Abstract
Ataxia-telangiectasia and rad3 (ATR)-related Seckel syndrome is associated with growth retardation and premature aging features. ATR-Seckel fibroblasts have a reduced replicative capacity in vitro and an aged morphology that is associated with activation of stress-associated p38 mitogen-activated protein kinase and phosphorylated HSP27. These phenotypes are prevented using p38 inhibitors, with replicative capacity restored to the normal range. However, this stressed phenotype is retained in telomerase-immortalized ATR-Seckel fibroblasts, indicating that it is independent of telomere erosion. As with normal fibroblasts, senescence in ATR-Seckel is bypassed by p53 abrogation. Young ATR-Seckel fibroblasts show elevated levels of p21WAF1, p16INK4A, phosphorylated actin-binding protein cofilin, and phosphorylated caveolin-1, with small molecule drug inhibition of p38 reducing p16INK4A and caveolin-1 phosphorylation. In conclusion, ATR-Seckel fibroblasts undergo accelerated aging via stress-induced premature senescence and p38 activation that may underlie certain clinical features of Seckel syndrome, and our data suggest a novel target for pharmacological intervention in this human syndrome.
Key Words: Progeroid syndromes, Telomeres, p53, ATR, Premature aging, Werner syndrome, Fragile sites, Chromosome instability, Replication stress, Caveolin-1.
Segmental progerias are human genetic syndromes that accelerate some, but not all, of the features of human aging and have long been used to explore genotype–phenotype relationships in human aging (1). In previous studies, we have focused on one of the most dramatic progeroid syndromes, that is, Werner syndrome (WS). Lack of WRNp (Werner syndrome RecQ helicase-like protein) leads to premature fibroblast senescence resulting from activation of the stress-associated p38 mitogen-activated protein (MAP) kinase, which is thus termed stress-induced premature senescence or SIPS (2). This SIPS has been proposed to underlie some of the premature aging features seen in WS (3). We previously demonstrated that the dramatic shortening of WS cellular replicative life span observed in vitro could be rescued by treatment with p38 MAP kinase inhibitors (4). We hypothesized that loss of WRNp results in replication stress, activation of p38 MAP kinase, and premature whole-body aging as a result of upregulation of p38-dependent inflammatory molecules (inflamm-aging) and/or p38-driven premature senescence (5).
Subsequently, we explored a range of non-WS progerias and demonstrated that activation of p38 MAP kinase was not a widespread phenomenon (6,7). Many of these progeroid syndromes show defects in DNA repair and genome stability, indicating that p38 activation in WS is not simply the result of generalized compromised genome integrity. We have also shown that patient-derived cells from Rothmund–Thomson syndrome, a mild progeroid syndrome caused by mutations in another RecQ helicase, show activation of p38 MAP kinase, albeit without a dramatic effect on replicative life span (8). This suggests that activation of p38 in progerias is closely linked with a subset of biological processes related to genome stability.
Given the DNA replication defects observed in WS that include the stalling of DNA replication forks (9), and our hypothesis that such defects are an upstream driver of p38 activation in WS (5), we sought to examine additional progeroid syndromes carrying mutations in the same axis to ask whether they also showed activation of p38, and in this study, we focused on the ataxia-telangiectasia and rad3 (ATR)-related checkpoint kinase. Although ATR is an essential gene, viable mutations in ATR are found in a subset of human Seckel syndrome individuals (10).
Seckel syndrome is a rare heterogeneous autosomal recessive disorder found in less than 1 in 10,000 live births (11). Mutations have been found in five genes in Seckel syndrome that includes the ATR gene (SCKL1) in the ATR-Seckel variant (12). The rarity of ATR-Seckel patients is consistent with the observation that null mutations in ATR appear to be lethal; no human examples of viable homozygous ATR null mutations have been identified and experimental-induced null mutations of ATR are lethal in the mouse (13). ATR-Seckel thus appears to be a rare viable hypomorphic allele (10).
Seckel syndrome is characterized by intrauterine growth retardation, developmental delay, bird-like facies, severe microcephaly, and short stature, as well as brain size reduction, cysts, and agenesis of the corpus callosum (11,14). Other symptoms include sparse hair, café-au-lait spots, impaired cardiovascular function, and age-related disorders such as type II diabetes, all features seen in normal aging, and Seckel syndrome individuals die earlier than normal individuals (15). Thus, although Seckel syndrome appears to be primarily a “failure of growth” disorder, it is associated with mild progeroid features (10,16). This segmental progeroid nature of ATR-Seckel is not untypical of several of the recognized progeroid syndromes, where the premature aging features are not the primary phenotypic manifestations (1). It should also be noted that there appear to be no genes that specifically “cause aging”; the processes that affect aging involve gene products that have diverse additional functions in the body, so mutations in such genes will have broad-ranging phenotypic consequences. However, premature aging is a primary feature seen in the ATR-Seckel mouse model (13). Human WS is also associated with growth retardation, as WS individuals fail to show the pubertal growth spurt and are short in height (17). Thus, ATR-Seckel shares with WS two phenotypic characteristics, that of premature aging and growth retardation.
ATR-Seckel was chosen for this study because of the hypothesized role of replication stress as a driver of the premature aging phenotype of WS fibroblasts. An important function of ATR is the coordination of checkpoint control responses to replication fork stalling, which arises during normal replication, particularly at DNA sites that are difficult to replicate, including the so-called fragile sites (10,18,19). ATR-Seckel fibroblasts are reported to grow slowly, have slow cycling time and increased chromosomal instability (CIN), especially at fragile sites (10,20,21), and show increased replication fork stalling (22). These features are replicated in a mouse ATR-Seckel model, with ATR S/S mouse embryonic fibroblasts (MEFs) showing slow growth, premature cellular senescence, and CIN at fragile sites and ATR S/S mice showing growth retardation and premature aging (13). Human WS fibroblasts also show slow growth rates and premature senescence (4), an increase in replication fork stalling (9), and CIN at fragile sites (23). Common fragile sites are observed as nonstaining gaps or breaks in metaphase chromosomes of cells cultured under conditions of replicative stress. These reproducible nonrandom fragile regions of chromosomes observed in vitro correspond to regions where specific DNA instability has been observed in vivo in various human cancers (24). WRNp deficiency recapitulates ATR defects in terms of fragile site instability either when cells are exposed to aphidicolin or under unperturbed conditions (23). According to the model proposed by Casper and colleagues (20), ATR is activated after replication stress to stabilize and rescue stalled replication forks. Similarly, WRNp appears to be essential for fruitful rescue from replication fork arrest (25–27) and is targeted for ATR phosphorylation upon replication arrest (28). It appears that ATR collaborates with and recruits WRNp to replication fork stalls in a DNA damage pathway that responds to replication stress, particularly due to problems inherent in the replication of fragile site regions to aid replication fork recovery and to restart DNA synthesis (29). This idea is supported by the observation that ATR deficiency in WS fibroblasts does not increase the frequency of fragile site expression (ie, ATR and WRNp do not synergize), which is suggestive of a common pathway (23).
The interaction between ATR and WRNp in a common signalling pathway, the resemblance between WS and ATR-Seckel cells, and the potential involvement of aberrant DNA replication in both syndromes led us to hypothesize that the premature aging seen in both syndromes may reflect an overlap in causal mechanisms. To address this hypothesis, we examined the mechanisms leading to cellular senescence in ATR-Seckel by determining the growth characteristics and replicative capability of ATR-Seckel fibroblasts and the role of p53 using shRNA abrogation in replicative senescence. In addition, we investigated the role played by p38 MAP kinase using a combination of molecular profiling and small molecule inhibitor use. Furthermore because telomere shortening is a major mechanism driving fibroblast senescence and ATR deficiency results in telomere fragility (30), we have also used ectopic expression of human telomerase to determine whether replicative senescence in ATR-Seckel fibroblasts is telomere dependent.
Materials and Methods
Cells and Cell Culture
The primary dermal fibroblasts used in this work were obtained from the Coriell Cell Repository (Camden, NJ); ATR-Seckel strain GM18366 that carries a hypomorphic ATR allele (31); three normal dermal fibroblast strains (NDFs) AG06234, AG13152, and AG16409; and the WS strain AG05229.
All cells were grown in Earle’s Modified Eagle medium (EMEM; Gibco) supplemented with 10% fetal calf serum (Autogen Bioclear, Witshire, UK) in an atmosphere of 20% O2 and 5% CO2, and passaged every 4–5 days exactly as described previously (4).
Protein Kinase Inhibitors
SB203580 was obtained from Tocris Chemical Co. (Bristol, UK). BIRB 796 and VX-745 were synthesized according to Bagley and colleagues (32,33). For experiments using inhibitors, growth medium was supplemented with SB203580 and BIRB 796 at 2.5 µM and VX-745 at 0.5 µM. For controls, an equivalent volume of dimethyl sulfoxide was added to the medium. SB203580 at 2.5 µM is in the range used routinely for studying the effects of SB203580 on p38 activity in cell biological systems (6) and that does not inhibit the related JNK1/2 kinases (34). BIRB 796 at 2.5 µM is the maximum concentration that inhibits p38 without inhibiting the related JNK1/2 kinases (34). VX-745 at 0.5 µM is the minimal concentration needed to maximally inhibit p38 (33). To maintain maximal p38 inhibition, growth medium was replaced daily with fresh EMEM-containing p38 inhibitors.
Retroviral Gene Transfer
The ectopic expression of human telomerase protein and the expression of an shRNA against p53 in ATR-Seckel cells were exactly as described previously (8,35).
Immunofluorescence Microscopy
Actin staining with fluorescein isothiocyanate-conjugated (FITC)-phalloidin for immunofluorescence microscopy was performed exactly as described (4).
Immunoblot Analysis
Immunoblot analysis was done exactly as previously described (4). All antibodies have been previously described except anti-phospho(Y14)-caveolin-1 and anti-caveolin-1 (Cell Signalling, New England Biolabs, Hitchin, UK [4,8,35,36]).
Results
Effects of p38 Inhibitors on the Growth of Normal and ATR-Seckel Fibroblasts
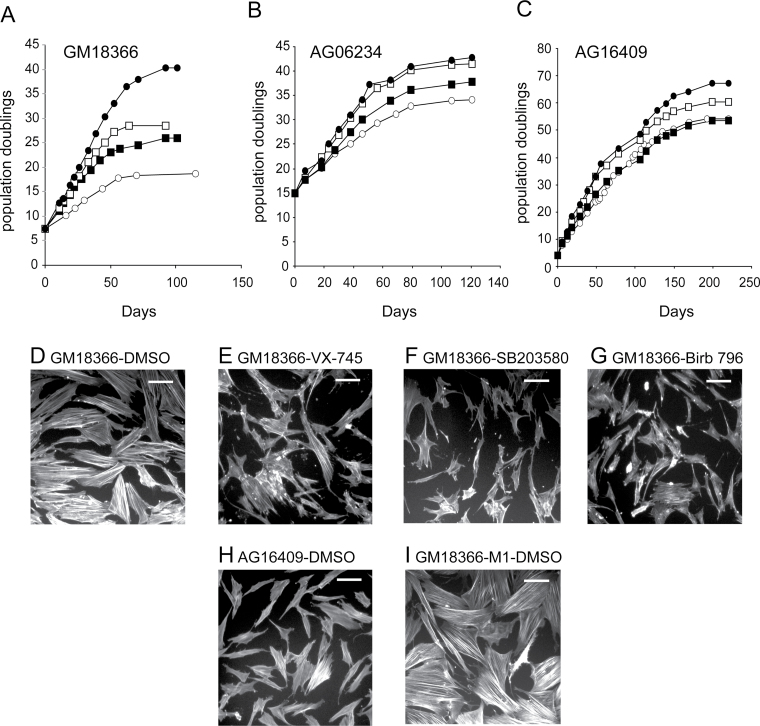
ATR-Seckel GM18366 fibroblasts were grown in triplicate to replicative senescence in the presence or absence of p38 inhibitors. As shown in Figure 1A, GM18366 control cells had a replicative capacity of 19.3±0.6 population doublings (PDs) that was not statistically shorter than the mean of three NDFs (Table 1; Figure 1B and C). The GM18366 replicative capacity increased with each p38 inhibitor used with VX-745 having the smallest and BIRB 796 the largest effect (Figure 1A; Table 1). With BIRB 796, the GM18366 replicative capacity was within the range of BIRB 796–treated NDFs (Table 1). The percentage increases in replicative capacity of GM18366 cells for each inhibitor compared with NDFs were all highly statistically significant (p < .0001; z test).
Figure 1.
Growth characteristics of ataxia-telangiectasia and rad3 (ATR)-related Seckel and normal dermal fibroblast (NDF) strains with or without p38 inhibitor treatment. (A) GM18366 cells, (B) AG06234 cells, and (C) AG16409 cells. Fibroblasts were grown in Earle’s Modified Eagle medium (EMEM) supplemented with 0.1% (v/v) dimethyl sulfoxide (DMSO; ○) or the p38 inhibitors VX-745 (■), SB203580 (□), and BIRB 796 (●). Growth is measured as population doublings (PDs) vs days. Observation of F-actin stress fibers in (D–G) ATR-Seckel cells, (H) AG16409 cells, and (I) ATR-Seckel cells at M1. The conditions for growth are indicated on the panels. Bar = 100 μm.
Table 1.
Life Span of Fibroblasts Grown in the Presence or Absence of p38 Inhibitors
| Strain | PD at Start* | PDs Achieved† | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | VX-745 | %‡ | SB203580 | %‡ | BIRB 796 | %‡ | ||
| Normal | ||||||||
| AG06234 | 15.0 | 36.8 | 41.6 | 22.0 | 44.4 | 34.8 | 45.6 | 40.4 |
| AG13152 | 6.0 | 28.0 | 31.7 | 16.8 | 35.6 | 34.5 | 38.3 | 46.8 |
| AG16409 | 4.0 | 54.3 | 54.3 | 0 | 61.9 | 15.1 | 67.3 | 25.8 |
| Mean ± SD | 39.7±13.3 | 42.5±11.3 | 12.9±11.5 | 47.3±13.4 | 28.1±11.3 | 50.4±15.1 | 37.7±10.8 | |
| ATR-Seckel§ | ||||||||
| GM18366 ± SD | 7.5 | 19.3 ± 0.6 (3) | 26.8 ± 0.6 (3) | 63.6 | 28.3 ± 0.3 (3) | 76.3 | 39.1 ± 1.8 (3) | 167.8 |
| Probability (p)|| | >.063 | >.083 | <.0001 | >.079 | <.0001 | >.22 | <.0001 | |
Notes: PD = population doubling; SD = standard deviation.
*Reported PDs of cell strain when they were obtained from Coriell Repository.
†Total PDs achieved (this includes PDs at start plus PDs achieved during these growth experiments).
‡The percentage increase in replicative capacity is determined with reference to starting PD, for example, for AG06234, the replicative capacity increase using VX-745 is (41.6 PDs −15 PDs)/(36.8 PDs −15 PDs) = 1.22 or a 22.0% increase in experimental replicative capacity.
§All ataxia-telangiectasia and rad3-related (ATR)-Seckel cultures are grown in triplicate.
||Probability that this is same as for normal cells; z test.
Visualization of F-Actin Stress Fibers in ATR-Seckel Fibroblasts
Low PD GM18366 cells stained with FITC-phalloidin showed many cells that were enlarged with numerous visible F-actin stress fibers (Figure 1D); in contrast, low PD AG16409 NDFs were smaller with few F-actin stress fibers (Figure 1H). When grown in the presence of p38 inhibitors, the morphology of GM18366 cells more resembled that of young NDFs (Figure 1E–G). The three inhibitors were not equally effective, however, with VX-745 having the smallest effect with several enlarged cells with F-actin fibers remaining (Figure 1E). In contrast, the inhibitors had little effect on NDFs (not shown). When GM18366 cells reached M1, all the cells were enlarged with extensive stress fibers (Figure 1I) and p38 treatment had no effect on this (not shown). Similar results were seen for AG16409 cells at M1 (not shown).
ATR-Seckel Fibroblasts Have Activated p38 and Stress Signalling
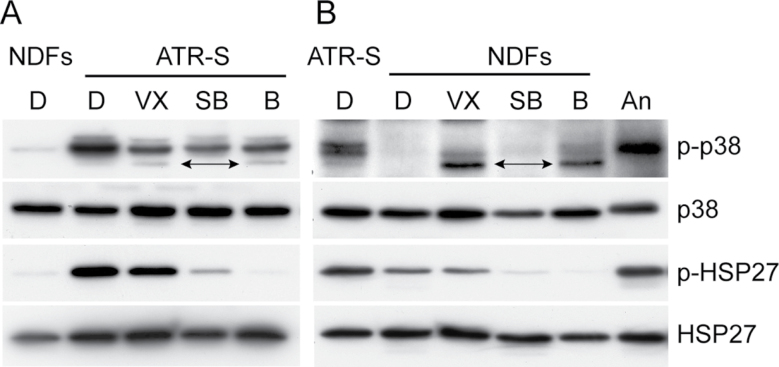
Activated (phosphorylated) p38 (p-p38) was detected by immunoblot assay in GM18366 young primary fibroblasts but not in young AG16409 cells (Figure 2). All three p38 inhibitors reduced the level of p-p38 in GM18366 cells to some extent but didn’t abolish it (Figure 2A). The ability of p38 inhibitors to partially prevent p38 activation has been reported previously for VX-745 and SB203580 at the concentrations used here (33). BIRB 796 is reported to completely prevent phosphorylation of p38 at 10 µM but not at 1 µM (32,37); thus, it may be expected that BIRB 796 would only partially prevent p38 activation at the concentration of 2.5 µM used here. In contrast, p38 inhibitors had no effect on the very low p-p38 levels in the AG16409 cells (Figure 2B). When the GM18366 cells reached M1, the levels of p-p38 increased (Figure 4F).
Figure 2.
Immunoblot analysis of p38 and HSP27. Protein lysates were prepared from GM18366 (ataxia-telangiectasia and rad3-related Seckel [ATR-S]) and AG16409 (normal dermal fibroblast strains [NDFs]) cells. Expression levels were compared for phosphorylated p38 (p-p38), p38, phosphorylated HSP27 (p-HSP27), and HSP27. Loadings were normalized for p38. For each series A and B, a control lane has been added (with ATR-S used as control for NDFs and vice versa). Lanes are D, dimethyl sulfoxide (DMSO); VX, VX-745; SB, SB203580; B, BIRB 796. “An” are AG16409 cells treated with 30 μM anisomycin for 45min to activate p38 and phosphorylate HSP27. The bands marked with arrows in lanes VX and B of A are nonspecific bands that are also seen in B.
Figure 4.
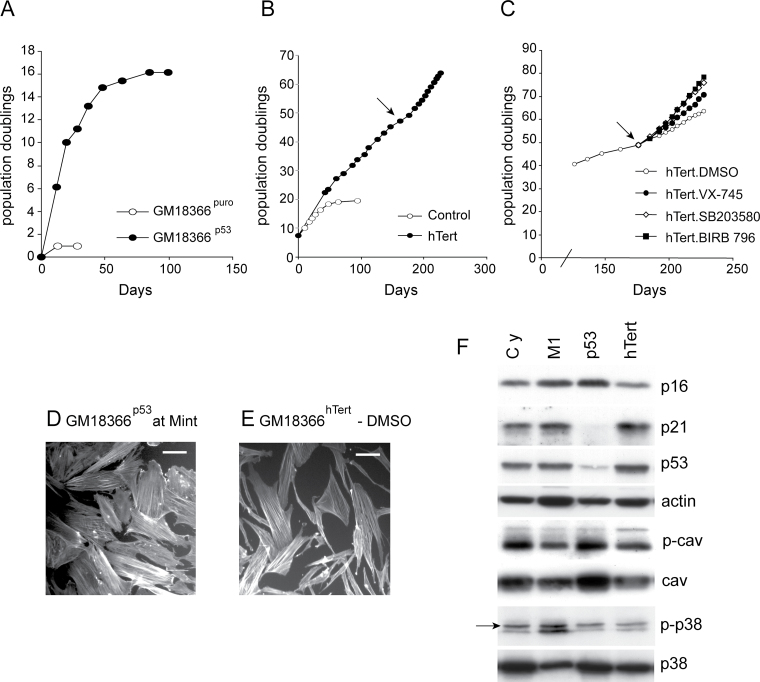
Effects of p53 abrogation and ectopic hTert expression on the growth of ataxia-telangiectasia and rad3 (ATR)-related Seckel cells. Growth in population doublings (PDs) vs days for (A) GM18366p53 cells, (B) GM18366hTert cells, and (C) GM18366hTert cells treated with p38 inhibitors as indicated. In B and C, the arrow indicates the point at which inhibitor treatment of GM18366hTert began. Observation of F-actin stress fibers in (D) GM18366p53 cells at Mint and (E) GM18366hTert cells. Bar = 100 μm. (F) Immunoblot analysis for GM18366 cells (proteins analyzed as for Figures 2 and 3; the arrow on the p-p38 row shows activated p38). Cy = low PD cycling cells; M1 = cells at senescence; p53 = GM18366 abrogated for p53; hTert = GM18366 cells infected with hTert.
HSP27, a downstream target of the p38 pathway, was phosphorylated in GM18366 fibroblasts and, to a lesser extent, in AG16409 cells (Figure 2). Phosphorylation levels were reduced by all three inhibitors in the young GM18366 cells; however, the inhibitors differed in their efficacy, with VX-745 having a small effect and BIRB 796 almost completely abolishing p-HSP27 levels. A similar effect was seen with AG16409 cells, although in this case, the p-HSP27 levels were much lower in the AG16409 cells under all conditions tested. The immediate p38 target MK2 is activated in young GM18366 cells as indicated by the doublet, and this was reduced in inhibitor-treated cells (Figure 3A). MK2 is the major HSP27 kinase (38).
Figure 3.
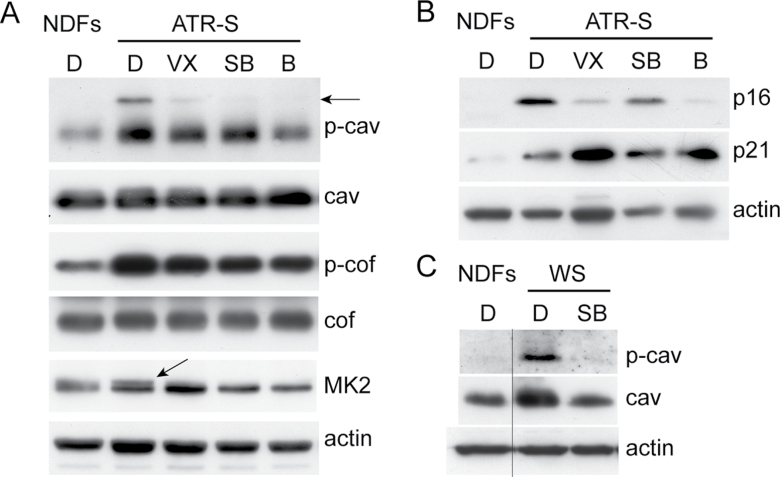
Immunoblot analysis of cell cycle proteins and caveolin-1. Protein lysates were prepared from low population doubling (PD) GM18366 (ataxia-telangiectasia and rad3-related Seckel [ATR-S]), AG16409 (normal dermal fibroblast strains [NDFs]), and AG05229 (Werner syndrome [WS]) cells. Expression levels were compared for p16INK4A, p21WAF1, phospho-caveolin-1 (p-cav), caveolin-1 (cav), phospho-cofilin (p-cof), cofilin (cof), and MK2. Actin is used as loading control for each panel. Lanes are D, dimethyl sulfoxide (DMSO); VX, VX-745; SB, SB203580; and B, BIRB 796. The horizontal arrow indicates the 24kDa p-cav band and the diagonal arrow indicates activated MK2. The vertical line in C indicates that the single lanes have been cut and pasted from the same gels as the rest of the samples, however, the images have been handled in the same manner otherwise.
The LIN-11/Isl1/MEC3-domain (LIM) kinase pathway is thought to be regulated by p38 signalling under some circumstances and regulates F-actin stress fiber expression via phosphorylation and inactivation of the actin depolymerizing factor cofilin (39). In low PD GM18366 cells, cofilin was phosphorylated compared with low PD AG16409 cells, and the level of p-cofilin was only slightly reduced by p38 inhibitors (Figure 3A), suggesting that the stress fiber phenotype is not via the LIM kinase pathway.
Expression of Cell Cycle Proteins in ATR-Seckel Cells
Low PD GM18366 cells showed elevated levels of the cyclin-dependent kinase inhibitors (CdkIs) p21WAF1 and p16INK4A compared with NDFs (Figure 3B). The effects of p38 inhibitors on this expression in GM18366 cells were mixed, with little effect seen on the levels of p21WAF1; however, the levels of p16INK4A were much reduced by all inhibitors used with SB203580 having the smallest effect. When the GM18366 cells reached M1, both CdkIs appeared to show small increases (Figure 4F). The tumor suppressor p53 protein was present at similar levels in both low PD GM18366 cells and GM18366 cells at M1 (Figure 4F).
ATR-Seckel Cells Have Phosphorylated Caveolin-1
Although elevated caveolin-1 expression is associated with premature fibroblast senescence (40), low PD ATR-Seckel cells did not show elevated caveolin-1 levels compared with low PD NDFs and p38 inhibitors had no effects on this expression (Figure 3A). However, ATR-Seckel cells had activated (phosphorylated) caveolin-1 compared with low PD AG16409 cells (Figure 3A) as shown by elevated levels of the 21kDa protein and the presence of the 24kDa protein (indicated by arrow). Treatment of ATR-Seckel cells with p38 inhibitors decreased the levels of both phosphorylated protein variants with BIRB 796 treatment reducing the level of p-caveolin-1 to that seen in AG16409 cells. When GM18366 cells reached M1, the levels of p-caveolin-1 were reduced compared with low PD cells, whereas no changes in the levels of caveolin-1 were seen (Figure 4F). These data contrast with WS cells in that the levels of both caveolin-1 and p-caveolin-1 are elevated in low PD WS fibroblasts compared with NDFs and both are reduced with the p38 inhibitor SB203580 (Figure 3C).
Abrogation of p53 Allowed ATR-Seckel Cells to Bypass Senescence
Presenescent GM18366 fibroblasts at PD 17 were infected with amphotropic retroviral vectors encoding a puromycin resistance (puro) gene alone, or both puro and an shRNA to p53. Analysis of puromycin-resistant cultures (designated GM18366puro and GM18366p53, respectively) began 2–3 weeks after infection.
Cells expressing puro alone ceased proliferating within the first 2 weeks and entered a senescent-like state after achieving 1 PD (Figure 4A). The GM18366puro cells at this stage appeared essentially identical to uninfected GM18366 cells at senescence (not shown). In contrast, expression of the p53 shRNA resulted in evasion of senescence and generated rapidly growing cultures. Eventually, approximately 15 PDs beyond M1, the GM18366p53 cells entered a senescence-like state termed Mint that has been described previously for p53-abrogated human fibroblasts (41) with the cells being enlarged with extensive F-actin stress fibers (Figure 4D).
In the GM18366p53 cells at Mint p53, protein levels were very low compared with cells at M1, showing that the shRNA had successfully abrogated p53 (Figure 4F). In addition, the level of the p53 target p21WAF1 was very low, whereas p16INK4A levels had increased compared with M1 (Figure 4F). Interestingly, the levels of both caveolin-1 and p-caveolin-1 increased in the GM18366p53 cells compared with cells at M1.
Extension of the Replicative Capacity of ATR-Seckel Syndrome Cells By Ectopic hTert Expression
GM18366 cells were infected with retroviruses expressing puromycin resistance and hTert or puromycin resistance only. Drug-resistant cultures were selected and designated as GM18366hTert and GM18366puro.
GM18366 cells were infected at PD 7 and the GM18366puro control managed 19 PDs before reaching M1 (Figure 4B). The GM18366hTert culture achieved greater than 65 PDs and showed little sign of senescing, indeed these lines are still growing today. However, although apparently immortalized, GM18366hTert cells retained many of the characteristics of young GM18366 cells, in that many were enlarged with F-actin stress fibers (Figure 4E), and treatment of GM18366hTert cells with any of the p38 inhibitors corrected this (not shown). As well as correcting the morphology, p38 inhibitors increased the growth rate of GM18366hTert cells (Figure 4C), with VX-745 having the smallest effect and BIRB 796 the greatest effect, similar to that seen with primary GM18366 cells. This is compatible with the observation that p38 was still active in GM18366hTert cells (Figure 4F).
The levels of p16INK4A, p21WAF1, p53, caveolin-1, and p-caveolin-1 in GM18366hTert cells were all similar to that seen in low PD GM18366 cells (Figure 4F).
Discussion
Our data demonstrate that replicative senescence in ATR-deficient fibroblasts is qualitatively similar to that seen in normal human fibroblasts. Senescent cells feature irreversible growth arrest involving the upregulation of cell cycle inhibitors such as p21WAF1 and p16INK4A and have a characteristic enlarged and flattened morphology (42). Replicative senescence in NDFs is due to telomere shortening that activates the p53-signalling pathway (43). Replicative senescence in ATR-Seckel cells is p53 dependent, although p16INK4A and p21WAF1 levels were not elevated at M1 compared with low PD cells. This may be due to these CdkIs already being elevated in low PD ATR-Seckel cells with p16INK4A being elevated further at Mint (as is seen in NDFs). A further similarity is that replicative senescence in ATR-Seckel cells is telomere dependent; ectopic expression of human telomerase–enabled ATR-Seckel cells to bypass senescence and proliferate continuously.
However, despite the normal replicative senescence mechanism, ATR-Seckel cells had a reduced replicative capacity compared with NDFs, an observation that is novel to this work. Although the GM18366 replicative capacity was not significantly reduced compared with the three NDFs used here, it was significantly reduced (p < .036; z test) when the replicative capacity of a further five previously studied NDFs (8) that were grown under the same conditions were added to the data (Supplementary Table 1).
As with WS fibroblasts, many ATR-Seckel fibroblasts displayed features suggestive of activation of p38, such as an enlarged morphology with extensive F-actin stress fibers, and molecular profiling indeed revealed both activated p38 and phosphorylated HSP27 (a downstream target of p38). Furthermore, the replicative capacity of ATR-Seckel cells was significantly increased by treatment with p38 inhibitors, with the replicative capacity using BIRB 796 now within the range seen for normal fibroblasts, and the senescent (aged) morphology reverted to that seen in NDFs. The effectiveness of each inhibitor on replicative capacity and cellular morphology correlated with the degree to which the p38 pathway was inhibited, as assessed by the level of pHSP27. Overall this indicates that, like WS fibroblasts, ATR-Seckel cells undergo some degree of p38-dependent SIPS.
Molecular profiling provided further insights as to the potential mechanism whereby p38 activation leads to cell cycle arrest. The CdkIs p16INK4A and p21WAF1 were both upregulated in low PD ATR-Seckel cells. Although p38-dependent SIPS can be transduced by either CdkI (4,44,45), and p38 is known to activate p21WAF1 via direct phosphorylation, or by activation of p53 (46), p38 inhibition in ATR-Seckel cells reduces p16INK4A levels but not p21WAF1, suggesting that p38-dependent SIPS in ATR-Seckel cells is transduced, at least in part, via p16INK4A. Thus, the SIPS process in ATR-Seckel cells is similar to that seen in cells prematurely senesced by expression of oncogenic ras that results in upregulation of p16INK4A (44,47) and contrasts with WS where p38-dependent SIPS is transduced by p21WAF1(4).
Another pathway that appears to be differently implicated in senescence in WS and ATR-Seckel cells involves caveolin-1. This is the principal component of caveolae, which are 50–100nm flask-shaped invaginations of the cell membrane found in many cell types including fibroblasts (48). Caveolin-1 acts as a scaffolding protein to compartmentalize and functionally regulate signalling molecules within caveolar membranes (40). Caveolin-1 upregulation plays a key role in SIPS in MEFs and human chondrocytes under serum starvation, oxidative stress, or IL-1β treatment (48–50). SIPS can be prevented using siRNA caveolin-1 knockdown or MEFs from caveolin-1 null mice (51). Caveolin-1 is thought to activate the p53/p21WAF1 signalling pathway (48), and in turn it is regulated by p38 via both increased caveolin-1 protein and elevated phosphorylation (52). In ATR-Seckel cells, p-caveolin-1 levels were high, and this was reduced by treatment with p38 inhibitors, although elevated caveolin-1 protein was not observed. However, the observation that p21WAF1 levels were not reduced by p38 inhibition suggests that the caveolin-1 phosphorylation seen in ATR-Seckel cells does not induce senescence via p21WAF1. This contrasts with the situation in low PD WS cells, where caveolin-1, p-caveolin-1, and p21WAF1 are all regulated by p38.
In summary, our data support the hypothesis that the replication stress due to lack (or low levels) of ATR seen in ATR-Seckel cells results in SIPS via p38-dependent upregulation of p16INK4A and potentially via phosphorylated caveolin-1. Furthermore, this SIPS appears to be independent of telomere erosion, as immortalized GM18366hTert cells maintain a stressed phenotype showing p38 activation and levels of p16INK4A and p21WAF1 similar to that seen in low PD GM18366 cells. As replicative senescence in ATR-Seckel cells appears qualitatively normal, p38 activation and/or SIPS synergizes with the normal telomere-dependent senescence to yield the reduced replicative capacity seen in ATR-Seckel cells. This SIPS results in many young ATR-Seckel cells having an aged phenotype and molecular profile that resembles cells at M1, thus, ATR-Seckel cells undergo accelerated aging.
Overall, these results suggest a strong overlap in the cellular phenotype of WS and ATR-Seckel cells as related to senescence-related phenotypes. In both WS and ATR-Seckel fibroblasts, replicative senescence is telomere driven and p53 dependent, and they show high levels of activated p38 and SIPS (this work and [4,35]). A further similarity is that SIPS in both WS and ATR-Seckel cells is independent of telomeres but synergizes with telomere-dependent senescence to reduce the replicative capacity. As WRNp and ATR interact in a common signalling pathway, we hypothesize that both WS and ATR-Seckel fibroblasts undergo SIPS resulting from increased replication stress. This SIPS may lead to aspects of the whole-body phenotypes of both ATR-Seckel and WS such as growth retardation and premature aging due in part to a reduction in cellular division capacity and an accelerated rate of build up of senescent cells (3,10). The chronic activation of p38 may also contribute to accelerated aging and the disease predisposition spectrum of these patients—so-called inflamm-aging (53).
Although the similarities between the two syndromes are marked at the cellular level, an important question remains as to why, if ATR and WRNp share a common signalling pathway, there are so many nonoverlapping phenotypic symptoms? This may relate to ATR having a wider and more pivotal role in cell physiology; ATR is an essential protein, whereas WRNp is not. It would be surprising, therefore, for ATR and WS to yield identical phenotypes when mutated. A further complication is that ATR-Seckel individuals appear to have shorter lives than WS individuals (15,17), so perhaps have insufficient time to develop as dramatic a progeroid phenotype as seen in WS. Nevertheless, the multiple observations of replication stress–driven p38 activation in a subset of human progerias strengthen the potential relevance of this mechanism to human aging (4,8). Although ultimately ATR-Seckel and WS are “private” mechanisms of aging (insofar as they are driven by mutations not found in normal individuals), we would note that both pathways rapidly converge on a core signalling pathway that is subject to substantial regulation by cell intrinsic and extrinsic factors. This in turn raises the possibility that normal human aging might be affected, even if temporarily, by differential activation of the p38 pathway as a result of other activating circumstances. Finally, we would note that the accelerate cell aging phenotype of both ATR-Seckel and WS fibroblasts can be abrogated by small molecule drugs that target p38.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by grants from the Economic and Social Sciences Research Council New Dynamics of Aging Initiative (RES-356-25-0024), the Medical Research Council (PhD Studentship to H.S.E.T.) and the Cardiff Undergraduate Research Opportunities Program (CUROP). None of the funders played a role in study designs, collection, analysis or interpretation of data, or in manuscript preparation.
Conflict of Interest
There are no commercial affiliations or conflicts of interest to disclose.
Supplementary Material
References
- 1. Hofer AC, Tran RT, Aziz OZ, et al. Shared phenotypes among segmental progeroid syndromes suggest underlying pathways of aging. J Gerontol A Biol Sci Med Sci. 2005;60:10–20 [DOI] [PubMed] [Google Scholar]
- 2. Toussaint O, Dumont P, Remacle J, et al. Stress-induced premature senescence or stress-induced senescence-like phenotype: one in vivo reality, two possible definitions? ScientificWorldJournal. 2002;2:230–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kipling D, Davis T, Ostler EL, Faragher RG. What can progeroid syndromes tell us about human aging? Science. 2004;305:1426–1431 [DOI] [PubMed] [Google Scholar]
- 4. Davis T, Baird DM, Haughton MF, Jones CJ, Kipling D. Prevention of accelerated cell aging in Werner syndrome using a p38 mitogen-activated protein kinase inhibitor. J Gerontol A Biol Sci Med Sci. 2005;60:1386–1393 [DOI] [PubMed] [Google Scholar]
- 5. Davis T, Kipling D. Werner Syndrome as an example of inflamm-aging: possible therapeutic opportunities for a progeroid syndrome? Rejuvenation Res. 2006;9:402–407 [DOI] [PubMed] [Google Scholar]
- 6. Davis T, Kipling D. Assessing the role of stress signalling via p38 MAP kinase in the premature senescence of ataxia telangiectasia and Werner syndrome fibroblasts. Biogerontology. 2009;10:253–266 [DOI] [PubMed] [Google Scholar]
- 7. Tivey HSE, Brook AJC, Rokicki MJ, Kipling D, Davis T. p38 MAPK stress signalling in replicative senescence in fibroblasts from progeroid and genomic instability syndromes. Biogerontology. 2012.10.1007/s10522-012-9407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis T, Tivey HSE, Brook AJC, Grimstead JW, Rokicki MJ, Kipling D. Activation of p38 MAP kinase and stress signalling in fibroblasts from the progeroid Rothmund-Thomson syndrome. Age. 2012.10.1007/s11357-012-9476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodríguez-López AM, Jackson DA, Iborra F, Cox LS. Asymmetry of DNA replication fork progression in Werner’s syndrome. Aging Cell. 2002;1:30–39 [DOI] [PubMed] [Google Scholar]
- 10. Alderton GK, Joenje H, Varon R, Børglum AD, Jeggo PA, O’Driscoll M. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum Mol Genet. 2004;13:3127–3138 [DOI] [PubMed] [Google Scholar]
- 11. Seckel HPG. Bird Headed Dwarfs: Studies in Developmental Anthropology Including Human Proportions. Springfield, IL: Thomas, C.C.; 1960. [Google Scholar]
- 12. O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501 [DOI] [PubMed] [Google Scholar]
- 13. Murga M, Bunting S, Montaña MF, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shanske A, Caride DG, Menasse-Palmer L, Bogdanow A, Marion RW. Central nervous system anomalies in Seckel syndrome: report of a new family and review of the literature. Am J Med Genet. 1997;70:155–158 [DOI] [PubMed] [Google Scholar]
- 15. Rauch A. The shortest of the short: pericentrin mutations and beyond. Best Pract Res Clin Endocrinol Metab. 2011;25:125–130 [DOI] [PubMed] [Google Scholar]
- 16. O’Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair (Amst). 2004;3:1227–1235 [DOI] [PubMed] [Google Scholar]
- 17. Martin GM, Oshima J, Gray MD, Poot M. What geriatricians should know about the Werner syndrome. J Am Geriatr Soc. 1999;47:1136–1144 [DOI] [PubMed] [Google Scholar]
- 18. Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196 [DOI] [PubMed] [Google Scholar]
- 19. Ozeri-Galai E, Schwartz M, Rahat A, Kerem B. Interplay between ATM and ATR in the regulation of common fragile site stability. Oncogene. 2008;27:2109–2117 [DOI] [PubMed] [Google Scholar]
- 20. Casper AM, Nghiem P, Arlt MF, Glover TW. ATR regulates fragile site stability. Cell. 2002;111:779–789 [DOI] [PubMed] [Google Scholar]
- 21. Casper AM, Durkin SG, Arlt MF, Glover TW. Chromosomal instability at common fragile sites in Seckel syndrome. Am J Hum Genet. 2004;75:654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mokrani-Benhelli H, Gaillard L, Biasutto P, et al. Primary microcephaly, impaired dna replication, and genomic instability caused by compound heterozygous atr mutations. Hum Mutat. 2012;10.1002/humu.22245 [DOI] [PubMed] [Google Scholar]
- 23. Pirzio LM, Pichierri P, Bignami M, Franchitto A. Werner syndrome helicase activity is essential in maintaining fragile site stability. J Cell Biol. 2008;180:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Keefe LV, Richards RI. Common chromosomal fragile sites and cancer: focus on FRA16D. Cancer Lett. 2006;232:37–47 [DOI] [PubMed] [Google Scholar]
- 25. Baynton K, Otterlei M, Bjørås M, von Kobbe C, Bohr VA, Seeberg E. WRN interacts physically and functionally with the recombination mediator protein RAD52. J Biol Chem. 2003;278:36476–36486 [DOI] [PubMed] [Google Scholar]
- 26. Pichierri P, Franchitto A, Mosesso P, Palitti F. Werner’s syndrome protein is required for correct recovery after replication arrest and DNA damage induced in S-phase of cell cycle. Mol Biol Cell. 2001;12:2412–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakamoto S, Nishikawa K, Heo SJ, Goto M, Furuichi Y, Shimamoto A. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells. 2001;6:421–430 [DOI] [PubMed] [Google Scholar]
- 28. Pichierri P, Rosselli F, Franchitto A. Werner’s syndrome protein is phosphorylated in an ATR/ATM-dependent manner following replication arrest and DNA damage induced during the S phase of the cell cycle. Oncogene. 2003;22:1491–1500 [DOI] [PubMed] [Google Scholar]
- 29. Ammazzalorso F, Pirzio LM, Bignami M, Franchitto A, Pichierri P. ATR and ATM differently regulate WRN to prevent DSBs at stalled replication forks and promote replication fork recovery. EMBO J. 2010;29:3156–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNees CJ, Tejera AM, Martínez P, et al. ATR suppresses telomere fragility and recombination but is dispensable for elongation of short telomeres by telomerase. J Cell Biol. 2010;188:639–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stokes MP, Rush J, Macneill J, et al. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci USA. 2007;104:19855–19860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagley MC, Davis T, Dix MC, Widdowson CS, Kipling D. Microwave-assisted synthesis of N-pyrazole ureas and the p38alpha inhibitor BIRB 796 for study into accelerated cell ageing. Org Biomol Chem. 2006;4:4158–4164 [DOI] [PubMed] [Google Scholar]
- 33. Bagley MC, Davis T, Dix MC, et al. Gram-scale synthesis of the p38α MAPK-inhibitor VX-745 for preclinical studies into Werner syndrome. Future Med Chem. 2010;2:1417–1427 [DOI] [PubMed] [Google Scholar]
- 34. Bagley MC, Davis T, Rokicki MJ, Widdowson CS, Kipling D. Synthesis of the highly selective p38 MAPK inhibitor UR-13756 for possible therapeutic use in Werner syndrome. Future Med Chem. 2010;2:193–201 [DOI] [PubMed] [Google Scholar]
- 35. Davis T, Singhrao SK, Wyllie FS, et al. Telomere-based proliferative lifespan barriers in Werner-syndrome fibroblasts involve both p53-dependent and p53-independent mechanisms. J Cell Sci. 2003;116(Pt 7):1349–1357 [DOI] [PubMed] [Google Scholar]
- 36. Davis T, Haughton MF, Jones CJ, Kipling D. Prevention of accelerated cell aging in the Werner syndrome. Ann N Y Acad Sci. 2006;1067:243–247 [DOI] [PubMed] [Google Scholar]
- 37. Kuma Y, Sabio G, Bain J, Shpiro N, Márquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–19479 [DOI] [PubMed] [Google Scholar]
- 38. Shi Y, Kotlyarov A, Laabeta K, et al. Elimination of protein kinase MK5/PRAK activity by targeted homologous recombination. Mol Cell Biol. 2003;23:7732–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25:713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volonte D, Galbiati F. Caveolin-1, cellular senescence and pulmonary emphysema. Aging (Albany NY). 2009;1:831–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bond JA, Haughton MF, Rowson JM, et al. Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol Cell Biol. 1999;19:3103–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116 [DOI] [PubMed] [Google Scholar]
- 43. d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198 [DOI] [PubMed] [Google Scholar]
- 44. Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144 [DOI] [PubMed] [Google Scholar]
- 46. Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–29802 [DOI] [PubMed] [Google Scholar]
- 47. Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem. 2004;279:1050–1059 [DOI] [PubMed] [Google Scholar]
- 48. Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galbiati F, Volonté D, Liu J, et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001;12:2229–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–831 [DOI] [PubMed] [Google Scholar]
- 51. Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee SH, Lee YJ, Park SW, Kim HS, Han HJ. Caveolin-1 and integrin β1 regulate embryonic stem cell proliferation via p38 MAPK and FAK in high glucose. J Cell Physiol. 2011;226:1850–1859 [DOI] [PubMed] [Google Scholar]
- 53. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.