Abstract
The cells that are responsible for detecting magnetic fields in animals remain undiscovered. Previous studies have proposed that pigeons employ a magnetic sense system that consists of six bilateral patches of magnetite containing dendrites located in the rostral subepidermis of the upper beak. We have challenged this hypothesis arguing that clusters of iron-rich cells in this region are macrophages, not magnetosensitive neurons. Here we present additional data in support of this conclusion. We have undertaken high resolution anatomical mapping of iron-rich cells in the rostral upper beak of pigeons, excluding the possibility that a conserved six-loci magnetic sense system exists. In addition we have extended our immunohistochemical studies to a second cohort of pigeons, confirming that iron rich cells in the upper beak are positive for MHCII and CD44, which are expressed by macrophages. We argue that it is important to critically assess conclusions that have been made in the past, while keeping an open mind as the search for the magnetoreceptor continues.
Keywords: magnetite, magnetoreception, pigeons, anatomical mapping, navigation
Introduction
The cells and molecular mechanisms that underlie the detection of magnetic fields in animals remain an unsolved scientific mystery. Two complementary theories have arisen which attempt to explain the basis of this remarkable sensory phenomenon. The first, a light dependent mechanism argues that the spin state of free radicals is influenced by the local magnetic environment which affects the reactivity of photosensitive molecules, that in turn alters neuronal activity.1,2 The second hypothesis argues that magnetic information is transduced into neuronal impulses by employing a magnetite (Fe3O4) based magnetoreceptor. This theory, commonly referred to as the magnetite theory of magnetoreception, gained credence following the discovery of aquatic bacteria and multicellular prokaryotes that employ intracellular magnetite to guide their directional swimming.3,4
A series of papers published by Fleissner and colleagues investigated the magnetite dependent hypothesis in pigeons.5-8 They undertook histological investigations employing the Prussian blue reaction that stains ferric (Fe3+) iron bright blue in color. They claim to have identified a magnetic sense system that resides in the upper beak of pigeons, that is a common sensory apparatus in avian species.7 It has been claimed that this system consists of six specific patches of magnetite containing dendrites that are located at bilateral locations in the rostral subepidermis of the pigeon beak.6 They have argued that these patches are about 350 μm long and 200 μm in diameter, vary only slightly in size and shape, always occur at specific sites near the lateral margin of the upper beak, are always in bilateral symmetry and are orientated in particular planes.5 When stained with Prussian blue these “dendrites,” which reside in the stratum laxum of the subepidermis, are characterized by light-blue background staining and a set of 10–15 dark-blue spherules. It is the contention of Fleissner and colleagues that these dark-blue spherules are only found in terminals positive for neuronal markers.6
We have recently undertaken an extensive, detailed and laborious attempt to replicate the claims of Fleissner and colleagues. Following the creation of a 3D anatomical blue-print of the pigeon beak, we undertook serial sectioning and manual counting of PB positive cells from the rostral concha to the tip of the pigeon beak. We counted cells on every 12th section, which represents a sampling distance of 120 μm. We failed to identify the reported 6 bilateral clusters that are claimed to constitute a magnetic sense system, and instead found an unexpected variation in the distribution and number of PB positive cells. We replicated this finding in a second population of unrelated birds, however it remains hypothetically possible that the patches of iron-rich cells are of smaller size in our cohorts and therefore escaped detection. Here we investigate this possibility by undertaking high-resolution anatomical mapping of the pigeon beak. In addition we provide further evidence that clusters of iron-rich cells in the subepidermal region of the pigeon beak are macrophages by undertaking additional immunohistochemical studies on a second cohort of pigeons employing sera against MHCII and CD44.
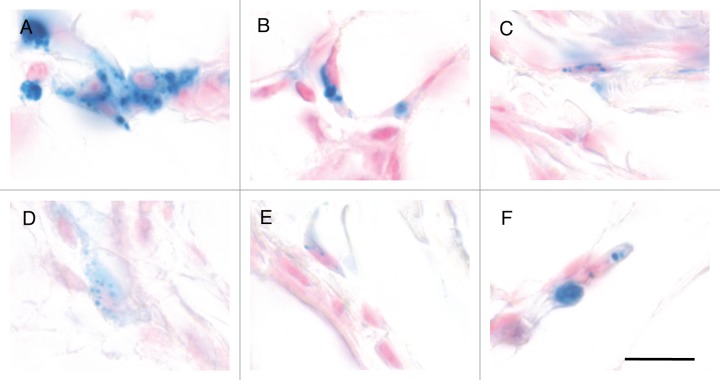
Results
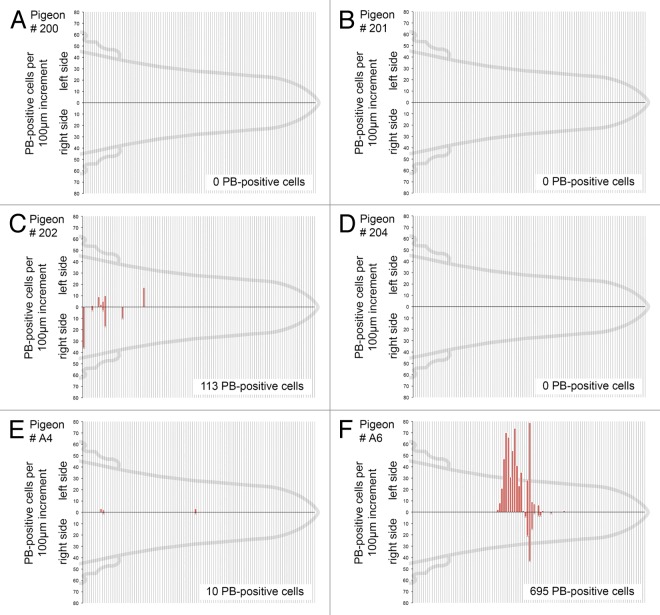
To explore the possibility that our serial sectioning had failed to identify the six-loci magnetic sense system reported by Fleissner and colleagues we undertook histological fine mapping employing the Prussian Blue reaction. In performing these experiments we drew on our anatomical blue-print for the pigeon beak, staining all sections from landmark 3 to the tip of the beak.9 We chose landmark 3 because Fleissner and colleagues have stated that the magnetic sense system lies rostral to the end of the pigeon cere. Landmark 3, which is defined by the anatomical position where the small lateral buds of the nasal cavity disappear, is caudal to this position.6 We performed PB staining on all sections rostral to landmark 3 on a total of 6 birds (4 from our Nuremberg cohort and 2 from our Vienna Cohort). PB positive cells were found in clusters in the stratum laxum of the subepidermis and were characterized by the presence of a nucleus, constellations of dark blue spherules (0.25 μm to 5 μm in diameter) and/or light blue cytoplasmic staining (Fig. 1A–F). These cells were indistinguishable from those in more caudal regions of the beak (i.e., from landmarks 1 to 3). Following counting of PB positive cells on all slides, we mapped their location onto a normalized pigeon beak (Fig. 2A–F). These results mirrored our previous estimation of PB positive cells when sampling every 12th section.9 We found a complete absence of PB cells in 3 birds (P200, P201, P204), and a varied distribution in three others (P202, A4 and A6). In those birds where PB positive cells were identified their location was not bilateral, nor were they found in six distinct patches. These results confirm our previous observations that the distribution of PB positive cells in the upper beak of pigeons is not conserved.
Figure 1. Examples of PB positive cells in the rostral subepidermis. (A–F). Light microscopy images of iron-rich cells in the rostral subepidermis of the pigeon upper beak stained with nuclear fast red and Prussian Blue. These cells are indistinguishable from those observed in caudal regions, containing multiple dark blue spherules (0.25 μm to 5 μm in diameter) and/or light blue cytoplasmic staining. Note also the presence of a nucleus shown by the pink staining. Scale bar shows 10 μm.
Figure 2. High resolution mapping of PB positive cells in the rostral subepidermis. (A–F). The distribution of PB positive cells along the rostro-causal axis between landmark 3 and the tip of the beak are shown for six birds (P200, P201, P202, P204, A4, A6). Red bars indicate the number of PB positive cells in the subepidermis, on the left and right sides in 100 μm increments. Total PB positive cell counts for each bird are shown in the bottom right corner. We found no evidence to support the existence of a six-loci magnetic sense system.
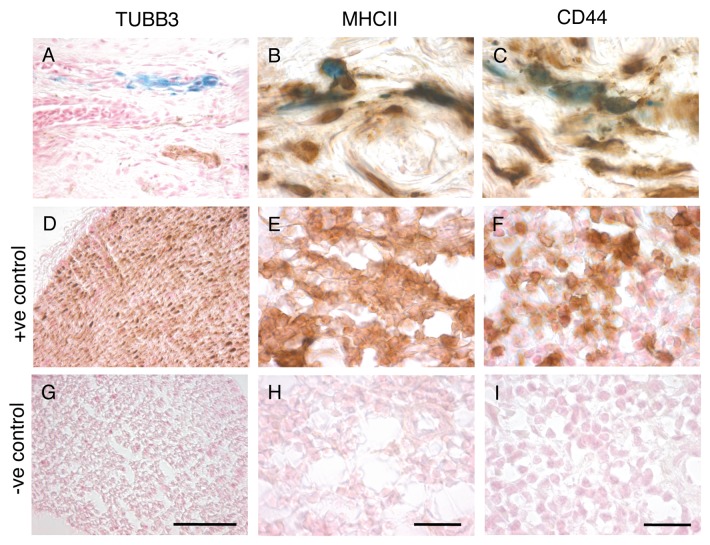
Our previous work on our Nuremberg cohort of pigeons has revealed that only a tiny fraction of PB positive cells in the subepidermal region co-localize with neuronal markers (e.g., TUBB3; 0.06%), whereas almost all PB positive cells co-localize with the antigen presenting marker MHCII (95%). We considered the possibility that this result might be unique to our Nuremberg strain of pigeons. To address this issue we repeated our immunohistochemical investigations on a second population of pigeons (our Austrian cohort) employing triple staining, with PB, NFR and anti-sera for TUBB3 or MHCII. Consistent with our previous work, we found that only 0.23% of PB positive cells co-localize with TUBB3 (n = 4 birds, 992 cells) (Fig. 3A, D and G), and that 97.2% co-localize with MHCII (n = 7 birds, 253 cells) (Fig. 3B, E and H). To provide further evidence that these cells are leukocyctes, we employed sera against CD44, which is a transmembrane glycoprotein expressed on T-cells, B-cells and macrophages.10 We found that 93.9% of PB positive cells co-localized with CD44 (n = 8 birds, 382 cells) (Fig. 3C, F and I).
Figure 3. Immunohistochemistry on Austrian Cohort. (A–C) Representative images of sections stained with PB and sera against (A) TUBB3, (B) MHCII or (C) CD44. Quantitative analysis revealed just 0.23% of PB-positive cells co-localize with TUBB3 (n = 4 birds, 992 cells). In contrast, 97.2% of PB positive cells co-localized with MHCII (n = 7 birds, 253 cells) and 93.9% with CD44 (n = 8 birds, 382 cells). (D–F) Positive controls for immunostaining. (D) Shows the ophthalmic branch of the trigeminal, a positive controls for TUBB3 staining. (E–F) Shows spleen sections, which served as positive controls for MHCII (E) and CD44 (F) staining. (G–I) Negative controls (the primary antibody was absent) for TUBB3, MHCII and CD44 experiments shows no background staining. Scale bars for MHCII and CD44 staining shown in H and I indicate 10 µm. Scale bar for TUBB3 staining shown in G indicates 50 µm.
Discussion
Here we present additional data supporting our conclusion that clusters of iron-rich cells in the subepidermal region of the pigeon beak are macrophages not magnetosensitive neurons. We have undertaken fine mapping of the rostral beak analyzing every section and find no evidence of a six-loci magnetic sense system. If anything our results overestimate the number of PB positive cells in the upper rostral beak as we employed particle counting in preference to the dissector method.11,12 In addition we have replicated our immunohistochemical studies in a second cohort of pigeons confirming that the vast majority of PB positive cells in the subepidermis (> 97%) are of hemopoietic origin. We do not, however, observe 100% co-localization with MHCII. What might be the explanation for this? Might a small percentage of PB-positive cells in the subepidermal region actually be magnetoreceptors? While conceivably possible, we think this is manifestly unlikely. The simplest, and most likely explanation for the small fraction of PB-positive MHCII negative cells, is that the antibody did not fully penetrate the tissue when performing the immunostaining. This explanation is supported by our observation that those few PB-positive cells that were negative for MHCII were not isolated, but rather were surrounded by PB-positive cells positive for MHCII which were morphologically indistinguishable. Similarly, those few cells that appear to be TUBB3 positive were again found within clusters of PB-positive cells that did not co-localize with the marker. The very small amount (0.23%) of TUBB3 co-localization we report is best explained by the nature of the Prussian Blue stain, which is sometimes diffuse. As a consequence, when a neuron is in close proximity to a macrophage, they are not readily distinguishable from one another, particularly if they are in the same vertical plane.
Our conclusion that clusters of iron-rich cells in the subepidermal region of the pigeon beak are macrophages, and not magnetosensitive neurons, is a stark contrast to that reached by Fleissner and colleagues.6,7 Why have we come to such different conclusions, particularly given that both groups (when employing the Prussian Blue reaction) report clusters of iron-rich cells with punctate dark blue spherules and a light blue cytoplasmic background in the subepidermis? First, we have undertaken a rigorous quantitative analysis of PB positive cells in the pigeon upper beak, taking great care to normalize the distribution to a uniform standard. We have performed this analysis in not one, but two, cohorts of racing pigeons. This has revealed large variations in the distribution and number of PB positive cells when comparing birds of the same age and sex. Our approach differs from Fleissner and colleagues who have not published any quantitative data that supports their assertion of a six-loci magnetic sense system that is arranged in bilateral clusters with “dendrites” orientated in three planes (X,Y,Z).
Second, we have investigated whether PB positive cells are neurons by employing several different neuronal antibodies (neurofilament, MAP1B, TUBB3), analyzing thousands of cells (> 2,500) in multiple birds.9 Cell counting blind to the antibody employed revealed that only a fraction of cells co-localize with neuronal makers (less than 1%), whereas almost all cells co-localize with markers found on white blood cells (e.g., MHCII). Moreover, in this manuscript we have replicated these findings in a second cohort of pigeons. Our approach contrasts with Fleissner’s and colleagues who relied on just one antibody, did not undertake a quantitative analysis, did not publish any controls, but nonetheless conclude that dark-blue spherules can only be found inside neurofilament-immunoreactive terminals (i.e., 100% co-localization).6 Furthermore, it should be noted that they adopted the unorthodox practice of stacking 10 optical planes to artificially amplify their antibody signal, raising the likely prospect that their assertions are based on false positives.
Third, when performing their electron microscopy studies, Fleissner and colleagues adopted a practice that invites contamination. Following dissection, fixation and embedding, they prepared 5 μm semithin sections and then stained every second section with PB.6 Those sections neighboring PB positive sections were then re-embedded and then 120 nm ultrathin sections are prepared. This re-embedding process results in poor image quality and invites contamination—contamination that appears to be part of the sample because it is surrounded by the same epon matrix. To avoid these pitfalls, we adopted a form of correlative light and electron microscopy (CLEM), which relies on taking alternative semithin (2 μm) and ultrathin (70–120 nm) sections. Semithin sections are stained with PB, and then the neighboring ultrathin section examined with transmission electron microscopy (TEM). This method, which does not involve re-embedding, is far more laborious than that adopted by the Fleissner group, but yields higher quality images limiting potential artifacts. It is plausible that the unusual large iron structures reported by the Fleissners (and re-published on multiple occasions) are the result of environmental contamination.6,13-15
Independent behavioral and neuronanatomical studies support the existence of a magnetoreceptor associated with the opthamlic branch of the trigeminal,16,17 however, our work has revealed that the sensory cells associated with this nerve remain undiscovered. An observer is left wondering: Where might these cells reside? One possibility is that magnetosensitive cells lie in more caudal regions of the pigeon beak, such as the olfactory epithelium, which has been implicated in magnetoreception in the rainbow trout,18,19 or alternatively nearby the olfactory bulbs. Beason and Nichols have previously reported PB positive structures in a thin layer of tissue nearby the olfactory bulbs in the Bobolink, Dolichonyx oryzivorus20 and it has previously been shown by Finger and colleagues that in rodents, some trigeminal branches extend into the olfactory bulbs.21 Wherever the cells reside, it is commonly assumed the trigeminal-based magnetoreceptor is an intensity detector which is reliant on magnetite,22,23 however, to our knowledge there is no conclusive evidence to support this contention. The assumption is problematic because it fails to consider the possibility of alternative mechanisms, such as a light-based magnetoreceptor in the beak.24,25 Nature is littered with examples of unexpected evolutionary adaptions which have confounded both prediction, and expectation. We think it is important to critically assess conclusions that have been made in the past while keeping an open mind as the search for the magnetoreceptor continues.
Materials and Methods
Prussian Blue staining and mapping
For Prussian Blue staining, we employed the method previously described.9 Specifically, we perfused pigeons with 4% PFA (pH 7.4), post-fixed for 18 h, before dehydration and paraffin embedding. We employed ceramic-coated blades to produce 10 μm sections that were mounted on electrostatic slides. All sections rostral to landmark 3 were then deparaffinated and stained in 5% potassium hexacyanoferrate in 10% HCl for 20 min, before washing (3x H20) and a 2 min counterstain with nuclear fast red (Sigma, 60700). All PB positive cells were then counted on every slide from Landmark 3 to the tip of the beak. The tip of the beak was defined by the last section where the intermaxillary bone was present. The number of PB positive cells was then grouped into 100 μm increments and normalized to established landmarks.
Immunohistochemistry
TUBB3 staining slides were de-paraffinated, washed in PBS (pH 7.4) and incubated with the primary antibody (Covance, MMS-435P) overnight at a concentration of 1:1000 in 0.1% Triton PBS with 2% milk. The next day, slides were washed in PBS (3 x 5 min) and incubated for 2 h with a biotinylated secondary antibody (1:500), before exposure to an avidin-biotin conjugate for 1 h (Vector Labs, PK-4002). Staining was visualized with the chomophore DAB (3,5-diaminobenzidine, Vector Labs, SK-4105, pH 7.4). For MHCII and CD44 staining, cryosections were prepared (12 μm) from tissue samples that had been fixed for 6 h in 4% PFA (pH 7.4). Slides were quenched in 2% H202 in PBS for 30 min, before incubation overnight with the primary antibody. For MHCII (Santa Cruz, SC-59323), the following conditions were used: 1:1000, 4% milk in 0.1% triton PBS. For CD44 (Southern Biotechnology, 8400-01) a concentration of 1:50 was used in 0.1% triton PBS with 2% milk. An ImmPress Reagent Kit (Vector Labs, MP-7402), followed by DAB incubation (Vector Labs, SK-4105, pH 7.4) was used to visualize CD44 and MHCII positive cells. Co-localization was then assessed by M.S. blind to the antibody employed. The average rate of co-localization for TUBB3, MHCII and CD44 staining was determined by calculating the rate of co-localization per bird and ascertaining the mean.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24859
References
- 1.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–18. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–8. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakemore R. Magnetotactic bacteria. Science. 1975;190:377–9. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 4.Scheffel A, Gruska M, Faivre D, Linaroudis A, Plitzko JM, Schüler D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature. 2006;440:110–4. doi: 10.1038/nature04382. [DOI] [PubMed] [Google Scholar]
- 5.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–42. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 6.Fleissner G, Holtkamp-Rötzler E, Hanzlik M, Winklhofer M, Fleissner G, Petersen N, et al. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol. 2003;458:350–60. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- 7.Falkenberg G, Fleissner G, Schuchardt K, Kuehbacher M, Thalau P, Mouritsen H, et al. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE. 2010;5:e9231. doi: 10.1371/journal.pone.0009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanzlik M, Heunemann C, Holtkamp-Rötzler E, Winklhofer M, Petersen N, Fleissner G. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. Biometals. 2000;13:325–31. doi: 10.1023/A:1009214526685. [DOI] [PubMed] [Google Scholar]
- 9.Treiber CD, Salzer MC, Riegler J, Edelman N, Sugar C, Breuss M, et al. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature. 2012;484:367–70. doi: 10.1038/nature11046. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–20. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- 11.Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- 12.Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(SICI)1096-9861(19970915)386:1<2::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Winklhofer M, Holtkamp-Rotzler E, Hanzlik M, Fleissner G, Petersen N. Clusters of superparamagnetic magnetite particles in the upper-beak skin of homing pigeons: evidence of a magnetoreceptor. Eur J Mineral. 2001;13:659–69. doi: 10.1127/0935-1221/2001/0013-0659. [DOI] [Google Scholar]
- 14.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–42. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 15.Stahl B, Fleissner G, Falkenberg G, Fleissner G. Magnetite nanoparticles alone are not able to explain ironmineral-based magnetoreception in birds. In: Kyriakopoulos A, Michalke B, Graebert A, Behne D, eds. Metalloproteins and Metalloidproteins. München: Utz, 2006. [Google Scholar]
- 16.Mora CV, Davison M, Wild JM, Walker MM. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature. 2004;432:508–11. doi: 10.1038/nature03077. [DOI] [PubMed] [Google Scholar]
- 17.Heyers D, Zapka M, Hoffmeister M, Wild JM, Mouritsen H. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc Natl Acad Sci USA. 2010;107:9394–9. doi: 10.1073/pnas.0907068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker MM, Diebel CE, Haugh CV, Pankhurst PM, Montgomery JC, Green CR. Structure and function of the vertebrate magnetic sense. Nature. 1997;390:371–6. doi: 10.1038/37057. [DOI] [PubMed] [Google Scholar]
- 19.Eder SH, Cadiou H, Muhamad A, McNaughton PA, Kirschvink JL, Winklhofer M. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc Natl Acad Sci USA. 2012;109:12022–7. doi: 10.1073/pnas.1205653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beason RC, Nichols JE. Magnetic Orientation and Magnetically Sensitive Material in a Transequatorial Migratory Bird. Nature. 1984;309:151. doi: 10.1038/309151a0. [DOI] [Google Scholar]
- 21.Schaefer ML, Böttger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444:221–6. doi: 10.1002/cne.10143. [DOI] [PubMed] [Google Scholar]
- 22.Wiltschko R, Schiffner I, Fuhrmann P, Wiltschko W. The role of the magnetite-based receptors in the beak in pigeon homing. Curr Biol. 2010;20:1534–8. doi: 10.1016/j.cub.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 23.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:675–93. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 24.Mouritsen H. Sensory biology: Search for the compass needles. Nature. 2012;484:320–1. doi: 10.1038/484320a. [DOI] [PubMed] [Google Scholar]
- 25.Mouritsen H, Hore PJ. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr Opin Neurobiol. 2012;22:343–52. doi: 10.1016/j.conb.2012.01.005. [DOI] [PubMed] [Google Scholar]