Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor (PAC1) play a critical role in biological processes that mediate stress response and have been implicated in psychological outcome following trauma. Our previous work [Ressler et al. (2011); Nature 470:492–497] demonstrated that a variant, rs2267735, in the gene encoding PAC1 (ADCYAP1R1) is associated with post-traumatic stress disorder (PTSD) in a primarily African-American cohort of highly traumatized females. We sought to extend and replicate our previous finding in a similarly trauma-exposed, replicate sample of 1,160 African-American adult male and female patients. Self-reported psychiatric measures were collected, and DNA was obtained for genetic analysis. Using linear regression models to test for association with PTSD symptom severity under an additive (allelic) model, we found a genotype × trauma interaction in females (P< 0.001), but not males (P> 0.1); however, there was no main effect of genotype as in our previous study. The observed interaction suggests a genetic association that increases with the degree of trauma exposure in females only. This interaction remained significant in females, but not males, after controlling for age (P< 0.001), income (P< 0.01), past substance abuse (P< 0.001), depression severity (P= 0.02), or child abuse (P< 0.0005), and all five combined (P= 0.01). No significant effects of genotype (or interactions) were found when modeling depression severity when controlling for comorbid PTSD symptom severity (P> 0.1), demonstrating the relative specificity of this variant for PTSD symptoms. A meta-analysis with the previously reported African-American samples revealed a strong association between PTSD symptom severity and the interaction between trauma and genotype in females (N = 1424, P< 0.0001).
Keywords: PACAP, PAC1R, ADCYAP1R1, gene, PTSD, gene × environment, sex differences, anxiety
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a severe psychiatric disorder that affects twice as many women as men (~10% women, ~4% men) [True et al., 1993]. PTSD has a prevalence of approximately 6%, but can occur in up to 25% of subjects who have experienced severe psychological trauma, such as combat veterans, refugees, and assault victims [Kessler et al., 2005; Koenen and Widom, 2009; Breslau et al., 2012; McLay et al., 2012]. The differential risk determining those who do versus those who do not develop PTSD is due in part to: (1) genetics, with ~30–40% heritability for PTSD risk following trauma; (2) gender, with women having approximately twice the risk as men to develop PTSD following trauma; and (3) trauma history, including adult and childhood trauma, and psychological factors which may differentially mediate fear and emotion regulation [Stein et al., 2002; Koenen et al., 2009; Roberts et al., 2011; Sartor et al., 2011]. From a public health perspective, determining who is at risk for developing and treating PTSD is critical as patients suffering from PTSD are at increased risk of major depression [Breslau et al., 2000], substance dependence [Breslau et al., 2003], impaired functioning, reduced life course opportunities, including unemployment and marital instability [Kessler, 2000], and significant health-related problems [Farley and Patsalides, 2001; Simpson, 2002; Zayfert et al., 2002].
Although initially identified among combat veterans, research now indicates that a number of populations are at high risk for PTSD based on high levels of trauma exposure. One of these populations is low-income African-Americans living in urban environments [Goldmann et al., 2011]. Over the last 5 years we have examined the prevalence and comorbidity of PTSD in over 4,000 low-income, primarily African-American men and women in Atlanta, documenting an extremely high prevalence of trauma exposure (>85%) and of PTSD. Specifically we have found clinically significant levels of current PTSD in 18–20% of the studied population and a lifetime rate of PTSD which approaches 40% [Gillespie et al., 2009]. Consistent with prior research on impairment associated with PTSD, our data suggest that within this population PTSD is associated with a number of adverse health and mental health outcomes including increased risk for suicide attempts (30%) and substance abuse (39% past/9% current). Additionally, a growing literature implies that untreated trauma-related disorders may contribute to an intergenerational cycle of PTSD [Roberts et al., 2012]. Understanding the biological vulnerability that differentiates those who develop PTSD from those who do not is critical for understanding the mechanisms of PTSD and for improving therapeutic and preventative strategies.
Despite evidence of genetic vulnerability to PTSD from twin studies, little progress has been made in identifying variants in specific genes that may influence female-specific vulnerability. Our group has recently shown that pituitary adenylate cyclase-activating polypeptide (PACAP) and its PAC1 receptor (PAC1R) may be critical mediators of abnormal processes in women following psychological trauma [Ressler et al., 2011]. Specifically, we found a sex-specific association of a single nucleotide polymorphism (SNP) residing in a putative estrogen response element within the ADCYAP1R1 gene (PAC1R) that predicts fear response, PTSD diagnosis, and symptoms in highly traumatized females (N = 763; P<2 × 10−5). In addition to the genetic association within the PACAP receptor, we found that levels of PACAP protein in the blood was associated with increased PTSD symptoms in women, and that there was differential methylation within the PAC1R correlated with PTSD symptoms. Finally, we found that in an entirely different postmortem cohort, the rs2267735 risk allele was associated with differential ADCYAP1R1 gene expression in the brain in females but not males. These data were recently supported by results from a cohort of male and female children of traumatized mothers demonstrating that the risk allele of the same SNP, rs2267735, in ADCYAP1R1 was also associated with dark-enhanced startle, and indicator of PTSD symptomology [Jovanovic et al., 2012]. Moreover, Uddin et al. [2012] found an interaction with ADCYAP1R1 genotype and childhood maltreatment in a cohort of females from the Detroit Neighborhood Health Study; however, another study failed to replicate this association when examining main effects of SNP rs2267735 among presumably less traumatized, African-American and Caucasian populations [Chang et al., 2012]. Their analyses accounted for trauma and age at worst trauma as predictors of PTSD; however, it is unclear whether they utilized a genotype × environment interaction between specific trauma types and rs2267735 genotype. Thus, determining the specific characteristics of the study cohorts that influence the impact of PAC1 genotype on PTSD susceptibility is important for a further mechanistic understanding of these pathways.
We sought to replicate our previous finding by genotyping rs2267735 in an additional sample of females and males to explore possible main and interacting effects of ADCYAP1R1 and trauma/ abuse on risk of PTSD. As a secondary analysis, we conducted a meta-analysis on a combined sample of African-Americans from the original sample [Ressler et al., 2011] plus the replication sample in order to tease apart the main and interacting effects of ADCYAP1R1 and variation in trauma load and/or child abuse on risk of PTSD in a larger overall high-risk sample. It is important to further extend the genetic studies examining this pathway with regard to PTSD and to elucidate the biological and social/environmental variables that may differentially mediate the risk associated with ADCYAP1R1 and PTSD. Establishing well-defined risk criteria for early intervention in the treatment of PTSD is a necessary advancement in development of preventative public health measures for traumatized populations.
METHODS
Sample and Sample Recruitment
The data from this study were collected as part of a larger study investigating the roles of genetic and environmental factors in predicting response to stressful life events in a predominantly African-American, urban population of low socioeconomic status (SES). This is a replication and extension of our original publication demonstrating PAC1R polymorphisms with PTSD [Ressler et al., 2011]. Research participants were approached while either waiting for their medical appointments or while waiting with others who were scheduled for medical appointments, in the waiting rooms of the primary care clinic or obstetrical–gynecological clinic of a large urban, public hospital. Subjects who indicated willingness to participate provided written informed consent, participated in a verbal interview, and provided a salivary sample for DNA extraction (described below). A percentage of individuals provided blood samples as part of a follow-up visit. The replication sample set analyzed were unique SIDs and did not include any subjects included in the first manuscript. Many of the study participants in the replication sample set were recruited at a later date compared to those included in the original sample set. In the replication sample, there was less recruitment from OB/GYN (59% vs.70%) clinics and more from primary care relative to the original group (33% vs. 23%); however, these differences were not statistically significant.
The data presented in this manuscript are limited to individuals self-identifying as African-American (92% of total genotyped) to decrease possible confounding factors due to ethnic stratification; thus only the African-American samples for whom we also had complete trauma load data (N = 911) from the original manuscript [Ressler et al., 2011] were used in the meta-analysis. A comparison of subject demographics between both sample cohorts is presented in Table 1. All procedures in this study were approved by the Institutional Review Boards of Emory University School of Medicine and Grady Memorial Hospital.
TABLE 1.
Original and Replication Sample Demographics Stratified by Sex
| Demographic (all self-identified African-American/Black) |
Total sample, N (%) |
Original sample, N (%) |
Replication sample, N (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| Total sample size (N) | 2,070 (100) | 646 (31.2) | 1,424 (68.8) | 911 (100) | 345 (37.9) | 566 (62.1) | 1,159 (100) | 301 (26.0) | 858 (74.0) |
| Agea,b,c | 2,067 (99.9) | 909 (99.8) | 1,158 (99.9) | ||||||
| 18–24 | 415 (20.1) | 67 (10.4) | 348 (24.4) | 182 (20.0) | 35 (10.1) | 147 (26.0) | 233 (20.1) | 32 (10.6) | 201 (23.5) |
| 25–34 | 393 (19.0) | 87 (13.5) | 306 (21.5) | 174 (19.1) | 50 (14.5) | 124 (21.9) | 219 (18.9) | 37 (12.3) | 182 (21.2) |
| 35–44 | 401 (19.4) | 136 (21.1) | 265 (18.6) | 189 (20.8) | 77 (22.3) | 112 (19.8) | 212 (18.3) | 59 (19.6) | 153 (17.9) |
| 45–54 | 569 (27.5) | 217 (33.6) | 352 (24.7) | 256 (28.2) | 120 (34.8) | 136 (24.0) | 313 (27.0) | 97 (32.2) | 216 (25.2) |
| 55–64 | 260 (12.6) | 124 (19.2) | 136 (9.6) | 96 (10.6) | 56 (16.2) | 40 (7.1) | 164 (14.2) | 68 (22.6) | 96 (11.2) |
| ≥65 | 29 (1.4) | 15 (2.3) | 14 (1.0) | 12 (1.3) | 7 (2.0) | 5 (0.9) | 17 (1.5) | 8 (2.7) | 9 (1.1) |
| Education | 2,067 (99.9) | 910 (99.9) | 1,157 (99.8) | ||||||
| <12th grade | 474 (22.9) | 144 (22.3) | 330 (23.2) | 215 (23.6) | 74 (21.4) | 141 (24.9) | 259 (22.4) | 70 (23.4) | 189 (22.0) |
| High school graduate | 806 (38.9) | 253 (39.2) | 553 (38.8) | 354 (38.9) | 136 (39.4) | 218 (38.5) | 452 (39.1) | 117 (39.1) | 335 (39.0) |
| Grad. equivalency diploma | 104 (5.0) | 37 (5.7) | 67 (4.7) | 53 (5.8) | 20 (5.8) | 33 (5.8) | 51 (4.4) | 17 (5.7) | 34 (4.0) |
| Some college/tech grad. | 525 (25.3) | 151 (23.4) | 374 (26.3) | 230 (25.3) | 87 (25.2) | 143 (25.3) | 295 (25.5) | 64 (21.4) | 231 (26.9) |
| College grad./grad. school | 158 (7.6) | 58 (9.0) | 100 (7.0) | 58 (6.4) | 27 (7.8) | 31 (5.5) | 100 (8.6) | 31 (10.4) | 69 (8.0) |
| Employment | 2,066 (99.8) | 910 (99.9) | 1,156 (99.7) | ||||||
| Currently unemployed | 1,447 (69.9) | 468 (72.4) | 979 (68.8) | 640 (70.3) | 254 (73.6) | 386 (68.2) | 807 (69.8) | 214 (71.6) | 593 (69.1) |
| Currently employed | 619 (29.9) | 175 (27.1) | 444 (31.2) | 270 (29.7) | 90 (26.1) | 180 (31.8) | 349 (30.2) | 85 (28.4) | 264 (30.8) |
| Household monthly incomec | 2,014 (97.3) | 882 (96.8) | 1,132 (97.7) | ||||||
| 0–499 US$ | 740 (35.7) | 247 (38.2) | 493 (34.6) | 350 (39.7) | 136 (40.7) | 214 (39.1) | 390 (34.5) | 111 (38.0) | 279 (33.2) |
| 500–999 US$ | 563 (27.2) | 163 (25.2) | 400 (28.1) | 259 (29.4) | 95 (28.4) | 164 (29.9) | 304 (26.9) | 68 (23.3) | 236 (28.1) |
| 1,000–1,999 US$ | 489 (23.6) | 136 (21.1) | 353 (24.8) | 188 (21.3) | 66 (19.8) | 122 (22.3) | 301 (26.6) | 70 (24.0) | 231 (27.5) |
| ≥2,000 US$ | 222 (10.7) | 80 (12.4) | 142 (10.0) | 85 (9.6) | 37 (11.1) | 48 (8.8) | 137 (12.1) | 43 (14.7) | 94 (11.2) |
| Substance abusea,b | 2,052 (99.1) | 902 (99.0) | 1,150 (99.2) | ||||||
| Past | 561 (27.1) | 265 (41.0) | 296 (20.8) | 263 (28.9) | 145 (42.3) | 118 (21.1) | 298 (25.9) | 120 (40.3) | 178 (20.9) |
| Current | 102 (0.5) | 58 (9.0) | 44 (3.1) | 46 (5.0) | 27 (8.0) | 19 (3.4) | 56 (4.9) | 31 (10.4) | 25 (2.9) |
Significant differences between males and females in the original set.
Significant differences between males and females in the replication set.
Significant differences between original and replication sample sets in females only.
Procedure
Participants completed a battery of verbal self-report measures, all which took 30–90 min to complete (dependent in large part on the extent of the participant’s trauma history and symptoms). We read the instruments to participants to guard against relatively high rates of impaired literacy. Each person was paid $15.00 for their time for participation in this phase of the study. The measures used in the current study are described below.
PTSD symptom scale (PSS)
The PSS is a psychometrically valid 17-item self-report scale assessing the extent of PTSD symptom frequency and severity over the prior 2 weeks [Falsetti et al., 1993; Coffey et al., 1998; Foa and Tolin, 2000]. Consistent with prior literature, we summed the PSS frequency items (“0: not at all” to “3: >5 times a week”) to obtain a continuous measure of PTSD symptom severity ranging from 0 to 51. DSM-IV diagnosis of PTSD was made on the basis of presence of DSM-IV criteria A–E based on response to the PSS questionnaire. The PSS had a standardized alpha coefficient of 0.92 (M= 12.44, SD = −12.40).
Beck depression inventory (BDI)
Depressive symptoms were assessed with the 21-item BDI [Beck et al., 1961], a well-validated, commonly used continuous measure of level of depressive symptoms. In this sample, the BDI had a standardized alpha coefficient of 0.93 (M= 13.88, SD = 12.04).
Traumatic events inventory (TEI)
The TEI [Schwartz et al., 2005,2006] is a 14-item instrument for lifetime history of traumatic events. For each event, the TEI assesses experiencing, witnessing, and confrontation of traumatic events where appropriate. For our analysis, we used the total TEI in addition to just the TEI items assessing trauma exposure after childhood since we used the CTQ to assess childhood trauma exposure.
Childhood trauma questionnaire (CTQ)
The CTQ [Bernstein and Fink, 1998] is a self-report inventory assessing three types of childhood abuse: sexual, physical, and emotional. Studies have established the internal consistency, stability over time, and criterion validity of both the original 70-item CTQ and the current brief version [Bernstein et al., 2003]. The CTQ yields a total score and subscale scores for each of the types of child abuse. Our CTQ data demonstrated high internal reliability (physical abuse: alpha = 0.80, M = 8.05, SD = 3.95; sexual abuse: alpha = 0.94, M = 7.66, SD = 4.99; emotional abuse: alpha = 0.84, M = 8.84, SD = 4.76; total of the three scales: alpha = 0.92, M = 24.55, SD = 11.49).
DNA Extraction and Genotyping
DNA from saliva was collected in Oragene vials (DNA Genotek Inc., Ontario Canada) or from whole blood collected in EDTA tubes. DNA from saliva was extracted using the DNAdvance extraction kit (Beckman Coulter Genomics, Danvers, MA), and DNA from blood was extracted using the E.Z.N.A. Blood DNA Midi Kit (Omega Bio-tek, Norcross, GA). All DNA for genotyping was quantified by gel electrophoresis using Quantity One (BioRad, Hercules, CA) and normalized to a concentration of 10 ng/µl. DNA concentrations that fell below5 ng/µl were not used. DNA was plated into 384 plates at 10 or 20 ng for Taqman or Sequenom genotyping, respectively. All DNAs were dried down prior to performing the reactions. Genotyping of rs2267735 (ADCYAP1R1) was done using both Sequenom and Taqman. Taqman reactions were performed using Taqman SNP Genotyping Assays along with Taqman Genotyping Master Mix (Applied Biosystems Inc., Foster City, CA). Alleles were discerned using the 7900HT Fast Real-Time PCR system. Seque-nom genotypes were collected using the iPLEX chemistries and the MassARRAY system (Sequenom, Inc., San Diego, CA). Genotypes represent forward strand alleles (C/G). Within- and across-plate duplicates were used for quality control of DNA plating. Negative controls were used to assess assay integrity. Both passed QC measures. We also performed across-method duplications to confirm accuracy of calls by either genotyping platform. Eighteen percent of the total samples in the replication sample set were genotyped using both Taqman and Sequenom. Only one discordant sample was identified resulting in a discordance rate of 0.4%. Fifteen percent of all the samples genotyped for the replication set had also been genotyped in the original, published cohort. These duplicates were used to confirm calls but were excluded from the analyses in this manuscript such that all samples in the replication set are unique relative to the original dataset. The concordance rate for these duplicates was 100%. The genotype call rate was >95% for either genotyping method. Genotypes for the replication set did not deviate significantly from the expected proportions under Hardy–Weinberg equilibrium (HWE; P=0.13).
Statistical Analyses
All statistical analyses were performed using SAS (v. 9.3) or R (v. 2.15.1). The distribution of the primary study variables was calculated using means with standard deviations for continuous variables, and frequencies (and percents) for categorical variables. To compare demographic variables between males and females or between the original (African-American subjects from Ressler et al. [2011]) and replication samples, Wilcoxon–Mann–Whitney tests were performed for continuous variables, and Chi-square tests were performed for categorical variables. For genetic association studies, we used linear regression to assess whether ADCYAP1R1 genotype was associated with the outcome, either PTSD symptom severity or depression severity. In all cases, we tested for association under an additive (allelic) model where the number of copies of the C allele was hypothesized to influence the outcome variable linearly. For the original and replication datasets, we first assessed the main effect of genotype on PTSD symptom severity, and subsequently included a main effect for trauma and the interaction between genotype and trauma as additional predictors in the model. We also fit the same model in the replication dataset with additional covariates for age, income, past substance abuse, depression, child abuse, or all five variables. To assess the specificity of the ADCYAP1R1 variant, we also tested the same models as above in the replication dataset but with depression levels (using BDI score) as the outcome measure and controlled for comorbid PTSD symptom severity. For the meta-analyses, we combined the original and replication datasets and included an indicator variable (coded as 0 for the original sample or 1 for the replicate sample) as a covariate in the regression analyses. To adjust for potential confounding due to admixture in our all African-American sample, we conducted principal components analysis using Illumina Human Omni1-Quad genome-wide data that was recently collected for a subset of our subjects as a part of a separate study. We then used the top 10 principal components as covariates in our main analysis (regressing ADCYAP1R1 genotype and trauma load on PTSD symptom severity), in the original and replication samples separately.
To ensure that our interaction test results did not depend on linearity or distributional assumptions, we computed robust standard errors and verified the key result with permutation and bootstrapping. To address the possibility that standard errors could be underestimated due to departure from the assumed linear model, we used the robust function in R [Cookson, 2011] to estimate robust (heteroscedasticity-consistent) standard errors [White, 1980] as suggested by Voorman et al. [2011]. For the permutation procedure, we used R to carry out 10,000 permutations after mean-centering the genotype and trauma variables as described in Buzkova et al. [2011]. In each permutation, trait values were randomly shuffled across individuals, the association test was re-performed, and the t-statistic for the interaction between genotype and trauma was recorded. Permutation P-values were then estimated as the proportion of permutations for which the t-statistic for the interaction term exceeded the original in magnitude. Because this permutation test is dependent on the assumption that genotype and trauma are independent, we also carried out a bootstrapping procedure similar to that described in Buzkova et al. [2011]. Briefly, we fit a model with no interaction term to obtain null-hypothesis parameter estimates. We then drew samples of the residuals from this model, with replacement, and used each set of residuals along with the parameter estimates to create 10,000 datasets simulated under the null hypothesis of no interaction. Interaction t-statistics from each simulated dataset were recorded, and the bootstrapped P-value was computed as the proportion of t-statistics from the simulated data that exceeded the original in magnitude.
RESULTS
Demographic information describing SES for the original and replication samples are listed in Table 1. There were no significant differences between the sexes with respect to the SES variables of education, employment, or income; however, males were older (z = 7.58, P< 0.0001) and had more substance abuse than females (past: χ2=46.10, P< 0.0001; current: χ2=26.69, P< 0.0001) in the replication sample. We found significant differences between the sexes with respect to the psychiatric variables of total trauma load (TEI total score, z = 5.11, P< 0.0001), adult trauma load (TEI score for non-childhood items only, z=6.78, P<0.0001), childhood trauma load (CTQ score, z= −2.78, P<0.01), and depression severity (z = −2.04, P < 0.05), but not PTSD symptom severity (PSS total score), our main psychiatric outcome measurement. We found that males had higher total and adult trauma loads, whereas females had higher childhood trauma loads and depression symptom severity (BDI total score) in the replication sample. Table 2 illustrates these findings, as well as more detailed subscale measures for both adult and childhood traumas across sexes from both cohorts.
TABLE 2.
Distribution of the Main Psychiatric Variables for Each Sample Set, Stratified by Sex
| Variable | Original sample, Mean ± SD (N) |
Replication sample, Mean ± SD (N) |
||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| PTSD symptom severity | 12.70 ± 12.45 (911) | 12.96±12.1 (345) | 12.54±12.6 (566) | 1275±12.4 (1159) | 12.30 ±12.6 (301) | 12.91 ±12.3 (858) |
| Total trauma load | 4.83 ±3.5 (911)*** | 5.60 ±3.4 (345) | 4.36 ±3.4 (566) | 472 ±3.2 (1,159)*** | 5.44 ±3.1 (301) | 4.47 ±3.3 (858) |
| Experiencing attack | 3.28±4.39 (911)*** | 4.13 ±4.99 (345) | 276±3.88 (566) | 3.29 ±4.19 (1,159)*** | 4.04 ±4.21 (301) | 3.02±4.16 (858) |
| Witnessing attack | 5.93±7.18 (761)*** | 7.99±8.19 (304) | 4.55±6.05 (457) | 5.54±6.59 (1,111)*** | 7.18±7.00 (290) | 4.96±6.34 (821) |
| Experiencing a natural disaster |
0.42 ±0.95 (911)*** | 0.58±1.01 (345) | 0.33 ±0.89 (566) | 0.42 ±0.95 (1,159)*** | 0.59 ±1.18 (301) | 0.36±0.85 (858) |
| Experiencing a sudden illness/injury |
0.29 ±070 (911) | 0.33 ±076 (345) | 0.28±0.67 (566) | 0.29 ±0.69 (1,159) | 0.35±0.80 (301) | 0.27 ±0.64 (858) |
| Military combat exposure | 0.06±0.51 (911) | 0.16±0.82 (345) | N/A | 0.03±0.23 (1,159) | 0.08 ±0.42 (301) | 0.01±0.10 (858) |
| Adult trauma load | 4.24 ±3.0 (911)*** | 5.18±3.0 (345) | 3.67 ±2.8 (566) | 4.11 ±2.8 (1,159)*** | 5.01 ±2.8 (301) | 3.80 ±2.8 (858) |
| Childhood trauma load | 41.60 ±17.2 (909)* | 38.93 ±137 (343) | 43.21 ±18.8 (566) | 40.54±17.2 (1,142)** | 37.19±13.4 (300) | 4174 ±18.3 (842) |
| Sexual abuse | 7.52±4.92 (909)*** | 5.85 ±2.59 (343) | 8.55±5.67 (566) | 774±5.03 (1,142)*** | 5.83 ±2.56 (300) | 8.42 ±5.50 (858) |
| Physical abuse | 8.30 ±4.06 (909) | 8.27 ±3.56 (343) | 8.32 ±4.34 (566) | 8.05 ±3.94 (1,142) | 7.92±3.19 (300) | 8.10±4.18 (858) |
| Emotional abuse | 9.07 ±479 (909)* | 8.47 ±4.21 (343) | 9.43 ±5.07 (566) | 878 ±474 (1,142)* | 8.09 ±4.09 (300) | 9.03 ±4.93 (858) |
| Depression severity | 14.08±12.1 (911) | 13.16±117 (330) | 14.59 ±12.3 (535) | 14.25±12.3 (1,159)* | 13.36±12.6 (287) | 14.57 ±12.1 (812) |
P< 0.05,
P< 0.01,
p< 0.0001 for differences between the sexes.
Comparing the original and replication samples, we found no significant differences between the datasets for the measurements of education, employment, and substance abuse (Table 1). However, we did find that females were older in the replication sample than the original sample (z= −2.16, P< 0.05), but males were generally older than females in both datasets (z = 10.69, P< 0.0001). Though there were marginal sex differences between the original and replication sample in terms of income (χ2= 8.00, P< 0.05), there was a difference between income in females in the original sample compared to the replication sample (χ2 = 9.06, P < 0.05) with more females making <$500/month in the original sample. There were also more males than females with substance abuse in both datasets (past: χ2=92.01, P< 0.0001; current: χ2 =33.21, P< 0.0001). Moreover, there were no significant differences between the total sample datasets with respect to the psychiatric and trauma exposure variables; however, there were sex differences in both datasets for total trauma load, adult trauma load, and childhood trauma load (see Table 2).
The allele frequencies of ADCYAP1R1 for the replication and original samples are listed in Table 3. There was no statistically significant difference in the frequencies of each genotype between the sample sets (χ2= 1.97, P>0.1).
TABLE 3.
Genotype Distribution in the Original and Replication Samples (All African-American)
| PACIR genotype | Original |
Replication |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| GG | 117 (12.8%) | 42 (12.2%) | 75 (13.3%) | 127 (11.0%) | 36 (12.0%) | 91 (10.6%) | 244 (11.8%) | 86 (12.2%) | 195 (12.1%) |
| GC | 415 (45.6%) | 164 (47.0%) | 253 (44.7%) | 551 (47.5%) | 150 (49.8%) | 401 (46.7%) | 966 (46.7%) | 342 (48.4%) | 742 (46.0%) |
| CC | 379 (41.6%) | 141 (40.9%) | 238 (42.0%) | 481 (41.5%) | 115 (38.2%) | 366 (42.7%) | 860 (41.5%) | 279 (39.4%) | 675 (41.9%) |
ADCYAP1R1 Genotype Interacts With Trauma Load to Predict PTSD Symptom Severity in the Original and Replication Samples
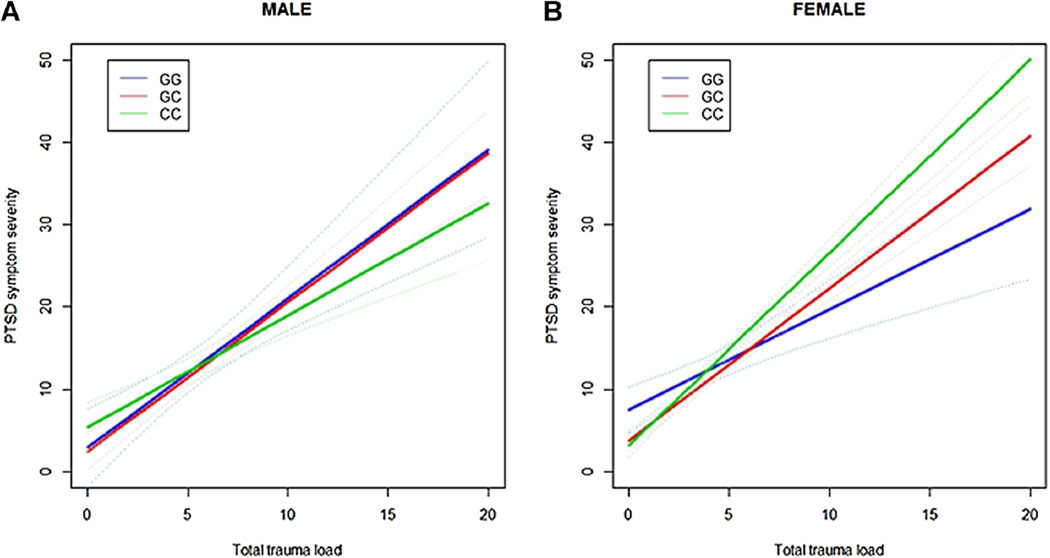
Using linear regression and an additive effect of genotype in the original sample (i.e., the African-American subset of data published in Ressler et al. [2011]), we found a significant association between PTSD symptom severity and ADCYAP1R1 genotype in females (N = 566, t=3.14, P= 0.0018) as in the original publication [Ressler et al., 2011]. In a separate model including the trauma load variable, we found a genotype × trauma interaction in females (Fig. 1A, Table 4), but not males (N = 345, P= 0.27), with carriers of the C allele associating with greater ZPTSD symptom severity (t = 2.38, P=0.0174).
FIG. 1.
Regression lines with 95% confidence intervals (dotted lines) showing predicted PTSD symptom severity based on total trauma load. These lines illustrate that there is an interaction between total trauma load and risk genotype (CC) in females, but not males (P > 0.2 in both), in the (A) original (N=566, P=0.0174) and (B) replication (N = 858, P=0.0006) samples.
TABLE 4.
Parameter Estimates in the Model Predicting PTSD Symptom Severity in Females Only
| Sample set | Parameter | Estimate | Standard error | t-Statistic | P-value |
|---|---|---|---|---|---|
| Original (N = 566) | rs2267735×total trauma load | 0.44 | 0.18 | 2.38 | 0.0174 |
| Replication (N = 858) | rs2267735×total trauma load | 0.63 | 0.18 | 3.46 | 0.0006 |
In the replication sample, we did not find a significant association between PTSD symptom severity and ADCYAP1R1 genotype in females as in the original sample; however, we did find a genotype × trauma interaction that differed by sex. When stratifying by sex, there was an ADCYAP1R1 genotype × trauma interaction (t= 3.46, P = 0.0006) in females (N = 858) (Fig. 1B, Table 4). As shown in Figure 1B, the C allele is more associated with PTSD symptom severity for females with high trauma load. In males (N = 301) on the other hand, there was no ADCYAP1R1 genotype -× trauma interaction (P = 0.47) or main effect of genotype, similar to that presented in Figure 1A. The genotype × trauma interaction in females remained when adjusting for age (t= 3.44, P = 0.0006), income (t= 3.21, P = 0.001), past substance abuse (t=3.56, P = 0.0004), depression severity (t= 2.30, P = 0.02), or childhood trauma load (t= 3.76, P = 0.0002), and all five (t= 2.50, P = 0.01). Moreover, when controlling for comorbid PTSD symptom severity, we did not find a significant association of ADCYAP1R1 genotype on depression severity nor a trauma × genotype interaction (P> 0.1).
The main finding of an ADCYAP1R1 genotype × trauma interaction on PTSD symptom severity was tested for statistical robustness and verified with permutation and bootstrapping methods. Computing heteroscedasicity-consistent standard errors as sug-gested by Voorman et al. [2011], we obtained a P-value of 0.0006, suggesting that the observed association was not due to model misspecification. To compute empirical P values, we applied two approaches suggested by Buzkova et al. [2011]. Conducting 10,000 permutations after mean-centering the genotype and trauma variables, we obtained a P-value of 0.001. Using bootstrapping methods to generate 10,000 datasets, we obtained a bootstrapped P-value of 0.0005. The consistency of these results suggests that the originally observed association was not dependent on asymptotic assumptions.
ADCYAP1R1 Genotype Interacts With Trauma Load to Predict PTSD Symptom Severity in the Meta-Analysis
In the combined sample, we found a strong association between PTSD symptom severity and a trauma × genotype interaction in females (N = 1424, t=4.30, P<0.0001) but not males (N = 646, P> 0.1) (Fig. 2, Table 5). Thus, at higher trauma levels, carriers of the C allele are associated with PTSD symptom severity. Again, the genotype × trauma interaction in females remained when adjusting for age (t=4.29, P< 0.0001), income (t=4.44, P< 0.0001), past substance abuse (t=4.31, P< 0.0001), depression severity (t=2.57, P=0.01), or childhood trauma load (t=4.16, P< 0.0001), and all five (t=2.85, P= 0.004).
FIG. 2.
Regression lines with 95% confidence intervals (dotted lines) showing predicted PTSD symptom severity based on total trauma load. These lines illustrate that there is an interaction between total trauma load and risk genotype (CC) in females (N = 1424, P < 0.0001), but not males (N = 646, P > 0.1), in the meta-analysis of both the original and replication samples.
TABLE 5.
Parameter Estimates in the Model Predicting PTSD Symptom Severity for the Meta-Analysis in Females Only (N = 1424)
| Parameter | Estimate | Standard error | t-Statistic | P-value |
|---|---|---|---|---|
| Intercept | 6.28 | 1.04 | 6.03 | <0.0001 |
| Sample (0 = original, 1 = replication) | −0.16 | 0.57 | −0.28 | 0.7830 |
| rs2267735 | −173 | 0.69 | −2.50 | 0.0125 |
| Total trauma load | 1.27 | 0.19 | 6.80 | <0.0001 |
| rs2267735×total trauma load | 0.55 | 0.13 | 4.30 | <0.0001 |
Controlling for Admixture Within the Sample Populations
To adjust for potential confounding due to admixture in our self-reported African-American samples, we conducted principal components analysis on subjects for which genome-wide data was available (56.5% of the original sample and 66.3% of the replication sample). We re-fit our main model (ADCYAP1R1 genotype × trauma interaction on PTSD symptom severity) both including and excluding the first 10 principal components as covariates. In each case, the estimated effect size did not change substantially with inclusion versus exclusion of the principal components, suggesting that the associations reported above are not the result of confounding due to admixture.
DISCUSSION
In this study, we sought to replicate our previous finding that genetic variation in ADCYAP1R1 associates with risk of PTSD in highly traumatized females. Although we did not replicate the main effect of ADCYAP1R1 genotype on PTSD risk in females, we did find a trauma × genotype interaction, with higher trauma predicting PTSD symptom severity in carriers of the “C” allele in this replicate sample. Indeed, the same trauma × genotype interaction was also found in the original African-American only sample of highly traumatized females [Ressler et al., 2011]. When combining African-American subjects from our prior study [Ressler et al., 2011] with the new replication sample in a meta-analysis, we found an even stronger trauma × genotype association in females only, but no main effect of ADCYAP1R1 genotype on PTSD risk. We did find differences between the original and replication sample sets (among females only) in the variables of income and age. How income could play a role in disease outcome related to genetic risk in not readily evident. However, a model for the effect of age on disease susceptibility, given genotype, may be plausible especially in the case of ADCYAP1R1 in light of its sensitivity to estrogen levels [Ressler et al., 2011]. Further investigation is warranted in regards to the relationship among genotype, estrogen levels, and ADCYAP1R1 genotype. Furthermore, with larger sample sets, it is also possible that some of the trend differences between cohorts and trauma exposure variables (Table 2) would be found to be important for the gene × environment effects.
Our current study identifies gene × environment interactions as the most robust model of the effect of the ADCYAP1R1 gene in females on PTSD symptoms. Upon re-analysis, the original published dataset [Ressler et al., 2011] also revealed a trauma × genotype interaction, in addition to a main effect of ADCYAP1R1 genotype, which we did not identify initially. Moreover, a recent publication also supports a trauma × genotype interaction in females; the trauma relates to childhood maltreatment [Uddin et al., 2012], which is likely incorporated in the total trauma load variable reported here. Although Chang et al.[2012] failed to replicate the ADCYAP1R1 genotype-PTSD association in two independent samples, the direction of the reported effects for females was in the expected direction. It is unclear, however, whether they incorporated gene × environment interactions in their analyses. Further testing is necessary to quantify exact differences between non-replicating sample sets.
In our sample populations, it is clear that at higher trauma levels genotype associates with PTSD symptom severity in African-American females as demonstrated by the plots of both the original and replication datasets. Thus the main effect reported in the previous report [Ressler et al., 2011] is likely an interaction with trauma load given that the association was found in females with higher trauma levels. The complexity of the effects of psychiatric variables, especially trauma history, is inherently difficult to measure. Thus, it is quite possible that to observe the effect of this genotype on PTSD symptoms, analyses must account for the number of traumatic events, type and quality of events, and the relative severity of each event. If a study examined the effect of ADCYAP1R1 genotype were to primarily include subjects with few (e.g., <5 compared to ≥5 as seen in the Grady Trauma Project cohort) total types of lifetime trauma, one might not expect to observe the “risk” allele association as we have in this study of highly traumatized individuals.
Our current work follows on a recent replication of this effect in a small sample size (N = 50), physiology study of children sympto-mology [Jovanovic et al., 2012]. This manuscript demonstrated that the CC genotype was associated with heightened dark-enhanced startle in children, which replicated our previous findings in females [Ressler et al., 2011]. Notably, however, this finding in children was irrespective of gender, consistent with a hypothesis that pubertal/ adolescent hormonal factors are associated with the possible increased association in females at later ages. Note that in rodent models, PACAP, the ligand for PAC1R, has now been associated with increased startle within brain areas, including the bed nucleus of the stria terminals (BNST), that are associated with fear and anxiety [Hammack et al., 2009,2010]. Furthermore, recent data [Hashimoto et al., 2011; Stroth et al., 2011b; Tsukiyama et al., 2011; Hill et al., 2012; Smith and Eiden, 2012] in rodent models has confirmed the role of the PACAP pathway controlling HPA-axis activation during stress responses, activation of adrenal medulla activation in the periphery, and corticosterone regulation.
In summary, the PACAP—PAC1R pathway has been associated with numerous anxiety and fear-related phenotypes in animal models [Hashimoto et al., 2011; Stroth et al., 2011a]. Its role in regulating the hypothalamic—pituitary axis appears critical in this regard [Grinevich et al, 1997; Agarwal et al., 2005; Norrholm et al., 2005; Stroth and Eiden, 2010; Stroth et al., 2011b; Smith and Eiden, 2012]. A further understanding of the role of this pathway, particularly ADCYAP1R1, the gene encoding the PAC1 receptor, will enhance progress toward one possible mechanism underlying the fear dysregulation that accompanies PTSD. Our current work serves as a replication and extension of our previous finding in a highly traumatized human cohort. However, more work needs to be done with larger sample sizes and deeply phenotyped trauma histories to more fully understand the precise role of trauma exposure, frequency, quality, and severity in regulating the effect of ADCYAP1R1 genotype on PTSD development and symptoms. Overall our findings suggest that understanding the gene × environmental interactions of trauma load with ADCYAP1R1 is critical to understanding the specific ways in which this pathway may underlie stress-related disorders.
ACKNOWLEDGMENTS
Financial support was provided by NIH (R01MH096764, R01MH071537) and the Burroughs Wellcome Fund. We appreciate the technical support of all of the staff and volunteers of the Grady Trauma Project, in particular Emily Reiser, Dana Goodenough, Emily Krakoff, Jennifer Davis, Allen W. Graham, Angelo Brown. Most importantly, we are extremely indebted to and appreciative of the time and effort given from all of the participants of the Grady Trauma Project.
Footnotes
Conflicts of interest: All authors do not have any conflicts of interest.
REFERENCES
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder-major depression connection. Biol Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Breslau N, Troost JP, Bohnert K, Luo Z. Influence of predispositions on post-traumatic stress disorder: Does it vary by trauma severity? Psychol Med. 2012:1–10. doi: 10.1017/S0033291712001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzkova P, Lumley T, Rice K. Permutation and parametric bootstrap tests for gene-gene and gene-environment interactions. Ann Hum Genet. 2011;75:36–45. doi: 10.1111/j.1469-1809.2010.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, Oslin D, Purcell SM, Roberts AL, Smoller JW, Uddin M, Gelernter J, Koenen KC. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. 2012;17:239–241. doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Falsetti SA, Saladin ME, Brady KT. Screening for PTSD in a substance abuse sample: Psychometric properties of a modified version of the PTSD symptom scale self-report. Posttraumatic stress disorder. J Trauma Stress. 1998;11:393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- Cookson JA. Package ‘tonymisc’: Functions for Econometrics Output. R package version 1.1.1. 2011 [Google Scholar]
- Falsetti SA, Resnick HS, Resick P, Kilpatrick D. The modified PTSD symptom scale: A brief self-report measure of postraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- Farley M, Patsalides BM. Physical symptoms, posttraumatic stress disorder, and healthcare utilization of women with and without childhood physical and sexual abuse. Psychol Rep. 2001;89:595–606. doi: 10.2466/pr0.2001.89.3.595. [DOI] [PubMed] [Google Scholar]
- Foa EB, Tolin DF. Comparison of the PTSD symptomscale-interview version and the clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: The Detroit Neighborhood Health Study. J Trauma Stress. 2011;24:747–751. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor(BDNF)mRNA expression in the bed nucleus of the stria terminalis (BNST): Roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is implicated in the stress axes. Curr Pharm Des. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Lee SK, Samasilp P, Smith C. Pituitary adenylate cyclase-activating peptide enhances electrical coupling in the mouse adrenal medulla. Am J Physiol Cell Physiol. 2012;303:C257–C266. doi: 10.1152/ajpcell.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, Cross D, Smith AK, Ressler KJ, Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry [Epub ahead of print] 2012 doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry. 2000;61:4–12. 13–4; discussion. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Widom CS. A prospective study of sex differences in the lifetime risk of posttraumatic stress disorder among abused and neglected children grown up. J Trauma Stress. 2009;22:566–574. doi: 10.1002/jts.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: An update. J Trauma Stress. 2009;22:416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Webb-Murphy J, Hammer P, Volkert S, Klam W. Post-traumatic stress disorder symptom severity in service members returning from Iraq and Afghanistan with different types of injuries. CNS Spectr. 2012;17:11–15. doi: 10.1017/S1092852912000016. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local micro-infusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regul Pept. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41:71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Cerda M, Wright RJ, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder across two generations: Concordance and mechanisms in a population-based sample. Biol Psychiatry. 2012;72:505–511. doi: 10.1016/j.biopsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PA, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttrau-matic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56:212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, Garlow SJ, Ressler KJ. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47:136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL. Women’s treatment utilization and its relationship to childhood sexual abuse history and lifetime PTSD. Subst Abus. 2002;23:17–30. doi: 10.1080/08897070209511472. [DOI] [PubMed] [Google Scholar]
- Smith CB, Eiden LE. Is PACAP the major neurotransmitter for stress transduction at the adrenomedullary synapse? J Mol Neurosci. 2012;48:403–412. doi: 10.1007/s12031-012-9749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothala-mus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: A master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci. 2011a;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Liu Y, Aguilera G, Eiden LE. Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol. 2011b;23:944–955. doi: 10.1111/j.1365-2826.2011.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, Tanida M, Tajiri M, Hazama K, Ogata K, Hashimoto H, Baba A. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC. ADCYAP1R1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety [Epub ahead of print] 2012 doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorman A, Lumley T, McKnight B, Rice K. Behavior of QQ-plots and genomic control in studies of gene–environment interaction. PLoS ONE. 2011;6:e19416. doi: 10.1371/journal.pone.0019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. A Heteroskedasticity-consistent covariance-matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- Zayfert C, Dums AR, Ferguson RJ, Hegel MT. Health functioning impairments associated with posttraumatic stress disorder, anxiety disorders, and depression. J Nerv Ment Dis. 2002;190:233–240. doi: 10.1097/00005053-200204000-00004. [DOI] [PubMed] [Google Scholar]