Abstract
Biosimilars, although not identical to their originator product, are expected to become essential in reducing health care costs and improving access to lifesaving drugs. The FDA must find a way to balance rigorous testing to ensure quality, as is done for generic chemical drugs, with providing a cost-efficient way to expedite approvals of these products.
This is the first in a series of two articles about biosimilars. Part 2 will discuss the potential issues and challenges that P&T committees will face when evaluating biosimilars.
Introduction
Patents for many branded biologics will expire during the next few years, allowing biosimilars manufacturers to seek FDA approval for generic versions of these agents.1 The Biologics Price Competition and Innovation Act (BPCIA) of 2009, which was passed as part of the health care reform legislation enacted into law in 2010, authorizes the FDA to establish a long-awaited regulatory pathway for biosimilars. In 2012, the FDA issued draft guidance summarizing the proposed criteria for this pathway; this guidance is yet to be finalized.
These criteria have inspired debate and the emergence of several critical issues, such as to what extent the biosimilars pathway should be abbreviated, how much clinical data should be required for approval, or when an agent should be designated as comparable or interchangeable with an originator biologic.2 These parameters will determine the ease and cost for a manufacturer to develop and market a biosimilar and will also ultimately influence the price of these medications.2
The availability of biosimilars is eagerly anticipated, because these agents are expected to improve affordability and promote wider and earlier access to critical, often lifesaving therapeutic interventions.3,4 Ideally, the FDA’s finalized guidelines will establish a regulatory path for biosimilars that will ensure patient safety, control development costs, and encourage innovation by manufacturers.2
The Expanding Role of Biologics in Health Care
What Are Biologics and Biosimilars?
According to the U.S. Federal Code of Regulations (CFR), the definition of a biologic is “any virus, therapeutic serum, toxin, antitoxin, or analogous product applicable to the prevention, treatment, or cure of disease or injuries of man.”5 Biologics were first developed in the 1980s using recombinant techniques to copy or improve on naturally occurring complex peptides, proteins, and glycoproteins.1,3,4 Since then, even more complex products, such as monoclonal antibodies, have been produced through the manipulation of the DNA in bacteria, yeast, or mammalian cells to produce therapeutic or diagnostic agents.1,6–8 Biologic therapies available today include enzymes, vaccines, human insulins, interferons, interleukins, erythropoietins, gonadotropins, granulocyte–colony-stimulating factors (G–CSFs), human growth hormones, monoclonal antibodies, blood coagulation modifiers, and tissue plasminogen activators.7
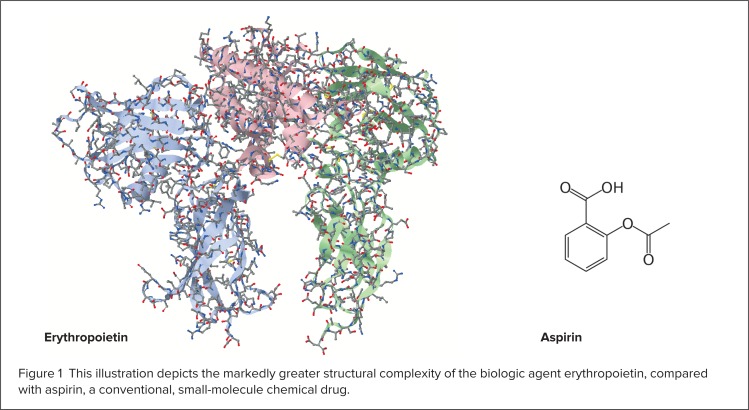
Biologics are much more complex than conventional “chemical” drugs because they are larger and have more complicated structures (Figure 1).5,7,8 Many biologics have become increasingly well characterized, including their mechanisms of action.3 The structure–function relationships of biologics are very sensitive, because modifications of primary or higher-order (secondary, tertiary, or quaternary) configurations may affect safety, purity, and/or potency.6 During the manufacturing process for biologics or biosimilars, primary amino acid sequences can become modified through glycosylation, changing the shape of a protein because of alterations in the way it folds.5 These post-translational modifications are not controlled by the recombinant DNA inserted into the host cell but are affected by the cell line and the environment in which the cell line is grown.3
Figure 1).
This illustration depicts the markedly greater structural complexity of the biologic agent erythropoietin, compared with aspirin, a conventional, small-molecule chemical drug.
Every manufacturer of biologics or biosimilars uses a unique cell line and a proprietary process to produce a particular biologic agent, so it is impossible to produce biosimilars that are identical to the originator drug.1,5,8 By contrast, conventional chemical drug molecules are much smaller, have a simpler structure, and can be easily manufactured using a controlled and predictable chemical process that generates identical copies. Table 1 (see page 272) presents a comparison of biosimilars and generic chemical drugs.5,8
Table 1.
Summary of Key Differences Between Biosimilars and Generic Chemical Drugs Compared With Originator Products
| Area | Biosimilars | Generic Chemical Drugs |
|---|---|---|
| Chemical structure | The amino acid sequence is the same, but slight differences are expected in terms of protein folding and glycosylation | The active drug is chemically identical to the reference product |
| Analytical characterization | The final structure cannot be fully defined based on current analytical techniques; therefore, the degree of structural similarity to the reference product is unknown | Current techniques are available to ensure that the active drug in the generic product is identical to the reference product |
| Manufacturing complexity | Very complex; produced in living cells and involves several stages of purification, production, and validation of the final product | Relatively simple; uses organic medicinal chemistry reactions |
| Impact of a change in manufacturing process | Small changes in process may alter the final structure and function of the protein | Likely to be negligible because the end product is identical |
| Legislation approving an abbreviated pathway | The Biologics Price Competition and Innovation Act of 2009 establishes a framework for an abbreviated approval pathway for biosimilars; final guidance has yet to be released by the FDA | Hatch–Waxman Act allows generics to be approved through an Abbreviated New Drug Application (ANDA) |
Reprinted with permission from Zelenetz AD, et al. JNNCN–Journal of the National Comprehensive Cancer Network 2011;9(Suppl 4):S1–S22.19 Created from data by Nowicki M. Kidney Blood Press Res 2007;30:267–272; and Kuhlmann M, Covic A. Nephrol Dial Transplant 2006;21(Suppl 5):v4–v8.
Conversely, for biologics, even minor modifications in the manufacturing process can result in a different end product.3,5 Therefore, the therapeutic efficacy, safety, and quality of a bio-similar could vary from the originator, or “reference,” biologic, because the end product is highly dependent on a proprietary manufacturing process that differs for each manufacturer.5 The inability to produce an exact copy of an originator biologic is the reason for the term “biosimilar” rather than “biogeneric” or “bioidentical.”3
The Increasing Clinical Use and Cost of Biologics
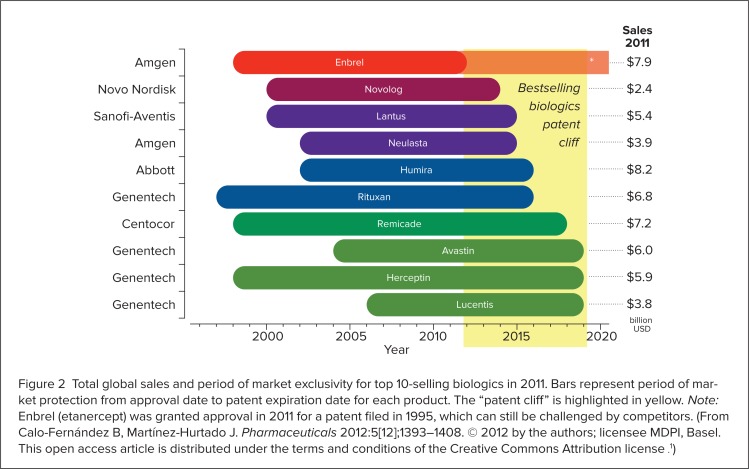
Biologics were a pivotal innovation by the pharmaceutical industry, because they successfully addressed previously unmet therapeutic needs.1 Since their introduction, biologics have become increasingly significant in terms of new product development, clinical use, and health care expenditures.3 In 2010, these agents were the fastest-growing segment of the pharmaceutical market as a result of expanding indications, increased utilization, and a burgeoning biologics development pipeline.8 During that year, biologics accounted for 32% of the products in drug development, 7.5% of marketed medications, and 10% of pharmaceutical expenditures.8 In 2011, worldwide biologic sales reached $142 billion, with 37.6% of this amount garnered by the top 10 selling products (Figure 2, page 273).1
Figure 2).
Total global sales and period of market exclusivity for top 10-selling biologics in 2011. Bars represent period of market protection from approval date to patent expiration date for each product. The “patent cliff” is highlighted in yellow. Note: Enbrel (etanercept) was granted approval in 2011 for a patent filed in 1995, which can still be challenged by competitors. (From Calo-Fernández B, Martínez-Hurtado J. Pharmaceuticals 2012:5[12];1393–1408. © 2012 by the authors; licensee MDPI, Basel. This open access article is distributed under the terms and conditions of the Creative Commons Attribution license .1)
The cost of biologics is also rapidly rising. Biologics are much more costly to develop and manufacture than conventional chemical drugs.4 Biologics firms spend about 30% of their revenues on research and development (R&D), among the highest percentages of any industry in the U.S.3,4 On average, the R&D for a biologic agent costs $1.2 billion, compared with $500 to $800 million for a conventional chemical drug.4 The investment of time to develop a biologic is also greater, usually between 10 and 15 years, compared with 7 to 10 years for a conventional chemical drug.4,9
Unfortunately, these increased investments by biologics manufacturers also translate into higher costs for consumers.4 Biologics are much more expensive than conventional chemical drugs.4,5 In 2012, the average cost of a branded biologic was estimated to be $34,550 per year and was even higher for some treatments—for example, as much as $200,000 per year for imiglucerase (Cerezyme) to treat Gaucher’s disease or $50,000 per year for adalimumab (Humira, Abbott) to treat rheumatoid arthritis or Crohn’s disease.4 The rate at which biologics prices have increased has also far exceeded the overall rate of inflation.4 The cost of biologics rose by 14.2% in 2009, by 17.2% in 2010, and by more than 13.6% in 2011, compared with the much smaller changes of −0.4%, 1.6%, and 3.2%, in the Consumer Price Index (CPI) during the corresponding years.4,10,11
The ‘Patent Cliff’ and Other Drivers of Biosimilars
The impending expiration of the patents for many branded biologic agents is a significant driver of biosimilars development.14 Since the 1980s and 1990s, the developers of originator biologics have been awarded patents granting a 20-year period of exclusivity.7 This exclusivity period has given rise to the “patent cliff,” the term used to describe the clustering of numerous branded biologics patent expirations occurring between 2011 and 2019 (see Figure 2, page 273).1,12 The expiration of these patents will compel competing manufacturers to develop biosimilars for these biologics, creating a market that is expected to grow at an annual rate of 20% going forward.2,12
Along with the patent cliff, the high cost of branded biologics is also a significant driver of biosimilars development. Cost pressures facing both public and private third-party payers, as well as the desire to improve patient access through decreased drug costs, creates a demand for biosimilars.1,8,12 Biosimilars are expected to be a cost-effective alternative to high-priced branded biologics, offering significant and much needed cost savings to both payers and patients.4,5 A 2008 Congressional Budget Office (CBO) report estimated that biosimilars will reduce federal spending for biologic drugs by $25 billion by 2018.5 Other drivers of biosimilars development include the long-awaited establishment of a new, expedited regulatory pathway for biosimilars by the FDA; advances in manufacturing techniques; and the expansion of biologics indications to include larger patient populations.1,8,12
Defining a Regulatory Pathway for Biosimilars
The Biologics Price Competition and Innovation Act of 2009
Traditionally in the U.S., biologics are approved under the Public Health Service Act (PHSA), whereas conventional chemical drugs are approved under the Federal Food, Drug, and Cosmetic Act (FDCA).2,3,5 Under the PHSA, originator biologics receive approval through a Biologics License Application (BLA), also known as the “351a pathway.” This process requires significant preclinical and clinical data to prove the efficacy, safety, and quality of the agent.2,4,5 In 1984, the Hatch–Waxman Act amended the FDCA to include expedited 505(j) and 505(b) (2) regulatory pathways, which allow generic chemical drugs to be approved through an Abbreviated New Drug Application (ANDA).3 By contrast, the PHSA does not define an approval process for biosimilars.2,5
Unlike the U.S., the European Medical Agency (EMA) has had a regulatory pathway for the review and approval of biosimilars, which is based on comparability to originator biologics, in place since 2006.5 The U.S. followed Europe’s lead by drafting the BPCIA in 2009.1 The BPCIA was included as part of the Patient Protection and Affordable Care Act (PPACA), which was enacted into law by Congress in March 2010.2,3,13 The BPCIA amends the PHSA to include an abbreviated 351k pathway for the approval of biosimilars of originator biologics that have previously been licensed through a BLA.2,3 This legislation also authorizes the FDA to designate a biosimilar as being either “comparable” or “interchangeable” with the reference product and to establish the evidentiary requirements and process for this purpose.2,5 The details regarding these and other requirements for the biosimilars pathway have not yet been finalized by the FDA.2
The BPCIA grants 12 years of exclusivity to originator or reference biologics; therefore, by law, the FDA cannot approve a biosimilar until this period has elapsed.2,3,5,13 The exclusivity period is intended to ensure economic incentives for biologic manufacturers to continue to invest in R&D for new, originator biologics.2,3 In exchange for the 12-year exclusivity period, biosimilar manufacturers are granted access to the 351k pathway, or the abbreviated BLA pathway, which will expedite the approval of biosimilars.3,5,13,14
FDA Draft Guidance on the Biosimilars Regulatory Pathway
In February 2012, the FDA issued three draft guidance documents regarding the regulatory requirements for biosimilars:4,14–16
Scientific Considerations in Demonstrating Biosimilarity to a Reference Product
Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product
Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009
These documents clarify the requirements of the BPCIA and discuss the scientific and quality considerations involved in evaluating the comparability of a biosimilar with a reference product.14
According to these draft documents, when reviewing biosimilars applications, the FDA will take a facts-focused, risk-based approach dependent on product data and clinical experience with the reference drug.14 Biosimilarity will be demonstrated through the submission of data derived from analytical, animal, and clinical studies.14 The guidance states that clinical studies must include an assessment of pharmacokinetics, pharmacodynamics, and immunogenicity and should also address one or more indications licensed for the reference product.14 The FDA will also consider product formulation, complexity, and stability when evaluating biosimilarity.14 Methods of evaluating safety beyond traditional clinical trials, such as pharmacovigilance, will also be used to monitor a biosimilar after it is on the market.2
Despite these statements, the FDA is granted discretion in the amount and type of data that it will require for the approval of biosimilars.5 After manufacturing changes are made to a branded biologic, the FDA and other regulatory agencies generally require only a “comparability exercise” (analytic data, with preclinical and clinical evidence required only when necessary).3 This approach acknowledges that a biosimilar is not unlike a product that would result after a change is made to the manufacturing process for a branded biologic.3 Therefore, the proposed FDA regulatory pathway for biosimilars resembles a comparability exercise rather than a new product-development program.3 This concept enables the agency to approve a bio-similar without requiring a full complement of clinical trials, which in turn facilitates lower product development costs, expedites regulatory approval, and eases market entry for biosimilars manufacturers.16
FDA Draft Guidance on Biosimilarity
The FDA recognizes that a biosimilar cannot be structurally identical to the originator (reference) product because differences in the manufacturing process alter the end product.7 Rather than requiring that a biosimilar be structurally identical to an originator biologic, the FDA requires that a biosimilar not be “clinically different.”7 According to the BPCIA, a biologic product is deemed biosimilar to the already approved, originator biologic if the available data show that it is highly similar to the reference product, “notwithstanding minor differences in clinically inactive components, and there are no clinically significant differences between the biologic product and the reference product in terms of safety, purity, and potency of the product.”1,14,16 Because the efficacy and safety of the reference product have already been demonstrated, a manufacturer must provide evidence only that a biosimilar is not significantly different.5
The goal is to use smaller-scale, direct comparisons and extrapolation instead of relying on replicating clinical trials that are presumed unnecessary.5 This is partially achieved through data derived from analytical studies that demonstrate that the biological product is “highly similar” to the reference product.15 The draft guidelines also acknowledge that biosimilarity can be demonstrated even though there are “formulation or minor structural differences, provided that the sponsor provides sufficient data and information demonstrating that the differences are not clinically meaningful and the proposed product otherwise meets the statutory criteria for biosimilarity.”15 For instance, certain post-translational modifications (such as alterations to C and N terminals) or changes to excipients are not expected to preclude a finding of biosimilarity.15
However, structural variability is very subtle and diverse, and currently available analytical techniques can be insufficient in fully characterizing biological products.5,7 So the FDA takes what it calls a “totality of evidence” approach, stating that analytical, physicochemical, and biological characterization should be extensive, utilizing comparisons between the biosimilar and reference product, including structure, function, and animal data, as well as human pharmacokinetics (PK), pharmacodynamics (PD), clinical immunogenicity, efficacy, and safety studies.5,14,15 Clinical studies demonstrating the efficacy and safety of the biosimilar in one or more of the reference product indications for which the biosimilar will be licensed are also required.5 However, the FDA has the flexibility to determine that some of these studies aren’t necessary, allowing the agency to define the best approach for specific biosimilar products and classes.5
Characterization of Biosimilars Through Analytical Studies
In the draft guidance provided by the FDA, the agency uses analytical studies to serve as the foundation for establishing comparability to the reference biologic, similar to a comparability exercise that is required for an originator biologic after a manufacturing change.3 The confidence in biosimilarity, as shown analytically, also provides the basis for the regulatory relief with respect to preclinical and clinical studies, which facilitates an abbreviated biosimilars-approval process.3,17 Analytical studies are also useful in determining the type and amount of animal and clinical data that should later be included in the biosimilar-approval application.5,17
However, first the sponsor must show that a candidate product is highly similar to the originator reference product at the analytical level, including structural characteristics.3 The analytical data required for this purpose could include studies showing the amino acid sequence, higher-order structures, glycosylation, pegylation, and so on, and should also include an analysis of lot-to-lot variability.5 Structure–function relationships can be analyzed by evaluating pharmacological activity via in vitro or in vivo experiments, with comparisons being made to the reference product.5 Sophisticated, high-tech tests such as spectroscopy can be used to measure physiochemical and functional similarity to the originator biologic.17 Nuclear magnetic resonance or mass spectroscopy can be used to distinguish differences in tertiary and quaternary structures, and gel electrophoresis and reverse-phase, high-performance liquid chromatography (HPLC) can be used to identify disparities in glycosylation patterns, aggregation, and purity.1 Product-specific colorimetric assays, such as enzyme-linked immunosorbent assays (ELISAs) and size exclusion chromatography, can determine the molecular weight and size differences between biosimilars and innovator reference biologics.1
The sponsor company must also perform a detailed analysis of the originator reference product for comparison.18 Because the originator product will have varied over its lifetime as a result of manufacturing changes (a phenomenon known as “drift”), multiple batches of the reference drug must be acquired, and analyses should be conducted across the shelf life of each of them.18 These data are then used to create the boundaries, or “goalposts,” of acceptable features for the biosimilar.18
After the biosimilar product attributes are within these boundaries, the sponsor can conclude that their candidate is “highly similar” to the originator reference product.18 Any parameter for the biosimilar that is outside the goalposts of the reference product must be demonstrated to have no impact on the clinical attributes of the final product.18 The draft guidance recommends that the sponsor company describe any differences between the biosimilar and the reference product in detail, explaining how they might potentially affect the safety and purity of the agent.14
Validation of Biosimilars Through Preclinical and Clinical Studies
The FDA also has the flexibility to decide which, if any, pre-clinical and clinical data are required to support a biosimilar application.15 When analytical data alone are insufficient to judge whether a biosimilar is comparable to the reference product, the FDA and sponsor company will determine which preclinical and clinical studies are necessary for validation of comparability.3,14 The FDA draft guidelines state that “analytical studies and at least one human PK and/or PD study against the reference product licensed under section 351(a) will be required to support a demonstration of biosimilarity.”3,5,14,15 The extent of the preclinical and clinical development program for a biosimilar depends on the degree of comparability that the agent demonstrated analytically.3,14
Comparative clinical efficacy and safety studies also will not always be needed for the approval of a biosimilar.5,14,15 The draft guidelines state that as
a scientific matter, comparative safety and effectiveness data will be necessary to support a demonstration of biosimilarity if there are residual uncertainties about the biosimilarity of the two products based on structural and functional characterization, animal testing, human pharmacokinetic and pharmacodynamic data, and clinical immunogenicity assessment.5,15
The draft guidelines suggest that, in some circumstances, PD data could suffice as evidence of comparable efficacy.15 However, for many monoclonal antibodies, clinical efficacy trials will likely be required as a rule, because good PD efficacy markers do not exist for these therapies.15 Comparative clinical efficacy and safety studies will also likely be mandatory for other large, structurally complex, heterogeneous biologics (such as fusion proteins) in order to confirm comparable efficacy and minimize the risk of adverse outcomes.6
The FDA draft guidance does explicitly mention the need for sponsor companies to assess immunogenicity in a clinical study unless this requirement is waived by the FDA.3 Specifically, the draft guidance states that the FDA recognizes that “immunogenicity remains a critical factor when assessing biosimilarity,” and the agency provides reassurance that it “will evaluate immunogenicity in a risk-based manner.”15 The biggest concern regarding immunogenicity is that changes in the production process could produce an end product that provokes an immune response in patients.2 Therefore, when the FDA deems the risk of immunogenicity to be high, large clinical studies will likely be required to assess the risk of the rare life-threatening events associated with this response.15 However, assessment of immunogenicity could also be achieved through a small pre-submission clinical program, supplemented by postmarketing immunogenicity studies.15
Regarding indications, after comparability has been demonstrated, the efficacy and safety of the biosimilar must be justified or demonstrated separately for each indication that has been approved for the originator biologic.3,7 Specifically, the FDA draft guidelines state that
the potential exists for the proposed product to be licensed for one or more additional conditions of use for which the reference product is licensed.15 However, the sponsor will need to provide sufficient scientific justification for extrapolating clinical data to support a determination of biosimilarity for each condition of use for which licensure is sought.15
Therefore, the number of approved indications for a biosimilar might be reduced, compared with the reference product.14 However, in some cases, the FDA does allow data supporting biosimilarity in one indication to be extrapolated to support the licensing of a biosimilar for one or more additional indications for which the reference product is approved.14,15 This could be accomplished by extrapolating data regarding the mechanism(s) of action, pharmacokinetics and drug distribution, and expected toxicities and immunogenicities, as well as other factors that might affect the efficacy or safety for each indication and for different patient populations.14,15 However, extrapolating data from one indication to support another will probably be the exception rather than the rule, even though doing so would eliminate the need for large comparative trials for each indication.3,14,15,18
With respect to the off-label use of biologics, whether data demonstrating the efficacy of a biosimilar can be extrapolated to off-label indications is not clear.18 In Europe, such extrapolation is allowed under the premise that if the biosimilar is comparable to the innovator product for one indication, it is likely to be comparable for another.18 However, if the mechanism of action differs between indications, additional clinical data might be needed to assess whether the off-label use of a biosimilar is appropriate.18
FDA Draft Guidance on Biosimilar Interchangeability
Although comparability might be proven by showing that the biosimilar and reference product have no clinically significant differences, the FDA defines “interchangeability” more stringently.2 This is because a designation of interchangeability would allow a biosimilar to be substituted for a brand-name biologic without the prescriber’s approval.16 According to the FDA guidance, the standard of interchangeability can be achieved by proving that a biosimilar can produce the same clinical results as the reference product “in any given patient.”14,16 The guidance states that
for a biological product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch.4,5,8,13,14
The FDA draft guidance advises that sponsor companies may apply for an interchangeability designation for a biosimilar; however, it also warns that it would be
difficult ... as a scientific matter to establish interchangeability in [the abbreviated] 351(k) application given the statutory standard for interchangeability and the sequential nature of that assessment. [The] FDA is continuing to consider the type of information sufficient to enable [it] to determine that a biological product is interchangeable with the reference product.15,16
To provide data proving interchangeability, difficult-to-recruit-for and expensive crossover studies might be required.4 Postmarketing studies conducted to demonstrate patient outcomes in large patient populations and pharmacovigilance programs to evaluate adverse event reports will also probably be necessary.13,14
Controversies Regarding Biosimilars
While final FDA guidance regarding the biosimilar regulatory pathway is pending, key issues are being debated.12,16 Topics of discussion include to what extent the biosimilars regulatory pathway should be abbreviated, how much and which types of clinical and interchangeability data should be required, and even which convention biosimilar product names should follow.12
Regulatory authorities and sponsor companies generally agree that if a biosimilar undergoes a comparability exercise showing that it is as close to the originator product as the originator product is to itself after manufacturing changes, then an abbreviated clinical trial program can be justified.3 However, although the development of a biosimilar may, in theory, resemble a change in manufacturing process for a biologic, these endeavors are in fact quite different.6
One important consideration is that the development of a manufacturing process for a biosimilar is performed entirely without full access to the documentation regarding the evolution of the innovator product.6 Therefore, there is a much greater potential for differences between an innovator biologic and a biosimilar than for a branded biologic after a manufacturing change.6 The FDA has acknowledged this issue by stating in its draft guidance:
Demonstrating that a proposed product is biosimilar to a reference product typically will be more complex than assessing the comparability of a product before and after manufacturing changes made by the same manufacturer. This is because a manufacturer who modifies its own manufacturing process has extensive knowledge and information about the product and the existing process, including established controls and acceptance parameters. In contrast, the manufacturer of a proposed product will likely have a different manufacturing process (e.g., different cell line, raw materials, equipment, processes, process controls, and acceptance criteria) from that of the reference product and no direct knowledge of the manufacturing process for the reference product. ... Therefore, in general, more data and information will be needed to establish biosimilarity than would be needed to establish that a manufacturer’s post-manufacturing change product is comparable to the pre-manufacturing change product.6
Current analytical techniques and abbreviated clinical studies might not be able to detect all of the potential differences in clinical outcomes between a biosimilar and the reference product.8 One problem with analytic studies is that they measure specific variables and are not able to predict all biological activity in patients, leaving open the possibility of overlooking characteristics of the proposed biosimilar that may signal safety or other problems.17 For example, even sophisticated in vivo models are not able to provide definitive conclusions regarding immunogenicity, because many immune responses are species-specific.1 There are also published examples in which unexpected clinical findings were observed following a major manufacturing process change for a biologic.6 Such examples demonstrate the need for clinical studies to assess the efficacy and safety of a biosimilar, especially when analytical studies are insufficient for assessing risks.5,6
Another concern is that the cost-savings accrued by an abbreviated biosimilars approval pathway won’t make up for the possible resultant losses in the efficacy, safety, or quality of these agents.3 However, requiring biosimilars to undergo a full development program (including extensive clinical trials) is not considered to be a viable approach, especially for lower-priced agents.3 If sponsor companies aren’t granted significant regulatory relief regarding submission requirements for preclinical and clinical studies, the biosimilars pathway may reach an impasse.3 The value offered by the biosimilar regulatory pathway would then be in question, particularly since the sponsor company could achieve 12 years of product exclusivity by instead taking the standard BLA route.15 Despite concerns regarding potential negative effects of an abbreviated regulatory pathway on product quality, the experience in Europe has been that biosimilars that have undergone the expedited approval process provide cost savings and improved patient access without compromising therapeutic or safety outcomes.3
To date, American companies have been hesitant to aggressively pursue a biosimilar pipeline without formal clarification of the data that the FDA will expect to see in 351(k) applications.16 When the FDA finalizes the guidance for the biosimilar regulatory pathway, the approval process will probably take at least 2 years; therefore, the entry of biosimilars into the U.S. market is also expected to be delayed until 2015 at the earliest.4
Conclusion
Biosimilars are expected to be an essential component in reducing health care costs and enhancing patient access to important, often lifesaving medications.15 It is hoped that the FDA will soon finalize these regulatory guidelines, clarify unanswered questions, and establish a biosimilars pathway that is based upon sound scientific principles.12,15 In doing so, the agency will need to find the proper balance between rigorous data and testing requirements and providing a cost-efficient, expedited pathway for biosimilar approval.16 Robust evidence is critical to ensure drug efficacy and safety, but in order to encourage the availability of biosimilars, it cannot be too burdensome to dissuade company sponsors from developing and introducing biosimilars to the market.2,4
References
- 1.Calo-Fernández B, Martínez-Hurtado J. Biosimilars: Company strategies to capture value from the biologics market. Pharmaceuticals. 2012;5(12):1393–1408. doi: 10.3390/ph5121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch BR, Lyman GH. Biosimilars: Are they ready for primetime in the United States? J Natl Compr Cancer Network. 2011;9:934–943. doi: 10.6004/jnccn.2011.0076. [DOI] [PubMed] [Google Scholar]
- 3.McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91(3):405–417. doi: 10.1038/clpt.2011.343. [DOI] [PubMed] [Google Scholar]
- 4.Blackstone E, Fuhr J. Innovation and competition: Will biosimilars succeed? Biotechnol Healthcare. 2012;9(1):24–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman JM, Li E, Stevenson JG. Preparing for biosimilars: Scientific, regulatory, and practice management issues for pharmacists. Live webcast, 47th ASHP Midyear Clinical Meeting and Exhibition; Las Vegas. December 3, 2012; Available at: www.ashpadvantagemedia.com/downloads/handout_biosimilars.pdf. Accessed February 26, 2013. [Google Scholar]
- 6.Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: Considerations for the healthcare provider. Curr Med Res Opin. 2012;28(6):1053–1058. doi: 10.1185/03007995.2012.686902. [DOI] [PubMed] [Google Scholar]
- 7.Simoens S, Verbeken G, Huys I. Biosimilars and market access: A question of comparability and costs? Target Oncol. 2012;7(4):227–231. doi: 10.1007/s11523-011-0192-7. [DOI] [PubMed] [Google Scholar]
- 8.Simoens S. Biosimilar medicines and cost-effectiveness. Clinicoeconomic Outcomes Res. 2011;3:29–36. doi: 10.2147/CEOR.S12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogbru O. Why drugs cost so much. Available at: www.medicinenet.com/script/main/art.asp?articlekey=18892. Accessed February 26, 2013.
- 10.Hoffman JM, Li E, Doloresco F, et al. Projecting future drug expenditures—2012. Am J Health Syst Pharm. 2012;69(5):405–421. doi: 10.2146/ajhp110697. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Available at: ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed February 26, 2013. [Google Scholar]
- 12.Rana JB, Chang DY. Pharmacist perception of biosimilar agents in the U.S. Pharmacy Purchasing Products. 2012;9(7):8. [Google Scholar]
- 13.Barlas S. President Obama reopens debate on patented biologics: Will the FDA quickly define an abbreviated pathway for biosimilars? P&T. 2011;36(4):178. [PMC free article] [PubMed] [Google Scholar]
- 14.Calvo B, Zuniga L. The U.S. approach to biosimilars: The long-awaited FDA approval pathway. Biodrugs. 2012;26(6):357–361. doi: 10.2165/11635830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Nick C. The U.S. Biosimilars Act: Challenges facing regulatory approval. Pharm Med. 2012;26(3):145–152. [Google Scholar]
- 16.Barlas S. FDA readies new guidance and user fee program for biosimilars: Drug interchangeability and user fees are contentious issues the FDA must resolve: Is the FDA up to the task? Biotechnol Healthcare. 2012;9(2):28–29. [PMC free article] [PubMed] [Google Scholar]
- 17.Reinke T. Biosimilars might not measure up to health plan expectations. Manag Care. 2012;21(10):12–13. [PubMed] [Google Scholar]
- 18.American Pharmacists Association. The biosimilar pathway: Where will it lead us? Pharmacy Today. 2011 Dec;:67–76. [Google Scholar]
- 19.Zelenetz AD, Ahmed I, Braud EL, et al. NCCN biosimilars white paper: Regulatory, scientific, and patient safety perspectives. J Natl Compr Cancer Network. 2011;9(Suppl 4):S1–S22. doi: 10.6004/jnccn.2011.0136. [DOI] [PubMed] [Google Scholar]