Abstract
The purpose of the study was to determine if an intensive pre- season training program modifies the inflammatory status in professional soccer players and if this inflammatory profile may be associated with the physical state. We compared plasma protein biomarkers, using proteomics, and the physiological state and cardiac function in 12 professional soccer players and 9 recreational soccer players. Reduced cardiac low frequency [LF] after the pre- season training program previous competition with respect to recreational soccer players was found. No differences were found in cardiac high frequency, cardiac high frequency/low frequency ratio, tension index and oxygen volume consumption. Alpha-1-antitrypsin isotype-3, fibrinogen-gamma isotypes-1, 2 and 3 and vitamin-D-binding protein isotype-1 were reduced in professionals players compared with those in recreational players. However, an increased content of alpha-1-antitrypsin isotype-6 and alpha-1-antichymotrypsin 1 and 4 were found in professional soccer players. Spearman’s analysis showed a positive correlation between LF and fibrinogen-gamma chain isotype 3; but LF was negatively correlated with alpha-antichymotrypsin isotype 4. Professional soccer players submitted to an intensive training showed differences in the content of plasma proteins associated with inflammatory/oxidative stress and thrombosis with respect to recreational soccer players. Proteomics analysis in combination with the analysis of cardiac function assessment may be useful to know more in depth molecular processes associated with sport and intensive exercise.
Key points.
Proteomics allow us to find differences in the plasma protein content in sportsmen.
Just after pre-season training program, professional soccer players showed lower content of circulating proteins associated with inflammation compared to recreational soccer players.
Proteomic analysis in combination with the analysis of cardiac function may be useful to know more in depth molecular inflammatory and oxidative processes associated with the sport.
Key words: Inflammation, proteomics, soccer, physical performance
Introduction
Regular practice of moderate aerobic exercise is associated with a reduced risk of cardiovascular-related disease (Shephard and Balady, 1999). Professional soccer players spend a variable time period previous to the season to improve their physical capacity. Intensive training intervention to improve cardiac output, and thereby aerobic endurance, has been proved positive in soccer performance in terms of distance covered, contacts with the ball and number of sprints during the match (Hoff, 2005). Indeed, the sportsmen subject to the highest levels of physical stress are the soccer players (Robson-Ansley et al., 2009). Several studies have reported that intensive exercise impairs the immune system, induces the synthesis of reactive oxygen species and increases the plasma concentration of pro-inflammatory cytokine such as interleukin-1 and -6 (IL-1, IL-6) and inflammatory-related acute phase reactants (Boluyt et al., 2006; Guelfi et al., 2006; Moldoveanu et al., 2000; Petersen and Pedersen, 2005). This acute-inflammatory reaction may favour an increasing number of muscle injuries, muscular overload and fatigue sensation that it could reduce the optimal physical performance of soccer players (Robson-Ansley et al., 2009; Lequesne et al., 1997). Although an extensive research has been developed to analyze the effect of acute exercise on inflammation and also if regular exercise may modify the inflammatory response associated with disease, less is known about the effect of regular training in healthy sportsman on the systemic expression of inflammatory-related biomarkers (Kim et al., 2009; Markovitch et al., 2008, Woods et al., 2009).
Until now, it has been difficult to monitor changes in several proteins at the same time in a single plasma sample. In the last years, a technology termed as proteomics has emerged, based in the use of two dimensional electrophoresis (2-DE) and mass spectrometry analysis. Proteomics provide us a useful methodology to quantify and identify changes in the content of multiple proteins and protein isotypes, which may be useful for understanding the molecular mechanisms involved in several processes, including the changes induced in the content of circulating proteins by exercise. Therefore, our hypothesis was that an intensive pre-season training program may modify the systemic inflammatory response in professional soccer players and that this inflammatory protein profile may be associated with the physical state of the athletes. Therefore, the aim of the present study was to compare plasma protein biomarkers, using proteomics, in professional soccer players after a pre-season training program with respect to recreational soccer players.
Methods
Study population
12 professional men soccer players (25 ± 4 years-old) from primer league of Spanish soccer who have been on pre-season training program and 9 sex and age matched subjects (26 ± 3 years-old) who have not practiced regular exercise training and only practice exercise during 3-4 hours/week were included in the study. The pre-season training program of the professional soccer players consisted of 20 hours/week during 3-4 weeks of moderate-heavy regular training. Exclusion criteria included the presence of an acute or chronic inflammatory disease, infection or injury and use of anti-inflammatory medication or supplements containing iron. In this regard, until the date in which blood samples were collected, professional soccer players as well as recreational soccer players did not take any additional nutritional supplements such as antioxidants and vitamins.
Blood samples for both experimental groups were obtained at rest time between 8:30 to 9:30 by peripheral venopuncture. Plasma was obtained by centrifugation and the samples were frozen at -80°C until the proteomic analysis was performed. This work is included within a genetic study to analyze genetic alterations associated with cardiovascular disease that was approved by the local Ethics Committee of Hospital Clínico San Carlos. All patients gave informed consent.
Physiological parameters
In order to evaluate the physical functional status, the Omegawave System (Omegawave Technologies Porland, OR, USA) was used. The Omegawave Sport Technology System is a non-invasive method to evaluate a number of physiological parameters in athletes, including the heart rate variability, aerobic and anaerobic capacity. Omegawave System is based in the measure of heart rate variability (HRV) that is a validated assessment of cardiac autonomic parameters comparable to standard 24-hour monitoring (Berkoff et al. , 2007; Sánchez et al., 2009). The Omegawave devices consists of a software package. The computer is connects to the ECG leads. Data were recorded for 2.5 minutes. The digitized signal was filtered according to common standards for ECG reading. R-R intervals were identified and were measured in milliseconds (ms) with an accuracy of ± 2 ms. All subjects were tested while lying supine on a padded table and were allowed to rest for up to 5 minutes until they reached a resting heart rate. Data were collected early morning (between 8 a.m. to 9 a.m.).
Proteomic analysis
For 2-dimensional electrophoresis, 500 g of plasma proteins were diluted in a buffer containing 8 mol·L-1 urea, 2% CHAPS (wt/vol), 40 mol·L-1 dithiothreitol, 0.2% Bio-Lyte ampholyte (Bio-Rad Labs, Hercules, CA), and 0,01 % (wt/vol) bromophenol blue. The samples were loaded on immobilized pH gradient gel strips (pH 4 to 7), and isoelectric focusing was performed using a Protean IEF cell system (Bio-Rad Labs). As previously reported (Alonso-Orgaz et al., 2006; López-Farré et al., 2007) the gels were actively rehydrated at 50 V for 60 hours followed by rapid and linear voltage ramping steps limited by a maximum current of 50 mA per gel. In the second dimension, the proteins from the strips were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels using a Protean II XL system (Bio-Rad Labs). Afterwards, the gels were fixed and silver-stained using the Silver Staining Kit Protein PlusOneTM (Amersham Biosciences) according to the manufacturer’s instructions.
Image acquisition analysis
As previously reported, (Sacristán et al., 2008) the stained gels were scanned in an Umax Powerlook III Scanner operated by the software Magic San V 4.5. Intensity calibration was carried out using an intensity stepwedge prior to gel image capture. Image analysis was carried out using the Quantity One 4.2.3 software (Bio-Rad). Image spots were initially detected, matched and then, manually edited. Each point intensity volume was processed by background subtraction.
Mass spectrometry (MS)
As reported in previous works (Mateos-Cáceres et al., 2004; Sacristán et al., 2008) the spots of interest were manually excised from the gels using biopsy bunches. In brief, for identification of each spot, spots from three different gels were obtained and analyzed after trypsin digestion. After, the peptides were purified using C18 Zip tips (Millipore). MS analysis was performed with 1μl of purified extracts mixed with 1μl of α-cyano 4-hydroxy-transcinnamic matrix (Sigma) in 50% acetoniltrile and the mixture (1μl) was spotted on to a MALDI plate. As reported, (Mateos-Cáceres et al., 2004; Sacristán et al., 2008) MS analyses were performed in a 4700 Proteomic Analyzer (Applied Biosystem) and operated in a reflector positive mode. All mass spectra were calibrated using a standard peptide mixture (Applied Biosystem). Peptides with a signal-to-noise greater than 20 were considered in the Mascot Database for protein identification. As reported (Mateos-Cáceres et al., 2004; Sacristán et al., 2008; Mateos-Cáceres et al., 2010) MASCOT database 1.9 (http://www.matrixscience.com) was used as algorithm to match the peptides obtained by MS. Identifications were accepted based on a tripartite evaluation that takes into account significant molecular weight search (Mowse) scores, spectrum annotation and observed versus expected migration on the 2-DE gel.
Determinations of Interleukin-6 (IL-6) and soluble intercellular adhesion molecule-1 (sICAM-1)
Interleukin-6 (IL-6) and soluble intercellular adhesion molecule-1 (sICAM-1) were measured in systemic plasma by enzyme-linked immunoabsorbent assays (ELISA) kits. The ELISA kits were purchased from Bender Med Systems (Vienna, Austria) for sICAM-1 and from R&D Systems [Minneapolis, US] for IL-6. The sensitivity of the ELISAs were 2.2 ng·mL-1 for sICAM-1 and 0.70 pg·mL-1 for IL-6. Duplicate analyses for each sample were performed, and the arithmetic average was used for data analyses.
Statistical analysis
Results are expressed as means ± standard deviation. To determine statistical differences between both groups the Mann-Whitney’s test was used. For correlations, Spearman’s analysis was performed. A p value <0.05 was considered statistically significant.
Results
Physical functional state
In the two studied groups, the following physiological parameters were analyzed: cardiac high frequency (HF), cardiac low frequency (LF), cardiac high frequency low frequency ratio (HF/LF), tension index, maxime oxygen volume consumption (VO2max), the standard deviation of the NN intervals (SDSD) and the square root of the mean squared differences of successive NN intervals (RMSSD). As table 1 shown, LF was significantly reduced in professional with respect to recreational soccer players. No statistical differences were found in any of the other physiological recorder parameters between the professional and recreational soccer players (Table 1).
Table 1.
Physiological parameters. Values are represented as means (±standard mean error).
| Parameter | Recreational soccer players | Professional soccer players |
|---|---|---|
| HF (m·s-2) | 473.1 (84.4) | 571.1 (190.6) |
| LF(m·s-2) | 745.5 (107.6) | 317.0 (84.4) * |
| LF/HF | 1.72 (.37) | 1.32 (.44) |
| VO2max (ml·Kg-1·min-1) | 57.3 (1.0) | 53.5 (1.2) |
| Tension Index | 75.8 (12.0) | 102.8 (17.2) |
| SDSD (ms) | 62.9 (6.6) | 77.3 (15.8) |
| RMSSD (ms) | 49.9 (5.5) | 59.7 (12.1) |
Abbreviations: Cardiac high frequency (HF), cardiac low frequency (LF), cardiac high frequency low frequency ratio (HF/LF), tension index, maxim oxygen volume consumption (VO2max), the standard deviation of the NN intervals (SDSD) and the square root of the mean squared differences of successive NN intervals (RMSSD).
* P value < 0.05.
Proteomic analysis
In the proteomic analysis, all the identified spots were al least expressed in 65% of the 2-DE gels within each of the two groups of patients.
We identified and quantified in the 2-DE gels the following proteins: alpha-1 antitrypsin isotypes 1 to 6, fetoprotein, three fibrinogen gamma chain isotypes, three vitamin D binding protein isotypes, five alpha-1 antichymotrypsin isotypes, six haptoglobin isotypes, six serotransferrin isotypes, tropomyosin (Table 2). In previous works from our group the identity of the above mentioned proteins were identified by mass spectrometry. In these previous published works mass spectrometry characteristics of the identified plasma proteins and protein isotypes were shown (Mateos-Cáceres et al., 2004; Sacristán et al., 2008). Plasma from professional soccer players showed a lower protein content of alpha-1 antitrypsin (ATT-1) isotype 3, fibrinogen gamma chain isotypes 1, 2 and 3 and vitamin D binding protein isotype 1 with respect to those observed in the plasma from recreational soccer players (Table 2). Moreover, a significative higher plasma content of alpha-1 antitrypsin isotype 6 and alpha-1-antichymotrypsin isotypes 1 and 4 were found in the plasma from professional soccer players with respect to those in the recreational group (Table 2).
Table 2.
Results of the plasma protein isoforms analyzed using proteomics. Data are means (±standard mean error).
| PROTEIN | Recreational Soccer Players [A.U.] |
Professional Soccer Players [A.U.] |
|---|---|---|
| Albumin | 16702 (3506) | 8509 (2324) |
| Alpha-1 antitrypsin | ||
| Isotype 1 | 542 (196) | 306 (85) |
| Isotype 2 | 288 (89) | 336 (62) |
| Isotype 3 | 1027 (249) | 315 (54) * |
| Isotype 4 | 747 (237) | 561 (182) |
| Isotype 5 | 266 (77) | 327 (93) |
| Isotype 6 | 207 (52) | 552 (125) * |
| Fetoprotein | 134 (22) | 220 (32) |
| Fibrinogen gamma chain | ||
| Isotype 1 | 557 (121) | 203 (44) * |
| Isotype 2 | 724 (221) | 250 (40) * |
| Isotype 3 | 494 (110) | 236 (36) * |
| Vitamin D binding protein | ||
| Isotype 1 | 683 (274) | 103 (20) * |
| Isotype 2 | 235 (102) | 236 (59) |
| Isotype 3 | 219 (71) | 42 (6) |
| Alpha-1 antichymiotrypsin | ||
| Isotype 1 | 65 (26) | 227 (52) * |
| Isotype 2 | 241 (62) | 398 (99) |
| Isotype 3 | 249 (44) | 428 (85) |
| Isotype 4 | 121 (28) | 376 (65) * |
| Isotype 5 | 72 (13) | 130 (23) |
| Haptoglobin | ||
| Isotype 1 | 403 (125) | 905 (221) |
| Isotype 2 | 356 (93) | 446 (193) |
| Isotype 3 | 433 (87) | 507 (159) |
| Isotype 4 | 624 (263) | 550 (136) |
| Isotype 5 | 366 (95) | 520 (80) |
| Isotype 6 | 173 (74) | 221 (42) |
| Serotransferrin | ||
| Isotype 1 | 480 (61) | 413 (68) |
| Isotype 2 | 1233 (290) | 1131 (240) |
| Isotype 3 | 954 (165) | 997 (201) |
| Isotype 4 | 812 (58) | 606 (109) |
| Isotype 5 | 557 (91) | 441 (131) |
| Tropomyosin | 328 (99) | 195 (41) |
A.U. densitometric arbitrary units.
* p < 0.05.
Non-significant differences were found between the two experimental groups in the plasma protein content of fetoprotein, the six haptoglobin isotypes, serotransferrin isotypes 1, 2, 3, 5 and tropomyosin (Table 2).
Circulating plasma levels of Interleukin-6 and soluble intercellular adhesion molecule-1
The plasma levels of the pro-inflammatory proteins IL-6 and sICAM-1 were measured in both groups of soccer players. Non statistical differences were found in the circulating plasma levels of IL-6 and sICAM-1 between professional and recreational soccer players (Table 3).
Table 3.
Circulating plasma levels of interleukin-6 (IL-6) and soluble Intracellular Adhesion Molecule-1 (sICAM-1). Data are means (±standard mean error).
| PROTEIN | Recreational Soccer Players |
Professional Soccer Players |
|---|---|---|
| IL-6 (pg·mL-1) | 1.10 (.96) | 1.58 (.27) |
| ICAM-1 (pg·mL-1) | 36.8 (3.6) | 35.8 (4.6) |
Relationship between the changes in the content of plasma proteins and the physical functional state
The relationship between the protein content in the plasma and the physical functional state of the soccer players was analyzed. Spearman’s analysis showed a positive correlation between LF and fibrinogen gamma-chain isotype 3 (Figure 1). LF also showed a negative and significative correlation between the plasma content of alpha antichymotrypsin isotype 4 and LF (Figure 1).
Figure 1.
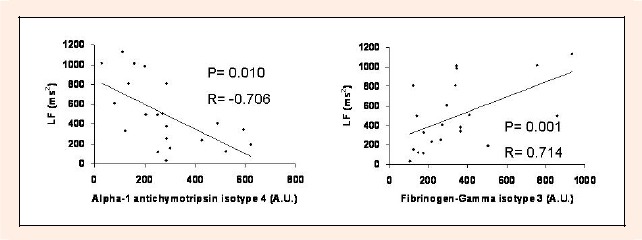
Scatter plots showing the relationship between low frequency and the plasma levels of fibrinogen-gamma isotype 3 and alpha-1 antichymotrypsin isotype 4. A.U.: densitometric arbitrary units
Discussion
The present study compared the plasma content of inflammatory-associated proteins, using proteomics, and the physiological state, using the Omegawave system, in professional soccer players after an intensive training program and before starting the league. A group of players who practice soccer at weekends were used as comparative group. The recreational soccer players have not been submitted to a specific training program previous the competition.
In the plasma proteomic map six isotypes of ATT-1 were identified. The plasma ATT-1 isotype 6 was founded increased, but ATT-1 isotype 3 decreased in professional soccer players compared with those in recreational players. ATT-1 is a powerful inhibitor of several proteolytic proteins, mainly released from neutrophils, and it is involved in the resolution of the inflammatory response (Brantly, 2002). Studies have shown that the level of ATT-1 was increased after the exercise probably to counterbalance the increased neutrophil elastase production which indirectly inhibits the complement activation induced during and / or after the exercise (Dufaux et al., 1991; Petibois et al., 2003; Semple et al., 2006). Therefore, the intensive and prolonged exercise may favour changes in the systemic content of specific ATT-1 isotypes. In this regard, it has not been established the role for each ATT-1 isotype, some ATT-1 genotypes have been associated with a reduced risk of ischemic cerebrovascular and heart diseases (Talmud and Stephens, 2004) and even with acute cardiovascular disease (Mateos-Cáceres et al., 2004) Professional soccer players showed an increased plasma level of alpha-1 antichymotrypsin isotypes 1 and 4. Alpha-1 antichymotrypsin is an anti-inflammatory factor that its main function is as serine protease inhibitor (Kalsheker et al., 2002). Works performed in patients with cardiovascular disease have demonstrated reduction of inflammatory- related proteins after supervised endurance training (Niessner et al., 2006). The fact that endurance training increased the plasma level of anti-inflammatory-associated proteins such as antichymotrypsin isotypes 1 and 4 anti may suggest that regular exercise reduced inflammation. However, in the present study, the circulating plasma levels of two pro-inflammatory biomarkers namely IL-6 and sICAM-1 were not modified in professional with respect to recreational soccer players. Therefore, the fact that the plasma content of antichymotrypsin isotypes were increased in the professional soccer players after the pre-season-training program might suggest that regular training favours an anti-inflammatory state without modifications in the synthesis of pro-inflammatory agents. Alpha-1 antichymotrypsin levels have been also associated with the control of oxidative damage (Licastro et al., 2001). Indeed, a soccer match increases the levels of oxidative stress and muscle damage throughout 72 hours-recovery period (Ascensão et al., 2008; Vollaard et all, 2005). Therefore, protective mechanism against the oxidative damage such as an increased plasma antichymotrypsin level may be favoured by regular exercise.
The proteomic analysis also revealed a decreased content of fibrinogen gamma-chain isotypes 1, 2 and 3 in the professional soccer players with respect to those in the recreational group. Fibrinogen is a glycoprotein involved in platelet aggregation since increases the binding of platelets, endothelial cells, and leukocytes between them (Bombeli et al., 1998; Fibrinogen Studies Collaboration et al., 2007). These findings may be in accordance with previous observations that moderate-intensity exercise training reduced platelet reactivity and enhances fibrinolysis at rest (Wang, 2006). However, some authors have also reported that these favorable effect of regular exercise training on thrombotic state return to a pre-training state after a period of deconditioning (Wang, 2006).
DBP isotype 1 was significantly decreased in the professional soccer players with respect to the recreational group. The DBP is a serum alpha-2-globulin that binds vitamin D (Gomme and Bertolini, 2004). Moreover, DBP has been also described as leukocyte activator (Zhang and Kew, 2004) and contributes to the sequestration of actin, stimulation of osteoclast activity, transport of fatty acids and also inhibits actin-induced platelet aggregation (Meier et al., 2006; Speeckaert et al., 2006). Therefore, the here reported decreasing of DBP isotype 1 content in professional soccer players may be also associated with the anti-thrombotic state that seem to be related to the intensive training (Wang et al., 2006).
Relationship between the changes in the content of plasma proteins and physical functional state
A non-significantly increasing in VO2max, cardiac high frequency, SDSD, RMSSD and tension index and a statistical significant decreasing of cardiac low frequency were observed in professional soccer players compared with control group. Previous studies have reported that aerobic training balance towards the vagal modulation which it reflected in heart rate variability parameters such as a decrease in the cardiac low frequency portions (Hautala et al., 2009). The individual variability in the physiological determined parameters may probably explain why non- statistical differences were found between the two experimental groups.
When all the study population was analyzed, a negative association between cardiac low frequency and the plasma content of alpha-1 antichymotrypsin isotype 4, and a positive association between cardiac low frequency and fibrinogen gamma-chain isotype 3 was found. Our results suggest that the cardiac functional state of soccer players may be correlated with these proteins. However, the facts that some proteins that their plasma content between professional and recreational players was changed did not correlate with the cardiac low frequency suggest that their plasma content was non- dependent of haemodinamic factors. In this regard, it is well established that the content on many proteins could be regulated by vascular shear stress. Vascular shear stress has been demonstrated increased by physical exercise particularly shear stress affects gene expression in endothelin (Niebauer and Cooke, 1996).
Conclusion
As conclusion, proteomic study has demonstrated differences in the content of proteins in the plasma associated with inflammatory/oxidative stress and thrombosis between professional and recreational soccer player. Particularly, professional soccer players showed a decreased content of circulating proteins associated with inflammation compared with those in recreational soccer players. In addition, these data suggest that proteomic analysis in combination with the analysis of cardiac function assessment may be useful to know more in depth molecular inflammatory and oxidative processes associated with the sport.
Acknowledgments
This work has been supported by Fondo de Investigaciones de la Seguridad Social (Redes temáticas de Cooperación Red Heracles RD06/0009/010). Petra J. Mateos-Cáceres is staff of Fundación para la Investigación Biomédica del Hospital Clínico San Carlos. José J Zamorano-León is a staff supported by Red Heracles. The authors thank the collaboration of the Science Research Center from Atlético de Madrid Foundation and Begoña Larrea and Miguel Dantart for their editorial assistance.
Biographies

Francisco J. Martín-Sánchez
Employment
Emergency Department from Hospital Clínico San Carlos de Madrid, Spain.
Degree
MD and PhD
Research interest
The systemic inflammatory response and functional status of recreational soccer players.
E-mail: fjjms@hotmail.com

José María Villalón
Employment
Head of Medical Department of Atlético de Madrid Football Club.
Degree
MD
Research interest
Sport medicine.
E-mail: josemaria_villalon@yahoo.es

José J. Zamorano-León
Employment
Cardiovascular Research Unit from Hospital Clínico San Carlos de Madrid, Spain
Degree
PhD
Research interest
Biologist with experience in molecular biology and proteomic technique.
E-mail: josejavier.zamorano.hcsc@salud.madrid.org

Luis Fernández Rosas
Employment
Head of Physiotherapy in the Medicine Faculty of San Pablo CEU University.
Degree
MD
Research interest
Sports medicine.
E-mail: luferro@ceu.es

Ricardo Proietti
Employment
Sports Science Consultant
Degree
Degree in physical activity and sports
Research interest
Designing training program in professional athletes, with a high experience in study of physical performance of professional athletes.
Email: r.proietti@omegawave.com

Petra J. Mateos-Caceres
Employment
Cardiovascular Research Unit from Hospital Clínico San Carlos de Madrid, Spain.
Degree
PhD
Research interest
The cardiovascular pathology, using proteomic technique.
Email: pjimenezm.hcsc@salud.madrid.org

Juan J. González-Armengol
Employment
Coordinator of Telemedicine from Hospital Clínico San Carlos de Madrid, Spain.
Degree
MD and PhD
Research interest
The systemic inflammatory response and functional status of recreational soccer players.
E-mail: jjgarmengol@hotmail.com

Pedro Villarroel
Employment
Head of Emergency Department from Hospital Clínico San Carlos de Madrid, Spain.
Degree
MD
Research interest
The systemic inflammatory response and functional status of recreational soccer players.
E-mail: pvillarroel.hcsc@salud.madrid.org

Carlos Macaya
Employment
Head of Cardiology Department from Hospital Clínico San Carlos de Madrid, Spain.
Degree
MD and PhD
Research interest
Cardiovascular diseases.
E-mail: cmacaya.hcsc@salud.madrid.org

Antonio J. López-Farré
Employment
Head of Cardiovascular Research Unit from Hospital Clínico San Carlos de Madrid, Spain.
Degree
PhD
Research interest
Cardiovascular diseases.
E-mail: lcarinv.hcsc@salud.madrid.org
References
- Alonso-Orgaz S., Moreno L., Macaya C., Rico L., Mateos-Cáceres P.J., Sacristán D., Pérez-Vizcaíno F., Segura A., Tamargo J., López-Farré A. (2006) Proteomic study of plasma from moderate hypercholesterolemic patients. Journal Proteome Research 5, 2301-2308 [DOI] [PubMed] [Google Scholar]
- Ascensão A., Rebelo A., Oliveira E., Marques F., Pereira L., Magalhães J. (2008) Biochemical impact of a soccer match - analysis of oxidative stress and muscle damage markers throughout recovery. Clinical Biochemistry 41, 841-851 [DOI] [PubMed] [Google Scholar]
- Berkoff D.J., Cairns C.B., Sanchez L.D., Moorman C.T., 3rd. (2007) Heart rate variability in elite American track-and-field athletes. Journal of Strength Conditioning Research 21, 227-231 [DOI] [PubMed] [Google Scholar]
- Boluyt M.O., Brevick J.L., Rogers D.S., Randall M.J., Scalia A.F., Li Z.B. (2006) Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein hsp20. Proteomics 6, 3154-3169 [DOI] [PubMed] [Google Scholar]
- Bombeli T., Schwartz B.R., Harlan J.M. (1998) Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. Journal Experimental Medicine 187, 329-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly M. (2002) Alpha1-antitrypsin: not just an antiprotease: extending the half-life of a natural anti-inflammatory molecule by conjugation with polyethylene glycol. American Journal Respiratory Cell Molecular Biology 27, 652-654 [DOI] [PubMed] [Google Scholar]
- Dufaux B., Order U., Liesen H. (1991) Effect of a short maximal physical exercise on coagulation, fibrinolysis, and complement system. International Journal of Sports Medicine 12(Suppl1), S38-42 [DOI] [PubMed] [Google Scholar]
- Fibrinogen Studies Collaboration Kaptoge S., White I.R., Thompson S.G., Wood A.M., Lewington S., Lowe G.D., Danesh J. (2007) Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. American Journal Epidemiology 166, 867-879 [DOI] [PubMed] [Google Scholar]
- Gomme P.T., Bertolini J. (2004). Therapeutic potential of vitamin D-binding protein. Trends Biotechnology 22, 340-345 [DOI] [PubMed] [Google Scholar]
- Guelfi K.J., Casey T.M., Giles J.J., Fournier P.A., Arthur P.G. (2006) A proteomic analysis of the acute effects of high-intensity exercise on skeletal muscle proteins in fasted rats. Clinical and Experimental Pharmacoly Physiology 33, 952-957 [DOI] [PubMed] [Google Scholar]
- Hautala A.J., Kiviniemi A.M., Tulppo M.P. (2009) Individual responses to aerobic exercise: the role of the autonomic nervous system. Neuroscience Biobehavioral Reviews 33, 107-115 [DOI] [PubMed] [Google Scholar]
- Hoff J. (2005). Training and testing physical capacities for elite soccer players. Sports Science 23, 573-582 [DOI] [PubMed] [Google Scholar]
- Kalsheker N., Morley S., Morgan K. (2002) Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochemical Society Transactions 30, 93-98 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee Y.H., Kim C.K. (2009) Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42195 km) and a ultra-marathon (200 km) race. European Journal of Applied Physiology 105, 765-770 [DOI] [PubMed] [Google Scholar]
- Lequesne M.G., Dang N., Lane N.E. (1997) Sport practice and osteoarthritis of the limbs. Osteoarthritis Cartilage 5, 75-86 [DOI] [PubMed] [Google Scholar]
- Licastro F., Pedrini S., Davis L.J., Caputo L., Tagliabue J., Savorani G., Cucinotta D., Annoni G. (2001) Alpha-1-antichymotrypsin and oxidative stress in the peripheral blood from patients with probable Alzheimer disease: a short-term longitudinal study. Alzheimer Disease Association Disorder 15, 51-55 [DOI] [PubMed] [Google Scholar]
- López-Farré A.J., Mateos-Cáceres P.J., Sacristán D., Azcona L., Bernardo E., de Prada T.P, Alonso-Orgaz S, Fernández-Arquero M., Fernández-Ortiz A., Macaya C. (2007) Relationship between vitamin D binding protein and aspirin resistance in coronary ischemic patients: a proteomic study. Journal Proteome Research 6, 2481-2487 [DOI] [PubMed] [Google Scholar]
- Markovitch D., Tyrrell R.M., Thompson D. (2008) Acute moderate-intensity exercise in middle-aged men has neither an anti- nor proinflammatory effect. Journal Applied Physiology 105, 260-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Cáceres P.J., Macaya C., Azcona L., Modrego J., Mahillo E., Bernardo E., Fernandez-Ortiz A., López-Farré A.J. (2010) Different expression of proteins in platelets from aspirin-resistant and aspirin-sensitive patients. Thrombosis Haemostasis 103, 160-170 [DOI] [PubMed] [Google Scholar]
- Mateos-Cáceres P.J., García-Méndez A., López Farré A., Macaya C., Núñez A., Gómez J., Alonso-Orgaz S., Carrasco C., Burgos M.E., de Andrés R., Granizo J.J., Farré J., Rico L.A. (2004) Proteomic analysis of plasma from patients during an acute coronary syndrome. Journal American College Cardiology 44, 1578-1583 [DOI] [PubMed] [Google Scholar]
- Meier U., Gressner O., Lammert F., Gressner A.M. (2006) Gc-globulin: roles in response to injury. Clin Chem 52, 1247-1253 [DOI] [PubMed] [Google Scholar]
- Moldoveanu A.I., Shephard R.J., Shek P.N. (2000) Exercise elevates plasma levels but not gene expression of IL-1beta, IL-6, and TNF-alpha in blood mononuclear cells. Journal Applied Physiology, 89, 1499-1504 [DOI] [PubMed] [Google Scholar]
- Niebauer J., Cooke J.P. (1996) Cardiovascular effects of exercise: role of endothelial shear stress. Journal American College Cardiology 28, 1652-1660 [DOI] [PubMed] [Google Scholar]
- Niessner A., Richter B., Penka M., Steiner S., Strasser B., Ziegler S., Heeb-Elze E., Zorn G., Leitner-Heinschink A., Niessner C., Wojta J., Huber K. (2006). Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: impact on plaque stabilization?. Atherosclerosis 186, 160-165 [DOI] [PubMed] [Google Scholar]
- Petersen A.M., Pedersen B.K. (2005) The anti-inflammatory effect of exercise. Journal Applied Physiology 98, 1154-1162 [DOI] [PubMed] [Google Scholar]
- Petibois C., Cazorla G., Poortmans J.R., Déléris G. (2003) Biochemical aspects of overtraining in endurance sports: the metabolism alteration process syndrome. Sports Medicine 33, 83-94 [DOI] [PubMed] [Google Scholar]
- Robson-Ansley P., Barwood M., Canavan J., Hack S., Eglin C., Davey S., Hewitt J., Hull J., Ansley L. (2009) The effect of repeated endurance exercise on IL-6 and sIL-6R and their relationship with sensations of fatigue at rest. Cytokine 45, 111-116 [DOI] [PubMed] [Google Scholar]
- Sacristán D., Marques M., Zamorano-León JJ., Luque M., Armengol J., del Castillo J., Martín J., Delpón E., Ramos-Mozo P., Tamargo J., Barrientos A., Macaya C., López-Farré A. (2008) Modifications by olmesartan treatment of the platelet protein profile of moderate hypertensive patients. Proteomics Clinical. Application 2, 1300-1312 [DOI] [PubMed] [Google Scholar]
- Sacristán D., López-Farré A.J., Zamorano-León J.J., Azcona L., Fernández-Ortiz A., Romero J., Farré J., Macaya C. (2008) Effects of coronary prestenting platelet inhibition on coronary poststenting inflammation. Journal Cardiovascular Pharmacology 51, 286-292 [DOI] [PubMed] [Google Scholar]
- Sánchez L.D., Pereira J., Berkoff D.J. (2009) The evaluation of cardiac complaints in marathon runners. Journal Emergency Medicine 36, 369-376 [DOI] [PubMed] [Google Scholar]
- Semple S.J., Smith L.L., McKune A.J., Hoyos J., Mokgethwa B., San Juan A., Lucia A., Wadee A.A. (2006) Serum concentrations of C reactive protein, alpha1 antitrypsin, and complement (C3, C4, C1 esterase inhibitor) before and during the Vuelta a España. British Journal Sports Medicine 40, 124-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard R.J., Balady G.J. (1999) Exercise as cardiovascular therapy. Circulation 99, 963-972 [DOI] [PubMed] [Google Scholar]
- Speeckaert M., Huang G., Delanghe J.R., Taes Y.E. (2006) Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clinica Chimica Acta 372, 33-42 [DOI] [PubMed] [Google Scholar]
- Talmud P.J., Stephens J.W. (2004). Lipoprotein lipase gene variants and the effect of environmental factors on cardiovascular disease risk. Diabetes Obesity Metabolism 6, 1-7 [DOI] [PubMed] [Google Scholar]
- Vollaard N.B., Shearman J.P., Cooper C.E. (2005) Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Medicine 35, 1045-1062 [DOI] [PubMed] [Google Scholar]
- Wang J.S. (2006). Exercise prescription and thrombogenesis. Journal Biomedicine Science 13, 753-761 [DOI] [PubMed] [Google Scholar]
- Woods J.A., Vieira V.J., Keylock K.T. (2009) Exercise, inflammation, and innate immunity. Immunology Allergy Clinics North America 29, 381-393 [DOI] [PubMed] [Google Scholar]
- Zhang J., Kew R.R. (2004) Identification of a region in the vitamin D-binding protein that mediates its C5a chemotactic cofactor function. Journal Biological Chemistry 279, 53282-53287 [DOI] [PubMed] [Google Scholar]


