Vascular calcification has long been a major area of interest in cardiovascular medicine. A great deal of data has emerged, ranging from descriptions of the extent of vascular calcification in ancient Egyptian mummies, to intricate dissections of complex molecular signaling pathways (1-2). Arguably, the strongest message to have arisen from the recent studies appears to be the sobering fact that vascular calcification is a complex pathobiologic process, about which we are only just beginning to gain a rudimentary understanding. As a prime example, while for a period there was general agreement that there were two principal types of vascular calcification – intimal (associated with atherosclerosis) and medial (also known as Mönckeberg's sclerosis or arteriosclerosis) (3); even this long-standing classification has now come into question, with data from patients with renal failure suggesting that these distinct classifications should be considered as a continuum (4). Another important new paradigm to emerge is the so-called ‘vascular calcification paradox’, being a robust inverse association between the degree of bone mineralization and the degree of vascular calcification (3, 5). However, at the present time we are far from an adequate unifying explanation that might explain this reciprocal link between vascular and skeletal calcification, and a great deal of work remains to be undertaken. At the molecular level, unexpected complexities have arisen, such as the possibility that removal of lipid deposits by the use of statins may trigger macrophages, osteoclast-like cells and vascular smooth muscle cells (VSMCs) to generate extracellular matrix and cause vascular calcification (2, 6). This may explain the paradox of an increase in calcium score as a parameter of disease, but during the process of vascular ‘healing’ (6-7). In corroboration, meta-analysis data has shown that statin use to reduce or slow vascular calcification is ineffective (8).
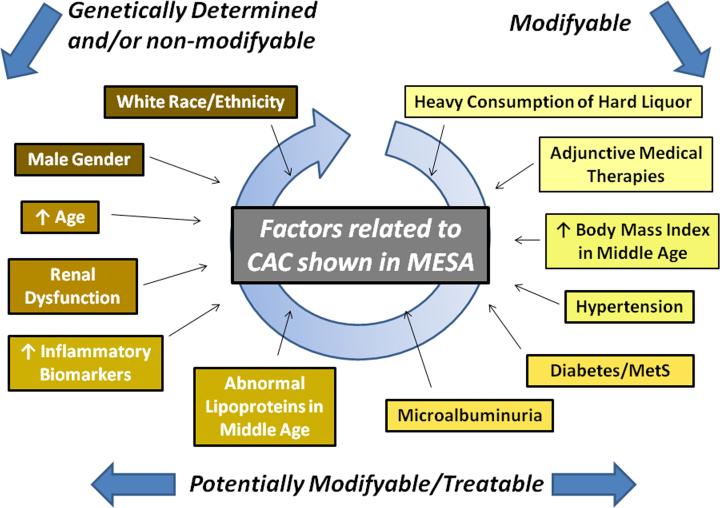
Amidst this flurry of activity, the Multi-ethnic study of atherosclerosis (MESA) has stood as a steadying reference beacon for almost a decade (9). In a series of seminal studies, the large and painstakingly collected MESA dataset has left an indelible impact on our understanding of coronary artery calcification (CAC), with a multitude of important factors shown to influence, or to at least be associated with, the incidence and progression of CAC (Figure) (9-17). In the process, MESA has raised the bar for population-level cardiovascular observational studies, collecting a wealth of parameters with a large sample size, thus permitting this rich suite of publications. In this issue of JACC Cardiovascular Imaging, Wong et al (17) add to this stable of MESA-derived manuscripts, focusing on the role of the metabolic syndrome (MetS) and diabetes (DM) in CAC development and progression. Here, they solve several individually important pieces of the vascular calcification puzzle. First, for patients with no CAC at baseline (as assessed by CT scanning), the relative risk of incident CAC at follow-up scanning (mean of 2.4 years later) was 1.7 – 1.9 for patients with the MetS, DM, or both conditions, as compared to patients without DM or MetS. Second and perhaps most interesting of their findings, in those with CAC at baseline there was a dose-response effect for the progression of CAC, which in broad terms was least for those without MetS/DM, intermediate for those with either MetS or DM, and highest for those with both conditions. Third, while an association between CAC progression and clinical cardiovascular events is already known to exist across all comers (DM and non-DM) (18), Wong et al (17) extended these data by showing that CAC progression is associated with subsequent coronary heart disease events in patients with MetS and DM.
Figure.
Factors shown to be associated with the incidence and/or progression of CAC from MESA (9-17). What remains unclear is whether calcific deposition is a secondary phenomenon to coronary disease in some or all of the factors outlined in the figure.
Inevitably, as with any study there are limitations, one being the lack of consideration of renal function. Thus, as a possible confounding factor, patients with DM and/or MetS might be expected to have a greater prevalence of renal dysfunction, which may have increased the burden of CAC as a secondary rather than a primary effect. Also, patients who suffered a cardiac event prior to follow-up scanning were excluded; while their progression of CAC is unknown, these patients had more severe clinical progression of their coronary artery disease than others in the study and their exclusion is a potential source of selection bias although, the number excluded was small (70 of 5,662). Finally, as pointed out, the use of statins may enhance vascular ‘healing’ but with increased vascular calcification. The current study does not provide such therapeutic information (statin use), however, the relatively low and generally equivalent lipid levels across the patient groups might suggest similar use of these agents. Nevertheless, we feel these caveats would appear unlikely to have made an appreciable impact on the overall results or conclusions.
What can we take away from this study? Certainly, the MESA investigators have now put the link between MetS, DM and CAC beyond question. Also, they have demonstrated that even among patients with MetS/DM, who are already known to have an increased risk of coronary heart disease events, those with CAC progression are at even greater risk of these adverse outcomes. This begs the question of whether clinicians should be performing serial CT scans to identify patients MetS/DM and significant CAC progression who may benefit from particularly aggressive medical therapy. While interesting in theory, in the current climate of cost containment and clinical use appropriateness criteria we believe that this use of resources would be unlikely to gain widespread acceptance. Indeed, current (2010) appropriate use criteria classify the repeat use of coronary CT scanning as ‘inappropriate’ in asymptomatic patients with known CAD and prior CT scanning (19). Nevertheless, documentation of rampant progression of CAC in occasional selected patients with MetS/DM, renal dysfunction, or other comorbidities may be of assistance in tailoring individual therapeutic regimens; in effect, a form of personalized medicine.
Progress in understanding CAC at the basic and clinical levels has been moving forwards at a steady pace. However, to put this in perspective, in the coming decades we are potentially facing a worldwide cardiovascular health epidemic, as it is predicted that the population will become significantly older, obesity will increase, and the prevalence of DM will rise significantly (20). Given this, in a small but important way the data of Wong et al (17) add to concerns of a looming cardiovascular health crisis, connecting the dots and highlighting that this growing number of people affected with MetS/DM will also be at increased risk for CAC and coronary heart disease events. Averting this global health crisis has now become a priority agenda item at the highest levels, including the United Nations and the Institute of Medicine of the US National Academy of Sciences (21-22). Among other things, we need to urgently engage in targeted research initiatives, to define novel therapies, and to implement global initiatives to combat obesity, DM, and other aspects of this major cardiovascular health concern (23).
Acknowledgments
No specific funding or grant was used to prepare this manuscript. Jason Kovacic is supported by National Institutes of Health Grant 1K08HL111330-01.
Footnotes
We have no financial disclosures or relationships to report.
REFERENCES
- 1.Allam AH, Thompson RC, Wann LS, Miyamoto MI, Thomas GS. Computed tomographic assessment of atherosclerosis in ancient Egyptian mummies. JAMA. 2009;302:2091–4. doi: 10.1001/jama.2009.1641. [DOI] [PubMed] [Google Scholar]
- 2.Byon CH, Sun Y, Chen J, et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–96. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg's sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–98. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 5.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Terry JG, Carr JJ, Kouba EO, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am J Cardiol. 2007;99:1714–1717. doi: 10.1016/j.amjcard.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Houslay ES, Cowell SJ, Prescott RJ, et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart. 2006;92:1207–1212. doi: 10.1136/hrt.2005.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int J Cardiol. 2011;153:31–35. doi: 10.1016/j.ijcard.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 12.Paramsothy P, Katz R, Owens DS, Burke GL, Probstfield JL, O'Brien KD. Age-modification of lipoprotein, lipid, and lipoprotein ratio-associated risk for coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2010;105:352–8. doi: 10.1016/j.amjcard.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okwuosa TM, Greenland P, Lakoski SG, et al. Factors associated with presence and extent of coronary calcium in those predicted to be at low risk according to Framingham risk score (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2011;107:879–885. doi: 10.1016/j.amjcard.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClelland RL, Bild DE, Burke GL, Mukamal KJ, Lima JA, Kronmal RA. Alcohol and coronary artery calcium prevalence, incidence, and progression: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88:1593–601. doi: 10.3945/ajcn.2008.26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmariah S, Delaney JA, O'Brien KD, et al. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong ND, Nelson JC, Granston T, et al. Metabolic Syndrome, Diabetes, and Incidence and Progression of Coronary Calcium: The Multiethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2012 doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011;123:1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 21.Fuster V, Kelly BB, Vedanthan R. Promoting global cardiovascular health: moving forward. Circulation. 2011;123:1671–1678. doi: 10.1161/CIRCULATIONAHA.110.009522. [DOI] [PubMed] [Google Scholar]
- 22.Fuster V, Kelly BB, editors. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. National Academies Press (US); Washington (DC): 2010. Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries. [PubMed] [Google Scholar]
- 23.Kovacic JC, Fuster V. From Treating Complex Coronary Artery Disease to Promoting Cardiovascular Health: Therapeutic Transitions and Challenges, 2010-2020. Clin Pharmacol Ther. 2011;90:509–518. doi: 10.1038/clpt.2011.173. [DOI] [PubMed] [Google Scholar]