Abstract
Study Objectives:
Oral appliance therapy has emerged as an important alternative to continuous positive airway pressure (CPAP) in treating patients with obstructive sleep apnea syndrome (OSAS). In this study we report about the subjective and objective treatment outcome of oral appliance therapy and CPAP in patients with OSAS.
Design:
Cohort study of a previously conducted randomized clinical trial.
Setting:
University Medical Center, Groningen, The Netherlands.
Patients or Participants:
One hundred three patients with OSAS.
Interventions:
CPAP and oral appliance therapy (Thornton Adjustable Positioner type-1, Airway Management, Inc., Dallas, TX, USA)
Measurements and Results:
Objective (polysomnography) and subjective (Epworth Sleepiness Scale, Functional Outcomes of Sleep Questionnaire, Medical Outcomes Study 36-item Short Form Health Survey [SF-36]) parameters were assessed after 1 and 2 years of treatment. Treatment was considered successful when the apneahypopnea index (AHI) was < 5 or showed substantial reduction, defined as reduction in the index of at least 50% from the baseline value to a value of < 20 in a patient without OSAS symptoms while undergoing therapy. Regarding the proportions of successful treatments, no significant difference was found between oral appliance therapy and CPAP in treating mild to severe OSAS in a 2-year follow-up. More patients (not significant) dropped out under oral appliance therapy (47%) compared with CPAP (33%). Both therapies showed substantial improvements in polysomnographic and neurobehavioral outcomes. However, CPAP was more effective in lowering the AHI and showed higher oxyhemoglobin saturation levels compared to oral appliance therapy (P < 0.05).
Conclusions:
Oral appliance therapy should be considered as a viable treatment alternative to continuous positive airway pressure (CPAP) in patients with mild to moderate obstructive sleep apnea syndrome (OSAS). In patients with severe OSAS, CPAP remains the treatment of first choice.
Clinical Trial Information:
The original randomized clinical trial, of which this study is a 2-year follow-up, is registered at ISRCTN.org; identifier: ISRCTN18174167; trial name: Management of the obstructive sleep apneahypopnea syndrome: oral appliance versus continuous positive airway pressure therapy; URL: http://www.controlled-trials.com/ISRCTN18174167.
Citation:
Doff MHJ; Hoekema A; Wijkstra PJ; van der Hoven JH; Slater JJRH; de Bont LGM; Stegenga B. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. SLEEP 2013;36(9):1289-1296.
Keywords: Continuous positive airway pressure, obstructive sleep apnea syndrome, oral appliances, treatment outcome
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a sleep related breathing disorder characterized by snoring and repetitive pharyngeal collapse.1 It is associated with excessive daytime sleepiness, a decreased quality of life, increased cardiovascular morbidity, and a higher risk of traffic accidents.2,3 The standard treatment, i.e., continuous positive airway pressure (CPAP), reduces upper airway obstructions and improves quality of life.4 However, because of the cumbersome nature of CPAP, patients often have difficulty adhering to or may even abandon treatment. Oral appliance therapy has been shown to be superior to CPAP regarding treatment success in patients with mild to moderate OSAS in the short term.5 Furthermore, many patients prefer oral appliance therapy to CPAP.6 Long-term outcomes of oral appliance therapy have been described in a few studies.7–11 In four studies, respiratory parameters deteriorated in some patients during the follow-up period, even in patients who were treated successfully at short-term follow-up.7,8,10,11 Some studies have been restricted to those patients with mild and moderate OSAS or included patients who had already undergone surgical treatment of OSAS. To our knowledge, no published parallel study has evaluated the 2-y outcome of oral appliance versus CPAP therapy in previously untreated patients with mild to severe OSAS.
The primary aim of this parallel cohort study was to evaluate the 2-y objective and subjective outcome of oral appliance and CPAP therapy in patients with OSAS, representing the entire spectrum of the disorder and to gain more insight into the specific indications for both treatments. In this study we report on the 2-y follow-up of a cohort of a previously conducted randomized controlled trial (RCT).5
METHODS
Patients and Study Protocol
After assessing 228 patients with OSAS, 103 participants were recruited (between September 2002 and August 2005) for the previously conducted and reported RCT (ISRCTN18174167)5 at the Department of Home Mechanical Ventilation of the University Medical Center Groningen, The Netherlands. Individuals older than 20 y who underwent polysomnography were eligible and were selected based on predefined medical, dental, and psychological criteria (supplemental material). This study was approved by the Groningen University Medical Center's ethics committee (METc2002/032). Written informed consent was obtained from all patients before enrollment. Block randomization was used to allocate participants to either oral appliance therapy or CPAP therapy.12 It was not possible to blind patients or clinicians to the treatment assignment. At baseline, each participant underwent a physical examination (supplemental material). Severity of disease was assessed based on the apneahypopnea index (AHI), i.e., the mean number of apneas and hypopneas per hour of sleep. Participants were classified as having nonsevere (AHI 5-30) or severe (AHI ≥ 30) OSAS. More detailed information is provided in the supplemental material.
Study Design
For this follow-up study, polysomnographic and subjective evaluations were carried out after 1 y (T15) and 2 y (T27) of treatment. Treatment was considered successful when the AHI was < 5 or showed a substantial reduction, defined as a reduction in the index of at least 50% from the baseline value to a value < 20 in a patient without subjective OSAS symptoms while undergoing therapy (no excessive daytime sleepiness or fewer than two subjective OSAS symptoms) (supplemental material). Treatment in participants not meeting these criteria at any follow-up review was considered nonsuccessful. Patients for whom oral appliance or CPAP therapy was successful continued the treatment. If either treatment was not successful at any time during the follow-up period, patients were offered the alternative therapy (CPAP or oral appliance, respectively), which was thereupon titrated in the same fashion as the initial therapy. Patients who discontinued treatment for any reason were considered nonadherent to treatment (worst-case scenario). More detailed information on the results of the 2-mo follow-up (T2) has been published previously.5
Interventions
The oral appliance (Thornton Adjustable Positioner type-1, Airway Management, Inc., Dallas, TX, USA) positioned the patient's mandible in a forward and downward position. This type of oral appliance is also known as a mandibular advancement device. By turning a propulsion screw, which was incorporated anteriorly into the appliance, patients could adjust the mandibular advancement in 0.2-mm increments. When initiating oral appliance therapy (T0), the mandible was set at 50% of the patient's maximal protrusion. After having adapted to this position during a 2-w period, patients were allowed to further adjust the oral appliance during a 6-w period. They were instructed to advance the mandible until symptoms abated or until further advancement caused discomfort. Subsequently, the treatment effect was assessed with a polysomnographic evaluation (T2). If necessary, the adjustment period was extended until the AHI was < 5 or until the adjustments became uncomfortable for the patient. The titration period ended with a final polysomnographic evaluation or when the patient discontinued treatment (e.g., because of poor tolerance). If necessary, the oral appliance was adjusted during the follow-up if OSAS symptoms appeared or when the patient experienced discomfort associated with wearing the oral appliance.
CPAP titration, aimed at abolishing all signs of apneas, hypopneas, and snoring, was performed during an afternoon nap.13
Polysomnography
Polysomnographic studies (Embla® A10 digital recorder, Medcare, Reykjavik, Iceland) for baseline and all follow-up reviews were conducted while participants slept at home and were evaluated according to standardized criteria (supplemental material). All polysomnographic evaluations were evaluated and scored by the same neurophysiologist (JH), who was unaware of the patient's treatment assignment.
Physical and Neurobehavioral Examinations
Physical examination at baseline and follow-up reviews included documentation of height, weight, neck circumference, alcohol consumption, tobacco use, and current medications. For the neurobehavioral examinations, patients completed the following questionnaires at all time points: the Epworth Sleepiness Scale (ESS), the Functional Outcomes of Sleep Questionnaire (FOSQ) addressing OSAS-related symptoms,14,15 and the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) evaluating health perceptions16 (supplemental material). Furthermore, compliance rates were scored at all time points using a questionnaire.
Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (version 16.0, SPSS Inc., Chicago, IL, USA). All continuous baseline variables were normally distributed and their means with standard deviations (SD) are reported. The AHI in the oral appliance and CPAP groups at baseline was normally distributed after logarithmic transformation. Medians and interquartile ranges are reported for variables with a skewed distribution. Details about the sample size calculation regarding the previously conducted 2-mo RCT have previously been described5 and are also described in the supplemental material.
The primary outcome measure of this follow-up study was the proportion of successful treatments at different time points using oral appliance or CPAP therapy (i.e., AHI was < 5 or showed a substantial reduction, defined as a reduction in the index of at least 50% from the baseline value to a value of < 20 in a patient without subjective OSAS symptoms while using therapy). Secondary outcome measures were polysomnographic and neurobehavioral outcomes at different time points. To compare polysomnographic and neurobehavioral outcomes at different time points, Student t-tests were performed for outcomes with a normal distribution and Mann-Whitney U tests for outcomes with skewed distributions. Although proper randomization is executed, a (small) difference in the average values of a determinant for the two treatment arms may occur. To statistically correct this regression-to-the-mean phenomenon in our analysis when comparing T27 values with baseline values, the baseline value was at all times included in the regression model (i.e., autoregression analysis model).
Comparison of the proportions of successful treatments between both treatments (chi-square test) was also performed as function of disease severity (prespecifi ed subgroup analysis). The difference in dropout rates between both treatments was analyzed, using the chi-square test. The signifi cance level α of all analyses was set at 5%.
RESULTS
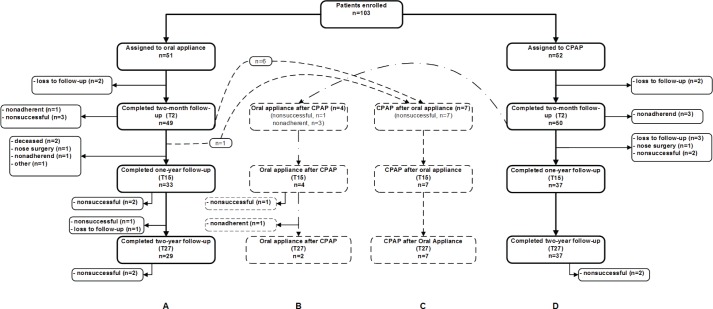
One hundred three patients were enrolled into the RCT. Randomization resulted in an oral appliance group of 51 patients and a CPAP group of 52 patients (Table 1). A fl ow diagram, summarizing the distribution of the patients, is shown in Figure 1. In the oral appliance group, 27 patients (53%) completed the 2-y follow-up successfully while using their oral appliance (Figure 1; group A). Seven more patients of this group completed the follow-up after switching to CPAP therapy (Figure 1; group C) because oral appliance therapy was considered nonsuccessful (Table S1, supplemental material). In the CPAP group, 35 patients (67%) completed the 2-y follow-up successfully while using CPAP (Figure 1; group D). Another four patients switched to oral appliance therapy during the follow-up period (three were considered nonad-herent to CPAP therapy, and treatment was nonsuccessful in one patient). Of these four patients, two completed the 2-y follow-up (Figure 1; group B). The seven patients (group C) who had switched to CPAP after oral appliance therapy had a higher baseline AHI (P = 0.17), body mass index (BMI) (P = 0.02), and age (P = 0.28) than the four patients (group B) who had switched from CPAP to oral appliance therapy (Table S1, supplemental material).
Table 1.
Characteristics of randomized patients
Figure 1.
Flow diagram of participants throughout each phase of the follow-up period.
In the oral appliance group, one patient switched to CPAP after the 2-mo follow-up because of his profession as a truck driver, although treatment with an oral appliance was considered successful (AHI decreased from 69 to 17). However, from a judicial point of view, an AHI of 17 is still considered as moderate OSAS and is not allowed in this patient's profession in The Netherlands. From that point the patient refused further participation in this study. In the CPAP group, one patient dropped out because of difficulty adhering to the therapy after the 2-mo follow-up, although treatment was effective. This patient was treated with an oral appliance from that point but refused further participation in this study. Demographic variables of the patients who completed the 2-y follow-up are provided in Table S2 of the supplemental material.
Notwithstanding the fact that weight loss was always encouraged, the BMI did not significantly change in both treatment groups during the follow-up period.
The follow-up period (including the RCT period) started in October 2002 and ended in October 2007. The mean follow-up for the oral group was 2.3 ± 0.2 y (mean ± SD) and 2.4 ± 0.3 y for the CPAP group. This difference was not signifi cant.
Compliance rates did not significantly differ between both treatments. CPAP and oral appliance mean use was 6.8 ± 0.8 and 6.7 ± 0.7 nights per week, respectively. Furthermore, CPAP and oral appliance therapy was used 6.9 ± 1.2 and 7.2 ± 0.8 h per night.
More patients (not significant) dropped out (including those who switched treatment) under oral appliance therapy (total n = 24; loss to follow-up n = 3, nonsuccessful n = 15; nonadherent n = 2; nose surgery during follow-up n = 1; deceased n = 2, other n = 1) compared with CPAP (total n = 17; loss to follow-up n = 5; nonsuccessful n = 5; nonadherent n = 6; nose surgery during follow-up n = 1). Reasons for discontinuing treatment are described in detail in Table S4 of the supplemental material and shown in Figure 1.
Polysomnographic Outcomes
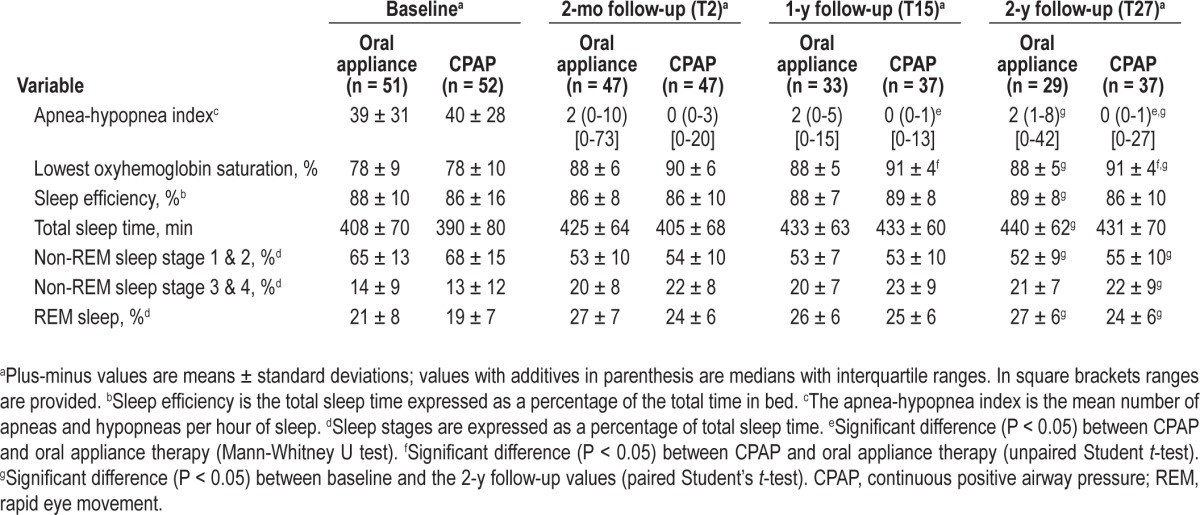
The AHI was significantly lower in the CPAP group compared with the oral appliance group after 1 y with CPAP (0.0 [0.0-13.0]) (median with range) versus oral appliance (2.0 [0.0-15.0]) and 2 y of treatment with CPAP (0.0 [0.0-27.0] versus oral appliance (2.0 [0.0-42.0]) (Figure 2, Table 2). This significant difference in advantage of CPAP therapy was also found for the lowest oxyhemoglobin saturation at the 1- and 2-y follow-up (at both time points 91 ± 4% with CPAP versus 88 ± 5% with oral appliance therapy).
Figure 2.
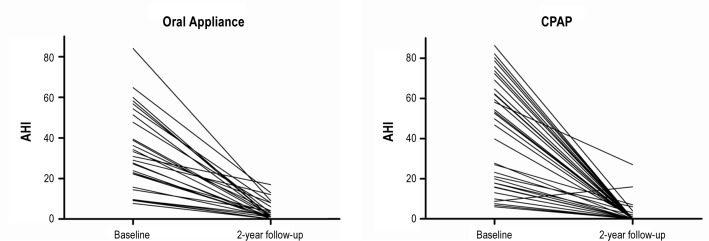
Individual values of the apneahypopnea index (AHI) of the patients who completed the entire follow-up in the randomized treatment group. CPAP, continuous positive airway pressure.
Table 2.
Polysomnographic outcomes at different time points of all patients who completed the entire follow-up period with the treatment that was assigned at baseline
Treatment Outcome
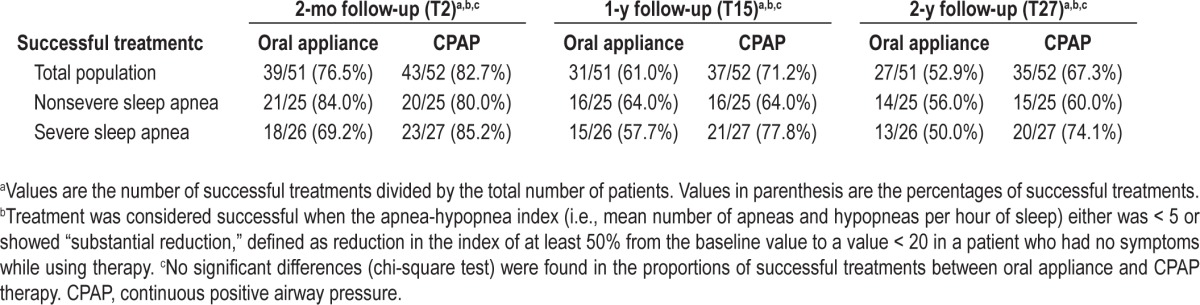
Table 3 shows the proportions of successful treatments at the different time points. After 2 y, treatment of the 51 patients randomized to the oral appliance group was successful for 27 patients (52.9%) (14 of 25 patients with nonsevere OSAS [56.0%] and 13 of the 26 patients with severe OSAS [50.0 %]). Of the 52 patients intentionally treated with CPAP, 35 (67.3%) were successfully treated (15 of 25 patients [60%] with nonsevere OSAS and 20 of 27 patients [74.1%] with severe OSAS). The differences in proportions of successful treatments between both treatments were not significant. Proportions of successful treatments after applying the criterion AHI < 5 are provided in Table S3 of the supplemental material.
Table 3.
Proportions of successful treatments with an oral appliance or with continuous positive airway pressure at different time points
Neurobehavioral Outcomes
Both treatments yielded comparable improvements in the ESS score, the FOSQ-score, and scores of the SF-36 at all time points (Table 4). Most of the variables improved significantly compared with the baseline values. No significant differences were found between both treatments. The mean values of the FOSQ total score at different time points for both treatments is shown in Figure 3.
Table 4.
Neurobehavioral outcomes at different time points for all patients who completed the 2-y follow-up period with the treatment that was assigned at baseline
Figure 3.
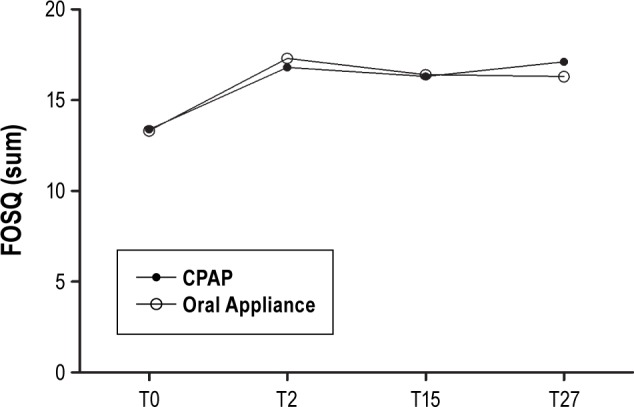
Functional outcomes of sleep questionnaire (FOSQ) total score at baseline (T0), after 2 mo (T2), after 1 y (T15) and 2 y (T27). Higher scores indicate better functioning. CPAP, continuous positive airway pressure.
Adverse Effects
Mild and transient adverse effects were commonly reported in the initial period of oral appliance therapy and include tooth pain, temporomandibular joint pain, myofascial pain, dry mouth, and excessive salivation. Furthermore, we found that long-term oral appliance therapy and CPAP may result in dental changes in patients with OSAS. Concerning this cohort, more detailed information regarding adverse effects, associated with long-term oral appliance therapy and CPAP, is provided in separate studies.17–19
DISCUSSION
The results of this study indicate that, regarding treatment success, there is no significant difference between oral appliance therapy and CPAP in treating mild to severe OSAS in a 2-y follow-up. CPAP, however, showed a tendency toward a higher (nonsignificant) overall success rate than oral appliance therapy. This tendency was most pronounced in patients with severe OSAS. Both therapies had positive effects on polysomnographic variables during follow-up but CPAP therapy was significantly more effective in improving the AHI and the lowest oxyhemoglobin saturation levels at 1- and 2-y follow-up. In this study, more people (not significant) dropped out in the oral appliance group compared with the CPAP group because treatment was classified as nonadherent or nonsuccessful. Both types of therapy seemed to be comparable in improving subjective sleepiness, functional outcomes, and health perceptions.
To our knowledge this is the first follow-up of an RCT, comparing the 2-y outcome of oral appliance and CPAP therapy in patients with mild to severe OSAS. From this current follow-up study, short-term (2 mo) results have previously been described,5 concluding that the outcome (in terms of proportions of successful treatments) of oral appliance therapy was not inferior to CPAP therapy. In that study, subgroup analysis showed a tendency toward a higher but not significant success rate of oral appliance therapy in patients with nonsevere OSAS (AHI 5-30) compared with CPAP (84.0% versus 80.0%). The follow-up of these patients, as described in this study, shows that CPAP therapy generally yielded more successful treatment (a not significant difference of 14.4%) after 2 y of therapy. This difference can first be explained by the fact that we included patients with severe OSAS. In a systematic review4 it is described that CPAP is more effective than oral appliance therapy in reducing respiratory disturbances, especially in patients with moderate and severe OSAS. In addition, patients switched to the alternative therapy because of adherence problems or nonsuccess of their treatment after the 2-mo follow-up. Several studies have identified specific predictors for treatment success with oral appliance therapy.20–22 A lower baseline AHI, lower BMI, and younger age were all associated with better treatment responses to oral appliance therapy. In this follow-up study, seven patients who were older, obese, and with predominantly severe OSAS switched from oral appliance therapy to CPAP therapy because oral appliance therapy appeared to be nonsuccessful. Obese patients show an enlargement of upper airway adipose tissue, assessed by magnetic resonance imaging.23,24 It was found that mandibular advancement increases the retropalatal airway space in nonobese patients, but not in obese patients.25 This may explain why the afore-mentioned seven patients were not treated successfully with an oral appliance. Notwithstanding this outcome, oral appliance therapy was still successful in 50.0% of the patients with severe OSAS who completed the 2-y follow-up. According to the latest practice parameters of the American Academy of Sleep Medicine,26 an oral appliance is indicated in patients with mild to moderate OSAS who prefer an oral appliance to CPAP, do not respond to CPAP, are unsuit-able candidates for CPAP, and in whom treatment attempts with CPAP fail. However, our results indicate that an oral appliance can also play an important role in treating specific patients with severe OSAS in the long term.
Oral appliance therapy had a tendency to be less stable during the 2-y follow-up, resulting in more patients who discontinued treatment compared with CPAP therapy. This finding is consistent with other studies, showing deterioration in success with oral appliance therapy, even in patients who were treated effectively in the short term.7,8,10 We currently assume that the working mechanism of an oral appliance is based on advancement of the mandible and its attached soft tissue structures and musculature, especially the genioglossus muscle, resulting in an increased tone with increased anteroposterior and lateral dimensions of the upper airway.27,28 Bearing the aforementioned considerations in mind, this deterioration in treatment success is possibly due to loosening and adaptation of soft tissue structures and musculature of the upper airway as a result of long-term overnight mandibular advancement. It could be hypothesized that patients with more severe OSAS need to protrude the mandible more extensively to gain the desired effect, in addition to a possible overstretching that negatively affects the morphology of the upper airway soft tissue structures and tonus of the musculature. Furthermore, it has been described that the muscle tone of the genioglossus is nega-tively correlated with age.29 Therefore, a second explanation is that the increasing age of our study group results in decreased success of oral appliance therapy. It is unknown to what extent both possibilities contribute to this change in outcome.
Subjective improvements in sleepiness, functional outcomes, and health perceptions were found in both treatment groups (no significant differences), underlining the therapeutic success of oral appliance and CPAP therapy at all time points during the follow-up, even in patients with severe OSAS. Similar findings in different studies using the same questionnaires (pooled) were reported in a review article by Chan et al.30 However, most of these studies included patients with mild and moderate OSAS. Gagnadoux et al.31 found comparative subjective improvements among patients treated with an oral appliance or CPAP using the Nottingham Health Profile questionnaire. Compared with the baseline values, significant improvements were found for four of the six domains with oral appliance therapy and for two of the six domains with CPAP. There was no significant difference between the two treatments. This study had a crossover design and had a follow-up of only 8 w for each treatment arm, which makes their findings only partial comparable with our results. In another study the objective and subjective efficacy of two different commercially available oral appliances (Silencer and Klearway) were compared. For both appliances significant improvements were found in the ESS scores and FOSQ scores but no significant differences were found between both appliances.32 Furthermore, the Respiratory Disturbance Index (RDI) was slightly lower with the Silencer but patients' preference for comfort was in favor of the Klearway.
In our study we used the Thornton Adjustable Positioner type-1, which is a two-piece adjustable appliance. In one study the objective and subjective efficacy of the Thornton Adjustable Positioner type-1 was compared with a modified Herbst appliance (IST®) in a 2-y follow-up.33 Although the Thornton Adjustable Positioner type-1 was more effective in lowering the AHI, both appliances seemed to be effective therapeutic devices for OSAS which is consistent with our findings. Currently, many types of oral appliances are commercially available for treating patients with OSAS. Therefore careful comparison of results from the current study with studies in which other types of oral appliances are used is important, as there can be differences in efficacy and patients' preferences.34
The current study has some potential limitations. Patients were allowed to switch therapy during follow-up if they were considered nonadherent or if treatment was considered nonsuccessful. These findings may have biased our results because patients could pretend to be nonadherent after randomization in a possible non-preferred study group. However, only small numbers of patients switched, and except for one patient, all patients switched after 2 mo of therapy. We also believe that a serious disorder, such as OSAS, should be treated as effectively as possible and the possibility to switch during the follow-up period provides a better picture of the true clinical situation. We furthermore considered all patients who discontinued treatment for any reason as a dropout, even if they were treated effectively (worst-case scenario). Success rates therefore may be underestimated.
Another limitation is the sample size that was determined for the 2-mo RCT.5 There was an a priori risk of having ended up with an insufficient number of patients for reliable long-term results as dropouts in the long term were not anticipated in the power analysis. Future studies should therefore focus and power on the long-term outcome of both oral appliance and CPAP therapy in prestratified treatment groups.
In conclusion, regarding the percentage of successful treatments, no significant differences were found between oral appliance therapy and CPAP in treating mild to severe OSAS in a 2-y follow-up. However, CPAP was more effective in lowering the AHI and showed higher oxyhemoglobin saturation levels compared with oral appliance therapy. Furthermore, CPAP proved to be more successful in patients with severe OSAS. However, even in a 2-y follow-up, oral appliance therapy seems to be a viable alternative to CPAP in the treatment of mild and moderate OSAS. Oral appliances may be considered as a long-term alternative in patients with severe OSAS who do not respond to CPAP or in whom treatment attempts with CPAP fail. Further research with larger groups of patients is needed to investigate which patients with severe OSAS can be treated successfully with an oral appliance.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1271.
SUPPLEMENTAL MATERIAL
SUPPLEMENTAL METHODS
Recruitment of Patients
The University Medical Center Groningen has a catchment area that includes most of the north- eastern part of The Netherlands. Patients suspected to have sleep apnea were referred to this medical center by general practitioners from the region and physicians from departments of pulmonary medicine, neurology, and ear, nose, and throat surgery in several regional hospitals to possibly participate in a previously conducted randomized controlled trial. We used the recommendation of the American Academy of Sleep Medicine to diagnose obstructive apnea1 and it is defined as:
1 or 2, plus criterion 3 of the following:
A clear decrease (> 50%) from baseline in the amplitude of a valid measure of breathing during sleep. Baseline is defined as the mean amplitude of stable breathing and oxygenation in the 2 min preceding onset of the event (in individuals who have a stable breathing pattern during sleep) or the mean amplitude of the three largest breaths in the 2 min preceding onset of the event (in individuals without a stable breathing pattern).
A clear amplitude reduction of a validated measure of breathing during sleep that does not reach the above criterion but is associated with either an oxygen desaturation of > 3% or an arousal.
The event lasts 10 sec or longer.
Each patient in whom obstructive sleep apnea1 has been diagnosed consulted our Department of Home Mechanical Ventilation for treatment.
Selection of Patients and Informed Consent
To screen for any underlying disease, all eligible patients underwent a comprehensive physical evaluation, spirometry, thoracic radiography, electrocardiography, and blood tests.
General criteria for inclusion in the study
Age older than 20 y
- Polysomnography showing an apneahypopnea index ≥ 5; i.e., the mean number of apneas and hypopneas per hour of sleep in combination with:
- Excessive daytime sleepiness that is not better explained by other factors or
- Two or more of the following that are not better explained by other factors:
- Choking or gasping during sleep
- Recurrent awakenings from sleep
- Unrefreshed sleep
- Daytime fatigue
- Impaired concentration
Medical and psychological criteria for exclusion from the study
Previous treatment of obstructive sleep apnea (continuous positive airway pressure (CPAP), oral appliance therapy, or uvulopalatopharyngoplasty)
Morphological airway abnormalities requiring treatment (a compromised nasal passage, enlarged tonsils or adenoids, upper airway or pulmonary neoplasm, or upper airway soft-tissue or craniofacial abnormality)
Endocrine dysfunction (hypothyroidism, acromegaly, or pituitary adenoma)
A reported or documented history of severe cardiac or pulmonary disease (daytime respiratory insufficiency, severe chronic obstructive pulmonary disease (Tiffeneau index < 40%),2 heart failure, coronary disease, or severe cardiac arrhythmias)
Moderate or severe periodic limb movement disorder (periodic limb movement index > 25).
A psychological condition precluding informed consent (mental retardation or psychiatric disorder; e.g., depression or schizophrenia)
Dental criteria for exclusion from the study
Extensive periodontal disease or tooth decay
Active temporomandibular joint disease (including severe bruxism)
Restrictions in mouth opening (< 25 mm) or advancement of the mandible (< 5 mm)
Partial or complete edentulism (fewer than eight teeth in upper or lower jaw)
Patients who qualified for inclusion were informed about the study and their questions were answered by the pulmonologist and dentist who evaluated the patients. Each patient was given a brochure with details about the study and had 1 w to decide whether or not they wanted to participate. Patients who decided to participate signed and returned an informed consent form.
Randomization Procedure
The clinical epidemiologist (BS) for the study made computer-generated randomization sequences, balancing for disease severity. The randomization sequences were used for selecting random permuted blocks with lengths of two, four, and six.3 The clinicians supervising oral appliance and CPAP therapy were not informed about how the randomization was performed. The randomization sequences were concealed and administered by the secretarial office of the Department of Oral and Maxillofacial Surgery. After each patient's serial number and diagnosis of disease severity were provided, the treatment was disclosed. Each serial number could be provided only once. It was not possible to blind patients or clinicians to treatment assignment.
Interventions, Conservative Measures, and Treatment Adjustments
Oral appliance therapy was initiated in the Department of Oral and Maxillofacial Surgery and was supervised by two experienced dentists (MD, AH). CPAP therapy was initiated in the Department of Home Mechanical Ventilation. Oral appliance therapy was initiated within a 4-w period and CPAP therapy within a 2-w period after a patient was enrolled in the study. Before treatment was initiated, all patients were instructed to adopt conservative measures; specifically, to avoid using depressants (e.g., alcohol or sleep medication) and to have at least 7-8 h of sleep each night. When indicated, patients were encouraged to give up smoking and lose weight.
The oral appliance used in this study consisted of two separate parts.4 The upper part was supported by the dentition of the maxilla and the lower part by the dentition of the mandible. By turning a propulsion screw incorporated anteriorly in the appliance, the patient could adjust the amount of mandibular advancement in 0.2-mm increments. The maximum advancement of the mandible was determined with a George-GaugeTM (H Orthodontics, Michigan City, IN, USA) before oral appliance therapy began. Initially, the mandible was set at 50% of the patient's maximum advancement. After patients became accustomed to the oral appliance during a 2-w period, they returned for a checkup visit. They were instructed to adjust the oral appliance over the following 6 w, until the second polysomnographic study was performed—advancing the mandible by turning the propulsion screw each night with one or two clockwise turns (i.e., 0.2-0.4 mm) until symptoms abated (i.e., snoring, apneas, hypopneas, or excessive sleepiness). If the second polysomnographic assessment indicated an apneahypopnea index ≥ 5, the oral appliance was adjusted, if possible, in an attempt to improve effectiveness. The oral appliance was adjusted to obtain the maximum advancement of the mandible with which the patient was comfortable. A third polysomnographic study was performed 4 w after that adjustment was made.
CPAP titration was performed during an afternoon nap.5 All patients using CPAP received detailed instructions about this procedure and correct use of CPAP from a skilled nursing consultant. Patients were fitted with a comfortable CPAP mask before titration. After CPAP titration, all patients received a similar CPAP device (Breas® PV10, Mölnlycke, Sweden). Patients were permitted to become accustomed to CPAP during a 2-w period, after which they returned for a checkup visit. After another 6 w of treatment, a second polysomnographic study was performed. If polysomnography indicated an apneahypopnea index ≥ 5, we adjusted the CPAP, if possible, with a rise in pressure by 1 or 2 cm H2O, depending on the residual apneahypopnea index with CPAP. A third polysomnographic study was performed 4 w after that adjustment was made (T2). Adjustments of CPAP therapy were continued until the apneahypopnea index was < 5 or until adjustments of CPAP became uncomfortable for the patient.
Patients for whom oral appliance or CPAP therapy was successful continued the treatment. Patients for whom treatment was not effective were offered the alternative CPAP or oral appliance therapy. After 1 y (T15) and 2 y (T27) of treatment another polysomnographic study was performed.
Polysomnography
Surface electroencephalography, submental electromyography, and left and right electrooculography were used to stage sleep by using standardized criteria.6 A pulsoximeter (Oximeter Flex Sensor – 8000J-3, Medcare, Reykjavik, Iceland) was used to record oxyhemoglobin saturation. Electrocardiography was used to monitor cardiac function. Oronasal airflow was recorded with a pressure cannula. Respiratory effort was monitored with thoracic and abdominal strain gauges. An anterior tibial electromyogram was recorded to screen for periodic limb movements. Each polysomnographic study started at 11:00 and ended at 09:00 the next morning. Polysomnographic outcomes were assessed during the night while the patient was asleep. Outcomes included total sleep time, sleep efficiency, apneahypopnea index, minimum oxyhemoglobin saturation, the percentage of nonrapid eye movement sleep during stages 1 and 2 and stages 3 and 4, and the percentage of rapid eye movement sleep. Baseline polysomnographic outcomes were those obtained at the time of diagnosis.
Characteristics of patients who switched to the alternative therapy during the follow-up
Characteristics of the patients included in the final analysis
Proportions of successful treatments with an oral appliance or with continuous positive airway pressure according to the criterion AHI < 5
Details regarding dropout of patients during the follow-up
Neurobehavioral Examination
Patients completed the Epworth Sleepiness Scale to assess their propensity to fall asleep in eight different situations.7 Patients completed the functional outcomes of sleep questionnaire to assess the effect of excessive sleepiness on a number of activities of everyday living.8 This questionnaire consists of 30 different questions that yield a score for five different subscales and a total score. Patients completed the Medical Outcomes Study 36-Item Short Form Health Survey to assess their perception of their health status.9 This questionnaire consists of 11 questions that yield a score for eight different dimensions and one item regarding changes in the patient's health.
Patients' usage of their therapy was evaluated by asking patients how many nights each week and how many hours each night they used their treatment. Whereas CPAP usage can be monitored covertly with a mechanism built into the device, oral appliance usage cannot be assessed covertly in any reliable way. To eliminate bias, we ensured that treatment usage was assessed in the same manner by basing the assessments on self-reports in both groups. Some patients did not use treatment during the entire week; for example, on weekends when they did not anticipate a strenuous day. Patients were considered nonadherent to therapy only if they voluntarily discontinued use of the therapy due to poor tolerance or any other reason.
Statistical Analysis
Calculation of the sample size was performed with the PASS 2000 software package (NCSS, Kaysville, UT). Statistical analyses were performed by using the Statistical Package for the Social Sciences (version 16.0, SPSS Inc., Chicago, IL, USA). The clinical epidemiologist (BS) who collected and analyzed all the data did not have any contact with the patients. Power analysis was done for a previously conducted 2-mo random-ized controlled trial10 of which this study is the 2-y follow-up. For that randomized controlled trial, a minimum of 46 patients would be required in each treatment group when a one-sided significance level of 5%, a power of 90%, and an assumed proportion of treatment effectiveness of 90% was applied.
SUPPLEMENTAL REFERENCES
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 3.Altman DG. Designing research. In: Altman DG, editor. Practical statistics for medical research. London: Chapman & Hall; 1991. pp. 74–106. [Google Scholar]
- 4.Pancer J, Al-Faifi S, Al-Faifi M, Hoffstein V. Evaluation of variable mandibular advancement appliance for treatment of snoring and sleep apnea. Chest. 1999;116:1511–8. doi: 10.1378/chest.116.6.1511. [DOI] [PubMed] [Google Scholar]
- 5.Hoekema A, Stegenga B, van der Aa JG, Meinesz AF, van der Hoeven JH, Wijkstra PJ. Nap-titration: an effective alternative for continuous positive airway pressure titration. Resp Med. 2006;100:705–13. doi: 10.1016/j.rmed.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Rechtschaffen AK, AA . Washington DC: U.S. Government Printing Office; 1968. Manual of standardized technology, techniques and scoring system for the sleep stages of human subjects. 0068; 204:1. [Google Scholar]
- 7.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 8.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 10.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87:882–7. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Barbe F, Pericas J, Munoz A, Findley L, Anto JM, Agusti AG. Automobile accidents in patients with sleep apnea syndrome. An epidemiological and mechanistic study. Am J Respir Crit Care Med. 1998;158:18–22. doi: 10.1164/ajrccm.158.1.9709135. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev (Online) 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87:882–7. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 6.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 7.Rose EC, Barthlen GM, Staats R, Jonas IE. Therapeutic efficacy of an oral appliance in the treatment of obstructive sleep apnea: a 2-year follow-up. Am J Orthod Dentofacial Orthop. 2002;121:273–9. doi: 10.1067/mod.2002.121006. [DOI] [PubMed] [Google Scholar]
- 8.Marklund M, Sahlin C, Stenlund H, Persson M, Franklin KA. Mandibular advancement device in patients with obstructive sleep apnea: long-term effects on apnea and sleep. Chest. 2001;120:162–9. doi: 10.1378/chest.120.1.162. [DOI] [PubMed] [Google Scholar]
- 9.Fransson AM, Tegelberg A, Leissner L, Wenneberg B, Isacsson G. Effects of a mandibular protruding device on the sleep of patients with obstructive sleep apnea and snoring problems: a 2-year follow-up. Sleep Breath. 2003;7:131–41. doi: 10.1007/s11325-003-0131-7. [DOI] [PubMed] [Google Scholar]
- 10.Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121:739–46. doi: 10.1378/chest.121.3.739. [DOI] [PubMed] [Google Scholar]
- 11.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011;82:162–8. doi: 10.1159/000324580. [DOI] [PubMed] [Google Scholar]
- 12.Altman DG. Designing research. In: Altman DG, editor. Practical statistics for medical research. London: Chapman & Hall; 1991. pp. 74–106. [Google Scholar]
- 13.Hoekema A, Stegenga B, van der Aa JG, Meinesz AF, van der Hoeven JH, Wijkstra PJ. Nap-titration: an effective alternative for continuous positive airway pressure titration. Resp Med. 2006;100:705–13. doi: 10.1016/j.rmed.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 17.Doff MH, Finnema KJ, Hoekema A, Wijkstra PJ, de Bont LG, Stegenga B. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on dental side effects. Clin Oral Investig. 2013;17:475–82. doi: 10.1007/s00784-012-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doff MH, Hoekema A, Pruim GJ, Huddleston Slater JJ, Stegenga B. Long-term oral-appliance therapy in obstructive sleep apnea: a cephalometric study of craniofacial changes. J Dent. 2010;38:1010–8. doi: 10.1016/j.jdent.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Doff MH, Veldhuis SK, Hoekema A, et al. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on temporomandibular side effects. Clin Oral Investig. 2012;16:689–97. doi: 10.1007/s00784-011-0555-6. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001;120:639–47. doi: 10.1067/mod.2001.118782. [DOI] [PubMed] [Google Scholar]
- 21.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 22.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 23.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–6. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 24.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 25.Isono S, Tanaka A, Tagaito Y, Sho Y, Nishino T. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87:1055–62. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 27.Gale DJ, Sawyer RH, Woodcock A, Stone P, Thompson R, O'Brien K. Do oral appliances enlarge the airway in patients with obstructive sleep apnoea? A prospective computerized tomographic study. Eur J Orthod. 2000;22:159–68. doi: 10.1093/ejo/22.2.159. [DOI] [PubMed] [Google Scholar]
- 28.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–9. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 30.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693–9. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 31.Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34:914–20. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier L, Laberge L, Beaudry M, Laforte M, Rompre PH, Lavigne GJ. Efficacy of two mandibular advancement appliances in the management of snoring and mild-moderate sleep apnea: a cross-over randomized study. Sleep Med. 2009;10:329–36. doi: 10.1016/j.sleep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Ghazal A, Sorichter S, Jonas I, Rose EC. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J Sleep Res. 2009;18:321–8. doi: 10.1111/j.1365-2869.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 34.Ahrens A, McGrath C, Hagg U. Subjective efficacy of oral appliance design features in the management of obstructive sleep apnea: a systematic review. Am J Orthod Dentofacial Orthop. 2010;138:559–76. doi: 10.1016/j.ajodo.2010.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients who switched to the alternative therapy during the follow-up
Characteristics of the patients included in the final analysis
Proportions of successful treatments with an oral appliance or with continuous positive airway pressure according to the criterion AHI < 5
Details regarding dropout of patients during the follow-up