Abstract
Background and Purpose
Carotid Intima-Media Thickness (cIMT) was a widely accepted ultrasound marker of subclinical atherosclerosis in the past. Although traditional risk factors may explain approximately 50% of the variance in plaque burden, they may not explain such a high proportion of the variance in IMT, especially when measured in plaque free-locations. We aimed this study to identify individuals with cIMT unexplained by traditional risk factors for future environmental and genetic research.
Methods
As part of the Northern Manhattan Study, 1,790 stroke-free individuals (mean age 69±9; 60% women; 61% Hispanic, 19% black, 18% white) were assessed for cIMT using B-mode carotid ultrasound. Multiple linear regression models were evaluated: (1) incorporating pre-specified traditional risk factors; and (2) including less traditional factors, such as inflammation biomarkers, adiponectin, homocysteine and kidney function. Standardized cIMT residual scores were constructed to select individuals with unexplained cIMT.
Results
Mean total cIMT was 0.92±0.09 mm. The traditional model explained 11% of the variance in cIMT. Age (7%), male sex (3%), glucose (<1%), pack years of smoking (<1%), and LDL-cholesterol (<1%) were significant contributing factors. The model including inflammatory biomarkers explained 16% of the variance in cIMT. Adiponectin was the only additional significant contributor to the variance in cIMT. We identified 358 (20%) individuals with cIMT unexplained by the investigated risk factors.
Conclusions
Vascular risk factors explain only a small proportion of variance in cIMT. Identification of novel genetic and environmental factors underlying unexplained subclinical atherosclerosis is of outmost importance for future effective prevention of vascular disease.
Keywords: carotid ultrasound, carotid intima-media thickness, risk factors
Introduction
Atherosclerosis and cardiovascular disease (CVD) are the leading causes of death and disability in industrialized nations [1]. Carotid intima-media thickness (cIMT) was a widely accepted imaging marker of subclinical atherosclerosis in the past [2,3,4], however it is increasingly clear that IMT is a separate phenotype from carotid plaque, which is a focal lesion most likely determined by a set of different biological and genetic factors [5,6].
Early detection of risk factors of cIMT and their early modification may have a significant impact on the prevention of atherosclerotic disease. Traditional and common vascular risk factors such as hypertension, diabetes, dyslipidemia and smoking have been associated with increased cIMT [2,3,5,7-9]. Although these traditional vascular risk factors account for less than 50% of the variance of atherosclerotic plaque burden [10-13], they may not explain such a high proportion of the variance in IMT, especially when measured in plaque free-locations [4,14]. The contribution of other less traditional factors such as homocysteine [15,16], kidney function [17,18], and adiponectin [19] to cIMT is less clear. Furthermore, since atherosclerosis is considered an inflammatory disease [20], factors involved in inflammatory processes may be important determinants of increased cIMT, including white blood cell count (WBC) [21], CRP [22], IL-6 [23], serum amyloid A (SAA) [24] and others.
Discovery of important contributing factors of atherosclerosis, either protective or deleterious, may help in the improvement of treatment and prevention of CVD. In addition, identification of individuals in whom traditional CVD risk factors do not predict the observed level of subclinical atherosclerosis may lead to the detection of novel genetic and environmental factors. Selective genotyping of these individuals with “unexplained atherosclerosis” would allow for more efficient genetic studies and discoveries of therapeutic targets without loss of statistical power [25].
The aim of this study was to assess the contribution of traditional and less traditional vascular risk factors to the variance in cIMT and to identify individuals whose cIMT is not explained by these factors to serve as a resource for future genetic and environmental research.
Methods
Subjects
Subjects were participants in the NIH-funded Northern Manhattan Study (NOMAS), an ongoing, prospective, population-based study of stroke incidence and vascular risk factors and concurrently enrolled in the NIH-funded Oral Infections and Vascular Disease Study (INVEST) cohort [26,27]. Since 1998, 1,790 consecutive stroke-free subjects have been enrolled in the carotid imaging ancillary study. These individuals underwent high-resolution two-dimensional (2D) carotid ultrasound for assessment of cIMT. Details on subject ascertainment, extensive assessments, and methods used in NOMAS and INVEST are described elsewhere [5,13,19,21,26,27]. The high reliability of cIMT measurements in our laboratory was reported previously [23]. Both studies were approved by the IRBs of Columbia University, NY and the University of Miami, FL. All subjects gave written consent.
Evaluation of Risk Factors
Data were collected through interviews of the participants using standardized data collection instruments, review of the medical records, and physical and neurologic examinations. Race–ethnicity was based on self-identification through a series of questions modeled after the US Census. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or a patient’s self-report of a history of hypertension or use of antihypertensive medications. Cigarette smoking was categorized as non-smoker, former, or current smoker (within the last year) and the pack-years of smoking were calculated. Completion of high school was used as a proxy for socioeconomic status. Fasting total cholesterol and HDL-cholesterol were measured using a Hitachi 705 automated spectrophotometer (Boehringer Mannheim, Mannheim, Germany). Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the patient’s self-report of such a history or use of insulin or hypoglycemic medications [5,21]. Adiponectin was measured as previously described [19]. Fasting serum homocysteine was measured by licensed methods for commercial use [28]. Serum inflammatory markers (IL-6, CRP, SAA, TNF) were measured using enzyme-linked immunosorbent assay utilizing monoclonal antibodies (Biosource International, Camarillo, CA) [29]. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula [30].
Assessment of carotid IMT
Carotid ultrasound was performed according to the standard scanning and reading protocols by a trained and certified sonographer as detailed previously [23,27]. Our cIMT protocol is in the alignment with the Mannheim consensus, which recommends to measure cIMT in the segments free of plaque [4]. The near and the far wall of the left and the right carotid bifurcations, and the internal and the common carotid arteries were measured off-line using an automated edge detection image analysis system M’Ath (Intelligence in Medical Technologies, Inc., Paris, France). cIMT was calculated as a composite measure of the mean IMT measured at each of the 12 carotid sites within an individual, averaged and expressed in mm.
Statistical Analysis
Univariate analysis was performed using the F-test for categorical variables and correlation scores for continuous variables to assess the associations of demographic and vascular risk factors with cIMT, whereas general linear regression modeling for categorical variables and partial correlation for continuous variables were conducted to evaluate their age-adjusted associations with cIMT. In order to validate the previously proposed model using traditional vascular risk factors [25], we first regressed cIMT on the traditional risk factors including age, sex, glucose level, pack years of smoking, LDL-cholesterol, HDL-cholesterol, blood pressure (BP), pule pressure (PP), and lipid-lowering and antihypertensive medications (Model 1: Traditional model), with forward stepwise modeling by setting the selection criterion of p < 0.1 for each term in the model. We then performed a multiple regression using a similar approach to investigate whether more variation of cIMT can be explained by adding other potentially important factors. In addition to the factors in Model 1, Model 2 (Modified model) included socioeconomics (race-ethnicity, education), traditional factors (body mass index-BMI, waist-to-hip-ratio-WHR, waist, alcohol, physical activity), and less traditional factors (adiponectin, homocysteine, kidney function and inflammatory biomarkers: white blood cell count-WBC, CRP, IL-6, SAA). To identify the individuals with largely unexplained cIMT we have taken the approach from the Spence and colleagues [6,12,25] and computed the standardized cIMT residual scores from Model 2. A “predicted cIMT” value was calculated by summing the product of each individual’s independent variables and the standardized parameter coefficients from a multiple linear regression. Subtracting an individual’s predicted cIMT value from actual cIMT yielded a residual cIMT value. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Carotid ultrasound was performed among 1,790 stroke-free subjects. Demographics of this group did not differ from the characteristics of the parent cohort. The mean age in the carotid population was 69±9 years; 60% were women; 61% Caribbean Hispanics, 19% black, 18% white. Mean total cIMT was 0.92±0.09 mm.
Population demographic characteristics together with traditional and less traditional factors and their relationship to cIMT (univariate and age-adjusted) are listed in Table 1. The following factors were significantly associated or correlated with cIMT in univariate analyses: age, sex, race-ethnicity, WHR, waist, pack-years of smoking, systolic BP, PP, fasting glucose level, WBC, estimated glomerular filtration rate (eGFR), adiponectin, and homocysteine. In age-adjusted analyses, male sex, moderate alcohol intake, increase in WHR, pack-years of smoking, fasting glucose, WBC, and lower levels of adiponectin remained significantly associated with cIMT.
Table 1.
Demographics and Clinical Characteristics of the Study Population and Relationships to Carotid Intima-media Thickness (cIMT)
| Characteristics | Sample N (%) |
IMT Mean ± SD |
p | Age-adjusted p |
|---|---|---|---|---|
| All | 1790 (100) | 0.92±0.09 | ||
| Sex | <0.0001 | <0.0001 | ||
| Female | 1074 (60) | 0.91±0.08 | ||
| Male | 716 (40) | 0.94±0.09 | ||
| Race-Ethnicity | 0.0004 | 0.24 | ||
| Black | 341 (19) | 0.93±0.08 | ||
| Hispanic | 1094 (61) | 0.91±0.09 | ||
| Other | 42 (2) | 0.9±0.07 | ||
| White | 313 (18) | 0.93±0.09 | ||
| High school completion | 0.24 | 0.47 | ||
| No | 943 (53) | 0.92±0.09 | ||
| Yes | 847 (47) | 0.92±0.08 | ||
| Moderate alcohol drinking | 0.39 | 0.04 | ||
| No | 1081 (60) | 0.92±0.09 | ||
| Yes | 709 (40) | 0.92±0.09 | ||
| Physical Activity | 0.09 | 0.63 | ||
| No | 767 (43) | 0.92±0.08 | ||
| Yes | 1003 (57) | 0.92±0.09 | ||
| Anti-hypertension medications | 0.83 | 0.44 | ||
| No | 1062 (59) | 0.92±0.09 | ||
| Yes | 728 (41) | 0.92±0.09 | ||
| Lipid-lowering medications | 0.87 | 0.58 | ||
| No | 1493 (83) | 0.92±0.08 | ||
| Yes | 297 (17) | 0.92±0.1 | ||
| Insulin or oral medications for diabetes | 0.10 | 0.11 | ||
| No | 1563 (87) | 0.92±0.09 | ||
| Yes | 227 (13) | 0.93±0.09 | ||
| Clinical Characteristics of the Study Population and Correlations with Carotid Intima-media Thickness (cIMT) | ||||
|
| ||||
| Mean±SD | Correlation | p | Age-adjusted p | |
|
| ||||
| Age, years | 69.4±9.3 | 0.27 | <0.0001 | N/A |
| Body mass index (BMI), kg/m2 | 28.16±5.03 | 0.01 | 0.85 | 0.06 |
| Waist-to-hip ratio (WHR) | 0.90±0.09 | 0.11 | <0.0001 | <0.0001 |
| Waist, inches | 36.85±4.76 | 0.09 | <0.0001 | <0.0001 |
| Smoking, pack-years | 12.16±23.06 | 0.09 | <0.0001 | 0.001 |
| Systolic blood pressure (SBP), mmHg | 140.97±20.21 | 0.07 | 0.003 | 0.44 |
| Diastolic blood pressure (DBP), mmHg | 83.01±10.93 | -0.03 | 0.14 | 0.66 |
| Pulse pressure (PP), mmHg | 57.96±16.35 | 0.11 | <0.0001 | 0.20 |
| LDL-C, mg/dL | 128.01±35.09 | 0.03 | 0.16 | 0.27 |
| HDL-C, mg/dL | 46.69±14.43 | -0.01 | 0.74 | 0.22 |
| Triglyceride (TG), mg/dL | 134.68±79.19 | -0.04 | 0.14 | 0.36 |
| Total cholesterol (TC), mg/dL | 201.28±38.60 | 0.01 | 0.68 | 0.98 |
| LDL/HDL ratio | 2.98±1.20 | 0.02 | 0.33 | 0.16 |
| Fasting glucose, mg/dL | 102.15±42.53 | 0.06 | 0.01 | 0.007 |
| White blood cell count (WBC), 1000/mm3 | 6.20±2.01 | 0.05 | 0.03 | 0.02 |
| Estimated glomerular filtration rate (eGFR), ml/min | 75.09±19.89 | -0.10 | 0.0002 | 0.52 |
| Adiponectin | 10.31±5.20 | -0.06 | 0.02 | <0.0001 |
| Homocysteine, mmol/L | 9.42±4.62 | 0.06 | 0.03 | 0.48 |
| C-reactive protein (CRP), mg/L | 4.68±7.21 | -0.02 | 0.47 | 0.95 |
| Interleukin 6 (IL-6), pg/mL | 34.93±400.84 | 0.036 | 0.28 | 0.24 |
| Serum amyloid A (SAA), mg/L | 8.42±20.98 | 0.01 | 0.75 | 0.73 |
After performing the stepwise multiple regression model with inclusion of traditional factors (Model 1; Table 2), the following factors were identified as significant contributors to the variance in cIMT: age (7%), male sex (3%), glucose (<1%), pack-years of smoking (<1%) and LDL cholesterol (<1%). Overall, these factors explained 11% of the variance in cIMT (the coefficient of determination, R2=0.108)
Table 2.
Variation of Carotid Intima-Media Thickness (cIMT) Explained by Traditional Vascular Risk Factor Model and Modified Risk Factor Model after Inclusion of Less Traditional Risk Factors
| Parameter estimate | SE | β | Partial R2 | P-value | |
|---|---|---|---|---|---|
| Traditional model (Model 1) | |||||
| Age | 0.0026 | 0.0002 | 0.276 | 0.074 | <.0001 |
| Male sex | 0.0261 | 0.0041 | 0.150 | 0.025 | <.0001 |
| Glucose | 0.0001 | 0.0000 | 0.069 | 0.005 | 0.003 |
| Smoking, pack-years | 0.0002 | 0.0001 | 0.046 | 0.002 | 0.054 |
| LDL-C | 0.0001 | 0.0001 | 0.041 | 0.002 | 0.077 |
| R2=0.108 | |||||
|
| |||||
| Modified model (Model 2) | |||||
| Age | 0.0031 | 0.0002 | 0.341 | 0.090 | <.0001 |
| Male sex | 0.0247 | 0.0043 | 0.149 | 0.026 | <.0001 |
| LDL-C | 0.0002 | 0.0001 | 0.091 | 0.009 | 0.0002 |
| BMI | 0.0014 | 0.0004 | 0.089 | 0.009 | 0.0005 |
| Glucose | 0.0002 | 0.0000 | 0.081 | 0.007 | 0.0008 |
| Adiponectin | -0.0012 | 0.0004 | -0.073 | 0.004 | 0.005 |
| Smoking, pack-years | 0.0002 | 0.0001 | 0.067 | 0.004 | 0.007 |
| Race, black | 0.0109 | 0.0050 | 0.053 | 0.003 | 0.029 |
| Lipid-lowering medications | -0.0109 | 0.0057 | -0.047 | 0.003 | 0.054 |
| BP-lowering medications | -0.0081 | 0.0042 | -0.048 | 0.002 | 0.055 |
| R2=0.157 | |||||
β: indicates standardized parameter estimate; R2: coefficient of determination; P-values are based on the multiple linear regression models with a forward selection; BP: bold pressure.
The modified model (Model 2; Table 2) was able to explain 16% of the variance (R2=0.157). The contributing factors in this model were age (9%), male sex (3%), LDL-cholesterol (0.9%), BMI (0.9%), and fasting glucose (0.7%). The contributions of adiponectin (0.4%), pack years of smoking (0.4%), and black race-ethnicity (0.3%) were low but significant, whereas those of lipid (0.3%) and blood pressure lowering medication (0.2%) were marginally significant. The addition of less traditional risk factors such as homocysteine, eGFR and inflammatory markers did not significantly contribute to the cIMT variance (not included in Table 2). The results remained the same after exclusion of 438 subjects with a history of CAD, PAD or MI.
We have calculated the cIMT residual scores for each participant by regressing cIMT on the significant contributors in Model 2 and identified 358 (20%) individuals with cIMT unexplained by these factors (Figure 1). There is no significant difference in the risk factors between these two groups, except in observed cIMT (Table 3).
Figure 1. Predicted cIMT Distribution versus Masured cIMT Distribution.
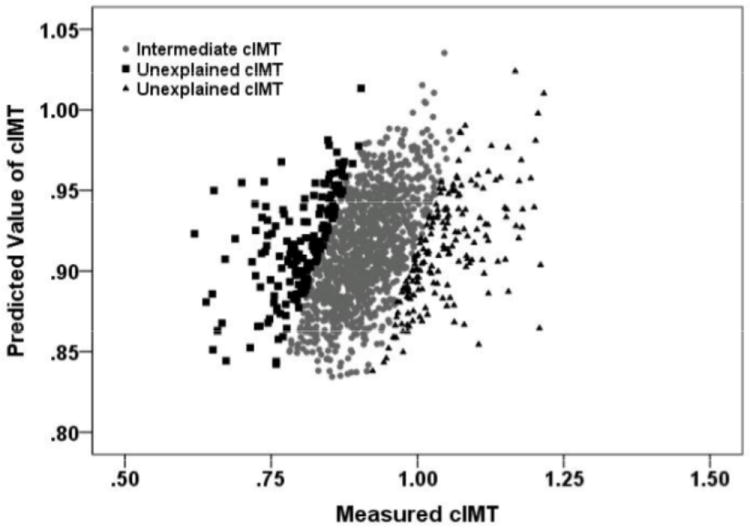
The three groups of individuals are distributed according to their residual scores computed using the approach from Spence and colleagues [6,12,25]. The solid gray circles represent individuals whose cIMT is explained by the final regression model (Intermediate cIMT), while the black squares (the bottom 10% of regression residuals) and black triangles (the top 10% of regression residuals) represents individuals in whom cIMT is unexplained by the factors included in the final model (Unexplained cIMT).
Table 3.
Traditional and Less Traditional Risk Factors among Individuals with Unexplained cIMT
| Characteristics | Unexplained cIMT (Bottom 10%) | Unexplained cIMT (Top 10%) | P Bottom 10% vs. Top 10% |
|---|---|---|---|
| N | 179 | 179 | |
| Mean Age, years±SD | 70.2±9.7 | 70.3±7.8 | 0.94 |
| Male sex | 67 (37) | 74 (41) | 0.45 |
| LDL-C | 132.8±32.5 | 132.9±34.9 | 0.96 |
| BMI | 28.2±4.8 | 28.3±5.3 | 0.83 |
| Glucose | 100.1±36.2 | 104.8±45.1 | 0.27 |
| Adiponectin | 10.3±4.9 | 9.9±4.0 | 0.44 |
| Smoking, pack-years | 11.8±21.6 | 11.1±22.8 | 0.78 |
| Race, black | 40 (22) | 37 (21) | 0.70 |
| Lipid-lowering meds | 28 (16) | 30 (17) | 0.77 |
| BP-lowering meds | 74 (41) | 67 (37) | 0.45 |
| Predicted cIMT (mm) | 0.91±0.03 | 0.91±0.03 | 0.49 |
| Observed cIMT (mm) | 1.05±0.06 | 0.80±0.05 | 2.37×10-133 |
Discussion
In this large, urban and multi-ethnic population, we report that traditional vascular risk factors explain only 11% of the variance in cIMT. The addition of other less traditional factors, including adiponectin, homocysteine and inflammation, explained an additional 5% of the cIMT variance, resulting in a total of 16% of the cIMT variance explained by all of these factors. Age and sex explain most of the variance in cIMT (about 10%). Therefore, most of cIMT variance in our study is not explained by traditional vascular risk factors commonly investigated in cerebrovascular research or assessed in vascular preventive clinics.
Our results are similar to previous findings from the Cardiovascular Health Study (CHS), where cholesterol levels, cigarette smoking, hypertension, diabetes, age, and sex contributed to 17% of the variance in cIMT in CCA and 18% in ICA (14), suggesting that cIMT less likely represents atherosclerosis. The contribution of traditional risk factors to the variance of cIMT in other populations however differed from our results [31,32,33,34] (Table 4). In the Framingham Offspring cohort, the risk factors in the Framingham score explained 28.6% of the cIMT variability in CCA and 27.5% in ICA [31], with age and sex being the strongest predictors of cIMT. In a population-based study from Mexico among low-income residents, there was a significant association of age, diabetes mellitus, systolic BP, total cholesterol (TC) and HDL cholesterol with cIMT accounting for 28% of cIMT variance in CCA, but only 12% in ICA [32]. Despite the differences between cIMT protocols and population characteristics of these studies, the majority of cIMT variance (over 70%) is not explained by traditional vascular risk factors. Age and sex are the highest contributors reported, while other contributors vary most likely due to different study populations, study designs, and measurements of cIMT in different carotid sites, e.g. CCA vs. ICA, the near vs. the far wall, inclusion of carotid plaques to cIMT measurements, or cIMT measured as a composite measure of all carotid segments outside a portion of plaque such as in our study.
Table 4.
Summary of the Carotid Intima-Media Thickness (cIMT) Protocols and Traditional Risk Factors Contributions in Selected Population-Based Studies (listed alphabetically)
| Study | Carotid segment measured | cIMT definition | Inclusion of plaque in cIMT measurements | Risk factors associated with IMT (and their contribution if available) |
|---|---|---|---|---|
|
| ||||
| ARIC [2] | CCA, ICA, Bifurcation; Far wall | Mean IMT | Yes | age, LDL, HDL cholesterol, hypertension, smoking, diabetes |
|
| ||||
| CHS [14] | CCA, ICA, Carotid bulb; Near and far wall | Max IMT | No | CCA IMT 18% |
| ICA IMT 17% from age, male sex, hypertension, diabetes, cholesterol levels, cigarette smoking | ||||
|
| ||||
| Epidemiological survey in Mexico City [32] | CCA, ICA; Near and far wall | Max IMT | No | CCA IMT: age, sex, triglycerides, TC, diabetes, HDL cholesterol, and SBP (all together 28 %) |
| ICA IMT: age, sex, triglycerides, TC, smoking, diabetes, and SBP (all together 12 %) | ||||
|
| ||||
| Framingham offspring cohort [31] | CCA, ICA, Carotid bulb; Far wall | Max IMT | Yes | CCA IMT: Total: 28.6%: age (19.4%), gender (4.1%), systolic BP (1.9%), HDL cholesterol (1.2%), smoking (0.9%), diabetes (0.8%), hypertension treatment (0.3%), and total cholesterol (0.002%). |
| ICA IMT: Total 27.5%: age (18.5%), gender (4%), smoking (1.6%), hypertension treatment (1.1%), systolic BP (0.8%), diabetes (0.8%), HDL cholesterol (0.6%), and total cholesterol (0.1%). | ||||
|
| ||||
| INVEST [27] | CCA, ICA, Bifurcation; Near and far wall | Mean IMT | No | Cumulative periodontal burden associated with IMT |
|
| ||||
| NOMAS [23] | CCA, ICA, Bif; Near and far wall | Mean IMT | No | Stromelysin-1 (MMP3), Interleukin-6 (IL6). Hepatic lipase (HL) (each 19%) |
CCA indicates common carotid artery; Bif, carotid bifurcation; ICA, internal carotid artery
Besides age and sex, only a small part of the cIMT variance (about 1-4%) is accounted by the remaining risk factors included in our study. Systolic BP, glucose, cholesterol and smoking were also small contributors to the cIMT in other reports [14,31,32-34]. The contribution of LDL-cholesterol in our study was marginal, whereas HDL-cholesterol, total cholesterol, triglycerides, and lipid-lowering medication did not have a significant effect. Other studies did not show a convincing contribution of LDL-cholesterol to the variance of cIMT either [14,31,33]. This may be substantiated by the results from the recent ENHANCE trial, where the addition of ezetimibe to a statin did not show any reduction of cIMT despite an obvious lowering effect on LDL-cholesterol [35]. Numerous lipid-levering interventional clinical trials have used cIMT as a surrogate measure of atherosclerosis with inconsistent and often conflicting results [36,37]. cIMT has not been affected to a large extend by the lipid metabolism, which could have been responsible for the “weak” results of the lipid lowering trials on cIMT.
Among the less traditional risk factors in our study, only adiponectin showed significant contribution to the cIMT variance, albeit a small one. Adiponectin was shown to be inversely correlated with cIMT [19,38]. This evidence underlines the role of adiponectin, an insulin-sensitizing adipocyte-secreted plasma protein, in maintenance of vascular homoeostasis through its vasoprotective actions. Evidence on the association of kidney dysfunction and cardiovascular disease is strong [39,40]. Our results, however, did not show a significant contribution of eGFR to cIMT variance. Accordingly, a relationship between eGFR and carotid plaque, but not IMT, has been documented, emphasizing again that cIMT and carotid plaque are different phenotypes [41]. A significant relationship between inflammatory markers and cardiovascular risk was reported [42] but their contribution to the cIMT variance was not found to be substantial in our as well as in other studies.
Our results of no apparent strong contribution of traditional and less traditional markers to the cIMT variance suggest that cIMT largely may not be a direct measure of atherosclerotic process. Carotid IMT may represent adaptive changes to biomechanical parameters with aging and not an indicator of atherosclerotic changes [43,44]. In addition, an increase in cIMT may be a consequence of hypertension with hypertrophy of the media layer of the arterial wall [43]. In our study, blood pressure parameters were not significant contributors to the variance in cIMT. Other vascular wall structure and function parameters (e.g., arterial diameter, stiffness) may be important contributors. Although cIMT was associated with vascular disease in prior reports [2-4,9,31,34], recent studies have argued that carotid plaque, not cIMT, was responsible for this effect [43,45,46].
Many unaccounted factors likely contribute to the variance of cIMT in a significant number of individuals as shown in our analyses of residual scores. Using our previous knowledge of traditional vascular risk factors and adding some novel factors, we have identified individuals whose cIMT is significantly greater or less than predicted, representing individuals with “unexplained cIMT”. These individuals would be ideal candidates for further investigations of genetic, lifestyle and novel environmental factors. Carotid IMT is a highly heritable trait [32,47] and genetic factors possibly attribute to a high proportion of the phenotypic variance of cIMT in CCA (66%) and in ICA (75%) [32,47,48]. Selective genotyping of extreme discordant phenotypes by identifying individuals with traits that cannot be explained by well-recognized risk factors may be a promising approach for discoveries of novel variants. With this approach, efficient and affordable genetic studies for identifying genetic variants and novel pathways of complex traits may be designed without loss of statistical power as elegantly showed in a study on extreme phenotypes of atherosclerotic plaque [25]. In addition, the influences of lifestyle factors such as dietary habits, moderate alcohol intake, and physical activity as well as occupational stress and psychosocial changes throughout life also have to be addressed in relation to cIMT in future studies. Lastly, the role of infection, inflammation and innate immunity has to be further investigated [27,49,50].
We acknowledge the limitations to our results. Our study included an elderly and predominantly Hispanic population and therefore our results may not be generalizable to other populations. Our results are cross-sectional and causality therefore cannot be inferred. Our selection of investigated risk factors might have been limited especially with the respect to sociocultural or socioeconomic characteristics, but we wanted to include traditional vascular risk factors with addition of several biologically plausible factors for atherosclerosis, which were also available in our cohort.
Conclusions
The variance of cIMT remains largely unknown. Traditional cardiovascular risk factors explain only a small part of the cIMT variance. Adiponectin is a novel factor, which has provided small but significant contribution to the cIMT variance in our study. Even though just a small part of variance of cIMT can be explained by traditional risk factors, adequate reduction and control of these factors are still the most important part of vascular disease prevention programs.
Acknowledgments
Sources of Funding:
This work was supported by the grants from the National Institutes of Health/National Institute of Neurological Diseases and Stroke R01 NS 065114 (Rundek, Blanton, Dong, Cabral, Sacco); K24 NS 062737 (Rundek); R37 NS 29993 (Elkind, Sacco, Rundek, Dong, Cabral); R01 DE-13094 (Desvarieux); and a Chair in Chronic Disease, École des Hautes Études en Santé Publique, France (Desvarieux)
Footnotes
Disclosures: None
References
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. American journal of epidemiology. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 3.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 4.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). Cerebrovascular diseases (Basel, Switzerland); An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences; Mannheim, Germany, 2004, and Brussels, Belgium, 2006. 2007. pp. 75–80. [DOI] [PubMed] [Google Scholar]
- 5.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spence JD. Technology Insight: ultrasound measurement of carotid plaque--patient management, genetic research, and therapy evaluation. Nature clinical practice. Neurology. 2006;2:611–619. doi: 10.1038/ncpneuro0324. [DOI] [PubMed] [Google Scholar]
- 7.Mannami T, Konishi M, Baba S, Nishi N, Terao A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke. 1997;28:518–525. doi: 10.1161/01.str.28.3.518. [DOI] [PubMed] [Google Scholar]
- 8.O’leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 9.Kuller L, Borhani N, Furberg C, Gardin J, Manolio T, O’leary D, et al. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. American journal of epidemiology. 1994;139:1164–1179. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 10.Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–587. doi: 10.1001/jama.285.5.581. [DOI] [PubMed] [Google Scholar]
- 11.Spence JD, Rundek T. Toward clinical applications of carotid ultrasound: Intima-media thickness, plaque area, and three-dimensional phenotypes. In: Nicolaides AE, editor. Ultrasound and Carotid Bifurcation Atherosclerosis. Springer-Verlag; London: 2012. pp. 431–448. [Google Scholar]
- 12.Spence JD, Barnett PA, Bulman DE, Hegele RA. An approach to ascertain probands with a non-traditional risk factor for carotid atherosclerosis. Atherosclerosis. 1999;144:429–434. doi: 10.1016/s0021-9150(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 13.Kuo F, Gardener H, Dong C, Cabral D, Della-Morte D, Blanton SH, et al. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43:1755–1760. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 15.Malinow MR, Nieto FJ, Szklo M, Chambless LE, Bond G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation. 1993;87:1107–1113. doi: 10.1161/01.cir.87.4.1107. [DOI] [PubMed] [Google Scholar]
- 16.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaka Y, Ishizaka N, Tani M, Toda A, Toda E, Koike K, et al. Relationship between albuminuria, low eGFR, and carotid atherosclerosis in Japanese women. Kidney & blood pressure research. 2008;31:164–170. doi: 10.1159/000131750. [DOI] [PubMed] [Google Scholar]
- 18.Kastarinen H, Ukkola O, Kesaniemi YA. Glomerular filtration rate is related to carotid intima-media thickness in middle-aged adults. Nephrology, dialysis, transplantation. 2009;24:2767–2772. doi: 10.1093/ndt/gfp172. [DOI] [PubMed] [Google Scholar]
- 19.Gardener H, Sjoberg C, Crisby M, Goldberg R, Mendez A, Wright CB, et al. Adiponectin and carotid intima-media thickness in the northern Manhattan study. Stroke. 2012;43:1123–1125. doi: 10.1161/STROKEAHA.111.641761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis is an inflammatory disease. American heart journal. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 21.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn R, Giral P, Bruckert E, Andre JM, Gonbert S, Bernard M, et al. Elevated C-reactive protein constitutes an independent predictor of advanced carotid plaques in dyslipidemic subjects. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1962–1968. doi: 10.1161/hq1201.099433. [DOI] [PubMed] [Google Scholar]
- 23.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie X, Ma YT, Yang YN, Fu ZY, Li XM, Huang D, et al. Polymorphisms in the SAA1/2 gene are associated with carotid intima media thickness in healthy Han Chinese subjects: the Cardiovascular Risk Survey. PloS one. 2010;5:e13997. doi: 10.1371/journal.pone.0013997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanktree MB, Hegele RA, Schork NJ, Spence JD. Extremes of unexplained variation as a phenotype: an efficient approach for genome-wide association studies of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:215–221. doi: 10.1161/CIRCGENETICS.109.934505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: the Northern Manhattan Stroke Study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 27.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Della-Morte D, Beecham A, Rundek T, Slifer S, Boden-Albala B, Mcclendon MS, et al. Genetic linkage of serum homocysteine in Dominican families: the Family Study of Stroke Risk and Carotid Atherosclerosis. Stroke. 2010;41:1356–1362. doi: 10.1161/STROKEAHA.109.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkind MS, Rundek T, Sciacca RR, Ramas R, Chen HJ, Boden-Albala B, et al. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis. 2005;180:181–187. doi: 10.1016/j.atherosclerosis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polak JF, Pencina MJ, Meisner A, Pencina KM, Brown LS, Wolf PA, et al. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. Journal of ultrasound in medicine. 2010;29:1759–1768. doi: 10.7863/jum.2010.29.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duggirala R, Gonzalez Villalpando C, O’leary DH, Stern MP, Blangero J. Genetic basis of variation in carotid artery wall thickness. Stroke. 1996;27:833–837. doi: 10.1161/01.str.27.5.833. [DOI] [PubMed] [Google Scholar]
- 33.Tan TY, Lu CH, Lin TK, Liou CW, Chuang YC, Schminke U. Factors associated with gender difference in the intima-media thickness of the common carotid artery. Clinical radiology. 2009;64:1097–1103. doi: 10.1016/j.crad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 34.O’leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. NEJM. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 35.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. NEJM. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 36.Smilde TJ, Van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 37.Crouse JR, 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clinical science. 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 39.Go AS, Chertow GM, Fan D, Mcculloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. NEJM. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, Abe Y, Furukado S, Miwa K, Sakaguchi M, Sakoda S, et al. Chronic kidney disease and carotid atherosclerosis. Journal of stroke and cerebrovascular diseases. 2012;21:47–51. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Choi SW, Kim HY, Lee YH, Ryu SY, Kweon SS, Rhee JA, et al. eGFR is associated with subclinical atherosclerosis independent of albuminuria: the Dong-gu Study. Atherosclerosis. 2010;212:661–667. doi: 10.1016/j.atherosclerosis.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 42.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 43.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis. Atheroscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 44.Glagov S, Vito R, Giddens DP, Zarins CK. Micro-architecture and composition of artery walls: relationship to location, diameter and the distribution of mechanical stress. J Hypertens Suppl. 1992;10:S101–S104. [PubMed] [Google Scholar]
- 45.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness. A systematic review and meta-anaysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 46.Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen ML, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 47.Juo SH, Lin HF, Rundek T, Sabala EA, Boden-Albala B, Park N, et al. Genetic and environmental contributions to carotid intima-media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke. 2004;35:2243–2247. doi: 10.1161/01.STR.0000142132.20442.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacco RL, Blanton SH, Slifer S, Beecham A, Glover K, Gardener H, et al. Heritability and linkage analysis for carotid intima-media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke. 2009;40:2307–2312. doi: 10.1161/STROKEAHA.109.554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. Journal of hepatology. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Mangili A, Polak JF, Quach LA, Gerrior J, Wanke CA. Markers of atherosclerosis and inflammation and mortality in patients with HIV infection. Atherosclerosis. 2011;214:468–473. doi: 10.1016/j.atherosclerosis.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


