Abstract
Background.
We sought to examine whether frailty is associated with dementia, Alzheimer’s disease (AD), and non-AD dementia risk.
Methods.
This is a prospective population-based cohort derived from an integrated health maintenance organization. The sample consisted of 2,619 participants aged 65 and older without dementia at baseline followed from 1994 to 2010. Frailty was defined as having at least 3 of the following criteria: weakness (grip strength), slowness (walking speed), weight loss, low physical activity, and self-reported exhaustion. Follow-up occurred every 2 years to identify incident dementia, possible or probable AD, and non-AD dementia using standard research criteria. Covariates came from self-report and study measures. We used adjusted Cox proportional hazards models to examine the association between frailty and each outcome.
Results.
Over a mean follow-up of 6.5 years, 521 participants developed dementia (of which 448 developed AD). In the model adjusted for age, sex, education, and race, the hazard ratio for frailty was 1.78 (95% confidence interval [CI] 1.32–2.40). In the fully adjusted models, the hazard ratio for frailty was 1.20 for all-cause dementia (95% CI 0.85–1.69), 1.08 for AD (95% CI 0.74–1.57), and 2.57 for non-AD dementia (95% CI 1.08–6.11). For all-cause dementia, we found an interaction between baseline cognitive score and frailty (p = .02); hazard ratio for frailty was 1.78 for those with higher global cognition (95% CI 1.14–2.78) and 0.79 for those with lower global cognition (95% CI 0.50–1.26).
Conclusion.
Frailty was associated with dementia when adjusting only for demographic variables but not in the fully adjusted model. Frailty was associated with higher risk of developing non-AD dementia but not AD. Although frailty was not associated with all-cause dementia in the entire sample, an association did exist in participants with higher cognitive scores. Mechanisms underlying these associations remain to be elucidated.
Key Words: Dementia, Alzheimer’s disease, Frailty, Epidemiology
IN the care of older adults, frailty is a term often used to describe a general health status characterized by increased vulnerability to stressors because of impairment in physiological reserve. Over the past several years, researchers and clinicians have attempted to develop a standard and measurable conceptualization of the frailty syndrome. There is no standard definition for frailty (1), but generally accepted components include muscle weakness, fatigue, slowness, low physical activity, and unintended weight loss (2–6).
Identifying frailty in older adults is important as it may help clinicians recognize those who are at increased risk for several adverse health outcomes including death, hospitalization, and disability (7). Furthermore, frailty has been associated with an increased risk of poor cognitive outcomes such as Alzheimer’s disease (AD) and mild cognitive impairment and also with neuropathologic markers of AD. Much of this prior work has been done using the Rush Memory and Aging Project, a study conducted in 40 residential facilities (8–10). In contrast, Avila-Funes and colleagues (11) reported an association between frailty and dementia in participants who had baseline cognitive impairment but not in those with intact cognition. Thus, results of prior observational studies are inconsistent. We are able to extend this research by examining data from a large, representative sample of community-dwelling older adults characterized by extended follow-up and a large number of incident dementia cases. We used data from the Adult Changes in Thought (ACT) study to examine the association between frailty and incident dementia, AD, and non-AD dementia.
Methods
Participants
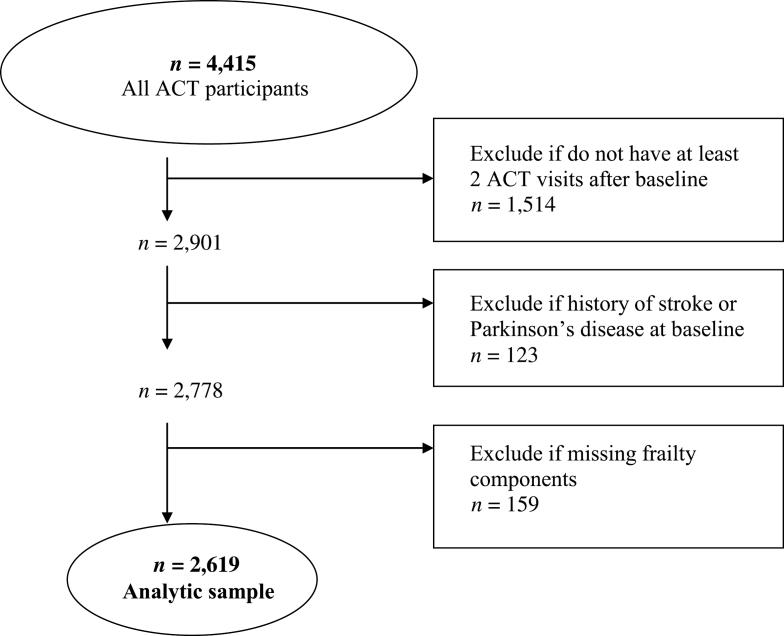
ACT is a population-based prospective cohort study of incident dementia and AD. ACT study methods have been described in detail elsewhere (12). Briefly, study participants, aged 65 years and older, were randomly sampled from Seattle-area members of Group Health, an integrated health care system in Washington state. The research protocol for this study was reviewed and approved by the Group Health and University of Washington institutional review boards. The original cohort of 2,581 participants without dementia was enrolled between 1994 and 1996. An additional 811 participants were enrolled between 2000 and 2002, and in 2004 the study began continuous enrollment (n = 1,023 as of September 30, 2010), to replace those who die or drop out from a total of 4,415 study participants. Figure 1 shows exclusion criteria for this analysis. Participants were required to have at least two biennial visits after the ACT enrollment visit for study inclusion. The first biennial follow-up visit was designated as the baseline year for this analysis so that we could define the weight loss component of frailty. Participants were excluded if they reported a history of stroke or Parkinson’s disease at baseline, or any of the components of the frailty measure were missing, yielding a cohort of 2,619 participants for analysis.
Figure 1.
Selection of study participants. ACT = Adult Changes in Thought study.
Ascertainment of Dementia and AD
At each ACT study visit, cognitive function was evaluated using the Cognitive Abilities Screening Instrument (CASI; 13). The CASI is a 40-item 100-point test of global cognitive functioning used in several population-based epidemiological studies. We used a cutoff value of 86 on the CASI to identify individuals for dementia evaluation because this score has a sensitivity and specificity of 96.5% and 92.0%, respectively (14). Participants whose CASI scores were less than 86 underwent a standardized dementia diagnostic evaluation, including a physical and neurological examination by a study neurologist, geriatrician, or internist and a 1-hour battery of neuropsychological testing. The neuropsychological testing included the clock drawing (15), verbal fluency (16), Mattis Dementia Rating Scale (17), Boston naming (16), verbal paired associations and recall, logical memory and recall (17), Word List Memory (16), Constructional Praxis and recall (16), Trails A and B (18), and Information and Comprehension subtest items (19). Relevant laboratory tests and brain CT or MRI studies were performed or results were obtained from Group Health records. Diagnoses were assigned at consensus diagnostic conferences using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for dementia (20) and criteria of the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association for AD (21). Frailty status was not considered at the consensus conference. Participants with new-onset dementia underwent at least one annual follow-up examination for verification of dementia status and subtype. The date of onset was assigned by convention as the midpoint between the ACT study visit that triggered a positive dementia evaluation and the preceding study visit. Accounting for interval censoring of dementia onset by using the midpoint of the interval in which onset occurred is robust to bias in time-to-event analyses (22,23). We examined three outcomes in these analyses: all-cause dementia, possible or probable AD, and non-AD dementia. The latter category consisted of all dementias not classified as possible or probable AD.
Frailty Definition
We defined frailty at baseline using modified criteria developed by Fried and colleagues (2). The components of frailty include weakness (grip strength), slowness (walking speed), low physical activity, weight loss, and self-reported exhaustion (Table 1). For each component, a participant was classified as positive for the component or not. When possible, we used the cut-points defined by Fried and colleagues (eg, grip strength, exhaustion).
Table 1.
Definition and Prevalence of Frailty Components (n = 2,619)
| Frailty Components | Frail, n (%) |
|---|---|
| Weakness (grip strength) | 789 (30.1) |
| Men | |
| Strength ≤ 29.0 for BMI ≤ 24.0 | |
| Strength ≤ 30.0 for BMI 24.1–26.0 | |
| Strength ≤ 30.0 for BMI 26.1–28.0 | |
| Strength ≤ 32.0 for BMI > 28.0 | |
| Women | |
| Strength ≤ 17.0 for BMI ≤ 23.0 | |
| Strength ≤ 17.3 for BMI 23.1–26.0 | |
| Strength ≤ 18.0 for BMI 26.1–29.0 | |
| Strength ≤ 21.0 for BMI > 29.0 | |
| Slowness (walking speed) | 241 (9.2) |
| Walking speed < 0.6 m/s | |
| Low physical activity* | 870 (33.2) |
| Exercised fewer than 3 times per week | |
| Weight loss | 197 (7.5) |
| Weight loss of ≥7.5% of body weight since prior study visit | |
| Exhaustion | 405 (15.5) |
| Self-reported positive response to one of two questions from the CESD scale: “I felt that everything I did was an effort”; “I could not get going.” |
Notes: BMI = body mass index; CESD = Center for Epidemiological Studies Depression.
*Participants were asked the number of days per week they did each of the following activities for at least 15min at a time: walking, hiking, bicycling, aerobics, swimming, water aerobics, weight training, stretching, or other exercise. Frequency of exercise per week was defined by summing across these activities.
Grip strength was assessed using a handheld dynamometer and measured to the nearest 0.1kg. Participants were asked to use their dominant hand to grip the handle with maximal effort for three attempts; the average of these three attempts was used. Weakness was defined using cut-points stratified by sex and body mass index. Walking speed was assessed by having participants walk a 10-foot distance at their usual speed using assistive devices if needed. Each participant was timed for two walks and the average time was used. Slow walking speed was defined as less than 0.6 m/s (24). Participants were asked about the number of days per week they did each of the following activities for at least 15 minutes at a time during the past year: walking, hiking, bicycling, aerobics or calisthenics, swimming, water aerobics, weight training or stretching, or other exercise. Participants were categorized as having low physical activity if they self-reported exercising fewer than three times per week (25). Weight loss was defined as losing more than 7.5% of body weight since the previous ACT visit. Exhaustion was assessed using two items from the 10-item Center for Epidemiological Studies Depression (CESD) scale (26): (a) “I felt that everything I did was an effort” and (b) “I could not get going.” Participants were categorized as positive for the exhaustion component if they answered yes to either of these two items. Participants were classified as frail (three or more components), prefrail (one or two components), or not frail (0 components).
Potential Confounders
At study baseline and each follow-up visit, information was collected on demographic factors, self-reported medical history, and health characteristics. Demographic factors included age, sex, race, and years of education. Participants were asked whether a physician had ever told them that they had congestive heart failure, hypertension, diabetes mellitus, or myocardial infarction. Health characteristics included smoking (past, never, current), body mass index calculated from measured weight and height, and self-rated health (excellent or very good, good, fair or poor). Potential depression was determined by presence of depressive symptoms (CESD summary score ≥ 10 vs < 10) and evidence of a prescription for an antidepressant in the 1 year prior to baseline.
Statistical Analyses
Descriptive analyses were stratified by baseline frailty status and presented as counts and percentages for categorical variables. Age-adjusted incidence rates of dementia, AD, and non-AD dementia per 1,000 person-years were calculated according to frailty status by the direct method of standardization, using the age distribution of the full cohort as the standard population. To analyze the association between frailty and risk of dementia, we estimated hazard ratios (HR) using Cox proportional hazards regression models with participant age as the time scale (27) and the event time taken to be the age at dementia onset. Individuals not diagnosed with dementia were censored when they were diagnosed with incident stroke or Parkinson’s disease, at the time of their last ACT study visit, or at time of disenrollment from Group Health, whichever was earliest. In addition, for the outcome of AD, individuals were censored at the time of non-AD dementia diagnosis. Similarly, for the outcome of non-AD dementia, individuals were censored at the time of AD diagnosis. Model 1 was adjusted for age at baseline, sex, education, and race. Model 2 was further adjusted for body mass index, depressive symptoms, antidepressant use (in year prior to baseline), self-rated health, hypertension, diabetes, myocardial infarction, congestive heart failure, and smoking status. Model 3 was further adjusted for baseline CASI score to examine further potential confounding by global cognitive functioning. We assessed proportional hazards assumption by testing for an interaction between frailty status and age. The assumption was satisfied for all models. We examined effect modification by sex, depression, and baseline CASI (< 92 and ≥ 92). This CASI threshold corresponds to the lowest quartile of CASI scores. In exploratory analyses, we examined the association between the individual frailty components and each outcome. All analyses were performed using Stata version 10 (StataCorp, College Station, TX).
Results
Table 2 provides baseline characteristics of the 2,619 eligible ACT participants according to baseline frailty status. The mean age at baseline was 76.8 (standard deviation [SD] 5.9) years, approximately 60% were female, and more than 60% had at least some college education. At baseline, 213 (8.1%) participants were frail. On average, frail participants were older, more likely to be female, to be obese, to have depressive symptoms, to rate their health as fair or poor, and to report several chronic health conditions.
Table 2.
Baseline Characteristics of the Cohort, Overall and by Baseline Frailty Status*,†
| Baseline Frailty | ||||
|---|---|---|---|---|
| Total (n = 2,619) | Not Frail (n = 1,021) | Prefrail (n = 1,385) | Frail (n = 213) | |
| Age, mean (SD) | 76.8 (5.9) | 75.2 (5.1) | 77.4 (5.8) | 80.6 (7.0) |
| Age, y | ||||
| <70 | 271 (10.4) | 158 (15.5) | 103 (7.4) | 10 (4.7) |
| 70–74 | 888 (33.9) | 403 (39.5) | 440 (31.8) | 45 (21.1) |
| 75–79 | 737 (28.1) | 273 (26.7) | 417 (30.1) | 47 (22.1) |
| 80–84 | 450 (17.2) | 132 (12.9) | 264 (19.1) | 54 (25.4) |
| 85+ | 273 (10.4) | 55 (5.4) | 161 (11.6) | 57 (26.8) |
| Female | 1,573 (60.1) | 566 (55.4) | 837 (60.4) | 170 (79.8) |
| White | 2,404 (91.8) | 941 (92.2) | 1,269 (91.6) | 194 (91.1) |
| Education | ||||
| Less than high school | 321 (12.3) | 101 (9.9) | 183 (13.2) | 37 (17.5) |
| Completed high school | 611 (23.4) | 210 (20.6) | 341 (24.6) | 60 (28.3) |
| At least some college | 1685 (64.3) | 709 (69.4) | 861 (62.2) | 115 (54.0) |
| Body mass index, mean (SD) | 27.2 (5.7) | 26.5 (4.3) | 27.3 (4.9) | 29.4 (12.1) |
| Body mass index | ||||
| <25 | 914 (34.9) | 382 (37.4) | 475 (34.4) | 57 (26.8) |
| 25–30 | 1,089 (41.6) | 458 (44.9) | 557 (40.3) | 74 (34.7) |
| 30+ | 613 (23.4) | 181 (17.7) | 350 (25.3) | 82 (38.5) |
| Smoking status | ||||
| Never | 1,276 (48.8) | 487 (47.8) | 664 (48.0) | 125 (58.7) |
| Former | 1,215 (46.5) | 498 (48.9) | 641 (46.4) | 76 (35.7) |
| Current | 124 (4.7) | 34 (3.3) | 78 (5.6) | 12 (5.6) |
| Depressive symptoms (CESD ≥ 10) | 216 (8.3) | 24 (2.4) | 134 (9.7) | 58 (27.2) |
| Antidepressant use in past year | 397 (15.2) | 120 (11.8) | 211 (15.2) | 66 (31.0) |
| Self-reported health status | ||||
| Excellent or very good | 1,343 (51.3) | 649 (63.6) | 657 (47.4) | 37 (17.4) |
| Good | 925 (35.3) | 311 (30.5) | 530 (38.3) | 84 (39.4) |
| Fair or poor | 350 (13.4) | 60 (5.9) | 198 (14.3) | 92 (43.2) |
| Hypertension | 1,182 (45.3) | 406 (39.9) | 652 (47.2) | 124 (59.3) |
| Diabetes | 281 (10.7) | 82 (8.0) | 162 (11.7) | 37 (17.4) |
| Myocardial infarction | 271 (10.4) | 97 (9.5) | 144 (10.4) | 30 (14.2) |
| Congestive heart failure | 121 (4.6) | 33 (3.2) | 65 (4.7) | 23 (10.9) |
| Any apolipoprotein Eε4 allele | 604 (25.5) | 234 (25.3) | 317 (25.4) | 53 (27.6) |
| CASI score | ||||
| 25th percentile | 92 | 93 | 91.9 | 89.7 |
| Median | 95 | 95.5 | 94.5 | 92.5 |
| 75th percentile | 97 | 97.4 | 97 | 96 |
Notes: CASI = Cognitive Abilities Screening Instrument; CESD scale = Center for Epidemiological Studies Depression scale; SD = standard deviation.
*Results presented as n (%) unless otherwise noted.
†Missing values: education (2), body mass index (3), smoking status (4), depressive symptoms (3), self-reported health (1), hypertension (11), diabetes (1), myocardial infarction (10), congestive heart failure (11), apolipoprotein Eε4 allele (253), and CASI (28).
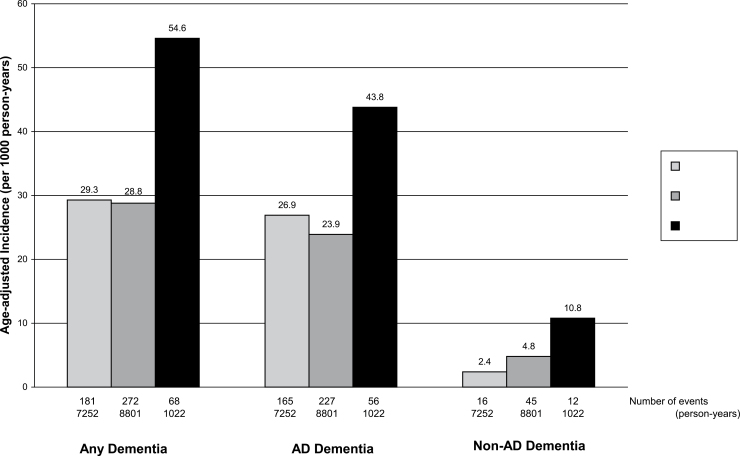
During the 17,075 person-years of follow-up (mean 6.5, SD 3.9 years), 521 cases of dementia were diagnosed, of which 448 were cases of probable or possible AD and 73 were cases of non-AD dementia. Age-adjusted incidence rates of these outcomes were considerably higher for those who were frail at baseline (Figure 2). The age-adjusted incidence of any dementia was 54.6 per 1,000 person-years for those who were frail at baseline and 29.3 per 1,000 person-years for those who were not frail.
Figure 2.
Age-adjusted rates of dementia per 1,000 person-years according to baseline frailty.
In the model adjusted for age, sex, education, and race, frailty was associated with an increased risk of dementia (Table 3; Model 1: adjusted HR 1.78, 95% confidence interval [CI] 1.32–2.40). The association was attenuated when adjusting for additional health status covariates (Model 2: HR 1.40, 95% CI 1.00–1.96) and further when adjusting for baseline cognitive score (Model 3: HR 1.20, 95% CI 0.85–1.69). In comparing the results of Model 3 with Model 2, further adjustment for baseline cognitive score attenuated the strength of the relationship between frailty and all dementia outcomes. The multivariate-adjusted HR for frailty was 1.08 (95% CI 0.74–1.57) for AD and 2.57 (95% CI 1.08–6.11) for non-AD dementia.
Table 3.
Risk of Incident Dementia, Alzheimer Disease (AD), and Non-AD Dementia Associated With Frailty
| Outcome | Model 1, HR (95% CI)* | Model 2, HR (95% CI)† | Model 3, HR (95% CI)‡ |
|---|---|---|---|
| Dementia | |||
| Not frail | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Prefrail | 0.98 (0.81–1.18) | 0.90 (0.74–1.09) | 0.84 (0.68–1.02) |
| Frail | 1.78 (1.32–2.40) | 1.40 (1.00–1.96) | 1.20 (0.85–1.69) |
| Possible or probable AD | |||
| Not frail | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Prefrail | 0.89 (0.72–1.09) | 0.82 (0.67–1.02) | 0.76 (0.62–0.95) |
| Frail | 1.55 (1.12–2.14) | 1.26 (0.87–1.82) | 1.08 (0.74–1.57) |
| Non-AD dementia | |||
| Not frail | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Prefrail | 1.94 (1.08–3.46) | 1.65 (0.91–3.00) | 1.59 (0.87–2.89) |
| Frail | 4.46 (2.03–9.80) | 2.90 (1.22–6.86) | 2.57 (1.08–6.11) |
Notes: CI = confidence interval; HR = hazard ratio.
*From Cox proportional hazards regression model with age as the time scale, adjusted for age at baseline, sex, education, and race.
†Further adjusted for body mass index, depressive symptoms, antidepressant use, self-reported health, hypertension, diabetes, myocardial infarction, congestive heart failure, and smoking status.
‡Further adjusted for baseline Cognitive Abilities Screening Instrument (CASI) score.
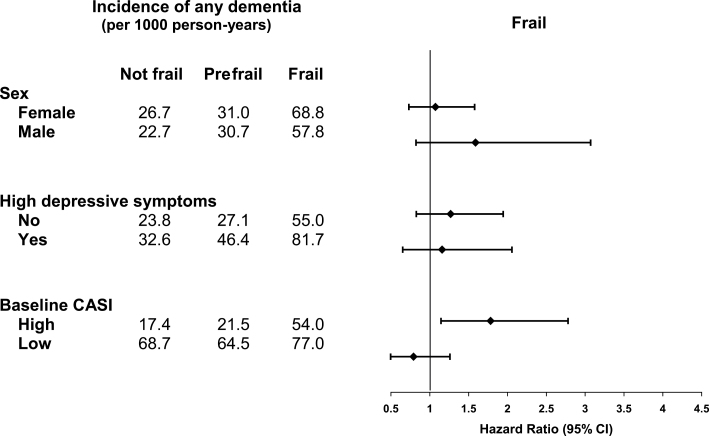
The relationship between baseline frailty and dementia did not differ according to sex or depression status (Figure 3) but did differ by baseline global cognition. The association between frailty and dementia was stronger for those with higher baseline cognitive scores (HR 1.78, 95% CI 1.14–2.78) than for those with lower cognitive scores (HR 0.79, 95% CI 0.50–1.26; p value for interaction = .02).
Figure 3.
Subgroup analysis of risk of dementia associated with frailty. Results are from Cox proportional hazards regression model with age as the time scale, adjusted for age at baseline, sex, education, race, body mass index, depressive symptoms, antidepressant use, self-reported health, hypertension, diabetes, myocardial infarction, congestive heart failure, and smoking status and baseline Cognitive Abilities Screening Instrument (CASI) score.
We conducted exploratory analyses to examine the association between the individual frailty components and each outcome as a way to understand our primary findings (Table 4). The individual components were not significantly related to risk for dementia or AD, however, the HRs for slow walking speed were slightly increased (dementia: HR 1.27; AD: HR 1.16). Slow walking speed was the only component significantly related to an increased risk for non-AD dementia (HR 2.13, 95% CI 1.09–4.16), whereas the adjusted HR for low grip strength was 1.28 and for exhaustion was 1.77.
Table 4.
Risk of Incident Dementia, Alzheimer Disease (AD), and Non-AD Dementia Associated With the Individual Frailty Components*
| Frailty Components | Dementia, HR (95% CI) | Possible or Probable AD, HR (95% CI) | Non-AD Dementia, HR (95% CI) |
|---|---|---|---|
| Weakness (grip strength) | 1.06 (0.87–1.29) | 1.04 (0.84–1.28) | 1.28 (0.77–2.11) |
| Slowness (walking speed) | 1.27 (0.96–1.69) | 1.16 (0.85–1.59) | 2.13 (1.09–4.16) |
| Low physical activity | 0.97 (0.80–1.17) | 0.95 (0.77–1.17) | 1.03 (0.62–1.69) |
| Weight loss | 0.88 (0.62–1.24) | 0.92 (0.63–1.33) | 0.72 (0.28–1.84) |
| Exhaustion | 1.08 (0.82–1.40) | 0.96 (0.72–1.30) | 1.77 (0.95–3.31) |
Notes: CI = confidence interval; HR = hazard ratio.
*From Cox proportional hazards regression model with age as the time scale, adjusted for age at baseline, sex, education, race, body mass index, depressive symptoms, antidepressant use, self-reported health, hypertension, diabetes, myocardial infarction, congestive heart failure, and smoking status and baseline Cognitive Abilities Screening Instrument (CASI) score.
Discussion
In this population-based study of older adults, frailty was not associated with all-cause dementia or AD but was associated with a 2.57-fold increased risk for non-AD dementia. Baseline global cognitive functioning was an important confounder and reduced the magnitude of associations between frailty and the three dementia outcomes.
An unexpected finding of this study was that the association between frailty and dementia varied according to baseline cognitive scores. This is the first study to our knowledge that has examined the association between frailty and incident dementia stratified by baseline cognitive scores. Frailty was associated with increased risk for dementia only for those in the upper three quartiles of global cognitive functioning. The explanation for this finding is unclear. A prior study found that frailty was associated with dementia in those with cognitive impairment (11). Thus, we had expected the increased risk to be evident in participants with lower cognitive scores rather than the higher cognitive functioning group because these individuals were closer to the threshold for cognitive impairment that triggered the evaluation for dementia. Perhaps those in the lower quartile of cognitive scores, even though considered within the “normal” range of cognition for this study, may include participants who had mild cognitive impairment or impending dementia that may overshadow any association that frailty might have with incident dementia. In previous work in the ACT cohort, separate components of the frailty construct (eg, lower performance on walking speed and grip strength) were related to increased risk for dementia even in those who had scores in the lower quartile of the CASI; thus, an interaction was not found by cognitive status when examining these individual performance measures (28).
We found that frailty was associated with non-AD dementia. Among the components of frailty, only slow walking speed was significantly related to non-AD dementia. However, risk was also nonsignificantly increased for exhaustion and low grip strength. The HR for the association between frailty and non-AD dementia was greater than that between walking speed and non-AD dementia, suggesting that the frailty and non-AD dementia relationship was not determined solely by walking speed but that multiple elements of the frailty phenotype contributed to this relationship. This outcome includes several types of dementia not meeting the possible or probable AD definition. In our cohort, 42% (31 of 73) of those with non-AD dementia had vascular dementia. Because non-AD dementia includes several dementia types and the overall event rate is low, this finding requires confirmation in a larger sample where vascular dementia events could be examined separately. Our results are supported by a study conducted in France, where frailty was associated with a 2.73-fold increase in vascular dementia (5).
Our findings are consistent with two longitudinal studies (5,11) but not with the other (10). Avila-Funes and colleagues (11) also did not find an association between frailty and risk of dementia in participants with intact cognition at baseline in a large French sample followed for 4 years. In contrast, Buchman and colleagues (10) reported that in a sample of 823 older adults followed for 3 years, increasing levels of frailty were associated with increased risk for incident AD. When these investigators used a categorical measure of frailty, similar to the frailty definition we used, frailty was associated with a twofold increase risk for AD after adjusting for age, sex, and education. Of note, we also found an association between frailty and AD in our minimally adjusted model including similar variables (HR 1.55, 95% CI 1.12–2.14), which was attenuated considerably in the fully adjusted model with several additional covariates including baseline cognition (HR 1.08, 95% CI 0.74–1.57). Thus, baseline cognition, even within the range that is considered intact should be considered as an important confounder in future research in this area. Slow walking speed as defined for the frailty phenotype was not related to overall dementia or AD. Other investigators have found an association with walking speed and dementia outcomes; however, different characterizations of walking speed were used (continuous, quartiles) and may explain the different findings (28,29).
Disentangling the relationship between physical frailty and impaired cognition is complex and the directional relationship between these two syndromes is not known. There is information to support the notion that frailty may precede dementia (8) and for the notion that impaired cognition (mild) may precede the development of frailty (30). Some have proposed that impaired cognition and physical frailty co-occur because of shared pathophysiology and risk factors. Experts have even suggested adding cognition to the frailty phenotype to improve the ability to predict poor health outcomes (1,11,31).
This study had several strengths, including a large, representative sample of community-dwelling older adults with extended follow-up and a large number of incident dementia cases. A few limitations should also be noted. The study population was predominantly white and well educated, which may limit generalizability. A standard definition of frailty does not exist, however, we have used a phenotype that has been well accepted by the research community (4). We were able to adjust for a number of important potential confounders. However, residual confounding is a concern with all epidemiological studies. The low number of cases of vascular dementia precluded further delineation of the findings related to the non-AD dementia outcome.
In conclusion, we found an association between frailty and non-AD dementia but not with overall dementia or AD. Frailty was associated with dementia when adjusting only for demographic variables but not in the fully adjusted model underscoring the importance of considering confounders. Frailty was associated with dementia in the subgroup with higher cognitive scores at baseline. The findings from this article illustrate the importance of adjusting for baseline cognition in studies examining the association between frailty and dementia outcomes. Future research in other samples is needed to explore the association with frailty and dementia subtypes.
Funding
This work was supported by National Institutes of Health (NIH) grant U01-AG-06781.
References
- 1. Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an Operational Definition of Frailty: a Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J Gerontol A Biol Sci Med Sci. 2012; 68: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 3. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005; 53: 1321–1330 [DOI] [PubMed] [Google Scholar]
- 4. Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011; 59: 2129–2138 [DOI] [PubMed] [Google Scholar]
- 5. Avila-Funes JA, Carcaillon L, Helmer C, et al. Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J Am Geriatr Soc. 2012; 60: 1708–1712 [DOI] [PubMed] [Google Scholar]
- 6. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012; 67: 1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med. 2010; 26: 275–286 [DOI] [PubMed] [Google Scholar]
- 8. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010; 58: 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer’s disease pathology. Neurology. 2008; 71: 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007; 69: 483–489 [DOI] [PubMed] [Google Scholar]
- 11. Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009; 57: 453–461 [DOI] [PubMed] [Google Scholar]
- 12. Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002; 59: 1737–1746 [DOI] [PubMed] [Google Scholar]
- 13. Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994; 6: 45–58; discussion 62 [DOI] [PubMed] [Google Scholar]
- 14. Graves AB, Teng EL, Larson EB, White LR. Education in cross-cultural dementia screening: applications of a new instrument. Neuroepidemiology. 1992; 11: 8 [Google Scholar]
- 15. Spreen O. Compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford UP; 1991; [Google Scholar]
- 16. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989; 39: 1159–1165 [DOI] [PubMed] [Google Scholar]
- 17. Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 18. Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 19. Weschler D. WMS-R: Weschler Memory Scale-Revised manual. New York: Psychological Corporation/HBJ; 1987. [Google Scholar]
- 20. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 1994: 886 [Google Scholar]
- 21. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984; 34: 939–944 [DOI] [PubMed] [Google Scholar]
- 22. Lindsey JC, Ryan LM. Tutorial in biostatistics methods for interval-censored data. Stat Med. 1998; 17: 219–238 [DOI] [PubMed] [Google Scholar]
- 23. Pan W, Chappell R. Estimation in the Cox proportional hazards model with left-truncated and interval-censored data. Biometrics. 2002; 58: 64–70 [DOI] [PubMed] [Google Scholar]
- 24. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003; 51: 314–322 [DOI] [PubMed] [Google Scholar]
- 25. Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006; 144: 73–81 [DOI] [PubMed] [Google Scholar]
- 26. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1: 385–401 [Google Scholar]
- 27. Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997; 145: 72–80 [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006; 166: 1115–1120 [DOI] [PubMed] [Google Scholar]
- 29. Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci. 2012; 67: 425–432 [DOI] [PubMed] [Google Scholar]
- 30. Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010; 65: 1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004; 59: 1310–1317 [DOI] [PubMed] [Google Scholar]