Abstract
Objective and design
The objective of this study was to determine changes in TLR responses of monocytes, myeloid dendritic cells and plasmacytoid dendritic cells during primary and chronic HIV-1 infection. Toll-like receptors (TLRs) serve as important innate receptors to sense pathogens, and have been implicated in mediating immune activation in HIV-1 infection. Studies assessing the consequences of HIV-1 infection on the ability of innate immune cells to respond to TLR stimulation have come to varying conclusions.
Methods
Using intracellular flow cytometry, cytokine production by cryopreserved PBMCs from healthy controls and HIV-1 infected individuals were examined after TLR stimulation.
Results
We observed that the effect of HIV-1 infection on TLR responses not only depended on the stage of HIV-1 infection, but was also dependent on the individual receptor and cell type examined. Monocyte and mDC responses to TLR8 stimulation were associated with HIV-1 viral load and CD4+ T cell count, while pDC responses to TLR7 stimulation were not. Responses to TLR2 stimulation were not affected by HIV-1 infection while responses to TLR9 stimulation were universally decreased in all HIV-1 infected individuals examined regardless of treatment or clinical parameters.
Conclusion
Responsiveness to TLR7/8 stimulation, which have been shown to recognize HIV-1 ssRNA, did not decrease in chronic infection, and may represent a contributing factor to ongoing T cell immune activation in the setting of chronic viremic HIV-1 infection.
Keywords: HIV-1, Toll-like receptor, Innate immunity, Pathogenesis, Dendritic Cells, Monocytes
Introduction
The innate immune system is the first line of defense and consists of innate immune cells which are able to respond to infections quickly through the recognition of pathogen-associated molecular patterns (PAMPs) by receptors such as toll-like receptors (TLRs) [1, 2]. It has been shown that HIV-1 ssRNA encodes for multiple PAMPs that can be recognized by TLR7 expressed in plasmacytoid dendritic cells (pDC) and TLR8 expressed in both monocytes and myeloid dendritic cells (mDC) [3, 4]. Stimulation of TLR7 on pDCs induces cytokines such as IFNα and TNFα which have been shown to activate a cascade of antiviral effector functions [5-7]. IFNα in particular has a dichotomy of functions both beneficial in the induction of antiviral activities [7, 8] but also detrimental in its contribution to chronic persistent immune activation and HIV-1 disease progression [9-11].
Although IFNα and type I interferon stimulated genes are upregulated in vivo in chronic HIV-1 infection [12, 13], in vitro stimulation of DCs isolated from HIV-1 infected individuals with TLR7/8 ligands has produced mixed results with some studies showing a lower level of IFNα production in chronically HIV-1-infected individuals as compared to healthy controls [14-19] while other studies showed an elevated IFNα response [12, 20, 21]. These studies included examining responses by mRNA expression [12], total cytokine secretion [15, 17-19, 21] as well as percentages of cytokine producing cells [14, 16, 20], and have been performed in heterogeneous populations of chronically HIV-1-infected individuals [14, 16, 18, 19]. In primary HIV-1 infection, responses to TLR7/8 stimulation have been reported to initially decline and then rebound as disease progresses to a chronic state [20-22]. Here we examined the changes in immune responses following stimulation of TLR7/8, which have been shown to recognize HIV-1 encoded ssRNA, TLR9, which utilizes the same pathway as TLR7 in inducing IFNα production, and TLR2, an important detector of mycobacterium tuberculosis and other mycobacterium, a rising co-infection of HIV-1. These responses were examined in both early and late stages of primary HIV-1 infection and various stages of chronic HIV-1 infection, including individuals defined as “elite controllers”. We observed that HIV-1 infection does not induce a universal alteration of TLR responses and that TLR-mediated immune responses are dependent on both viral load and the stage of infection.
Methods
Ethics Statement
All human samples used were from individuals recruited and enrolled at Massachusetts General Hospital (MGH) and all subjects gave written informed consent for participation in these studies. The study was approved by the Partners Institutional Review Board.
Characteristics of study subjects
Peripheral blood mononuclear cells (PBMCs) previously isolated from blood drawn in acid citrate dextrose tubes were cryopreserved in liquid nitrogen for a minimum of one year. These included samples from HIV-1-infected individuals (n=38) and healthy HIV-1 negative individuals (n=11). HIV-1-infected individuals included participants with chronic infection (n=27) which were subdivided into three different groups: elite controllers (untreated HIV-1-infected individuals maintaining HIV-1 viral load below detectable levels of 50 copies mL−1 for more than six months; n=10), treated chronically HIV-1-infected individuals (on antiretroviral treatment for more than one year with undetectable viral loads; n=8), and untreated chronically HIV-1-infected individuals (treatment naïve or off antiretroviral treatment for more than one year; n=9). Primary HIV-1-infected individuals who were estimated to be within the first two months of infection as defined by western blot been negative or indeterminate at enrollment were also recruited for the study (n=11; Table 1). CD4+ T cell counts were significantly higher in elite controllers as compared to treated and untreated chronically HIV-1-infected individuals (P=0.002 and P<0.001 respectively) and primary HIV-1-infected individuals (P<0.001). CD4+ T cell count were not different between the treated, untreated and primary HIV-1-infected individuals. Untreated HIV-1-infected subjects had significantly higher HIV-1 viral load than elite controllers or individuals on antiretroviral therapy (P<0.001 for all). The HIV-1 viral loads in the primary HIV-1-infected individuals were furthermore significantly higher than in the untreated chronically HIV-1-infected individuals (P<0.001). There were no significant differences by gender and age between the three chronically HIV-1-infected groups and individuals with known hepatitis C or B virus co-infection were excluded.
Table 1.
Demographic details of HIV-1 cohort
| HIV-1 uninfected | Elite controllers | Treated chronic HIV-1 infection | Untreated chronic HIV-1 infection | Primary HIV-1-infected | |
|---|---|---|---|---|---|
| Number of subjects | 11 | 10 | 8 | 9 | 11 |
| HIV-1 RNA; Median (range), copies/mL | N/A | <50 | <50 | 2.5 × 104 (3.0 × 103 – 5.2 ×105)a | 1.0 × 106 (9.6 × 104 – 4.6 ×107)a |
| CD4+ T cell count; Median (range), cells/μL | N/A | 966 (752-2008) | 623 (453-1026)b | 642.5 (320-743)b | 470 (165-1020)b |
| Sex (%Female) | 54.5% | 60% | 50% | 55.6% | 0% |
| Age; Median (range) | N/A | 50 (27-65) | 48 (41-56) | 44 (34-62) | 38 (24-44) |
HIV-1 RNA significantly higher than in elite controllers and treated chronic HIV-1 infection
CD4+ T cell count significantly lower than in elite controllers
N/A – not available
Measurement of cytokine production following TLR stimulation
Cryopreserved PBMCs were thawed at 37oC, washed and resuspended in media. 1.5 million PBMCs/mL were stimulated with 1 μg/mL CL097 (Invivogen), 5 μM ODN2216 (CpG; Invivogen), or 107 cells/mL heat killed listeria monocytogene (HKLM; Invivogen). 5 μg/mL Brefeldin A (BFA; Sigma) was added immediately following the addition of TLR ligands, except for CpG where BFA was added after five hours of stimulation. Delayed addition of BFA was required for CpG stimulation as TLR9 traffics through the Golgi complex to the endolysosomes to detect CpG DNA [23]. Unstimulated cells with BFA served as the negative control. Presence of intracellular cytokines, including IFNα (clone MMHA-11), in monocytes, mDCs, and pDCs were determined after 20 hours of total incubation with the respective TLR ligands, as previously described [10]. All samples were acquired on an LSRFortessa (BD Biosciences). The percentages of cytokine producing pDCs, mDCs, and monocytes were determined by subsequent analysis using FlowJo software (Treestar Inc.). The populations of interest were defined as monocytes (Lineage−HLA-DR+CD11c+CD14+), mDCs (Lineage−HLA-DR+CD11c+CD14−CD123−) and pDCs (Lineage−HLA-DR+CD123+CD303+CD11c−CD14−). The assay was optimized for use with frozen/thawed HIV-1 samples, and cytokine responses were detectable in all three populations examined (Supplementary Figure 1). During the assay optimization we observed up to 50% decline in TLR induced cytokine responses in the frozen/thawed samples as compared to matched fresh PBMCs. However, the responses between fresh and frozen/thawed PBMCs were significantly correlated (P<0.001, r=0.857; data not shown).
Statistical analysis
For the immunologic assays comparing HIV-1 negative individuals to HIV-1 positive individuals, or within HIV-1 positive subgroups, Wilcoxon Rank tests (Mann Whitney) were used to determine statistically significant differences. Given the multiple comparisons only data that reached the 1% nominal level of significance was included for this initial comparison. Spearman correlations were used to assess correspondence between responses to TLR stimulation, CD4+ T cell count and HIV-1 viral load. Correlations were compared without adjustments for multiple testing at the 5% nominal level of significance. Comparison of early and late primary HIV-1-infected samples was done by paired t-test. All statistical tests were two-sided exploratory.
Results
TLR responses of dendritic cells and monocytes are altered by primary and chronic HIV-1 infection
Cryopreserved PBMCs from healthy donors and HIV-1-infected study participants were stimulated using a panel of ligands that stimulated various TLRs – CL097 (TLR7/8), HKLM (TLR2) and CpG (TLR9). The production of cytokines in response to TLR stimulation was compared between primary and chronic HIV-1-infected individuals and HIV-1 negative controls. Background production of cytokines measured in the media control were on average less than 1% for each of the cell populations with no significant differences between the various cohorts (data not shown). The ability of pDCs, mDCs and monocytes to respond to TLR stimulation as measured by cytokine production were differentially altered during both the primary and chronic phases of HIV-1 infection regardless of treatment (Fig. 1).
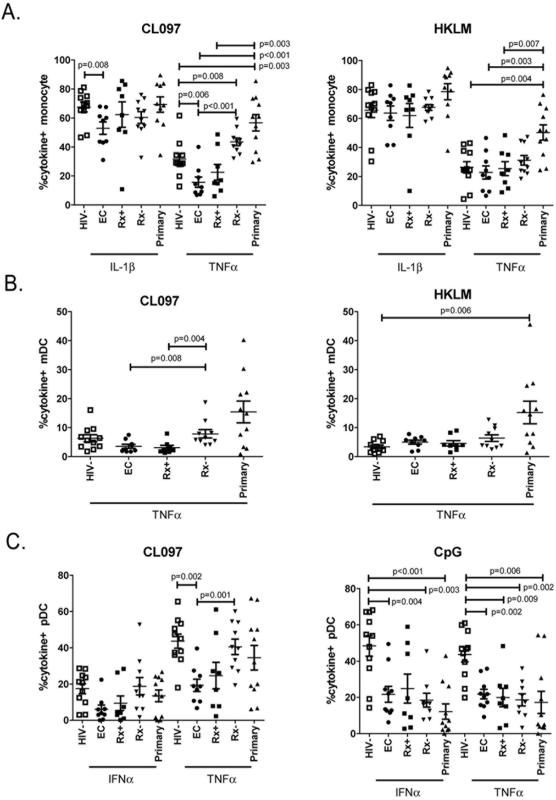
Figure 1. Monocyte and dendritic cell responses to TLR stimulation.
Cryopreserved PBMCs previously isolated from HIV-1 negative controls (HIV-, open squares), elite controllers (EC; closed circles), treated chronically HIV-1-infected individuals (Rx+, closed square), untreated chronically HIV-1-infected individuals (Rx-, inverted closed triangle), and individuals with primary HIV-1 infection (Primary, closed triangles), were stimulated with TLR7/8 (CL097), TLR2 (HKLM) and TLR9 (CpG) ligands. Intracellular cytokine production was measured in (A) monocytes, (B) myeloid dendritic cells (mDC), (C) and plasmacytoid dendritic cells (pDCs). Results are expressed as the percentage of cytokine producing cells. Means and standard deviations are shown in the graph.
Monocytes and mDCs both express TLR2 and TLR8 and responded to HKLM and CL097 stimulation. Neither HKLM nor CL097 induced significantly different cytokine responses when comparing healthy controls to the combined chronic HIV-1 infection groups. However, once chronically HIV-1-infected individuals were separated into the defined subgroups, differential responses to TLR stimulations were observed. Monocyte responses to CL097 stimulation were elevated in individuals who have primary HIV-1-infection with TNFα responses been significantly higher than in HIV-1 negative controls, elite controllers and treated chronically HIV-1-infected individuals (P=0.003, P<0.001 and P=0.003 respectively; Fig. 1A). Similarly monocyte TNFα responses to HKLM stimulation were also significantly higher in primary HIV-1 infection as compared to HIV-1 negative controls, elite controllers and treated chronically HIV-1-infected individuals (P=0.004, P=0.003 and P=0.007 respectively). Like individuals with primary HIV-1 infection, untreated HIV-1 chronically infected individuals also have significantly higher monocyte TNFα response to CL097 stimulation than HIV-1 negative controls (P=0.008). This was contrary to elite controllers who have significantly lower monocyte TNFα responses to CL097 than both HIV-1 negative controls and untreated chronically HIV-1-infected individuals (P=0.006 and P<0.001 respectively), and significantly lower IL-1β production when compared to healthy controls (P=0.008). The data suggests that HIV-1 viral control may be associated with reduced monocyte TNFα responsiveness to TLR8 stimulation as observed in elite controllers, while viremic HIV-1 infection is associated with an elevated responsiveness as compared to HIV-1 negative controls. None of our primary HIV-1 infected individuals became elite controllers during the first year of infection so whether the lowered level of monocyte responses observed in elite controllers was a consequence of innately lowered monocyte activation during primary HIV-1 infection, control of viral replication or both cannot be determined. Longitudinal follow-up of elite controllers starting in the primary infection may allow us to identify factors associated with innate control of HIV-1 infection.
Mimicking the monocyte responses, we also observed that mDCs from individuals with primary HIV-1 infection had a significantly higher response to HKLM stimulation than HIV-1 negative controls (P=0.006; Fig. 1B). In mDCs the elevated TNFα responses in untreated chronically HIV-1-infected individuals were more prominent and were significantly higher when compared to elite controllers and treated chronically HIV-1-infected individuals (P=0.008 and P=0.004 respectively). For both monocytes and mDCs, TLR2 responses to HKLM were not significantly different between the various chronically HIV-1-infected subgroups.
pDCs express TLR7 and TLR9 but do not express TLR2 and therefore do not respond to HKLM. TLR7 stimulation with CL097 in pDCs induced significantly lower TNFα responses in elite controllers than in both HIV-1 negative and untreated chronically HIV-1-infected individuals (P =0.002 and P = 0.001 respectively; Fig. 1C). This was in line with the TNFα responses observed in both monocyte and mDC as described above, however, the higher pDC TNFα responses observed in untreated chronically and primary HIV-1-infected individuals did not elevate above HIV-1 negative controls as was observed for the monocyte and mDCs. Interestingly CpG stimulation of pDCs resulted in diminished IFNα and TNFα responses in the combined chronically HIV-1-infected individuals (P<0.001 for both) and primary HIV-1-infected individuals (P<0.001 and P=0.006 respectively) when compared to HIV-1 negative controls. The reduced IFNα responses remained significantly lower than healthy donors for the elite controllers and untreated chronically HIV-1-infected individuals (P=0.004 and P=0.003 respectively) and TNFα responses remained significantly lower in all three chronically HIV-1-infected groups (P<0.01 for all) when compared to HIV-1 negative controls.
Taken together, these data demonstrated significant disparities between the various stages of chronic HIV-1 infection with untreated chronically HIV-1-infected individuals having a similar response to TLR7/8 stimulation as primary HIV-1-infected subjects, whilst elite controllers appeared to have a suppressed response to TLR7/8 stimulation. The difference to TLR7/8 stimulation between the various HIV-1 groups also diverged from the TLR2 responses that were elevated only in primary HIV-1-infected individuals, and from the TLR9 responses which were suppressed in all HIV-1-infected individuals.
TLR8 responses correlate with both CD4+ T cell counts and viral loads
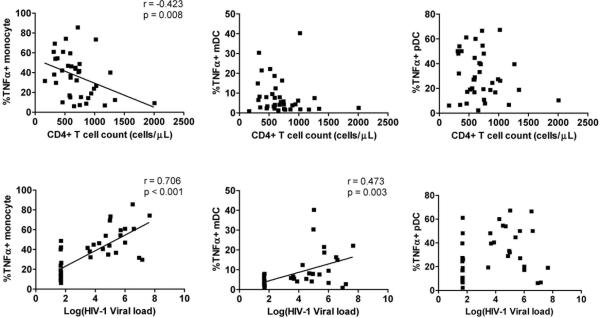
TLR7/8 stimulation with CL097 exposed the most differential response between the various HIV-1-infected study groups which were defined in part by their HIV-1 viral load and CD4+ T cell count. We therefore assessed whether differences in response to CL097 stimulation were associated with CD4+ T cell count or HIV-1 viral load (Fig. 2). Given that each HIV-1-infected group covered a unique range of CD4+ T cell count and HIV-1 viral load (Table 1), the analysis was performed on the combined group of all HIV-1-infected individuals. Correlations between responses observed in all HIV-1-infected individuals showed that the percentage of monocytes producing TNFα following CL097 stimulation was negatively correlated to CD4+ T cell count (P=0.008, r=-0.423) and positively correlated to HIV-1 viral load (P<0.001, r=0.706). Similarly mDC production of TNFα was also positively associated with HIV-1 viral load (P=0.003, r=0.473). HIV-1 viral load and CD4+ T cell count were as expected significantly negatively correlated with each other, potentially explaining the matched association observed (P<0.001, r=−0.613; data not shown). All the other cytokine responses did not display significant correlations with either CD4+ T cell count or with HIV-1 viral load (data not shown). Overall this shows that monocyte and mDC responses to CL097 were associated with HIV-1 viral load and may explain the differences observed between the various HIV-1-infected study groups. Although we also observed differences in the pDC responses following TLR7 stimulation this was not correlated to HIV-1 viral load or CD4+ T cell count (Fig. 2).
Figure 2. Correlation between monocyte and dendritic cells responses to TLR7/8 and CD4+ T cell count and HIV-1 viral load.
Monocyte, mDC and pDC production of TNFα in response to TLR7/8 ligand (CL097) stimulation were correlated to CD4+ T cell count (top graphs) and HIV-1 viral load (bottom graphs). Where a significant correlation was observed the line of best fit is shown with the correlation coefficient and P-values.
TLR responses in primary HIV-1 infection change as infection progresses
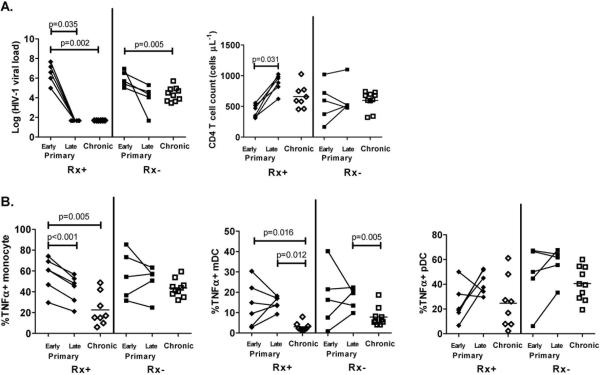
The eleven individuals identified during primary HIV-1 infection were followed longitudinally with a later sample (late) taken 23-58 weeks after the initial sample obtained within the first two months of infection (early). At the later time point six study participants had initiated antiretroviral therapy resulting in significant decrease in HIV-1 viral loads to undetectable levels (P=0.035) and significant increase in CD4+ T cell counts (P=0.031, Fig. 3A). The remaining five subjects with primary HIV-1-infection remained untreated and had a non-significant decline in HIV-1 viral load. There were no significant clinical differences between the primary HIV-1-infected individuals who initiated treatment or those who remained untreated when comparing their HIV-1 viral load or CD4+ T cell counts at the early treatment naïve time point. In the individuals who initiated therapy, monocyte TNFα response to CL097 also significantly diminished (P<0.001, Fig. 3B). These findings were consistent with the observed positive correlation between HIV-1 viral load and monocytes responses to TLR8 stimulation (Fig. 2). However, the ability of mDCs to produce TNFα in response to CL097 did not diminish at the later time point and remained significantly higher for both treated and untreated primary infections as compared to the corresponding treated and untreated chronic HIV-1 infections (P=0.012 and P=0.005 respectively). The pDC responses were also not significantly different between samples from the early versus the matching late samples. We did not observe any significant association between the responses to the other TLR ligands and either HIV-1 viral load or CD4+ T cell count (data not shown).
Figure 3. Monocyte and dendritic cells TLR7/8 responses in early and late sampling of individuals with primary HIV-1 infection.
(A) HIV-1 viral load and CD4+ T cell count of primary HIV-1-infected individuals who were treated (Rx+; solid diamonds) or remained untreated (Rx-; solid squares) during the early (within first two months of infection) and late (23-58 weeks later) time points are shown. In addition, data for treated (Rx+, Chronic) and untreated (Rx-, Chronic) chronically HIV-1-infected individuals were also included. (B) The corresponding TLR7/8 responses as measured by monocyte, mDCs and pDCs TNFα production were measured in individuals during the early and late primary HIV-1 infection and subjects with treated (Rx+) or untreated (Rx-) chronic HIV-1 infection. Differences were calculated using either paired t-test or Wilcoxon Rank tests (Mann Whitney).
Discussion
HIV-1 associated changes in the immune response of infected individuals have been well established, however the effect of HIV-1 infection on the responsiveness of the TLR pathways have shown conflicting results [12-14, 20, 24-28]. TLRs can recognize viruses such as TLR7/8-mediated recognition of HIV-1 itself but can also be stimulated by several other pathogens such as TLR2-mediated recognition of mycobacteria. We observed that the effect of HIV-1 infection on the innate immune responses not only depended on clinical status of the infected study subjects, but also differentially affected the various TLR responses both within and between the cell types examined.
We assessed the consequences of HIV-1 infection on the ability of antigen-presenting cells to respond to TLR stimulation, and observed that blood-derived monocytes and dendritic cells from both HIV-1-uninfected and infected individuals were capable of responding to TLR2, 7, 8 and 9 stimulation. When results from all individuals with chronic HIV-1 infection were combined, monocytes, mDCs and pDCs did not differ between infected and uninfected individuals in the production of cytokines following in vitro TLR7/8 stimulation. This is contrary to earlier published data describing significantly reduced IFNα production in chronic HIV-1 infections [14-19, 29]. However this study differed from previous publications in several aspects, most notably several studies used total quantity of cytokines in response to stimulation as the main method of measurement [15, 17-19, 21]. This method may be skewed by the decline in dendritic cell numbers during chronic HIV-1 infection [19, 29-34]. In our study, we assessed cytokine production by intracellular cytokine staining on the single cell level, and observed that the ability of the antigen-presenting cells that are present in the circulation to produce cytokines in response to TLR7/8 and TLR2 stimulation are not universally impaired in chronically HIV-1-infected individuals.
When chronically HIV-1-infected individuals were subdivided into those with treated and untreated chronic infections, as well as elite controllers, significant differences in TLR7/8 responses were observed, despite the relatively small cohort of individuals. The ability of monocytes, mDCs and pDCs to produce TNFα in response to TLR7/8 stimulation progressively increased from elite controllers to treated individuals with chronic HIV-1 infection to untreated viremic individuals with chronic infection. As CL097 stimulates cytokine production from all three cell types, some cross-stimulation may occur between the three innate immune cells examined. If cross-stimulation does occur, it would be unlikely to be due to secretion of soluble factors such as cytokines which should be blocked by the addition BFA immediately after stimulation. Only CpG stimulation was followed by a delayed addition of BFA; however, given that pDCs are the only cells that responded to CpG stimulation, it is unlikely that the pDC responses detected are modulated by cytokines produced by other cells. The role of non-soluble factors such as maturation and activation markers on monocytes and dendritic cells, however, were not examined and may explain the observed synchronized monocytes and dendritic cells responses to TLR7/8 stimulation. TLR7/8-stimulated cytokine production was positively correlated with HIV-1 viral load and negatively correlated to CD4+ T cell count contrary to the theory of HIV-1-induced anergy or HAART-induced recovery of TLR function as previously suggested [35, 36]. Our data showing enhanced TLR7/8-responsiveness of monocytes and mDCs in viremic infection are more in line with recently published data suggesting that HIV-1 does not induce the TLR anergy often observed with other persistent TLR stimulations [21, 37]. These findings suggest that direct HIV-1 stimulation of TLRs, in addition to indirect TLR stimulations as a consequences of microbial translocation in the gut [38], may drive chronic and persistent immune activation and subsequent disease progression [39].
It is of interest that the observed positive association between HIV-1 viral load and TLR7/8 responses by monocytes and mDCs was not universal to other TLR ligands, as we observed no significant differences between the subgroups of chronically HIV-1-infected subjects following stimulation via TLR2 or TLR9. Furthermore, we observed that HIV-1 infection universally reduced the ability of pDC to respond to CpG stimulation, a receptor that shares many of the downstream intracellular signaling pathways with TLR7 for the induction of IFNα and TNFα [40-42]. The lack of variation in IFNα production following TLR9 stimulation differs from that observed following TLR7 stimulation and suggests that there is no universal deregulation in pDC's ability to produce IFNα but instead that the ability to respond is still dependent on the stimulant. Although stimulation of TLR7 by HIV-1 can potentially inhibit the ability of TLR9 to respond to PAMPs [40], we did not observe a negative association between HIV-1 viral load and TLR9 responses. Instead TLR9 responses declined in all HIV-1 infected individuals regardless of their viral load. This may be in part explained by previously published data showing that the presence of gp120 can suppress TLR9 but not TLR7 responses via the activation of tyrosine phosphorylation in competition to CpG [43]. In contrast TLR2 recognition of HKLM was not altered in any of the subgroups of chronically HIV-1-infected individuals when compared to healthy controls suggesting that chronic HIV-1 viral infection does not necessary cause universal TLR disruption.
Contrary to chronic HIV-1 infection, early in primary HIV-1 infection the responses of monocytes and mDCs to TLR2 and TLR7/8 stimulations were elevated, and induced a significantly higher level of cytokine responses compared to uninfected controls and chronically HIV-1-infected individuals. This is consistent with the high level of immune activation and the cytokine storm described early in primary HIV-1 infections [5, 21]. Six of the 11 primary HIV-1-infected individuals initiated therapy soon after diagnosis and a second sample was obtained from both individuals that remained untreated and those that initiated therapy. We observed that following initiation of therapy only monocyte TNFα responses to CL097 declined to similar levels as those observed in the treated chronically HIV-1-infected individuals. This is in line with the strong positive correlation observed between HIV-1 viral load and monocyte TNFα responses to CL097. Although mDC TNFα responses to CL097 also declined following treatment of primary HIV-1 infection, they remained higher than in the treated chronic infection group. Potential explanations for this may be that the treated chronically HIV-1-infected individuals all initiated treatment during the chronic phase while the individuals in the primary infection group initiated treatment during the acute/early phase of infection. These data may suggests that early treatment of HIV-1 infection during primary infection rather than chronic infection may be beneficial in preventing disruption of the innate immune responses observed in chronic HIV-1 infection. Further longitudinal examination of primary HIV-1 infection will be needed to determine the kinetics of the innate immune response overtime, and their consequences for disease progression.
The diverse TLR responses observed here demonstrate the complexities in which HIV-1 differentially alters the innate immune responses at various stages of HIV-1 infection. The ability of the cells to still respond strongly to TLR7/8 stimulation especially in the setting of viremic HIV-1 infection means that ongoing chronic immune activation can be continuously driven by HIV-1 encoded PAMPs. The precise mechanism by which HIV-1 modifies these individual TLR responses will need to be further analyzed as the modulation of these pathways may have important implications not only for viral control but also HIV-1-associated immune pathogenesis.
Supplementary Material
Supplementary Figure 1. Flow Cytometry plot of CL097 stimulated cells using freeze/thawed PBMCs. Flow Cytometry plots of each of the three cell populations examined are shown from a HIV-1-infected individual following in vitro stimulation with CL097. Intracellular cytokine production was measured in CD14+ monocytes (top graphs), CD11c+ myeloid dendritic cells (mDC; middle graphs), and CD303+ plasmacytoid dendritic cells (pDCs, bottom graphs). Cytokines measured are TNFα in all three cell types (left graphs), IFNα in pDCs, and IL-1β in monocytes. This is representative of the other freeze/thawed PBMC samples collected.
References
- 1.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. Journal of leukocyte biology. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 8.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann C, Harper JM, Taubert D, Hartmann P, Fatkenheuer G, Jung N, et al. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2008;48:522–530. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- 13.Stylianou E, Aukrust P, Bendtzen K, Muller F, Froland SS. Interferons and interferon (IFN)-inducible protein 10 during highly active anti-retroviral therapy (HAART)-possible immunosuppressive role of IFN-alpha in HIV infection. Clin Exp Immunol. 2000;119:479–485. doi: 10.1046/j.1365-2249.2000.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-alpha but not proinflammatory cytokines. J Immunol. 2008;181:2887–2897. doi: 10.4049/jimmunol.181.4.2887. [DOI] [PubMed] [Google Scholar]
- 16.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 17.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 18.Ferbas J, Navratil J, Logar A, Rinaldo C. Selective decrease in human immunodeficiency virus type 1 (HIV-1)-induced alpha interferon production by peripheral blood mononuclear cells during HIV-1 infection. Clin Diagn Lab Immunol. 1995;2:138–142. doi: 10.1128/cdli.2.2.138-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 20.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 21.Sabado RL, O'Brien M, Subedi A, Qin L, Hu N, Taylor E, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 23.Chockalingam A, Brooks JC, Cameron JL, Blum LK, Leifer CA. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunology and cell biology. 2009;87:209–217. doi: 10.1038/icb.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilton JC, Manion MM, Luskin MR, Johnson AJ, Patamawenu AA, Hallahan CW, et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M., Jr. Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J Virol. 2009;83:10395–10405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 27.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 30.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 31.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 32.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 33.Conry SJ, Milkovich KA, Yonkers NL, Rodriguez B, Bernstein HB, Asaad R, et al. Impaired plasmacytoid dendritic cell (PDC)-NK cell activity in viremic human immunodeficiency virus infection attributable to impairments in both PDC and NK cell function. J Virol. 2009;83:11175–11187. doi: 10.1128/JVI.00753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 36.Bjorck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. Journal of immunology. 2004;172:5396–5404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. The Journal of clinical investigation. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 39.Chang JJ, Altfeld M. TLR-mediated immune activation in HIV. Blood. 2009;113:269–270. doi: 10.1182/blood-2008-10-184598. [DOI] [PubMed] [Google Scholar]
- 40.Berghofer B, Haley G, Frommer T, Bein G, Hackstein H. Natural and synthetic TLR7 ligands inhibit CpG-A- and CpG-C-oligodeoxynucleotide-induced IFN-alpha production. J Immunol. 2007;178:4072–4079. doi: 10.4049/jimmunol.178.7.4072. [DOI] [PubMed] [Google Scholar]
- 41.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 42.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 43.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flow Cytometry plot of CL097 stimulated cells using freeze/thawed PBMCs. Flow Cytometry plots of each of the three cell populations examined are shown from a HIV-1-infected individual following in vitro stimulation with CL097. Intracellular cytokine production was measured in CD14+ monocytes (top graphs), CD11c+ myeloid dendritic cells (mDC; middle graphs), and CD303+ plasmacytoid dendritic cells (pDCs, bottom graphs). Cytokines measured are TNFα in all three cell types (left graphs), IFNα in pDCs, and IL-1β in monocytes. This is representative of the other freeze/thawed PBMC samples collected.