Abstract
P&T committee members need a lot more information before they can evaluate the efficacy and safety, interchangeability, and cost-effectiveness of biosimilar products for inclusion on formularies.
This is the second part of a two-part series about biosimilar products. Draft guidance regarding the regulatory approval of biosimilars by the FDA was discussed in the May 2013 issue of P&T.
Introduction
P&T committees will have an important role in influencing the extent to which biosimilars are utilized by the health care system.1 The priority for P&T committees, when reviewing biosimilars, will be to evaluate the efficacy and safety, interchangeability, cost-effectiveness, pharmacovigilance requirements, reimbursement issues, and other factors regarding these agents.2 Institutional and managed care pharmacists generally view biosimilars as having significant potential to expand patient access to these important treatments; however, uncertainties remain regarding the value of these agents from an efficacy, safety, and quality-of-care perspective, compared with branded biologics.3 This article reviews the potential issues and obstacles that P&T committees will encounter when evaluating biosimilars for formulary inclusion.
Health Care Professionals Need More Information
Health care professionals, particularly those on P&T committees, will be very influential in driving the adoption of biosimilars by the health care system.1,4 By applying formulary and practice management tools and principles, P&T committee members will play a leadership role with respect to the use of these agents.4
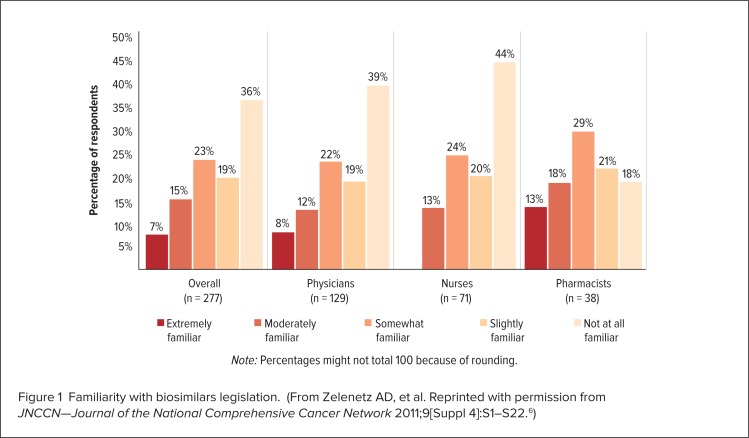
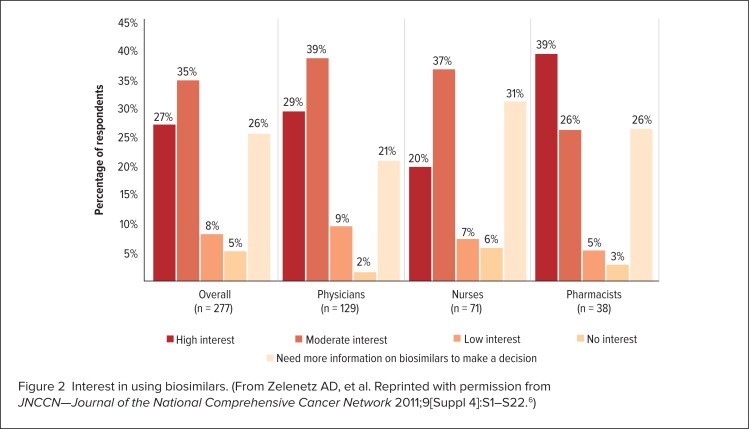
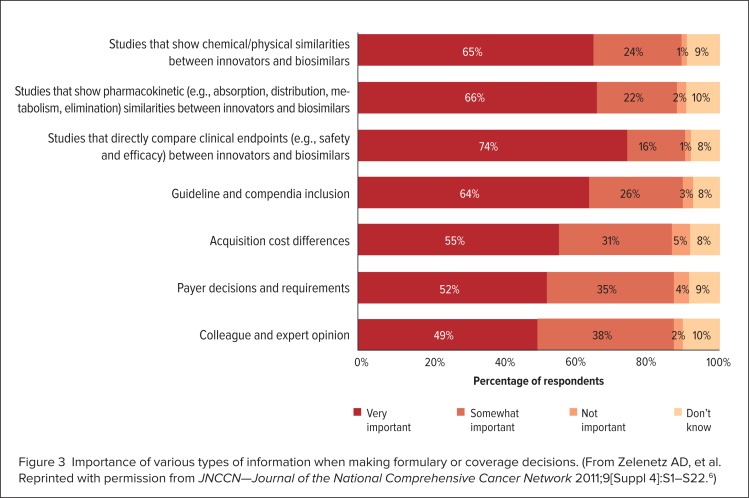
However, a lack of awareness and information regarding these agents on the part of health care professionals is one of the most significant problems that may hinder the adoption of biosimilars.5 A study conducted in 2011 at the 16th Annual Conference of the National Comprehensive Care Network (NCCN) surveyed 277 health care professionals, including pharmacists (n = 38), physicians (n = 129), and nurses (n = 71).4,6 The study found that only 13%, 8%, and 12% of these professionals, respectively, considered themselves to be “extremely familiar” with the abbreviated regulatory process for the approval of biosimilars, whereas 18%, 39%, and 44% considered themselves to be “not at all familiar” with this topic (Figure 1).4,6 This study also indicated that 26%, 21%, and 31% of pharmacists, physicians, and nurses, respectively, said they needed “more information before making a decision” regarding their interest in using biosimilars (Figure 2).4–6 Survey participants rated the results of clinical efficacy and safety, pharmacokinetic, and analytical studies comparing the biosimilar and originator biologic as being the most important information that they require (Figure 3, page 331).4,6
Figure 1.
Familiarity with biosimilars legislation. (From Zelenetz AD, et al. Reprinted with permission from JNCCN—Journal of the National Comprehensive Cancer Network 2011;9[Suppl 4]:S1–S22.6)
Figure 2.
Interest in using biosimilars. (From Zelenetz AD, et al. Reprinted with permission from JNCCN—Journal of the National Comprehensive Cancer Network 2011;9[Suppl 4]:S1–S22.6)
Figure 3.
Importance of various types of information when making formulary or coverage decisions. (From Zelenetz AD, et al. Reprinted with permission from JNCCN—Journal of the National Comprehensive Cancer Network 2011;9[Suppl 4]:S1–S22.6)
A lack of information about and familiarity with biosimilars was also revealed in a 2012 survey of institutional pharmacists. Only 44 participants (40.7%) knew that the Biologics Price Competition and Innovation Act (BPCIA), passed by Congress in 2010, had granted the FDA the authority to create an expedited approval pathway for biosimilars.3 A majority of the institutional pharmacists also expressed a desire for more information from the FDA, especially concerning the type and amount of clinical trial data required for biosimilar approval, as well as more technical information about comparability exercises and assays.3 Study participants also identified data regarding bioequivalence, long-term efficacy and safety, drug characteristics, head-to-head trials, and long-term health outcomes as being important when making formulary or coverage decisions regarding biosimilars.3
More than 57% of the institutional pharmacists surveyed also stated that confirmatory clinical trials comparing biosimilars to the branded originator biologic in each indication for which approval is sought, were necessary to prove biosimilarity.3 In addition, more than 61% of these respondents felt that crossover trials should be required to prove the interchangeability of biosimilars with branded biologics and that the distinction between “biosimilar” and “interchangeable” was important and clinically meaningful.3
Irrespective of the desire for this information, because the regulatory pathway for biosimilars will be abbreviated, it is unlikely that all of the data on which clinicians and formulary committees normally base their decisions will be available.4 Despite this challenge, it is expected that the P&T committee approach to evaluating biosimilars for formulary inclusion will be similar to that for reviewing conventional chemical drugs or branded biologics.4 Although some topics that P&T committees consider when evaluating biosimilars will differ, many will remain the same.1,4 These include relative efficacy and safety, immunogenicity, approved and non-approved indications, interchangeability, dose equivalence and conversion, nomenclature, tracking and information systems implications, pharmacovigilance requirements, reimbursement, and transition of care.1,4
An objective review of scientific evidence and the literature will remain the foundation of the P&T committee’s evaluation process.4 However, the fact that the regulatory pathway for biosimilars proposed by the FDA focuses heavily on analytical data might present a challenge for P&T committee members, who usually rely on evidence from randomized controlled trials and meta-analyses or systematic literature reviews as the foundation for drug evaluation.7 However, this information could be unavailable; therefore, P&T committees may instead need to acquire more expertise in laboratory methods and analytic techniques that are used for evaluating these agents.7 Because efficacy and safety vary among biologics, P&T committees will also need to ensure that they are aware of all class- and product-specific matters before approving a biosimilar product for formulary inclusion.1
Formulary committee best practices for reviewing biosimilars will involve proactively planning and establishing a system to evaluate these products.4 This process must include the active and direct involvement of physicians, pharmacists, and all other appropriate health care professionals.4 However, pharmacists should lead the efforts to educate health care providers and patients about these agents, particularly in terms of product interchange at the transition of care.4 Although biosimilars are expected to provide cost-savings, formulary decisions should be evidence-based and not focused solely on economics.4 Along with evaluating standard topics such as efficacy, safety, and cost-effectiveness, other ethical, legal, social, educational, and quality-of-life issues should also be considered to ensure optimal patient care.4 These and other important topics involved in the P&T committee review of biosimilars are listed in the American Society of Health-System Pharmacists (ASHP) policy guidelines on biosimilar approvals, summarized in Table 1.4
Table 1.
ASHP Policy Guidelines on Approval of Biosimilar Medications
|
Adapted from Hoffman JM, et al. December 2012.4
Manufacturing Challenges Can Alter Efficacy and Safety
When evaluating biosimilars, it is important for P&T committee members to understand the clinical implications inherent in the manufacturing challenges involved in biosimilar production.8 The regulatory pathway for biosimilars proposed by the FDA is based on the concept that the manufacturing process for a biosimilar is comparable to a manufacturing process change for a branded biologic.8 However, in reality, these processes differ because biosimilar development occurs without full access to the history of the branded agent, being that this information is proprietary and confidential.8,9 Further, biosimilars manufacturers don’t have access to the cell line that is used to produce the branded originator biologic.8,9 Because manufacturing and purification processes for biologics and biosimilars are very complex, differences in the quality, safety, and efficacy of the end product can occur even when minor alterations are made.9
Additional factors that may affect the integrity and quality of the end product include, but are not limited to, the source and type of raw materials used; conditions such as temperature, pH, and agitation; and contamination by chemical substances that can leach from containers and equipment.8 Because of these factors and many others, there is a greater potential for the biosimilar manufacturing process and the end product to differ more substantially from an originator biologic before and after a manufacturing change.8,9
Due to the sensitivity of the end products to changes in manufacturing processes, differences between branded originator biologics and biosimilars are unavoidable. A manufacturing process for a biosimilar could even yield an end product that has better (known as a “biobetter” agent), equal, or reduced efficacy compared with the branded originator biologic.9 Biosimilar manufacturing often uses the most modern techniques and equipment, so it is conceivable that the result could be an agent that is more effective than a branded biologic developed with 15- to 20-year-old production methods.9
Changes in manufacturing processes may also critically alter glycosylated proteins and monoclonal antibodies, which often consist of structural isoform variants.8 Some of these variants are considered to be impurities that can influence the efficacy and safety of the medication.8 To further complicate this matter, there is no single test that can detect and verify the ideal proportion or distribution of isoforms in a biologic or biosimilar formulation.8 Furthermore, current analytical techniques and the abbreviated clinical trials required for biosimilar approval are unable to predict all potential differences in therapeutic outcomes between a branded originator biologic and a biosimilar.9
Small changes in the manufacturing process for biosimilars can also have important safety ramifications.4 An immunogenicity reaction can cause serious clinical consequences and is therefore a major safety concern.10,11 The potential for a biologic to produce an immunogenicity reaction varies according to which type of therapeutic protein the agent represents.4 The more similar the structure of a biologic to a human protein, the less chance that immunogenicity will occur.4 While with insulin and human growth hormone an immunogenicity reaction is not expected, the human body could produce anti-epoetin antibodies in response to treatment with epoetin alfa (e.g., Epogen, Amgen; Procrit, Janssen), resulting in an antibody-mediated disease, such as pure red cell aplasia.4 This was observed when some patients taking recombinant epoetin alfa, produced after a subtle manufacturing change had been implemented, developed epoetin-resistant anemia.10,11 Other factors that can affect the immunogenicity of biologics and biosimilars include impurities, formulation changes (such as the removal of albumin), route of administration, dose, and immune status of the patient.4 Unfortunately, although scientific tools for detecting immunogenicity do exist in some cases, they are, for the most part, undeveloped.4
‘Biosimilar’ Does Not Always Mean Interchangeable
The FDA’s approval of a biosimilar does not necessarily mean that the medication is therapeutically equivalent to the corresponding branded originator biologic.3,4,11 For FDA approval of conventional chemical generic drugs, it is sufficient to show pharmaceutical equivalence (identical active substances) and bioequivalence (comparable pharmacokinetics) with respect to the branded agent without conducting formal efficacy and safety studies, but this is not the case for biosimilars.11
The FDA can, however, designate a biosimilar as “interchangeable.” This means that the biosimilar is not only comparable to the originator branded biologic; it is also expected to produce the same clinical results.3 One of the FDA’s criteria for an interchangeability designation is as follows:5,9,12
[For] a biological product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch.
The challenge for the FDA is to determine the depth and breadth of the clinical studies that will be necessary to establish the interchangeability and therapeutic equivalence of a biosimilar while still providing an abbreviated regulatory pathway.4 Because the biosimilar regulatory pathway is abbreviated, the clinical data required by the FDA will be limited.3 Therefore, it has been suggested that an “interchangeable” designation might be postponed until postmarketing data have shown that a biosimilar produces results that are identical to those of the branded originator biologic.12
Because a biosimilar might be similar (but not identical) to the branded biologic, the question of substituting one agent for another must be carefully considered.11 The distinction between biosimilars, with and without an interchangeable designation, is important, especially for pharmacists.11,13,14 An interchangeability designation might be sufficient to allow a pharmacist to automatically substitute a biosimilar for the corresponding branded biologic without receiving authorization from the prescribing health care provider.3 However, not all biosimilars will have an interchangeable designation; this means that biosimilars cannot always be substituted for a branded biologic at the pharmacy level.12 Instead, substitution must be evaluated on a drug-by-drug basis and should be verified as acceptable in a national compendium.14
In addition, although the FDA can designate a biosimilar as interchangeable, laws regarding drug substitution are under the authority of the states.1 States could theoretically pass laws that allow all biosimilars to be substituted for their corresponding branded biologics; this would increase the use of these agents but would prompt safety concerns.1 Alternatively, states could theoretically pass laws that prohibit substitution with biosimilars that are designated as interchangeable by the FDA, severely limiting the use of biosimilars.1 However, despite these possibilities, the most likely outcome is that the states will allow pharmacists to automatically substitute biosimilars designated as interchangeable by the FDA for branded biologics.7
Routine generic drug substitution practices cannot automatically be applied to all biosimilars, because some will lack an interchangeable designation; therefore, these agents present unique opportunities and responsibilities for P&T committees.4 The distinction between a designation of biosimilar and interchangeable is important, particularly when it comes to formulary decisions.3,7 This differentiation may limit the role of these agents, as well as the flexibility that institutions and health plans may have in formulary design and utilization management.7 Pharmacists on P&T committees should lead the objective evaluation of substituting biosimilars for branded biologic agents by applying the formulary process and therapeutic interchange guidelines and protocols, which involve asking questions about:4
therapeutic and dose equivalence
efficacy and safety risks when switching products.
cost advantages of one product over another.
the potential for a clear interchange process and understanding by prescribers.
the ability to opt out in specific circumstances.
the ability to monitor and assess efficacy and safety outcomes.
Pharmacovigilance Is Needed to Supplement Clinical Trial Data
Pharmacovigilance programs will be necessary to determine whether the safety profile of a biosimilar is comparable to that of the corresponding branded biologic.4,12 Pharmacovigilance will be critical to tracking immunogenicity reactions and other unforeseen adverse events that occur with biosimilar use.1,12,15,16 Although some clinical trials will be required for most biosimilars to receive FDA approval, because of the abbreviated nature of the regulatory pathway, the number of subjects enrolled in these studies is likely to be smaller than the quantity required for the approval of an originator biologic.1 A small study that enrolls a population of a few hundred patients is sufficient to demonstrate comparability; however, this is an inadequate sample size to thoroughly assess the adverse-event profile and the immunogenicity of an agent.1,15 Also, the time horizon for biosimilar clinical studies is not expected to be long enough to reveal all potential adverse events.9 Therefore, it is expected that postmarketing surveillance will be required to supplement the limited clinical trial programs that are suggested by the regulatory guidance on biosimilars proposed by the FDA.17 Ideally, besides assessing the efficacy and safety of biosimilars in standard populations and for approved indications, postmarketing surveillance will include monitoring the use of these agents in diverse patient populations and for off-label uses.1
Postmarketing surveillance can be accomplished by many means, including implementing patient registries and prospective or retrospective observational and epidemiological studies.1,4 The establishment of electronic health records by the Patient Protection and Affordable Care Act (PPACA), passed by Congress in 2010, may also assist pharmacovigilance efforts regarding biosimilars.15 Using Medicare claims data for this purpose is another promising strategy.14 Pharmacists can also contribute to postmarketing surveillance efforts by submitting MedWatch reports to the FDA.1 Whatever the approach to facilitating pharmacovigilance efforts, biosimilar agents will also need to be identified with unique nonproprietary names and National Drug Code (NDC) or billing codes in accounting and/or in electronic health records so that they can be traced; this capability may vary by setting.4
Establishing a Clear Naming System Avoids Confusion
The naming convention applied to biosimilars will be important for pharmacists, physicians, and payers.4 Although a naming system has not yet been decided, most stakeholders would like to see biosimilars identified by terms that correspond with their originator biologics.4,14 However, unlike conventional generic drugs, biosimilars are structurally different from corresponding branded biologics; therefore, they cannot be automatically assigned the same generic name.1 Multiple biosimilars for each branded biologic agent may also be available, each having unique characteristics, so it would not be appropriate for all of them to be known by the same generic name.1 Assigning all related biosimilars the same generic name would be especially confusing when prescribing and dispensing these agents, and it would also complicate postmarketing tracking and surveillance of these products.1
A simple naming system for biosimilars will provide many advantages, including the clear identification of biosimilar agents for the purpose of substitution, reimbursement, and tracking.4 However, the naming process for biologics and biosimilars is likely to be very complex. In the U.S., generic names are approved through the U.S. Adopted Names Council (USANC).1 Because generic chemical drugs are automatically assigned the same nonproprietary name as the branded agent, the USANC does not influence the naming process for these medications.1 However, the USANC has had an increasingly prominent role in developing naming rules and assigning names for biologic agents.1
According to the BPCIA, interchangeable biosimilars are considered to have the same active ingredient as the branded originator biologic, which suggests that they should be identified with the same generic name.17 However, it follows that this would mean that non-interchangeable biosimilars might be considered to have a new active ingredient and therefore should be given a generic name that differs from the branded agent to prevent inappropriate substitution.17 Furthermore, if a non-interchangeable biosimilar is granted interchangeable status, its generic name, presumably, would change to the same name as the originator biologic, although the product would not have changed.17 Lastly, if two non-interchangeable biosimilars are given different generic names, clinicians might be misled into thinking that these products are different, which could lead to a patient being unintentionally and inappropriately switched to a substantially similar product.17
To avoid this confusion, assigning a unique name to each biosimilar product is necessary.1 Otherwise, the biosimilar naming system would be a barrier to accurately differentiating and recording data regarding specific products in an information system, which is essential for effective tracking.4 Tracking will also require biosimilars to be assigned unique drug codes.12 To retain a relationship between interchangeability and nomenclature, it has been suggested that biosimilars should be assigned names that are unique but related to the corresponding branded biologic.1,17 This system would allow any adverse events to be tracked directly to the particular biosimilar and could also prevent inadvertent substitution for the branded biologic.1
For example, different numbers or Greek letters could be added to the root of the generic name to identify unique biosimilars.1 However, this could cause confusion for a product that already has a number and/or a Greek letter in its name, such as interferon beta-1b.1 Although the naming convention for biosimilars is yet to be decided, the FDA is expected to soon provide finalized guidance on this topic, including whether a unique generic name will be assigned to each biosimilar.1
Biosimilars Are Likely to Provide Significant Cost-Savings
Potential cost-savings are an important factor for P&T committees to consider when reviewing biosimilars.2 Biosimilars are expected to substantially reduce drug expenditures in hospitals and health systems, assuming that they achieve the same clinical results that branded agents do.1,3,5,15 However, it is difficult to estimate the cost-savings provided by biosimilars without finalized guidance from the FDA in terms of regulatory and data requirements, because these factors will influence manufacturing and development costs, which in turn will be reflected in market prices.2
Some experts speculate that biosimilars will not provide the same magnitude of cost-savings that generic chemical drugs do when compared with branded products.2 Differences of up to 80% in price between generic and branded chemical medications have been observed in the U.S.9,11 However, because biosimilars require much higher research and development costs, it is expected that these medications will be discounted by only 15% to 35%, compared with their corresponding branded biologic agents.1,9–11,15 For example, it takes 6 to 9 years and $10 to $250 million to develop a biosimilar, whereas it typically takes about 3 years and $1 to $2 million dollars to introduce a generic chemical drug.1,10
Although savings of 15% to 35% might not be as dramatic as the savings provided by generic chemical drugs, they will still be substantial in terms of dollar amount.1 A treatment course of a biologic product often costs tens of thousands of dollars per patient, so even a 20% discount on the billions spent on biologics ($100 billion in 2010) is significant and is expected to increase access and affordability for patients.1 The U.S. Congressional Budget Office estimates that the availability of biosimilars will save consumers $25 billion per decade.15 Decreased spending on branded biologics will also generate significant savings for federal Medicare and Medicaid programs, assuming that biosimilars produce the same clinical results.1,15 A 2007 Pharmaceutical Care Management Association report predicted that biosimilars will save Medicare Part B $3 billion by 2016.18 Additional cost-savings are expected to occur as the prices of biosimilars fall further because of gains in market share.9,11
Many health care systems and health plans support the development of biosimilars, because they believe that these agents can reduce expenditures and provide lower out-of-pocket costs and easier access to treatment for patients.3,7 The financial considerations taken into account by P&T committees that evaluate biosimilars will probably include:4
out-of-pocket costs for patients.
the financial impact on the health care system.
inpatient costs of administration.
outpatient margin.
potential additional monitoring costs when there is therapeutic interchange.
the influence of bundled contracting approaches and patient assistance programs on cost.
After biosimilars become available, it is also expected that most agents will have to be vetted through a cost-control method such as prior authorization or managed care pharmacy step-edit processes.3
P&T committees will have sufficient financial incentive to integrate biosimilars into drug formularies as long as adequate relative efficacy and safety data are available.1,9 If appropriately designed and powered clinical studies have demonstrated equivalent efficacy between a biosimilar and a branded agent, then a cost-minimization analysis will be conducted and the least expensive medicine will generally be chosen for the formulary.9,11 If efficacy differs, another economic evaluation will need to be conducted, such as a cost-effectiveness or a cost-utility analysis.9 Savings arising from the lower price of a biosimilar will need to be weighed against differences in efficacy and their impact on total health care costs.11 The potential differences between the long-term efficacy and safety of a biosimilar and branded agent could give rise to additional health care and productivity loss costs, reducing the cost-effectiveness of the biosimilar agent.9 A cost-effectiveness assessment for a biosimilar should be calculated at its introduction as well as at multiple time points throughout the life cycle of the product, given that uncertainty exists regarding the long-term safety and efficacy of these medications.9 Any databases or observational studies established by manufacturers to demonstrate the post-launch cost-effectiveness of a biosimilar will also aid analyses.9
P&T committees are likely to use established processes to assign biosimilars to an appropriate formulary tier.4 If a biosimilar is considered to be therapeutically equivalent to a branded biologic, the committee will assign it to the appropriate tier if it is decided that it should be covered at all.4 Health care plans are likely to use financial incentives to drive the use of biosimilars by patients.4 For example, requiring a 20% copayment for a biologic on the fourth tier and a smaller charge for a biosimilar on the third tier could mean the difference between an out-of-pocket expenditure for the plan member of $200 or more per month compared with $50 per month.4
However, it should be considered that patients and physicians might be reluctant to switch to a biosimilar on a lower tier for small cost-savings if the branded product is working.12 On the other hand, new, untreated patients might be more willing to try a biosimilar than those that are already receiving treatment.12 Branded biologics manufacturers might also provide payers with a discount or rebate program to forestall being placed in a higher formulary tier.7 A branded biologic manufacturer could also reduce the price of an agent by a fairly small amount, perhaps by 10% to 20%, to discourage buyers from switching to a corresponding biosimilar product.12
Data for Reimbursement Decisions Differ From Data for Regulatory Requirements
In addition to health care providers, third-party payers will also drive biosimilar use.1 However, regulatory approval data do not always correlate with the evidence needed by reimbursement authorities, complicating reimbursement decisions.9 In particular, data from adequately powered equivalence or non-inferiority studies; comparative efficacy studies in a real-world rather than in a clinical trial setting; and health outcome measures instead of surrogate endpoints are expected to be lacking for biosimilars.9 In addition to cost-effectiveness analyses, data comparing biosimilars with branded biologics also need to be considered for pricing and reimbursement purposes.11 Such studies will be available for some (but not all) biosimilars, especially since clinical trials submitted for regulatory approval usually use placebo as the comparator.9 This is significant, because reimbursement authorities require that the biosimilar be compared with the current standard treatment, which in most cases is the branded biologic.9,11 An indirect comparison can be set up using data from separate placebo-controlled trials of the branded biologic and biosimilar, but such comparisons are believed to be of lower methodological quality than direct head-to-head clinical trials of the agents.9
Regulatory authorities also require a study’s results to show efficacy in a structured clinical trial setting, whereas reimbursement authorities prefer data from a real-world setting.9,11 For example, strict patient inclusion and exclusion criteria, as well as the enrollment of healthy volunteers in clinical trials, restricts the generalizability of health care outcome results, limiting the use of such data for reimbursement decisions.9,11 In addition, differences in treatment regimens during clinical trials, compared with those prescribed in daily clinical practice, may have a clinically relevant influence on therapeutic outcomes.9,11
Finally, clinical trials conducted to obtain regulatory approval may utilize surrogate outcome measures, for example, the effect of the biosimilar epoetin alfa on patient hemoglobin levels.9 In contrast, reimbursement authorities prefer data based on primary health outcomes, such as mortality and quality of life.9 To overcome this issue, health economic modeling approaches can be used if there is evidence of a relationship between the surrogate endpoint and the therapeutic outcome.9
Economic evaluations conducted by reimbursement authorities aim to make safe and effective medications available while controlling health care expenditures.9 Reliance on rigorous economic evaluations by public and private payers is consistent with an overall trend toward evidence-based decision-making in health care.9 Illustratively, an increasing number of countries are demanding evidence about comparative effectiveness and cost-effectiveness and are conducting economic evaluations in order to guide pharmaceutical reimbursement decisions.9,11 To date, however, no reimbursement authority has issued guidelines regarding which technique should be used to calculate the cost-effectiveness of biosimilars.9 Given that there could be uncertainty about the relative effectiveness of a biosimilar, submissions to reimbursement authorities ought to include a cost-minimization, as well as a cost-effectiveness or a cost-utility analysis.9 In addition, such exercises might ideally also include sensitivity analyses that explore how changes in relative effectiveness influence the cost-effectiveness of biosimilars.9
Conclusion
Despite the many uncertainties that exist regarding biosimilars, the eventual availability of these agents in the U.S. is inevitable, and health care professionals must begin to understand how and when to use them.4,15 Biosimilars will no doubt become more routinely used when increasing experience and adequate time on the market convince clinicians that these products are safe and effective.1,15 In the meantime, P&T committee members must play a leadership role in adopting and using biosimilars appropriately by applying formulary and practice management tools and principles.4 Pharmacists must also help to ensure the safe and effective use of biosimilars by leading efforts to educate health care providers and patients about these agents.4
References
- 1.American Pharmacists Association. The biosimilar pathway: Where will it lead us? Pharmacy Today. 2011 Dec;:67–76. [Google Scholar]
- 2.Tadlock C. Challenges in the anticipation of biosimilars: How must a P&T committee gear up? Manag Care Interface. 2007;2012(4):14. [PubMed] [Google Scholar]
- 3.Rana JB, Chang DY. Pharmacist perception of biosimilar agents in the U.S. Pharmacy Purchas Prod. 2012;9(7):8. [Google Scholar]
- 4.Hoffman JM, Li E, Stevenson JG. Preparing for biosimilars: Scientific, regulatory, and practice management issues for pharmacists. Live Webcast, 47th American Society of Health-System Pharmacists Midyear Clinical Meeting and Exhibition; Las Vegas. December 3, 2012; Available at: www.ashpadvantagemedia.com/downloads/handout_biosimilars.pdf. Accessed February 26, 2013. [Google Scholar]
- 5.Hoffman JM, Li E, Doloresco F, et al. Projecting future drug expenditures—2012. Am J Health Syst Pharm. 2012;69(5):405–421. doi: 10.2146/ajhp110697. [DOI] [PubMed] [Google Scholar]
- 6.Zelenetz AD, Ahmed I, Braud EL, et al. NCCN biosimilars white paper: Regulatory, scientific, and patient safety perspectives. J Natl Compr Cancer Network. 2011;9(Suppl 4):S1–S22. doi: 10.6004/jnccn.2011.0136. [DOI] [PubMed] [Google Scholar]
- 7.Reinke T. Biosimilars might not measure up to health plan expectations. Manag Care. 2012;21(10):12–13. [PubMed] [Google Scholar]
- 8.Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: Considerations for the healthcare provider. Curr Med Res Opin. 2012;28(6):1053–1058. doi: 10.1185/03007995.2012.686902. [DOI] [PubMed] [Google Scholar]
- 9.Simoens S. Biosimilar medicines and cost-effectiveness. Clinico-economic Outcomes Res. 2011;3:29–36. doi: 10.2147/CEOR.S12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calo-Fernández B, Martínez-Hurtado J. Biosimilars: Company strategies to capture value from the biologics market. Pharmaceuticals. 2012;5(12):1393–1408. doi: 10.3390/ph5121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simoens S, Verbeken G, Huys I. Biosimilars and market access: A question of comparability and costs? Targ Oncol. 2012;7(4):227–231. doi: 10.1007/s11523-011-0192-7. [DOI] [PubMed] [Google Scholar]
- 12.Blackstone E, Fuhr J. Innovation and competition: Will biosimilars succeed? Biotechnol Healthcare. 2012;9(1):24–27. [PMC free article] [PubMed] [Google Scholar]
- 13.Barlas S. President Obama reopens debate on patented biologics: Will the FDA quickly define an abbreviated pathway for biosimilars? P&T. 2011;36(4):178. [PMC free article] [PubMed] [Google Scholar]
- 14.Calvo B, Zuniga L. The U.S. approach to biosimilars: The long-awaited FDA approval pathway. Biodrugs. 2012;26(6):357–361. doi: 10.2165/11635830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch BR, Lyman GH. Biosimilars: Are they ready for prime time in the United States? J Natl Compr Cancer Network. 2011;9:934–943. doi: 10.6004/jnccn.2011.0076. [DOI] [PubMed] [Google Scholar]
- 16.McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91(3):405–417. doi: 10.1038/clpt.2011.343. [DOI] [PubMed] [Google Scholar]
- 17.Nick C. The U.S. Biosimilars Act: Challenges facing regulatory approval. Pharm Med. 2012;26(3):145–152. [Google Scholar]
- 18.Barlas S. FDA readies new guidance and user fee program for biosimilars: Drug interchangeability and user fees are contentious issues the FDA must resolve. Is the FDA up to the task? Biotechnol Healthcare. 2012;9(2):28–29. [PMC free article] [PubMed] [Google Scholar]