Abstract
Dextromethorphan/quinidine (nuedexta) for pseudobulbar affect
INTRODUCTION
Pseudobulbar affect (PBA), also known as emotional lability, labile affect, or emotional incontinence, refers to sudden outbursts of involuntary crying or laughing in patients with neurological disorders, even though there might not be any sad or humorous event to trigger those emotions.1,2 Patients may have episodes of laughing, crying, or both, without an apparent motivating stimulus, or they may respond to stimuli that would not have elicited such an emotional response before the onset of the underlying neurological disorder.
A pathologically lowered threshold for exhibiting these responses is the cardinal feature of PBA. In some patients, the emotional response is exaggerated in intensity; for instance, a sad stimulus might provoke a pathologically exaggerated weeping response instead of a more typical sigh. For other patients, the emotional display can be incongruent with, and even contradictory to, the emotional valence of the provoking stimulus, or it may be incited by a stimulus with no clear valence. A patient may laugh in response to sad news or cry in response to stimuli with no emotional undertone. Alternatively, the episodes may switch from laughing to crying or vice versa.3
The symptoms of PBA can be severe, with persistent, unremitting episodes having a sudden or unpredictable onset. Some patients describe an episode as coming on like a seizure. An outburst might last a few seconds to several minutes or may occur several times a day.4
PBA occurs secondary to a brain injury or a neurological disorder. It is thought to result from disruptions of neural networks that control the generation and regulation of motor output of emotions. It is commonly observed in patients with traumatic brain injury, stroke, Alzheimer’s disease, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) and Parkinson’s disease.5–7 Up to 50% of ALS patients also exhibit PBA.8
The prevalence of PBA in the U.S. is estimated at more than 1 million people, but given the fact that it is relatively common among patients with neurological disorders, the actual prevalence may be higher.9,10 As a result of the characteristic persistent, unremitting episodes of involuntary crying or laughing, which may in turn lead to embarrassment, anxiety, depression, and social isolation, PBA can have a significant negative effect on patients, families, and caregivers.9
CHEMISTRY AND PHARMACOLOGY
The FDA approved dextromethorphan hydrobromide/quinidine sulfate capsules in October 2010 as Nuedexta (Avanir Pharmaceuticals) in a fixed-dose combination intended for oral use only. Each capsule contains the active ingredients of dextromethorphan hydrobromide (HBr) 20 mg and quinidine sulfate 10 mg. The inactive ingredients include croscarmellose sodium, microcrystalline cellulose, colloidal silicon dioxide, lactose monohydrate, and magnesium stearate.11
The chemical name of dextromethorphan HBr is morphinan, 3-methoxy-17-methyl-, (9α, 13α, 14α)-HBr monohydrate. Its empirical formula is C18H25NO • HBr • H2O, and the molecular weight is 370.33. The structural formula is presented in Figure 1.
Figure 1.
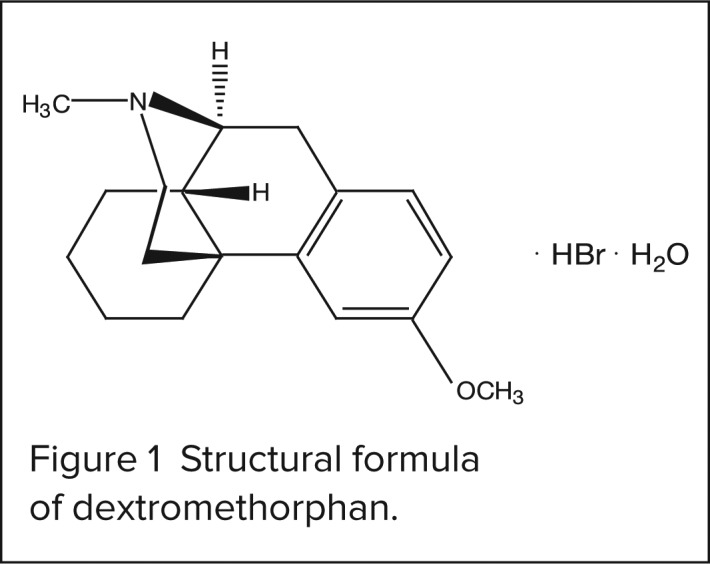
Structural formula of dextromethorphan.
The addition of quinidine sulfate, a specific inhibitor of cytochrome P450 2D6 (CYP2D6)–dependent oxidative metabolism, increases the systemic bioavailability of dextromethorphan. The chemical name of quinidine sulfate is cinchonan-9-ol, 6-methoxy-(9S) sulfate(2:1), (salt), dihydrate. Its empirical formula is (C20H24N2O2)2 • H2SO4 • 2H2O, and the molecular weight is 782.96. The structural formula is shown in Figure 2.11
Figure 2.
Structural formula of quinidine sulfate.
Dextromethorphan is a sigma-1 receptor agonist and a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist with an unknown mechanism of action in the treatment of PBA. By competitively inhibiting CYP2D6, quinidine increases plasma levels of dextromethorphan, thereby catalyzing a major biotransformation pathway for dextromethorphan.11
PHARMACOKINETICS AND PHARMACODYNAMICS
After oral administration, the mean peak concentration (Cmax) of dextromethorphan is elevated with escalating doses. By contrast, the mean Cmax of quinidine does not change substantially; dextromethorphan levels have been found to increase from 20 mg to 30 mg in patients with PBA.
After administration, the time to maximum concentration (Tmax) for dextromethorphan is reached in 3 to 4 hours with repeated doses of dextromethorphan 20 mg/quinidine 10 mg and dextromethorphan 30 mg/quinidine 10 mg. By contrast, the Tmax of quinidine is reached in 1 to 2 hours after repeated doses of dextromethorphan 20 mg/quinidine 10 mg and dextromethorphan 30 mg/quinidine 10 mg.
The mean area-under-the-curve (AUC) concentration of dextromethorphan is increased as the dose is increased from 20 mg to 30 mg. By contrast, a 20-fold increase occurs with dextromethorphan 30 mg/quinidine 10 mg. Dextromethorphan exposure is increased by about 20-fold, compared with dextromethorphan administered without quinidine.11
Absorption of the combination is not significantly affected by food. After the administration of dextromethorphan 20 mg/quinidine 10 mg, dextromethorphan is 60% to 70% protein-bound, whereas quinidine is 80% to 89% protein-bound. The liver is the primary site of metabolism.
Dextromethorphan is catalyzed by CYP2D6, whereas quinidine is metabolized by CYP3A4. Quinidine competitively inhibits the metabolism of dextromethorphan, thereby increasing and prolonging plasma concentrations of dextromethorphan.
The major metabolite of quinidine is 3-hydroxyquinidine, which is at least half as potent as quinidine in its cardiac effects, such as QT prolongation. Approximately 20% of quinidine is excreted unchanged in the urine when the urine pH is less than 7, but only about 5% is excreted in urine when the pH is more alkaline. The elimination half-life of dextromethorphan and quinidine in extensive metabolizers is approximately 13 and 7 hours, respectively, after administration of dextromethorphan 30 mg/quinidine 10 mg.11
PIVOTAL CLINICAL TRIALS
Study 1
A 12-week, randomized, double-blind, placebo-controlled study (n = 326) was conducted to evaluate the efficacy of dextromethorphan/quinidine in patients with pseudobulbar affect (PBA). Patients (ages 18 to 80) had clinically significant PBA, as determined by a score of at least 13 in the Center for Neurologic Study–Lability Scale (CNS–LS). Scores on the seven-item scale ranged from 19.8 to 21. Sample assessment questions include “I find myself crying very easily,” and “I find that I am easily overcome by laughter.”
Patients received dextromethorphan 30 mg/quinidine 10 mg (n = 110), dextromethorphan 20 mg/quinidine 10 mg (n = 107), or placebo (n =109) for 12 weeks. Baseline mean PBA episodes per day in the dextromethorphan 30-mg/quinidine 10-mg, the dextromethorphan 20-mg/quinidine 10-mg, and the placebo arms were 4.7 ± 9.5, 6.8 ± 12.9, and 4.5 ± 7.6 respectively.
Mean changes from baseline in the number of laughing and crying episodes per day in an intent-to-treat analysis of the primary outcome at week 12 were −4.1 (P = 0.0099) in the dextromethorphan 30-mg/quinidine 10-mg arm, −3.9 (P = 0.0048) for the dextromethorphan 20-mg/quinidine 10-mg arm, and −3 (P = 0.0099) in the placebo arm. The incremental reductions in the rate of PBA episodes were 46.9% and 49%, respectively, for dextromethorphan 30 mg/quinidine 10 mg and dextromethorphan 20 mg/quinidine 10 mg (P < 0.001 for each group compared with placebo).
There were more episode-free days with dextromethorphan 30 mg/quinidine 10 mg and dextromethorphan 20 mg/quinidine 10 mg compared with placebo except on day 15. Rates of remission of PBA, defined as the absence of episodes throughout the study’s final 14 days, were significantly greater in both groups (P < 0.005) compared with placebo.
The mean change in CNS–LS scores from baseline was −8.2 with dextromethorphan 30 mg/quinidine 10 mg (P = 0.0002), −8.2 with dextromethorphan 20 mg/quinidine 10 mg (P = 0.0113) and −5.7 with placebo.
Serious adverse events, including respiratory depression, ALS progression, and worsening muscle spasticity, were reported. Discontinuation rates attributable to adverse events were 5.5% with dextromethorphan 30 mg/quinidine 10 mg, 9.3% with dextromethorphan 20 mg/quinidine 10 mg, and 1.8% with placebo. No QTc interval was longer than 580 msec (with the Fridericia correction), and there was no change from baseline of greater than 60 msec reported in all dextromethorphan/quinidine-treated patients during the study.
Patients with any clinically significant abnormality on the electrocardiogram (ECG) or with a family history of a congenital prolonged QT interval syndrome were excluded from the study.12
Study 2
An 85-day randomized, double-blind study (n = 150) of treatment with dextromethorphan/quinidine significantly improved CNS–LS scores by at least 13 points (range, 7–35), compared with placebo, in MS patients who exhibited PBA. Patients received either dextromethorphan 30 mg/quinidine 30 mg (n = 76) or placebo (n = 74) twice daily. At baseline, mean CNS–LS scores were 20.3 ± 5 with treatment and 21.4 ± 5.1 with placebo.
The adjusted mean changes from baseline in CNS–LS scores were 7.7 (standard error [SE] = 0.6) and 3.3 (SE = 0.6) in the dextromethorphan/quinidine and placebo groups, respectively (P < 0.0001). The mean numbers of episodes per week of inappropriate crying and laughing were 4.7 ± 10.9 with the study drug and 11.5 ± 19.4 with placebo (P = 0.0002).
Complete remission, defined as no inappropriate crying or laughing during weeks 1 to 12, was achieved in 20.8% of the treated patients and in 6.9% of the placebo patients (P = 0.028). Response rates, defined as at least a 3-point decline in mean CNS–LS scores during treatment, were 83.6% for the study drug and 49.3% for placebo (P < 0.0001).
Dizziness was the most common adverse event observed with dextromethorphan/quinidine (26.3%) compared with placebo (9.5%) (P = 0.01). However, discontinuation rates caused by adverse events were 27.6% for dextromethor--phan/quinidine and 28.4% for placebo.13
Study 3
In a 29-day, randomized, double-blind, phase 3 study of ALS patients with PBA (n = 140) dextromethorphan/quinidine significantly improved CNS–LS scores of by least 13 points (range, 7–35) compared with either dextromethorphan alone or quinidine alone. Patients (ages 33 to 82 years) received dextromethorphan 30 mg/quinidine 30 mg (n = 70), dextromethorphan 30 mg (n = 33), or quinidine 30 mg (n = 37) twice daily for 28 days. Baseline CNS–LS scores in the dextromethorphan/quinidine, dextromethorphan, and quinidine groups were 20 ± 5.5, 21.4 ± 6.2, and 22.2 ± 5.2, respectively.
The adjusted mean changes (n = 125) from baseline in CNS–LS scores were 7.4 (SE = 0.6), 4.1 (SE = 0 .9; P = 0.001) and 3.7 (SE = 0.8; P < 0.001) for dextromethorphan/quinidine 10 mg, dextromethorphan, and quinidine, respectively. Each 1-point increase in CNS–LS scores is equivalent to an average episode rate increase of 12%.
The combined rates of laughing or crying episodes were 1.89, with a 95% confidence interval (CI) of 1.23 to 2.9 (P = 0.004) with dextromethorphan versus dextromethorphan/quinidine, and 2.13 (95% CI, 1.44–3.16; P < 0.001) with quinidine versus dextromethorphan/quinidine. Patients who were classified as poor dextromethorphan metabolizers, as identified by CYP2D6 genotyping, were not included in the study.14
SAFETY PROFILE
Contraindications
Dextromethorphan/quinidine is contraindicated in patients with a history of atrioventricular (AV) block (complete, without implanted pacemaker, or at a high risk of complete AV block), heart failure, a prolonged QT interval, congenital long-QT syndrome, or a history suggesting torsades de pointes. The drug should not be used concomitantly with other medications that contain quinidine, quinine, or mefloquine; that prolong the QT interval; or that are metabolized by the CYP2D6 isoenzyme, such as thioridazine (Mellaril, Novartis) and pimozide (Orap, Teva/Gate) because of the potential additive effects on the QT interval.
The combination is also contraindicated in patients with history of thrombocytopenia, hepatitis, bone marrow depression, or lupus-like syndrome induced by the quinidine-related drugs mentioned. The concomitant use of monoamine oxidase (MAO) inhibitors or the recent use of MAO inhibitors within 14 days is not recommended because of the potential risk of serious and fatal drug interactions, including serotonin syndrome. Patients with a hypersensitivity to dextromethorphan, as commonly seen in over-the-counter cough preparations such as Robitussin-DM, Mucinex-DM, Delsym, and others, should not take this drug.11
Warning and Precautions
Dextromethorphan HBr/quinidine sulfate may cause quinidine-induced thrombocytopenia that can be severe or fatal. Symptoms have included lightheadedness, chills, fever, nausea and vomiting. Dizziness and hepatitis have also been reported. Patients should be monitored for left ventricular dysfunction or hypertrophy, an increased risk of QT prolongation, and torsades de pointes. Quinidine may also induce lupus-like syndrome.11
Adverse Reactions
Serious adverse drug effects reported with dextromethorphan/quinidine in short-term clinical trials of ALS and MS are presented in Table 1. These reactions occurred in 3% or more of patients receiving dextromethorphan 20 mg/quinidine 10 mg twice daily and at an incidence of more than two times that of placebo.
Table 1.
Adverse Drug Reactions With Nuedexta and Placebo
| Nudexta (%) (N = 107) | Placebo (%) (N = 109 | |
|---|---|---|
| Diarrhea | 13 | 6 |
| Dizziness | 10 | 5 |
| Cough | 5 | 2 |
| Vomiting | 5 | 1 |
| Asthenia | 5 | 2 |
| Peripheral edema | 5 | 1 |
| Urinary tract infection | 4 | 1 |
| Influenza | 4 | 1 |
| Increased gamma-glutamyltransferase (GGT) levels | 3 | 0 |
| Flatulence | 3 | 1 |
From Nuedexta prescribing information.11
Long-term exposure with the combination product during open clinical trials is consistent with the safety profile observed during the placebo-controlled trials. Postmarketing safety reports mention drowsiness, dizziness, nervousness or restlessness, nausea, vomiting, and stomach pain with dextromethorphan, as well as convulsions, apprehension, and ataxia with quinidine.11
Cinchonism (elevated quinine levels), as characterized by nausea, vomiting, diarrhea, headache, hearing loss, vertigo, blurred vision, confusion, and delirium, is common with chronic quinidine toxicity. Other reported reactions with quinidine include depression, mydriasis, night blindness, optic neuritis, photosensitivity, and changes in skin pigmentation.11
Monitoring
It is recommended that patients be monitored for hypokalemia and hypomagnesemia before and during treatment with dextromethorphan/quinidine. For patients with risk factors for QT prolongation and torsades de pointes, including those with left ventricular dysfunction or hypertrophy, an ECG should be obtained 3 to 4 hours after the first dose of the combination drug. Patients should be re-evaluated if necessary. Patients with myasthenia gravis or other conditions that might be affected by anticholinergic effects of the combination drug should be monitored regularly.11
Drug Interactions
Because of potential serious and life-threatening drug–drug interactions, dextromethorphan/quinidine should not be used with MAO inhibitors or drugs from this class that have been taken within the previous 14 days. Concomitant use with drugs that prolong the QT interval and undergo extensive CYP2D6 metabolism (such as thioridazine and pimozide) may result in decreased efficacy or safety of the original drug.
Serotonin syndrome is a potential effect when the combination drug is taken with selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants.
The concomitant administration of quinidine (an inhibitor of P-glycoprotein) with digoxin (a P-glycoprotein substrate) may result in elevations of serum digoxin. Therefore, digoxin levels should be monitored in patients receiving both drugs. Patients should be cautioned about taking a combination of dextromethorphan and quinidine with alcohol and other drugs that act on the central nervous system.11
Pregnancy and Breastfeeding
Dextromethorphan/quinidine is a Pregnancy Category C drug. It should be used during pregnancy only if the potential benefit justifies the risk to the fetus. No adequate or well-controlled studies have been conducted in pregnant women.
It is not known whether the drug is excreted in human milk; therefore, caution should be used if this drug is prescribed to nursing mothers.11
DOSAGE AND ADMINISTRATION
The recommended starting dose for the treatment of PBA is dextromethorphan 20 mg/quinidine 10 mg orally once daily for 7 days. The frequency is then increased to every 12 hours. It is important to reassess patients periodically for spontaneous improvement of PBA and the need to continue the treatment. Dosage adjustments are not required in patients with mild or moderate renal and hepatic impairment.11
P&T COMMITTEE CONSIDERATIONS
Dextromethorphan/quinidine (Nuedexta) is the only FDA-approved therapy for PBA in patients with ALS and MS. Clinical trials have shown a decreased incidence of PBA episodes with therapy. It may be considered an addition to the formulary if other treatments, such as nonpharmacological strategies (e.g., relaxation and distraction techniques) and the off-label use of SSRIs and tricyclic antidepressants, are not an option for particular patients.
COST
Nuedexta is significantly more expensive than generically available dextromethorphan and quinidine. A price comparison is presented in Table 2.15,16
Table 2.
Cost of Drug Products Currently Used in the Treatment Of Pseudobulbar Affect
| Product | Cost Based on Average Wholesale Price |
|---|---|
| Dextromethorphan hydrobromide 20-mg/quinidine sulfate 10-mg capsules (Nuedexta) | $586 |
| Dextromethorphan hydrobromide 15-mg capsules, 7.5–15-mg lozenges, and various 5–15-mg/5-mL liquid products | $10–$32 |
| Quinidine sulfate 200 mg and 300 mg immediate-release tablets | $6–$12 |
CONCLUSION
Dextromethorphan/quinidine is indicated for the treatment of pseudobulbar affect (PBA) and has shown efficacy in patients with underlying ALS and MS. Dextromethorphan’s mechanism of action in treating PBA is not known. Quinidine is an antiarrhythmic agent that, when combined with dextromethorphan, results in an elevated dextromethorphan level.
SSRIs and tricyclic antidepressants are also used in an off-label fashion for PBA, but success in treating PBA exacerbations with these agents has been limited.17 The safety and effectiveness of the combination for other types of emotional lability have not been established. Further studies are needed to determine whether dextromethorphan/quinidine improves functional status or health outcomes, such as quality of life, and whether it is effective in treating other causes of PBA, such as stroke and dementia.
Footnotes
Disclosure: The author reports no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Arciniegas DB, Topkoff J. The neuropsychiatry of pathological affect: An approach to evaluation and treatment. Semin Clin Neuropsychiatry. 2000;5(4):472–479. doi: 10.1053/scnp.2000.9554. [DOI] [PubMed] [Google Scholar]
- 2.Cummings J, Arciniegas D, Brooks B, et al. Defining and diagnosing involuntary emotional expression disorder. CNS Spectr. 2006;11(6):1–7. doi: 10.1017/s1092852900026614. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Arciniegas DB, Bernardini GL, et al. Diagnosis and management of pathological laughter and crying. Mayo Clin Proc. 2006;81(11):1482–1486. doi: 10.4065/81.11.1482. [DOI] [PubMed] [Google Scholar]
- 4.Dark FL, McGrath JJ, Ron MA. Pathological laughing and crying. Aust NZ J Psychiatry. 1996;30(4):472–479. doi: 10.3109/00048679609065020. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: A link to the cerebellum. Brain. 2001;124(Part 9):1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 6.Robinson RG, Parikh RM, Lipsey JR, et al. Pathological laughing and crying following stroke: Validation of a measurement scale and a double-blind treatment study. Am J Psychiatry. 1993;150(2):286–293. doi: 10.1176/ajp.150.2.286. [DOI] [PubMed] [Google Scholar]
- 7.Lopez OL, Gonzalez MP, Becker JT, et al. Symptoms of depression and psychosis in Alzeimer’s disease and fronto-temporal dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1996;1996;9:154–161. [Google Scholar]
- 8.Gallagher JP. Pathologic laughter and crying in ALS: A search for their origin. Acta Neurol Scand. 1989;80:114–117. doi: 10.1111/j.1600-0404.1989.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 9.Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA): Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1–16. doi: 10.1017/s1092852900026602. [DOI] [PubMed] [Google Scholar]
- 10.Bradley W, Arnold R, Kaye R, et al. Involuntary emotional expression disorder (IEED): Prevalence and treatment. Presented at the American Academy of Neurology, 59th annual meeting; Boston. May 1, 2007. [Google Scholar]
- 11.Nuedexta (dextromethorphan hydrobromide and quinidine sulfate) oral capsules, prescribing information. Aliso Viejo, Calif.: Avanir Pharmaceuticals, Inc.; Oct, 2010. Revised August 2011. [Google Scholar]
- 12.Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693–702. doi: 10.1002/ana.22093. [DOI] [PubMed] [Google Scholar]
- 13.Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59(5):780–787. doi: 10.1002/ana.20828. [DOI] [PubMed] [Google Scholar]
- 14.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: A randomized trial. Neurology. 2004;63(8):1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 15.Drug Facts and Comparisons 4.0 (online) Conshohocken, Pa.: Wolters Kluwer Health; [Google Scholar]
- 16.Regence RxPrice Guide. Apr, 2011.
- 17.Schiffer R, Pope LE. Review of pseudo-bulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447–454. doi: 10.1176/jnp.17.4.447. [DOI] [PubMed] [Google Scholar]



