = -0.464; women, r = -0.446; both p < 0.001) in both men and women, while there were only weak correlations between age and other lower limb and trunk sites. Our results indicated that sarcopenia is observed as a site-specific loss of skeletal muscle mass, especially for the quadriceps and abdominal muscles, in Japanese men and women aged 20 to 95 years.
Key points.
It is not fully understood whether age-related changes in muscle size differ between the appendicular and trunk muscles and/or between muscle groups located in the anterior and posterior aspects of the body in a large population.
Age-related muscle loss is observed as a site-specific, especially of the quadriceps and abdominal muscles, in Japanese men and women aged 20 to 95 years.
The age-related muscle losses are not supported by the muscle activation pattern of normal daily activities evaluated by EMG activity.
Key words: Sarcopenia, muscle distribution, daily physical activity
Introduction
Sarcopenia, which is defined as the age-related loss of skeletal muscle mass (SMM) (Evans and Campbell, 1993), leads to an increased risk of several diseases and mortality (Rantanen, 2003), as well as reduced physical function, and may eventually result in the loss of functional independence (Rantanen et al., 2002; Sowers et al., 2005). The etiology of sarcopenia is complex. Several factors have been implicated and include declining anabolic hormone concentrations (Morley, 2003), nutritional deficiencies (Morley et al., 2001), chronic inflammation (Cesari et al., 2005), and insulin resistance (Guillet and Boirie, 2005). Decreased physical activity levels that occur with aging also contribute to sarcopenia. It is well established that hormonal receptors for mRNA and transcriptional activity are increased (Bamman et al., 2001; Willoughby and Taylor, 2004) and there is reduced insulin resistance (Black et al., 2010) in exercising muscles following an acute bout of exercise for sedentary individuals. Thus, there are many important interrelationships between physical activity levels and other etiologic factors associated with health and aging that may contribute in varying degrees to the age-related loss of SMM in older men and women.
Previous studies have reported that SMM loss with increased age is greater in the lower limbs than in the upper extremities (Janssen et al., 2000; Miyatani et al., 2003) and is primarily associated with decreased use of the lower extremities in activities, such as walking. The ability to complete normal daily physical activities, such as personal care, cleaning, shopping, etc, are highly dependent on different muscle groups located both centrally in the trunk and peripherally in the limbs of the upper and lower body. Consequently, age-related SMM loss can impact normal daily activities if tissue losses are site-specific.
Most previous studies have quantified the loss of muscle tissue primarily at appendicular sites by either assessing limb muscle mass or measuring the muscle cross-sectional area (CSA) (Gallagher et al., 1997; Janssen et al., 2000). Unfortunately, it is not fully understood whether age-related changes in muscle size differ between the appendicular and trunk muscles and/or between muscle groups located in the anterior and posterior aspects of the body in a large population. We hypothesized that the ability to characterize age-related, site-specific muscle loss would aid in understanding the various degrees of etiologic sarcopenia. Therefore, the purpose of the present study was to investigate the relationship between age and muscle size in both the appendicular and trunk regions of 1507 men and women aged 20 to 95 years.
Methods
Subjects
A total of 1507 subjects (722 men and 785 women, aged 20-95 years) were recruited from participants who had community based general health examination in several cities. Prior to obtaining informed consent, a written description of the purpose of the study and its safety was distributed to potential subjects, along with a lifestyle questionnaire. All subjects were free of overt chronic disease (diabetes, angina, myocardial infarction, cancer, stroke, etc) as assessed by self-report. Candidates with clinically relevant cardiovascular or musculoskeletal disease, as well as those with cancer or previous stroke, were excluded from the study. The study was approved by the Ethics Committee for Human Experiments of the University of Tokyo, Japan.
Assessments of body mass index and body composition
Body mass and standing height were measured to the nearest 0.1 kg and 0.1 cm, respectively, by using a height scale and an electronic weight scale. Body mass index (BMI) was defined as body mass (kg)/height2 (m2). Selected anthropometric measures were obtained bilaterally from all subjects, as described previously (Abe et al., 1994): humerus, femur, and tibial lengths, and hip and waist circumferences. Percent body fat was estimated from subcutaneous fat thickness using an ultrasound-derived prediction equation (Abe et al. , 1994), and fat-free mass (FFM) was calculated.
Measurements of site-specific muscle size
Muscle size was measured using B-mode ultrasound (Aloka SSD-500, Tokyo, Japan) at 8 anatomic sites on the anterior and posterior aspects of the body (biceps, triceps, abdomen, subscapula, quadriceps, hamstrings, tibialis anterior, and triceps surae), as has been described previously (Abe et al., 1994). The measurements were taken while the subjects stood with their elbows and knees extended and relaxed. A 5-MHz scanning head was placed on the measurement site without depressing the dermal surface. The subcutaneous adipose tissue-muscle interface and the muscle-bone interface were identified from the ultrasonic image, and the distance between the 2 interfaces was recorded as muscle thickness (MTH). Previous studies have reported that MTH is strongly correlated with muscle CSA in limb and trunk muscles (Abe et al., 1997). The MTH was expressed in terms relative to limb length (MTH/L) or standing height (MTH/Ht).
Estimation of muscle mass
Total and regional SMM was estimated from ultrasound- derived prediction equations (Sanada et al., 2006) that converted MTH to mass (kilogram) by using site-matched MTH × height. These equations were then used to calculate total body SMM. A strong correlation (R2 = 0.94) was observed between magnetic resonance imaging-measured SMM and ultrasound-predicted SMM (Sanada et al., 2006).
Lifestyles variables
Sociodemographic and lifestyle characteristics (age, gender, alcohol consumption, smoking status, and habitual physical activity) were considered potential confounders of the relationship between site-specific muscle loss and age in both genders.
Statistical analysis
The differences between men and women and between age groups (young [20-39 years], middle-aged [40-59 years], and old [60-95 years]) were tested for significance by 1-way analysis of variance (ANOVA). Pearson product correlations were performed to determine the relationships between relative muscle thickness (MTH/L or MTH/Ht) and age, body composition, and lifestyle variables within each gender. Results are expressed as means and standard deviation (mean [SD]) for all variables. The P values < 0.05 were considered statistically significant.
Results
Subject characteristics
The subjects varied in age (20-95 years) and BMI (15.7-36.6 kg·m-2). Mean ages for men (47.9 [15.2] years) and women (49.5 [15.9] years) were similar. However, the men were taller and heavier than the women. BMI was similar in both men and women. In men, a graded decrease in FFM was found in all age groups. In women, FFM was similar in the young and middle-aged groups, but was lower in the oldest group (Table 1). Age was significantly and inversely correlated with FFM in men (r = -0.358, p < 0.01), but not in women (r = -0.08). The rate of habitual physical activity (once or more a week) was 35.5% and 38.3%, respectively, in men and women. There were no significant correlations between alcohol consumption or smoking status and FFM in both genders.
Table 1.
Subject characteristics. Values are means (SD).
| Age Range, y | n | Height, m* | Weight, kg* | BMI, kg·m-2 | Fat, %* | FFM, kg* | SMM, kg* |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 20-39 | 211 | 1.70 (.06) | 66.8 (8.8) | 23.2 (2.9) | 19.6 (5.3) | 53.4 (5.4) | 24.2 (3.1) |
| 40-59 | 347 | 1.66 (.06) | 64.7 (8.0) | 23.6 (2.6) | 19.5 (3.8) | 51.9 (5.2) | 21.9 (2.7) |
| 60-95 | 164 | 1.61 (.06) | 58.7 (8.2) | 22.6 (2.5) | 18.2 (3.5) | 47.9 (5.7) | 18.6 (2.8) |
| All men | 722 | 1.66 (.07) | 63.9 (8.8) | 23.3 (2.7) | 19.2 (4.3) | 51.4 (5.8) | 21.8 (3.4) |
| Women | |||||||
| 20-39 | 207 | 1.58 (.05) | 52.0 (6.1) | 20.9 (2.2) | 24.3 (4.0) | 39.2 (3.7) | 14.4 (1.7) |
| 40-59 | 341 | 1.53 (.06) | 54.4 (7.6) | 23.2 (3.1) | 26.6 (4.6) | 39.7 (4.2) | 13.7 (1.6) |
| 60-95 | 237 | 1.49 (.06) | 52.5 (8.1) | 23.8 (3.3) | 26.1 (5.3) | 38.5 (4.5) | 12.2 (1.8) |
| All women | 785 | 1.53 (.07) | 53.2 (7.5) | 22.8 (3.1) | 25.9 (4.7) | 39.2 (4.2) | 13.4 (1.9) |
*p < 0.01, men vs. women. BMI indicates body mass index; FFM, fat-free mass; SMM, skeletal muscle mass.
Effects of age on MTH and total SMM
A graded decrease in SMM was observed in both men and women (Table 1). The relative SMM (divided by height) also decreased gradually with increased age in both groups. In men, the relative MTHs of the arm (biceps and triceps), lower leg (tibialis anterior and triceps surae), and hamstrings were similar in the young and middle-aged groups, but were lower (p < 0.01) in the oldest group. On the other hand, the relative MTHs of the quadriceps (young, 1.35 [0.19] cm·m-1; middle-aged, 1.23 [0.19] cm·m-1; old, 1.03 [0.20] cm·m-1) and trunk (abdomen and subscapula) decreased (p < 0.01) gradually with increased age. In women, the relative MTHs of the arm, subscapula, and hamstrings were greater (p < 0.05) in the middle-aged and old groups than in the young group. However, quadriceps (young, 1.25 [0.18] cm·m-1; middle-aged, 1.18 [0.20] cm·m-1; old, 1.00 [0.19] cm·m-1) and abdomen MTH decreased (p < 0.05) gradually with increased age.
Relationship between age and muscle size
In both men and women, age was strongly and inversely correlated with quadriceps MTH/L (r = -0.529 and r = -0.489, respectively, both p < 0. 001) and abdomen MTH/Ht (r = -0.464 and r = -0.446, respectively, both p < 0.001) (Figure 1 and 2). In men, however, there were only weak correlations between age and arm MTH/L (r = -0.136 and r = -0.243), subscapula MTH/Ht (r = -0.182), and lower leg MTH/L (r = -0.180 and r = -0.198), Hamstring MTH/L (r = -0.068) was not correlated with age. In women, 3 sites of MTH (triceps and 2 lower leg) were not correlated with age, and there were only weak and positive correlations between age and biceps (r = 0.229), subscapula (r = 0. 160), and hamstrings (r = 0.167) (Figures 2 and 3). There were no significant correlations between alcohol consumption or smoking status and MTH in both genders.
Figure 1.
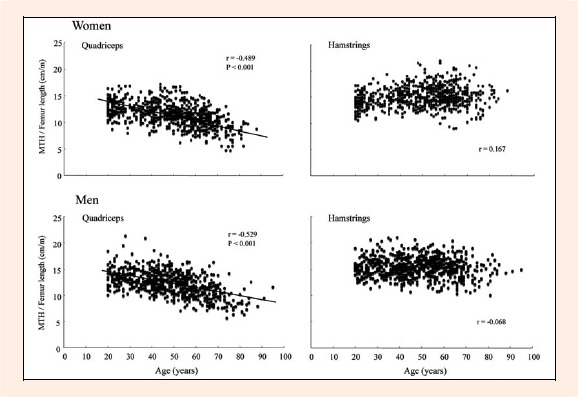
Relationships between age and quadriceps and hamstring MTH in Japanese men and women
Figure 2.
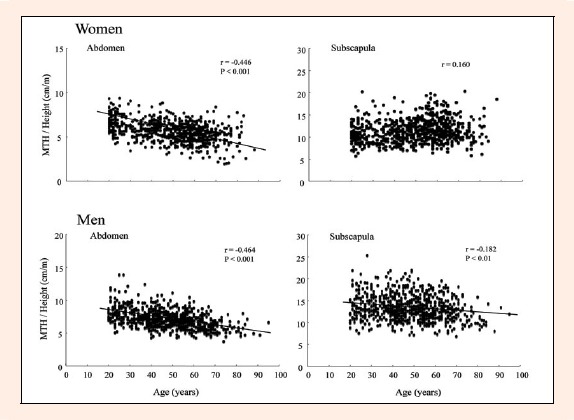
Relationships between age and abdomen and subscapula MTH in Japanese men and women
Figure 3.
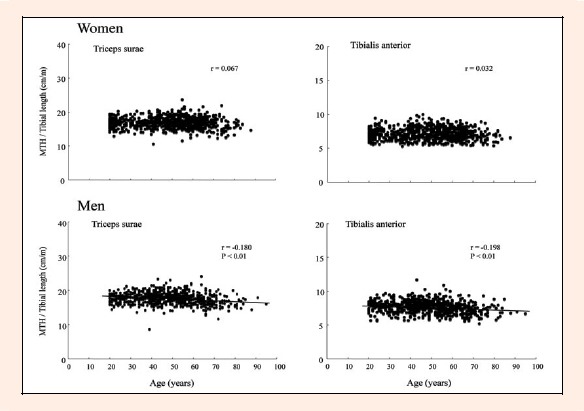
Relationships between age and triceps surae and tibialis anterior MTH in Japanese men and women
Discussion
To the best of our knowledge, this is the first study to demonstrate that age is correlated with site-specific loss of SMM in men and women aged 20 to 95 years. In the anterior and posterior regions of the thigh, age-related muscle loss was observed in quadriceps MTH but not in the posterior region. Our results coincide with a longitudinal study by Frontera et al., 2008, who reported significant reductions in total and anterior thigh muscle CSA after an 8.9-year follow- up, while the posterior muscle group did not change. In the present study, abdominal muscle mass decreased with increased age in both men and women, while the back muscles (subscapula MTH) were unchanged.
It is difficult to explain the site-specific muscle loss in the present study if one accepts the notion of a homogeneous loss of muscle tissue with increasing age owing to common etiologic factors. For instance, age-related declines in androgen concentrations may be a strong etiologic factor for sarcopenia (Morley, 2003), since circulating androgen acts to target tissue through specific hormone receptors that are up- regulated in exercising muscles but not in nonexercising muscles, following an acute bout of exercise. Our finding of an age-related, site-specific loss of SMM is probably associated with the pattern of muscle activation that occurs as normal daily activity begins to decline with increasing age.
Physiologic data concerning the intensity and duration of muscle activity in different muscles during normal daily activities are lacking. A limited number of studies (Sawai et al., 2006; Shirasawa et al., 2009) have reported that the electromyogram (EMG) activity patterns during ambulation differed among 3 lower-leg muscles (soleus, medial gastrocnemius, and tibialis anterior) even though the exercise intensity of those 3 muscles is similar (10%-20% of maximal voluntary effort). Muscle activation in the vastus lateralis and hamstrings muscles is also very low during ambulation. In the present study, lower-leg muscle size (both triceps surae and tibialis anterior) was only weakly correlated (r = -0.18 to -0.20) with age in both men and women (r = 0.03-0.07). Once again, in the lower body, only quadriceps muscle size was strongly correlated with age in both men and women.
To confirm the pattern of muscle activation, a total of 24 subjects (14 young and 10 old) in the present study were instructed to perform normal daily activities while EMG activity was recorded from the thigh muscles (Figure 4). The overall intensity associated with performing daily activities was higher in old subjects than in young subjects; however, both the hamstring (posterior thigh) as well as quadriceps (anterior thigh) muscles were comparably activated during each task. Thus, the age-related muscle losses seen in the current study are not supported by the muscle activation pattern measured in the study. While the cause of age-related, site-specific muscle loss is still unknown, it is suspected that a decline in habitual sport and physical activities, which require higher exercise intensities than most daily activities, may contribute to age-related loss in the size of the quadriceps. It should be noted that based on the findings of several previous studies, muscle mass and strength losses with increasing age can be attenuated by resistance exercise, at least in healthy older subjects (Frontera et al., 1988).
Figure 4.
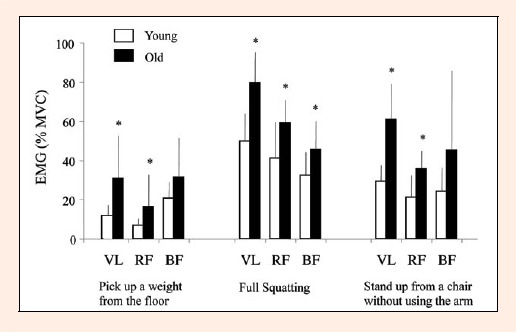
Electromyogram (EMG) activity patterns during normal daily activities. The EMG was recorded from the thigh muscles (VL, vastus lateralis; RF, rectus femoris; BF, biceps femoris). * p < 0.05, young vs. old
It has been documented that the loss of muscle mass is the single greatest contributing factor to strength declines observed in older men and women. In the present study, SMM in men and women decreased 23.1% and 15.3%, respectively, in the young (mean age 29 years) and old (mean age 68 years) groups. Our findings are consistent with those of previous studies (Gallagher et al., 1997; Janssen et al., 2000) that reported significantly greater muscle mass in men than in women at all ages and greater loss of muscle mass in men than in women with increasing age. In terms of site-specific muscle loss, a longitudinal study (Frontera et al., 2008) has provided evidence that reductions in thigh muscle CSA can be explained by losses in the anterior compartment since no changes were observed in muscle size and strength of the posterior compartment. The authors reported that the subjects maintained a similar level of physical activity during the 8.9 years that separated each testing period.
Muscle size has been determined for various limb and trunk muscle groups by ultrasound. Young et al. (1984; 1985) reported a 25% to 35% reduction in the ultrasound-measured quadriceps muscle CSA in old men and women compared with young controls, a finding that is consistent with the findings of the present study. However, it is not possible to measure muscle size by ultrasound without nonmuscle tissue. An increase in nonmuscle tissue in older muscle is a factor for changing muscle size. A previous study (Overend et al., 1992) reported increases in nonmuscle tissue of 59% for the quadriceps and 127% for the hamstrings in old subjects compared with young subjects. The absolute values of nonmuscle tissue in young and old subjects were 3.2 cm2 and 5.1 cm2, respectively, for quadriceps, and 2.2 cm2 and 5.0 cm2, respectively, for hamstrings. Although the relative change in nonmuscle tissue was larger in hamstrings, we cannot explain the site-specific muscle loss, especially the change in hamstring muscle size.
Conclusion
In conclusion, our results indicate that sarcopenia is observed as a site-specific loss of SMM, especially of the quadriceps and abdominal muscles, in Japanese men and women aged 20 to 95 years.
Acknowledgments
The authors thank the subjects who participated in this study. We also greatly appreciate the assistance of The University of Tokyo research staffs. This study was supported in part by The Ministry of Education, Science, Sports and Culture of Japan (grant No 05304048, No 11680043, No 15300221).
Biographies
Takashi Abe
Employment
Professor, University of Tokyo, Graduate School of Frontier Sciences, Japan
Degree
PhD
Research interest
Exercise physiology and sports performance, training science
E-mail: t12abe@gmail.com
Mikako Sakamaki
Employment
University of Tokyo, Graduate School of Frontier Sciences, Japan
Degree
PhD
Tomohiro Yasuda
Employment
University of Tokyo, Graduate School of Frontier Sciences, Japan
Degree
PhD
Michael G. Bemben
Employment
Professor, University of Oklahoma, Department of Health and Exercise Science, USA
Degree
PhD
Masakatsu Kondo
Employment
Professor, Nihon University, Japan
Yasuo Kawakami
Employment
Professor, Waseda University, Japan
Degree
PhD
Tetsuo Fukunaga
Employment
President, National Institute of Fitness and Sports in Kanoya, Japan
Degree
PhD
References
- Abe T., Kondo M., Kawakami Y., Fukunaga T. (1994) Prediction equations for body composition of Japanese adults by B-mode ultrasound. American Journal of Human Biology 6, 613-618 [DOI] [PubMed] [Google Scholar]
- Abe T., Kawakami Y., Suzuki Y., Gunji A., Fukunaga T. (1997) Effects of 20 days bed rest on muscle morphology. Journal of Gravitational Physiology 4, S10-S14 [PubMed] [Google Scholar]
- Bamman M.M., Shipp J.R., Jiang J., Gower B.A., Hunter G.R., Goodman A., McLafferty C.L., Jr, Urban R.J. (2001) Mechanical load increases muscle IGF-1 and androgen receptor mRNA concentrations in humans. American Journal of Physiology Endocrinology and Metabolism 280, E383-E390 [DOI] [PubMed] [Google Scholar]
- Black L.E., Swan P.D., Alvar B.A. (2010) Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. Journal of Strength and Conditioning Research 24, 1109-1116 [DOI] [PubMed] [Google Scholar]
- Cesari M., Kritchevsky S.B., Baumgartner R.N., Atkinson H.H., Penninx B.W., Lenchik L., Palla S.L., Ambrosius W.T., Tracy R.P., Pahor M. (2005) Sarcopenia, obesity, and inflammation–results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. American Journal of Clinical Nutrition 82, 428-434 [DOI] [PubMed] [Google Scholar]
- Evans W.J., Campbell W.W. (1993) Sarcopenia and age-related changes in body composition and functional capacity. Journal of Nutrition 123(2 suppl), 465-468 [DOI] [PubMed] [Google Scholar]
- Frontera W.R., Meredith C.N., O’Reilly K.P., Knuttgen H.G., Evans W.J. (1988) Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Journal of Applied Physiology 64, 1038-1044 [DOI] [PubMed] [Google Scholar]
- Frontera W.R., Reid K.F., Phillips E.M., Krivickas L.S., Hughes V.A., Roubenoff R., Fielding R.A. (2008) Muscle fiber size and function in elderly humans: a longitudinal study. Journal of Applied Physiology 105, 637-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D., Visser M., De Meersman R.E., Sepulveda D., Baumgartner R.N., Pierson R.N., Harris T., Heymsfield S.B. (1997) Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. Journal of Applied Physiology 83, 229-239 [DOI] [PubMed] [Google Scholar]
- Guillet C., Boirie Y. (2005) Insulin resistance: a contributing factor to age-related muscle mass loss?. Diabetes and Metabolism 31, 5S20-5S26 [DOI] [PubMed] [Google Scholar]
- Janssen I., Heymsfield S.B., Wang Z., Ross R. (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of Applied Physiology 89, 81-88 [DOI] [PubMed] [Google Scholar]
- Miyatani M., Kanehisa H., Azuma K., Kuno S., Fukunaga T. (2003) Site-related differences in muscle loss with aging: a cross-sectional survey on the muscle thickness in Japanese men and women aged 20 to 79 years. International Journal of Sport and Health Science 1, 34-40 [Google Scholar]
- Morley J.E. (2003) Hormones and the aging process. Journal of the American Geriatrics Society 51(7 suppl), S333-S337 [DOI] [PubMed] [Google Scholar]
- Morley J.E., Baumgartner R.N., Roubenoff R., Mayer J., Nair K.S. (2001) Sarcopenia. Journal of Laboratory and Clinical Medicine 137, 231-243 [DOI] [PubMed] [Google Scholar]
- Overend T.J., Cunningham D.A., Paterson D.H., Lefcoe M.S. (1992) Thigh composition in young and elderly men determined by computed tomography. Clinical Physiology 12, 629-640 [DOI] [PubMed] [Google Scholar]
- Rantanen T. (2003) Muscle strength, disability and mortality. Scandinavian Journal of Medicine and Science in Sports 13, 3-8 [DOI] [PubMed] [Google Scholar]
- Rantanen T., Sakari-Rantala R., Heikkinen E. (2002) Muscle strength before and mortality after a bone fracture in older people. Scandinavian Journal of Medicine and Science in Sports 12, 296-300 [DOI] [PubMed] [Google Scholar]
- Sanada K., Kearns C.F., Midorikawa T., Abe T. (2006) Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. European Journal of Applied Physiology 96, 24-31 [DOI] [PubMed] [Google Scholar]
- Sawai S., Sanematsu H., Kanehisa H., Tsunoda N., Fukunaga T. (2006) Sexual-related difference in the level of muscular activity of trunk and lower limb during basic daily life actions. Japanese Journal of Physical Fitness and Sports Medicine 55, 247-258 [Google Scholar]
- Shirasawa H., Kanehisa H., Kouzaki M., Masani K., Fukunaga T. (2009) Differences among lower leg muscles in long-term activity during ambulatory condition without any moderate to high intensity exercise. Journal of Electromyography and Kinesiology 19, e50-e56 [DOI] [PubMed] [Google Scholar]
- Sowers M.F., Crutchfield M., Richards K., Wilkin M.K., Furniss A., Jannausch M., Zhang D., Gross M. (2005) Sarcopenia is related to physical functioning and leg strength in middle-aged women. Journal of Gerontology 60A, 486-490 [DOI] [PubMed] [Google Scholar]
- Willoughby D.S., Taylor L. (2004) Effects of sequential bouts of resistance exercise on androgen receptor expression. Medicine and Science in Sports Exercise 36, 1499-1506 [DOI] [PubMed] [Google Scholar]
- Young A., Stokes M., Crowe M. (1984) Size and strength of the quadriceps muscles of old and young women. European Journal of Clinical Investigation 14, 282-287 [DOI] [PubMed] [Google Scholar]
- Young A., Stokes M., Crowe M. (1985) The size and strength of the quadriceps muscles of old and young men. Clinical Physiology 5, 145-154 [DOI] [PubMed] [Google Scholar]


