Abstract
Disorders of the gastrointestinal tract are common in the elderly people; however, the precise trait(s) of aging that contribute to the vulnerability of the gastrointestinal tract are poorly understood. Recent evidence suggests that patients with gastrointestinal disorders have increased intestinal permeability. Here, we address the hypothesis that disruption of the intestinal barrier is associated with aging. Our results demonstrated that permeability was significantly higher in colonic biopsies collected from old baboons compared with young baboons. Additionally, colonic tissue from the older animals had decreased zonula occluden-1, occludin, and junctional adhesion molecule-A tight junction protein expression and increased claudin-2 expression. Upregulation of miR-29a and inflammatory cytokines IFN-γ, IL-6, and IL-1β was also found in colonic biopsies from old baboons relative to young baboons. These results show for the first time that a pivotal contributing factor to geriatric vulnerability to gastrointestinal dysfunction may be increased colonic permeability via age-associated remodeling of intestinal epithelial tight junction proteins.
Key Words: Aging, Permeability, Tight junctions, MicroRNA, Cytokines
Aging significantly increases the vulnerability to gastrointestinal (GI) disorders with approximately 35%–40% of geriatric patients reporting at least one GI complaint during routine physical examination (1). Despite the need to further understand age-associated factors that increase the susceptibility to GI dysfunction, there is a paucity of studies investigating the key factors in aging that affect the GI tract. Thus, far studies in rodents have demonstrated that aging alters intestinal smooth muscle contractility (2), as well as the neural innervations of the GI tract musculature (3). Several studies in rodents have also reported an increase in intestinal permeability to macromolecules with age (4–7). Specifically, advancing age was shown to correlate with an enhanced trans- epithelial permeability of d-mannitol, indicating that there may be an age-associated decline in barrier function (8).
The gut barrier protects the body from potentially harmful compounds and microorganisms while permitting the absorption of nutrients, electrolytes, and water. A loss of gut barrier integrity can lead to increased intestinal permeability allowing for the passage of noxious substances across the intestinal mucosa (9–11). A key factor contributing to abnormalities in permeability is aberrant structuring of tight junction proteins that support gut barrier function (12). Tight junction proteins link adjacent epithelial cells, regulating the paracellular movement of substances across the small and large intestinal lumen. However, the efficiency of tight junctions in controlling paracellular transport is dependent on the composition of the tight junction proteins associated in the junction, which include various combinations of zonula occludens (ZOs), occludins, claudins, and junctional adhesion molecules (JAMs). Substrates that are known to induce aberrant structuring of tight junction proteins include enhanced expression of microRNAs (miRNAs) (13,14), decreased circulating concentrations of glutamine (15), and induction of inflammatory cytokines (16,17).
The overall goal of the current study was to investigate the role of aging on colonic permeability. The colon was specifically selected as the region of interest due to the technical feasibility and because many GI disorders associated with aging are predominantly colonic, that is, constipation, chronic diarrhea, and incontinence. To this end, we hypothesized that there is an increase in permeability with age that can be attributed, at least in part, to a structural decline in tight junction proteins. To test our hypothesis, we designed a comparative study to investigate the differences in permeability between colonic mucosal biopsies from young and old nonhuman primates, which have virtually identical rates and patterns of aging to humans (18).
Materials and Methods
Animals
Colonic mucosal biopsies were isolated from baboons (Papio anubis) housed at the University of Oklahoma Health Science Center’s Department of Comparative Medicine Annex, Oklahoma City, Oklahoma, which is an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. The animal studies were approved by the University of Oklahoma Health Science Center Institutional Animal Care and Use Committees and were performed in accordance with the Guide for the Care and Use of Laboratory Animals and National Research Council guidelines. All baboons were free from GI pathologies, and a full report of the pathologies of the baboon has been reviewed (19). Baboons aged 4–10 years were considered “young” baboons, whereas baboons aged 18 and older were considered “old” in this study. A total of 10 old and 10 young baboons were used for this study.
Collection of Mucosal Biopsies
Baboons were sedated with an intermuscular injection of ketamine 10 mg/kg, intubated and maintained under anesthesia with 1.25%–1.5% isoflurane in O2. During the biopsy collection, physiological parameters were monitored using a noninvasive blood pressure cuff, pulse oximeter, and visual observation of respiration. The depth of the anesthesia was confirmed by the absence of corneal reflex. To collect the biopsies, a colonoscope was inserted into the rectum of the baboon and mucosal biopsies (8mm) were obtained from the transverse colon at a distance of 60–70cm from the rectum using standard endoscopic forceps. Biopsy specimens were immediately placed in an iced oxygenated Krebs solution (pH 7.2–7.4; 120mM NaCl, 6mM KCl, 1.2mM MgCl2, 1.2mM NaH2PO4, 2.5mM CaCl2, 14.4mM NaHCO3, and 11.5mM of glucose), paraformaldehyde (4%), or flash frozen on dry ice. The colonoscope was removed at the end of the procedure, and the baboons were allowed to recover from anesthesia while being continually monitored by a veterinarian.
From each baboon, a total of eight colonic biopsies were collected. Immediately following collection, four out of the eight biopsies were mounted in individual Ussing chambers for permeability experiments. From the remaining four colonic biopsies, one was processed for routine histology and another for the assessment of myeloperoxidase (MPO) activity. Out of the remaining two biopsies, one was placed in 4% formaldehyde, paraffin embedded, and sectioned for tight junction histology, and the other was flash frozen in dry ice and stored at −80°C for extraction of RNA and protein.
Histology and MPO Activity
Formalin-fixed biopsies were paraffin embedded and sectioned at 10 μm. Hematoxylin and eosin staining was performed by Precision Histology (Oklahoma City, OK). A pathologist blind to the experiment performed examination of the histology for morphological characterization, using the validated Chiu score system for gut pathology (20). MPO activity was assessed using EnzChek MPO Activity Assay Kit (Life Technologies, Carlsbad, CA). Colonic biopsies were homogenized in five volumes of phosphate-buffered saline followed by three cycles of freeze and thawing and by sonication for 10 seconds. After 15 minutes of centrifugation at 14,000g at 4°C, the supernatant was collected and Amplex UltraRed reagent (phosphate-buffered saline, 2% dimethyl sulfoxide, and 0.005% of H2O2) was added for peroxidation detection. The fluorescent intensity of each sample was detected using an excitation of 530nm and emission at 590nm using a fluorescent plate reader. Each value was converted into MPO values by using curves obtained from a standard sample of human MPO provided in the kit.
Assessment of Permeability in Colonic Biopsies
Four biopsies were mounted in a modified Ussing chambers with an exposed tissue area of 2.0mm2 and bathed with 10 mL of Krebs buffer (pH 7.2–7.4; 120mM NaCl, 6mM KCl, 1.2mM MgCl2, 1.2mM NaH2PO4, 2.5mM CaCl2, 14.4mM NaHCO3, and 11.5mM of glucose). The Krebs solution was continuously stirred and oxygenated with a steady gas flow of 95% O2 and 5% CO2 and maintained at 37°C with a heating block system. Two pairs of agar–salt bridge electrodes were connected to an automatic voltage clamp apparatus (ECV4000, World Precision Instruments, Sarasota, FL) to monitor transmucosal potential difference and short-circuit current (I sc). Transmucosal resistance was calculated using Ohm’s law from the open-circuit potential difference and the I sc. The bathing solution on the mucosal side was then replaced with Krebs containing horseradish peroxidase (HRP type II; ~44kDa protein antigen). Two samples of 250 µL were withdrawn from the submucosal side of the chambers every 20 minutes for 100 minutes (t 0–t 5) and replaced with fresh Krebs buffer to maintain equal hydrostatic pressure. HRP concentration from the submucosal samples was quantified by spectrophotometer analysis of the reaction of HRP with 3,3′,5,5′-tetramethyl- benzidine liquid substrate. After 10-minute reaction period, a stop solution (0.1mM H2SO4) was added to the sample extracted from the submucosal chamber. The absorbance of the colorimetric reaction product was measured at wavelength 450nm and concentration was determined using a standard curve. HRP flux was expressed as quantity of HRP/20 min/2mm2 (Ussing chamber aperture) and recorded as pgHRP/min/mm2.
Expression of Tight Junction Proteins
A colonic biopsy from each baboon was placed in 4% formaldehyde, paraffin embedded, and sectioned. Sections were then deparaffinized in xylene and rehydrated with gradient ethanol dips. Antigen retrieval was performed using proteinase K digestion (20 µg/mL) in Tris–ethylenediaminetetraacetic acid buffer (50nM Tris, 1mM ethylenediaminetetraacetic acid, 0.5% Triton X-100, pH 8.0) for 10 minutes at 37°C and allowed to cool to room temperature and rinsed in Tris-buffered saline (TBS). Sections were then blocked with ImageIT signal enhancer (Life Technologies) for 30 minutes at room temperature. Sections were washed with TBS and incubated overnight with dual antibodies of either mouse anti-ZO-1 antibodies conjugated to Alexa Fluor 488 and rabbit anti-occludin or mouse anti-JAM-A and rabbit anti-claudin-2 (Life Technologies) at room temperature. Slides with no primary antibodies were used as negative controls. The following day, slides were rinsed in TBS and incubated in Alexa Fluor 546–conjugated anti-rabbit antibodies and Alexa Fluor 488–conjugated anti-mouse antibodies (Life Technologies) for 2 hours at room temperature. The slides were then rinsed again and cover slipped with ProLong Gold Anti-Fade solution (Life Technologies). Slides were imaged using a Zeiss LSM 510 confocal microscope at the Oklahoma Medical Research Foundation Imaging Core (Oklahoma City, OK). The negative control slides were used to calibrate the confocal microscope. Two randomly selected sections of 480,000 pixels at 40× magnification out of a total of six sections processed from each biopsy were analyzed with ImageJ software (NIH) for optical density (21). The densities were then averaged into a single n value used for statistical calculation.
Western Blot Analysis
Colonic tissue samples were homogenized in 20 volumes of lysis buffer (50mM Tris–HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150mM NaCl; 1 mM ethylenediaminetetraacetic acid; 1mM phenylmethylsulfonyl fluoride; 1mM Na3VO4; 1 mM NaF) containing a protease inhibitor cocktail (104mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 0.08mM aprotinin, 2.1mM leupeptin, 3.6mM bestatin, 1.5mM pepstatin A, and 1.4mM E-64; Sigma, St Louis, MO). Samples were then set on ice for 30 minutes and centrifuged at 14,000g for 20 minutes. The supernatants were collected and the protein concentration was determined by Bradford assay (Millipore, Billerica, MA). Approximately 30 μg of protein was separated on a 4%–20% gradient polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to a nitrocellulose membrane (Millipore). The membranes were blocked with 3% blocking solution in TBS-T for 1 hour. Blots were then incubated for 2 hours with the same antibodies used for immunohistochemistry in addition to anti-glyceraldehyde 3-phosphate dehydrogenase and anti-β-actin (Life Technologies) for normalization. Following incubation, the blots were washed in three changes of TBS-T and incubated for 1 hour with secondary anti-rabbit or anti-mouse antibodies (Millipore). Following three more washes in TBS-T, bands were visualized with ECL Western Blot Detection Kit (Amersham, Piscataway, NJ) and imaged using an Omega 12iC chemiluminescent imager (UltraLum, Claremont, CA).
Quantitative Reverse Transcription–Polymerase Chain Reaction
Whole tissue RNA and miRNA were extracted using RNeasy Mini Kit and miRNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Concentration of total RNA was determined by spectrophotometry (λ = 260nm). Extraction was followed by cDNA synthesis using RT2 First Strand cDNA Kit for mRNA and miScript II RT Kit for miRNA and qPCR using SYBR Green qPCR Mastermix or miScript SYBR Green in a total reaction volume of 25 μL (Qiagen). Samples were run in triplicates and “no template” conditions served as a negative control. The housekeeping gene ubiquitin-C was used to normalize mRNA samples and RNU-6B was used to normalize miRNA samples. Additionally, mRNA samples were normalized to the housekeeping gene 18S to validate results. The housekeeping gene β-actin was also used but found to be inconsistent between old and young baboon samples. The miRNA samples were also normalized to housekeeping genes SNORD-25 and SCARN-A7 to verify the results. The validated cytokine primers, 18S primers, and β-actin primers summarized in Table 1 were adapted from conserved sequences from multiple species (22,23) and acquired from Invitrogen (Carlsbad, CA). Human glutamine synthase (GLUL), ubiquitin-C, and miRNA Primer Assays were purchased from Qiagen. The reaction was performed on an Applied Biosystems StepOnePlus Real-Time PCR System Thermal Cycling Block (Life Technologies) with the initial denaturation at 95°C 15 minutes. Subsequent denaturations were at 94°C for 10 seconds, annealing at 60.0°C for 30 seconds, and extension at 72°C for 30 seconds, for a total of 40 cycles, and a final extension at 72°C for 10 minutes. Melting curves were performed at the end of each run from 72°C to 95°C for 90-second intervals and showed only a single peak for each gene analyzed. Calculation of the gene expression was performed as previously described (24). Relative quantity of mRNA from each sample was calculated as the difference in C(T) for target minus C(T) for housekeeping rRNA [ΔC(T)], and calibrated to C(T) of young control sample [ΔΔC(T)]. Fold change in transcription is expressed as 2[− ΔΔC(T)].
Table 1.
Baboon Primers
| Gene | Sense Primer | Antisense Primer | Product Size |
|---|---|---|---|
| IL-1β | TGTACGATCACTGAACTGCA | GAAGTCAGTTATATCCTGGC | 423 |
| IL-2 | AACTGGAGCATTTACTGCTG | GTGTTGAGATGATGCTTCGAC | 357 |
| IL-6a | ATGAAGTTCCTCTCTGCAAGAG | CACTAGGTTTGCCGAGTAGAT | 421 |
| IL-10 | AAACCACAAGACAGACTT | GATTTTGGAGACCTCTAATTTA | 427 |
| IL-12a | AGCAGCTGGTCATCTCTTGGTT | CCAGCATCTCCAAACTCTTTGA | 559 |
| TNF-α | TCTTCACTGGAAAGGACACCA | GAAGGAGAAGAGGCTGAGGAA | 540 |
| IFN-γ | TGCAGAGCCAAATTGTCTCCTTTTAC | GGGATGCTCTTCGACCTCGAAA | 453 |
| 18S | TCAAGACAAGTCGGAGG | GGACATCTAAGGGCATCACA | 489 |
| β-Actin | CTACAATGAGCTGCGTGTGG | AAGGAAGGCTGGAAGAGTGC | 528 |
Statistical Analysis
Data are represented as the mean ± SEM. Data from all Ussing chambers were averaged for each animal into a single n value. Means from old and young baboons were compared using a Student’s unpaired t test using GraphPad Prism Software (version 5.2; La Jolla, CA), and significance was defined as p < .05.
Results
The Effect of Aging on Colonic Mucosal Histology and MPO Activity
The histological analysis revealed no marked differences between the mucosal biopsies from young and old baboons (Figure 1A and B). Specifically, a review by a pathologist blind to the study indicated that there was no evidence of increased fibrosis, crypt atrophy, or acute inflammation in the aged samples when compared with the young samples. To further substantiate the histological observations, total MPO activity was measured to biochemically quantify levels of neutrophil infiltration (Figure 1C). Our results showed no difference in MPO activity between young and old baboons (p < .05).
Figure 1.
Hematoxylin and eosin staining revealed no histological differences between colonic biopsies from young and old baboons (A). Review by a pathologist blind to the study confirmed that there was no evidence of increased fibrosis, crypt atrophy, or acute inflammation in the aged samples when compared with the young samples. Quantification of myeloperoxidase (MPO) activity within the colonic biopsies showed no difference between young or old baboons (B). Statistical significance was *p > .05 by Student’s unpaired t test.
The Effects of Aging on Colonic Permeability
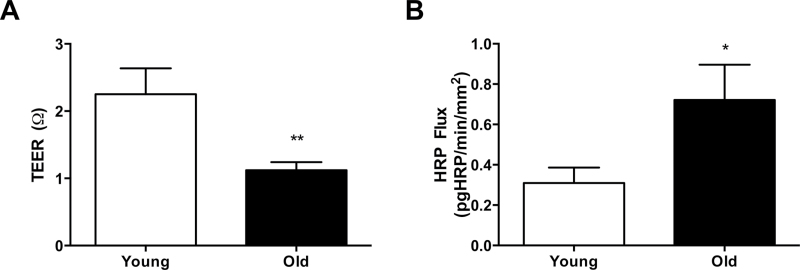
Transepithelial electrical resistance, an electrophysiological measure of the ionic gradient across the colonic mucosal biopsy, was recorded and summarized in Figure 2A. Overall, there was a lower transepithelial electrical resistance in old baboon colonic biopsies versus young baboon colonic biopsies (p < .01), suggesting an abnormality in the ability of the tissue from aged animals to maintain an ionic gradient. To further corroborate the electrophysiological finding, we assessed epithelial barrier function by quantifying the amount of HRP that traversed the epithelial barrier from the mucosal to the submucosal chamber. As illustrated in Figure 2B, we found that there was a greater HRP flux in colonic biopsies from old baboons compared with young baboons (p < .05) indicating an increase in colonic permeability to macromolecules in the tissue from aged animals.
Figure 2.
Aging increases colonic permeability. A reduction in transepithelial electrical resistance (TEER; A) and an increase in horseradish peroxidase (HRP) flux (B) was apparent in biopsies from old baboons compared with young baboons. Statistical significance was *p < .05 by Student’s unpaired t test.
The Effects of Aging on Tight Junction Protein Expression in Colonic Biopsies
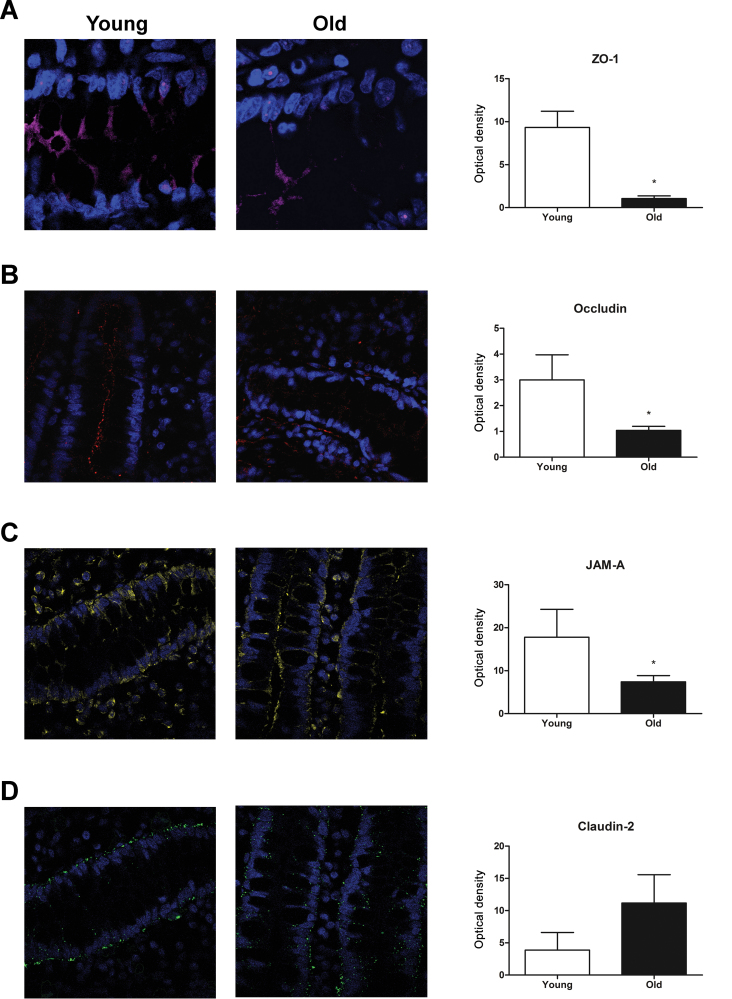
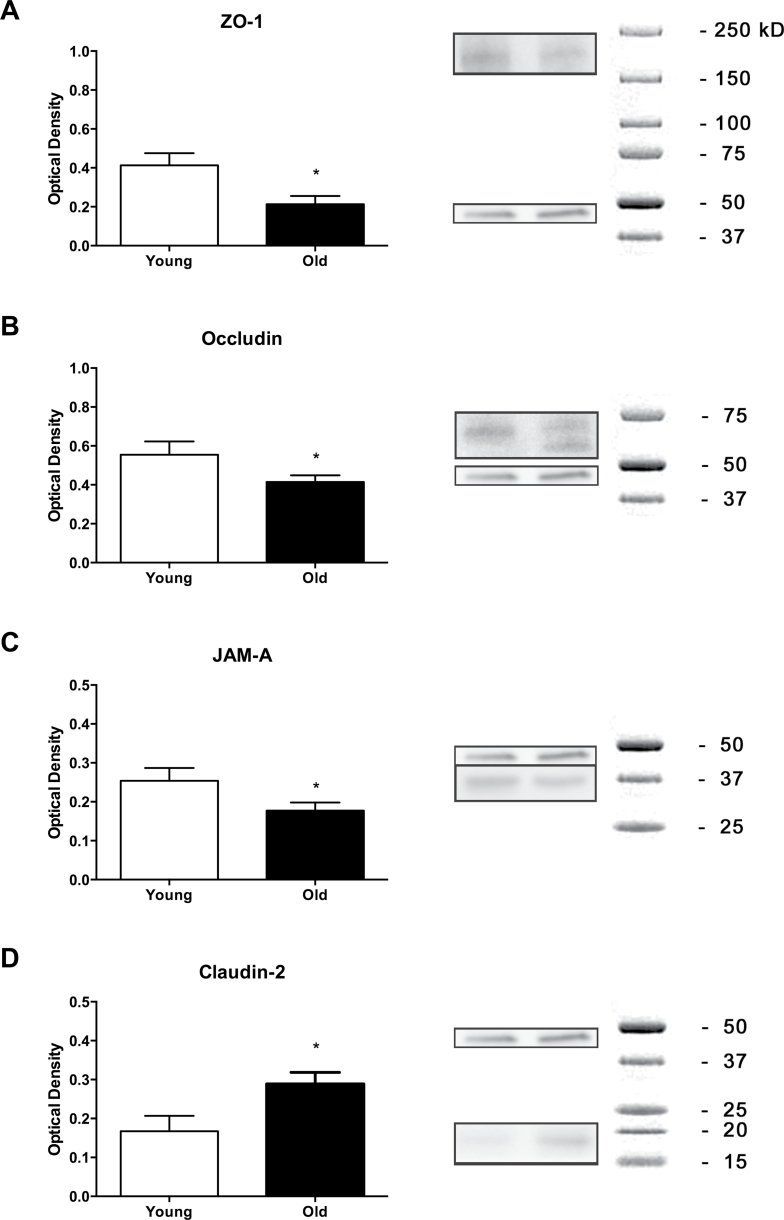
In the current study, four tight junction proteins were assessed: ZO-1, occludin, JAM-A, and claudin-2, all of which have been shown to play an important role in intestinal barrier function. Immunofluorescence was used to visualize the structure of the tight junctions in the epithelium of the colonic biopsies, and the fluorescent optical density was quantified (Figure 3). We found that aging decreased ZO-1, occludin, and JAM-A tight junction protein expression in the epithelium (p < .05). Although there was a trend toward an increase in claudin-2 protein expression, the changes were not statistically significant (p = .09). Western blot analysis was used to quantify the total expression levels in the colonic biopsies for each tight junction protein (Figure 4). In comparison with young baboon colonic biopsies, the old baboon biopsies exhibited significant decreases in ZO-1, occludin, and JAM-A (p < .05), in contrast to a significant increases in claudin-2 expression (p < .05).
Figure 3.
Aging restructures colonic epithelial tight junctions. Confocal micrographs of cross-sections imaged from colonic biopsies are shown for young (left) and old (middle) baboons in addition to a quantification of the fluorescent optical densities of the micrographs (right). Overall, there was a significant decrease in zonula occluden-1 (ZO-1; A), occludin (B), and junctional adhesion molecule (JAM)-A immunofluorescence (C). Although there was a trend toward increased claudin-2 expression, the change was not statistically significant (D). Statistical significance was *p < .05 by Student’s unpaired t test.
Figure 4.
Aging alters expression of tight junctions. Western blot was used to quantify total expression levels of tight junction proteins. Quantification of the optical densities is shown on the left panel with representative blots on the right. Detection by chemiluminescence revealed positive bands for the tight junction proteins zonula occluden-1 (ZO-1) at ~225kDa (A), occludin at ~65kDa (B), junctional adhesion molecule (JAM)-A at ~36kDa (C), and claudin-2 at ~22kDa (D). Glyceraldehyde 3-phosphate dehydrogenase (~36kDa) was used to normalize ZO-1, occludin, and claudin-2 optical densities, and β-actin (~42kDa) was used to normalize JAM-A. There was a significant decrease in ZO-1, occludin, and JAM-A expression in contrast to a significant increase in claudin-2 protein expression. Statistical significance is *p < .05 by Student’s unpaired t test.
The Effect of Aging on miRNAs and GLUL Expression in Colonic Biopsies
Previous studies have shown that tight junction proteins can be regulated by miRNAs (13–15) and glutamine (15,25). In the current study, miRNAs were measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and included miR-29a, miR-212, and miR-122a (Figure 5A). Overall, miR-29a was significantly increased in old baboon colonic tissue (p < .05), but no changes in miR-212 or miR-122a were observed with age (p > .05). GLUL mRNA expression was also measured by qRT–PCR and shown in Figure 5B. Our results showed no differences in GLUL expression between old and young colonic samples (p > .05).
Figure 5.
MicroRNA and glutamine synthase expression in colonic biopsies. With age there was a significant increase in miR-29a but not miR-122a or miR-212 expression within the colonic biopsies (A). There was no significant difference in GLUL expression in the same samples (B). Statistical significance was *p < .05 by Student’s unpaired t test.
The Effect of Aging on Cytokine Production in the Colonic Biopsies
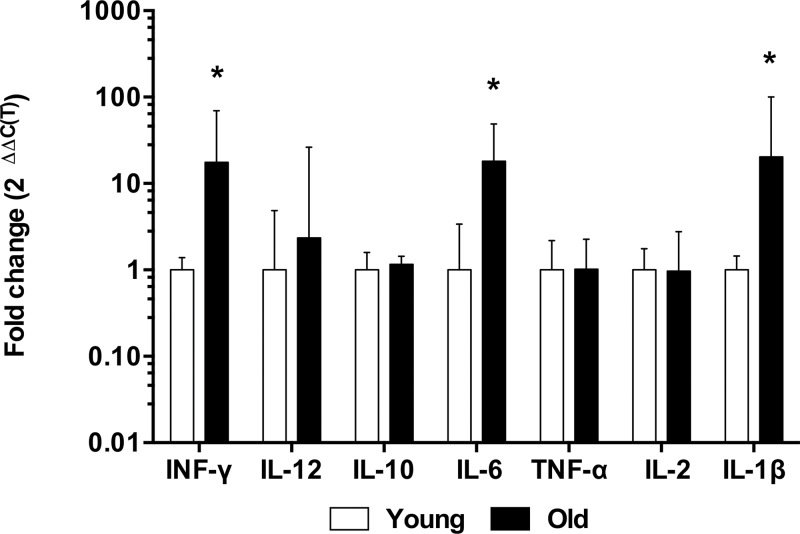
Inflammatory cytokines modulate intestinal permeability through regulation of tight junction protein expression and trafficking (16,17); thus, we measured cytokine expression by qRT–PCR in the colonic mucosal biopsies (Figure 6), which is the optimal method of detecting cytokine levels in isolated tissue (26). Because mRNA levels are more stable than protein, quantifying mRNA also eliminates confounding factors such as the short half-life of cytokines. Previous studies have also shown excellent correlation between cytokine mRNA levels quantified by qRT–PCR and protein levels quantified by enzyme-linked immunosorbant assays (27,28). Compared with young baboons, we found IFN-γ, IL-6, and IL-1β to be significantly increased (p < .05) in old baboon colonic biopsies without changes in IL-12 or TNF-α (p > .05). Anti-inflammatory cytokines IL-10 and IL-2 were undetectable by our methods.
Figure 6.
From the cytokine panel, there was a significant increase in IFN-γ, IL-6, and IL-1β expression in aged colonic tissue compared with young colonic tissue. There was also a trend toward increased IL-12 production that was not statistically significant. Statistical significance was *p < .05 by Student’s unpaired t test.
Discussion
The overarching goal of the current study was to delineate the effects of aging on intestinal barrier function. We found that epithelial permeability was greater in colonic biopsies isolated from older baboons. Supporting this observation, we discovered that there is significant tight junction remodeling including a decrease in ZO-1, occludin, and JAM-A proteins, and an increase in claudin-2 expression in old baboon colon compared with young baboon colon. Upon investigation of the potential mechanisms that may be responsible for age-associated changes in tight junction protein expression, we found an increase in miR-29a but no observable differences in GLUL expression. We also measured an elevated level of proinflammatory cytokines, in the absence of overt inflammation as assessed via routine histology and MPO activity, supporting the claim that inflammatory cytokines modulate intestinal permeability through regulation of tight junction protein expression and trafficking (16,17).
A growing body of evidence suggests that digestive disorders such as constipation, diarrhea, diverticular disease, and celiac disease may be the result of abnormal intestinal permeability due to compromised gut barrier function (9–11). Although elderly people often report abnormalities in bowel function, the etiology and pathophysiology are not well understood. To our knowledge, there is limited information on the effects of aging on the epithelial barrier; however, ischemic changes and an increase in the use of nonsteroidal anti-inflammatory drugs are thought to contribute to impaired epithelial barrier in the duodenum of elderly patients (29). Several studies in rodents have indicated an increase in overall absorption of macromolecules with age (4–7), as well as specifically in the colon (8), confirming that there may be an age-associated increase in intestinal permeability, at least in a rodent model. In the current study, we advanced these earlier observations by demonstrating in a nonhuman primate model that aging significantly increases colonic permeability. Specifically, in colonic biopsies, we found a decrease in transepithelial electrical resistance across the colonic epithelium in a nonhuman primate model implying that the intestinal barrier from aged animals is less effective at separating ionic charge, most likely due to increased mucosal permeability. Further substantiating these electrophysiological findings, we then went on to show that there was an increase in HRP flux across the epithelial membrane in old baboon colonic mucosal tissue compared with young baboon colonic mucosal tissue. Our experiments focused primarily on paracellular permeability of the colonic tissue, but it is important to note that transcellular movement also influences the overall permeability of the colon. The colonic epithelium contains ion channels, pumps, and carriers that actively transport vitamins, minerals, and electrolytes, and their functional capacity varies with ontogeny (30,31). Although there are limited studies evaluating transcellular mechanisms in the aging colon, investigations in the small intestine indicate that there is an overall decline in active absorption with age including carrier-mediated transport (32,33), and sodium-dependent transport of amino acids (34) and sugars (35).
Many studies have reported that the permeability of the intestinal epithelial barrier is dependent on a network of protein strands made up of ZOs, occludins, claudins, and JAMs. In the current study, we quantified tight junction proteins expression and, despite the lower resolution in the colonic biopsies when compared with whole intestinal mounts or cultured cells, we were able to visualize age-associated restructuring of the junctions. Specifically, our results indicated a significant decrease in ZO-1, occludin, and JAM-A in colonic biopsies from old baboons, indicating a disruption of tight junction barrier function. Whereas ZO-1, occludin, and JAM-A function in adhering the tight junctions together, claudin-2 creates “leaky” pores through the tight junctions (36). In the current study, although we found via Western blot analysis that claudin-2 levels were increased suggestive of enhanced gut permeability (36), this increase in claudin-2 expression was not confirmed via immunofluorescence. The discrepancy in the data remains to be resolved, but we postulate that the further activation of age-independent mechanisms may be required to induce insertion of claudin-2 proteins into the epithelial membrane. In summary, our results show that a pivotal factor contributing to the enhanced permeability in colonic biopsies from old baboons is senescence-induced remodeling of tight junction proteins, and taken together, these findings extend the previous studies in rodents, which demonstrated an increased intestinal permeability with age.
The mechanisms underlying these potential age-associated remodeling of tight junctions remain to be determined. However, recent evidence suggests that miRNAs are involved in regulating gut permeability via a decrease in the expression of tight junction proteins in response to specific environmental factors. For example, alcohol exposure increases gut “leakiness” by downregulating ZO-1 proteins though a mechanism involving increased miR-212 expression (37). Moreover, TNF-α has been shown to enhance intestinal permeability by inducing miR-122-mediated degradation of occludin mRNA (14). Our study showed a significant upregulation of miR-29a in colonic mucosal tissue from aged animals with enhanced gut permeability, which is supported by a previous study that found an intrinsic upregulation in miR-29a in normal aged mice when compared with young mice (38) and another study showing that miR-29a was increased in irritable bowel syndrome patients with enhanced gut permeability (15).
There is also evidence that suggests a role for glutamine in the regulation of gut permeability. Glutamine supplementation reverses abnormal paracellular permeability induced by nutrient deprivation in caco-2 cells (39) and in clinical experiments involving elderly patients (40). GLUL catalyzes the endogenous production of glutamine, and therefore changes in permeability may result from dysfunction in GLUL expression, as reported in irritable bowel syndrome patients with enhanced gut permeability (15). We therefore examined the expression of GLUL in our colonic biopsies taken from young and old baboons. In our study, there was no difference in intestinal GLUL expression in tissue taken from aged baboons compared with young, suggesting normal glutamine production.
Immune system–gut interactions, in particular cytokine expression in the epithelium, were examined as a potential mechanism for the enhanced permeability of the mucosal barrier observed in aged animals. Specific cytokines produced by the intraepithelial leukocytes, such as eosinophils, mast cells, macrophages, and TCRγδ-positive T cells (41,42), have been documented to regulate tight junction proteins (16,17). In support of neuroimmune-mediated mechanism accounting for the increase in gut permeability observed in response to aging, we found significant increases in IFN-γ, IL-6, and IL-β in colonic biopsies from aged animals. In other studies, IFN-γ exposure has been shown to (i) decrease epithelial resistance while increasing epithelial permeability (43) and (ii) induce endocytosis of tight junction proteins including occludin and JAM-A (44,45). Systemic IFN-γ has also been shown to increase with age in human plasma without the presence of inflammation or infection (46), supporting our data and implicating IFN-γ as an important factor in age-related changes in gut permeability. IL-6 has been shown to decrease the expression of ZO-1 in the intestine of mice (47) and increase claudin-2 expression (48). Furthermore, previous studies demonstrate that there is a significant increase in human and rhesus monkey plasma IL-6 with age without inflammation or infection, which supports the data in this study identifying IL-6 as an important mediator of age-associated increase in intestinal permeability (49). IL-1β is also a proinflammatory cytokine that causes an increase in intestinal epithelial permeability (50) and has been shown to increase with age in human plasma (51). Remodeling of tight junction proteins in response to IL-1β includes the downregulation of occludin (52), increased claudin-2 expression and hyperphosphor- ylation of occludin (53,54). Interestingly, not only does our data show decreased occludin and increased claudin-2 but we also show a second occludin band in our Western blot experiments in old baboon colonic tissue, which may represent differential phosphorylation states of occludin. Endogenous changes in cytokine production as a function of age can be caused by a number of factors including remodeling of the gut microbiome. In the colon, studies have shown that the microenvironment changes drastically with age (55,56), which can significantly affect local immunity (57). Although primary studies in humans have implicated a remodeling of the gut microbiota in facilitating a proinflammatory state in the elderly people (58), future studies in nonhuman primate models, beyond the scope of the current study, could more definitively determine the relationship between immunosenescence in the gut and the microbiome.
Other cytokines examined in the current study include IL-12 and TNF-α, which were previously demonstrated to affect tight junction proteins but were unchanged in our aging baboon model. Furthermore, we were unable to detect the anti-inflammatory cytokines IL-10 and IL-2, which function to decrease permeability. The inability to detect IL-10 and IL-2 may be because these cytokines are not constitutively present and expressed only upon activation such as in the case of IL-10 (59) or alternatively may be expressed in cells located in Peyer’s patches, which are deeper in the lamina propria and may not have been captured by the biopsies. It is important to note in the interpretation of our data that the increases in cytokines expression measured in the current study occurred in the absence of any inflammation within the colonic biopsies, which had normal histological appearance and levels of MPO activity resembling that in biopsies from younger animals. Moreover, because the cytokine levels measured were much lower than profiles from inflamed tissue (60), we can assume that the changes in cytokine expression are due to intrinsic changes in cytokine expression with age and not due to extrinsic induction of inflammation and immune infiltration.
In conclusion, our data in a nonhuman primate model suggest that normal aging increases intestinal permeability in colonic biopsies through the restructuring of tight junction proteins. Moreover, we have shown that via a mechanism involving increased miR-29a expression and/or enhanced inflammatory cytokine expression with age, there is increased intestinal permeability that may enhance the susceptibility to age-related GI disorders.
Funding
This work was supported by the National Center for Research Resources/National Institute of Health (P40RR012317).
Acknowledgments
The authors would like to acknowledge Gary L. White, DVM, MMS, for providing funding and Roman F. Wolf, DVM, for assistance in collecting the colonic biopsies. The authors would also like to acknowledge the Oklahoma Medical Research Foundation Imaging Core for their assistance and use of their confocal microscope, and Stan Lightfoot, MD, and Megan Lerner for the preparation and examination of the colonic histology.
References
- 1. Hall KE, Proctor DD, Fisher L, Rose S. American gastroenterological association future trends committee report: effects of aging of the population on gastroenterology practice, education, and research. Gastroenterology. 2005;129:1305–1338.10.1053/j.gastro.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 2. O’Mahony D, O’Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527 [DOI] [PubMed] [Google Scholar]
- 3. Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19.10.1016/j.autneu.2007.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollander D, Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology. 1985;31:133–137 [DOI] [PubMed] [Google Scholar]
- 5. Katz D, Hollander D, Said HM, Dadufalza V. Aging-associated increase in intestinal permeability to polyethylene glycol 900. Dig Dis Sci. 1987;32:285–288 [DOI] [PubMed] [Google Scholar]
- 6. Ma TY, Hollander D, Dadufalza V, Krugliak P. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol. 1992;27:321–333 [DOI] [PubMed] [Google Scholar]
- 7. Annaert P, Brouwers J, Bijnens A, Lammert F, Tack J, Augustijns P. Ex vivo permeability experiments in excised rat intestinal tissue and in vitro solubility measurements in aspirated human intestinal fluids support age-dependent oral drug absorption. Eur J Pharm Sci. 2010;39:15–22.10.1016/j.ejps.2009.10.005 10.1016/j.ejps.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 8. Mullin JM, Valenzano MC, Verrecchio JJ, Kothari R. Age- and diet-related increase in transepithelial colon permeability of Fischer 344 rats. Dig Dis Sci. 2002;47:2262–2270 [DOI] [PubMed] [Google Scholar]
- 9. Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416 [DOI] [PubMed] [Google Scholar]
- 10. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809.10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- 11. Camilleri M, Spiller R, Madsen K, Greenwood-Van Meerveld B, Verne N. Gut permeability in health and disease: an update. Neurogastroenterol Motil. 2012;24:503–512.10.111/j.1365–2982.01921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep. 2008;10:443–449 [DOI] [PubMed] [Google Scholar]
- 13. Vicario M, Martínez C, Santos J. Role of microRNA in IBS with increased gut permeability. Gut. 2010;59:710–712.10.1136/gut.2009.203695 [DOI] [PubMed] [Google Scholar]
- 14. Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333.10.1053/j.gastro.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784.10.1136/gut.2009.181834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871.10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci U S A. 2002;99:9591–9595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosanke SD. Pathology of baboons. In: ACVPand ASVCP, eds. 56th Annual Meeting of the American College of Veterinary Pathologists (ACVP) and 40th Annual Meeting of the American Society for Veterinary Clinical Pathology (ASVCP); Boston, MA Ithaca, NY: International Veterinary Information Service; (www.ivis.org); 2005:Document No. P2225.1205 [Google Scholar]
- 20. Quaedackers JS, Beuk RJ, Bennet L, et al. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proc. 2000;32:1307–1310 [DOI] [PubMed] [Google Scholar]
- 21. Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22:1093–1100.10.1111/j.1365-2826.2010.02051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morales-Montor J, Mohamed F, Baghdadi A, Baig S, Hallal-Calleros C, Damian RT. Expression of mRNA for interleukin-1beta, interleukin-6, tumor necrosis factor-alpha and macrophage migration inhibitory factor in HPA-axis tissues in Schistosoma mansoni-infected baboons (Papio cynocephalus). Int J Parasitol. 2003;33:1515–1524 [DOI] [PubMed] [Google Scholar]
- 23. Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology. 2004;145:959–966.10.1210/en.2003-1078 [DOI] [PubMed] [Google Scholar]
- 24. Tran L, Keele NB. P-chlorophenylalanine increases glutamate receptor 1 transcription in rat amygdala. Neuroreport. 2011;22:758–761.10.1097/WNR.0b013e32834ae2a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr. 2009;139:710–714.10.3945/jn.108.101485 10.3945/jn.108.101485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43 [PMC free article] [PubMed] [Google Scholar]
- 27. Hein J, Schellenberg U, Bein G, Hackstein H. Quantification of murine IFN-gamma mRNA and protein expression: impact of real-time kinetic RT-PCR using SYBR green I dye. Scand J Immunol. 2001;54:285–291 [DOI] [PubMed] [Google Scholar]
- 28. Blaschke V, Reich K, Blaschke S, Zipprich S, Neumann C. Rapid quantitation of proinflammatory and chemoattractant cytokine expression in small tissue samples and monocyte-derived dendritic cells: validation of a new real-time RT-PCR technology. J Immunol Methods. 2000;246:79–90 [DOI] [PubMed] [Google Scholar]
- 29. Meier J, Sturm A. The intestinal epithelial barrier: does it become impaired with age? Dig Dis. 2009;27:240–245.10.1159/000228556 [DOI] [PubMed] [Google Scholar]
- 30. Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289.10.1152/physrev.00026.2001 [DOI] [PubMed] [Google Scholar]
- 31. Pácha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633–1667 [DOI] [PubMed] [Google Scholar]
- 32. Yuasa H, Soga N, Kimura Y, Watanabe J. Effect of aging on the intestinal transport of hydrophilic drugs in the rat small intestine. Biol Pharm Bull. 1997;20:1188–1192 [DOI] [PubMed] [Google Scholar]
- 33. Barada KA, Atallah JB, Nassar CF. Age influence on intestinal taurine transport in mice. Comp Biochem Physiol A Physiol. 1997;118:159–163 [DOI] [PubMed] [Google Scholar]
- 34. Huang J, Nakajima T, Naito S. Age-related changes of intestinal absorption of α-aminoisobutyric acid, tyrosine and tyr-D-ala-gly in rats. Age. 1985;8:64–68.10.1007/BF02432073 [Google Scholar]
- 35. Freeman HJ, Quamme GA. Age-related changes in sodium-dependent glucose transport in rat small intestine. Am J Physiol. 1986;251(2 Pt 1):G208–G217 [DOI] [PubMed] [Google Scholar]
- 36. Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72.10.1136/gut.2006.094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364.10.1111/j.1530-0277.2007.00584.x [DOI] [PubMed] [Google Scholar]
- 38. Ugalde AP, Ramsay AJ, de la Rosa J, et al. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J. 2011;30:2219–2232.10.1038/emboj.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Bacquer O, Laboisse C, Darmaun D. Glutamine preserves protein synthesis and paracellular permeability in Caco-2 cells submitted to “luminal fasting.” Am J Physiol Gastrointest Liver Physiol. 2003;285:G128–G136.10.1152/ajpgi.00459.2002 [DOI] [PubMed] [Google Scholar]
- 40. Zhu M, Tang D, Zhao X, et al. Impact of glutamine of gut permeability and clinical prognosis on the aging patients undergoing gastric-intestinal operation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2000;22:425–427 [PubMed] [Google Scholar]
- 41. Austin LL, Dobbins WO., III Intraepithelial leukocytes of the intestinal mucosa in normal man and in Whipple’s disease: a light- and electron-microscopic study. Dig Dis Sci. 1982;27:311–320 [DOI] [PubMed] [Google Scholar]
- 42. Lundqvist C, Baranov V, Söderström K, et al. Phenotype and cytokine profile of intraepithelial lymphocytes in human small and large intestine. Ann N Y Acad Sci. 1995;756:395–399 [DOI] [PubMed] [Google Scholar]
- 43. Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727.10.1172/JCI113938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Utech M, Ivanov AI, Samarin SN, et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052.10.1091/mbc.E05-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933.10.1096/fj.04-3260com [DOI] [PubMed] [Google Scholar]
- 46. Bandrés E, Merino J, Vázquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(-)CD57(+) subpopulation. Clin Immunol. 2000;96:230–235.10.1006/clim.2000.4894 [DOI] [PubMed] [Google Scholar]
- 47. Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629.10.1152/ajpgi.00177.2003 [DOI] [PubMed] [Google Scholar]
- 48. Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271.10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ershler WB, Sun WH, Binkley N, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230 [PubMed] [Google Scholar]
- 50. Reinecker HC, Steffen M, Doehn C, et al. Proinflammatory cytokines in intestinal mucosa. Immunol Res. 1991;10:247–248 [DOI] [PubMed] [Google Scholar]
- 51. Cannon JG, Fiatarone MA, Meydani M, et al. Aging and dietary modulation of elastase and interleukin-1 beta secretion. Am J Physiol. 1995;268(1 Pt 2):R208–R213 [DOI] [PubMed] [Google Scholar]
- 52. Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097–4104 [DOI] [PubMed] [Google Scholar]
- 53. Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamoto T, Kojima T, Murata M, et al. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp Cell Res. 2004;299:427–441.10.1016/j.yexcr.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 55. Macfarlane GT, Macfarlane LE. Acquisition, evolution and maintenance of the normal gut microbiota. Dig Dis. 2009;27(suppl 1):90–98.10.1159/000268127 [DOI] [PubMed] [Google Scholar]
- 56. Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res Rev. 2010;9:107–116.10.1016/j.arr.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 57. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273.10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667.10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Braunstein J, Qiao L, Autschbach F, Schürmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCormack G, Moriarty D, O’Donoghue DP, McCormick PA, Sheahan K, Baird AW. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res. 2001;50:491–495 [DOI] [PubMed] [Google Scholar]