Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a frequent and often underdiagnosed condition that is associated with upper airway collapse, oxygen desaturation, and sleep fragmentation leading to cognitive dysfunction. There is meta-analytic evidence that subdomains of attention and memory are affected by OSA. However, a thorough investigation of the impact of OSA on different subdomains of executive function is yet to be conducted. This report investigates the impact of OSA and its treatment, in adult patients, on 5 theorized subdomains of executive function.
Design:
An extensive literature search was conducted of published and unpublished materials, returning 35 studies that matched selection criteria. Meta-analysis was used to synthesize the results from studies examining the impact of OSA on executive functioning compared to controls (21 studies), and before and after treatment (19 studies); 5 studies met inclusion in both categories.
Measurements:
Research papers were selected which assessed 5 subdomains of executive function: Shifting, Updating, Inhibition, Generativity, and Fluid Reasoning.
Results:
All 5 domains of executive function demonstrated medium to very large impairments in OSA independent of age and disease severity. Furthermore, all subdomains of executive function demonstrated small to medium improvements with CPAP treatment.
Discussion:
Executive function is impaired across all five domains in OSA; these difficulties improved with CPAP treatment. Age and disease severity did not moderate the effects found; however, further studies are needed to explore the extent of primary and secondary effects, and the impact of age and premorbid intellectual ability (cognitive reserve).
Citation:
Olaithe M; Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. SLEEP 2013;36(9):1297-1305.
Keywords: Obstructive sleep apnea, executive function, cognition, neuropsychology, review
INTRODUCTION
Obstructive sleep apnea (OSA) is a frequent and often underdiagnosed condition associated with upper airway collapse, oxygen desaturation, and sleep fragmentation leading to sleepiness, hypertension, increased risk of cardiac disease, and neurocognitive disturbance.1–3 Untreated OSA is associated with increased healthcare utilization, occupational injuries, motor vehicle accidents,4–6 and neurocognitive sequelae in memory, attention, and executive function.2,7 The gold standard treatment for OSA is continuous positive airway pressure (CPAP).8,9
To date, most reviews of cognitive functioning in OSA have inspected cognition as a whole, collapsing research findings into “memory,” “executive function,” or “attention” domains.10,11 As the evidence base grows, however, it is both possible and desirable to explore the cognitive burden of OSA within subcomponents. Based on current neurocog-nitive theory, there are functional and biological grounds for segregating cognitive domains or functional systems into such subcomponents.12,13 These subcomponents work in concert to produce what we colloquially know as memory, attention, and executive function.14,15
Recently, Wallace and Bucks16 divided episodic memory into theoretically driven subcomponents, revealing deficits in individuals with OSA in verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuo-spatial episodic memory (immediate and delayed recall), but not visual immediate recall or visuo-spatial learning. This theoretically driven division of memory reveals that not all components of memory are dysfunctional in OSA, and provides an explanation for the mixed findings in this field. A similar approach might prove fruitful when exploring executive dysfunction in OSA. In a review, Saunamäki and Jehkonen17 demonstrated that aspects of executive function may also be impaired or preserved in OSA. They divided executive functioning by test, demonstrating deficits in Digit Span Forwards, Corsi Block Tapping task, Double encoding task, Wisconsin Card Sorting Test, Phonemic fluency, Rey-Osterreith Complex Figure test, and Maze tasks. However, by meta-analyzing the data by test, this review did not aggregate executive functions using a theoretical framework. In addition, Saunamäki and Jehkonen included some tests that do not primarily measure executive function (i.e., Digit Span Forwards, Rey-Osterreith Complex Figure test, the Trail Making Test Part A, and the Corsi Block Tapping task), making it difficult to determine which subcom-ponents of EF, mapped by which tests, are impaired in OSA.
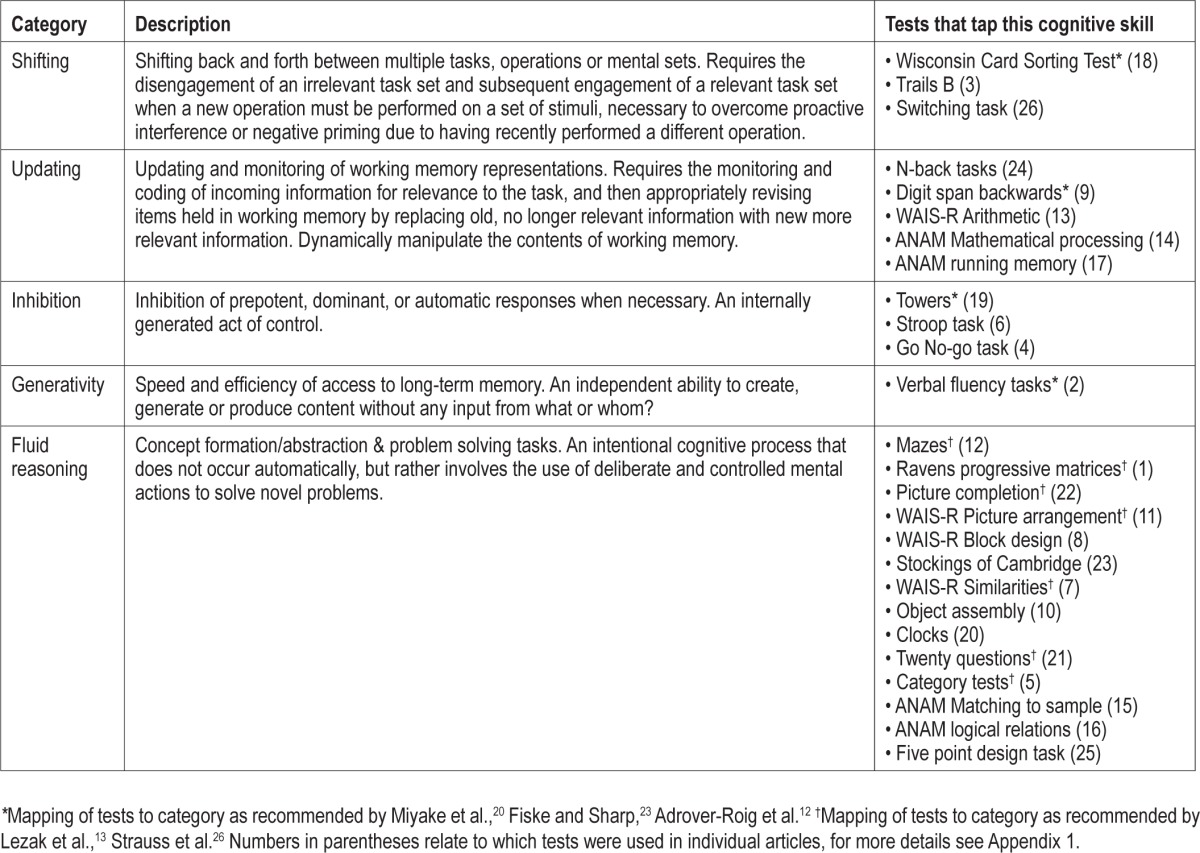
Executive function is an individually controlled and conscious effort to guide the operation of various cognitive processes and thereby regulate cognition.13,14,18–21 Like other cognitive domains, executive function is multidimensional.13,20–22 Miyake et al.,20 Fisk and Sharp,23 and Adrover-Roig et al.12 present an empirical basis for specifying how executive functions are organized, and what roles different subcomponents play. Miyake et al.20 divide executive functioning into (a) Shifting between tasks or mental sets, (b) Updating and monitoring of working memory representations and (c) Inhibition of dominant or prepotent responses. Fisk and Sharp23 and Adrover-Roig et al.12 utilized this same 3-factor structure, but proposed a fourth component; efficiency of access to long term memory (called Generativity in the present report, for brevity). This four-component structure has been confirmed in multiple populations with factor analysis (exploratory and confirmatory).12,23,24,25
Lezak et al.13 and Strauss et al.26 define a set of tasks that do not tap executive function per se, but rather an overarching system of reasoning and problem solving. These tasks involve complex, higher order abstraction, problem solving, and concept formation, and include tasks such as Porteus mazes and clock drawing tasks. The four-factor model defined above does not account for such tasks; however, they abound in OSA literature on executive function and are considered a part of executive functioning in neuropsychological theory.13,26 Hence, in the present paper a class of executive function tasks, called Fluid Reasoning, was created to capture this concept.
The present paper builds on past reviews and meta-analyses examining executive functioning in adults with OSA within current neuropsychological theory of executive function. No previous meta-analysis in OSA has assessed EF dysfunction, or the effect of treatment, within these 5, theoretically motivated domains: Shifting, Updating, Inhibition, Generativity, and Fluid Reasoning. We addressed three questions: (1) which specific executive functions are affected by the presence of untreated OSA?; (2) if executive functions are impaired, does treatment help to remediate these deficits?; and (3) are any of these effects moderated by publication status, sample source, study design, age, disease severity, or control screening?
METHODS
Search Strategy
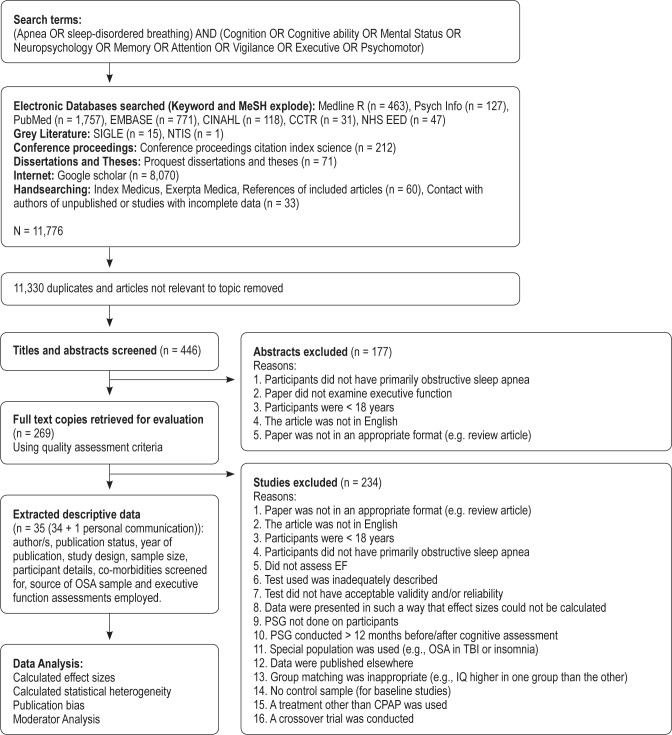
Data for this meta-analysis consisted of empirical articles published in peer-reviewed journals over the past 24 years (Jan 1987-Nov 2011). Details of the search methodology employed are outlined in Figure 1.
Figure 1.
Flow chart of search, retrieval and inclusion process
An extensive computer assisted literature search was conducted using electronic databases (Keyword and MeSH explode) for published articles (Medline R, PsychInfo, PubMed, EMBASE, CINAHL, CCTR, NHS EED), grey literature (SIGLE, NTIS), conference proceedings (Conference Proceedings Citation Index: Science), dissertations and theses (Proquest Dissertations and Theses), the Internet (Dogpile, Omni Medical search engine, Mednet), and via hand searching (Index Medicus, Exerpta Medica, references of included articles, contact with authors of unpublished studies). Unpublished studies were included in the search, to avoid publication bias.
The terms “apnea OR sleep-disordered breathing” were combined with “Cognition OR Cognitive ability OR Mental Status OR Neuropsychology OR Memory OR Attention OR Vigilance OR Executive OR Psychomotor.” The terms chosen covered a wide range of cognitive functions to capture tests that had been mislabelled or utilized to measure other cognitive domains.
Additional relevant articles were retrieved from the reference lists of studies included in the original search, conference proceedings and dissertations. Furthermore, key authors who have published articles on the relationship between OSA, cognition, and CPAP treatment were contacted asking if they were aware of any other relevant published or unpublished studies.
Study Selection Criteria
This review included studies that assessed executive function in adults with OSA as defined by an apnea-hypopnea index (AHI) > 5 per hour of sleep.27 In all instances except one, studies were excluded if OSA participants were not diagnosed using overnight polysomnography and/or if they did not include a control sample, if group matching was inappropriate (e.g., IQ statistically different between control and OSA groups), or there were no baseline data (participants were assessed after treatment only). The exception to this rule was Antic et al.,28 where participants were administered overnight oximetry instead of PSG. This paper was included as the oximetry was validated against in-laboratory PSG in a random selection of 50% of the participants.
Additionally, papers were excluded if the PSG was conducted more than 12 months before/after neuropsychological profiling was completed. These studies were excluded as individuals may lose or gain weight, or change their lifestyle habits (e.g., drink, smoke, or exercise more or less), which may alter the severity of their sleep apnea.29,30
This review considered only studies with adult participants (≥ 18 years), not from special populations (e.g., people with Down syndrome, insomnia, or traumatic brain injury). Research demonstrates that there are etiological differences between adult and childhood OSA31; thus the latter was not addressed here.
The present review aimed to delineate the pattern of executive deficits in OSA; hence, studies that included a majority of central or mixed sleep apnea patients were excluded. Research demonstrates that the pathophysiology, epidemiology, and clinical characteristics of central sleep apnea and OSA are distinct.32
Papers were excluded if the tests used were inadequately described such that acceptable validity and/or reliability could not be confirmed, the paper was a review paper not a study, if it reported data already included in the present review (in this instance the most complete data set was selected) or was a crossover trial. Crossover trials were excluded as research does not provide any definitive information regarding length of washout period required.33–35
The present study was only able to examine CPAP treatment, as after evaluating studies with the exclusion criteria, there remained an insufficient number of other treatment studies (no oxygen therapy, positional therapy, drug trial or weight loss studies, 1 surgical study, 3 mandibular advancement splint [MAS] studies, 3 studies with mixed treatments). Nor did the present review examine medication studies as these (e.g., modafinil and armodafinil) may alter alertness, cognitive function, and judgment without treating underlying nocturnal symptoms.36,37 Although research demonstrates that these medications can be helpful in conjunction with CPAP where there is residual sleepiness, the present study aimed to look at the effect of OSA on cognitive function, and such medications may have confounded these results.
Furthermore, studies were not considered if the data were presented in such a way that effect sizes could not be calculated even after contact with the author. We contacted 32 authors for further details on 33 research papers. Five authors or their representatives replied. Of these, 2 authors were deceased, 1 had no more detail to provide, and 2 emailed further data. Data received from N. Antic (personal correspondence, September 2012) were utilized in the present meta-review. We also received further data from M. Barnes38; however, these data were later excluded as they were from a crossover trial.
Given that it is difficult to keep participants and experimenters blinded to group (OSA or Control, Treatment, or No treatment) in OSA studies when assessing neuropsychological function,39 this review did not exclude unblinded studies.
Finally the present paper included studies in which controls were screened using PSG or with questionnaires. Despite the risk of undetected OSA in the control sample,40 evidence from Wallace and Bucks16 suggested that comparing OSA participants with controls within memory domains, with and without PSG screening of controls, did not dramatically reduce the significance of the effects found, and would have reduced the number of studies available per subcomponent. Rather, the present meta-analysis considered control screening method as a moderator instead.
Quality Assessment
The authors (MO, RSB) independently reviewed articles according to the selection criteria. Where there were disagreements about whether or not to include an article, the authors discussed and came to an agreement.
The data for all included studies were extracted and coded by the first author. The second author extracted and coded the data for 10 randomly selected studies. The intra-class correlation coefficient between the data extracted by the first and second author was r = 0.99 (CI: 0.99-1.00).
Study Categorization
Included studies (N = 35; 34 studies + 1 personal communication) were divided into two non-exclusive categories: (1) comparisons of pre-treatment OSA groups to controls were used to identify the specific pattern of executive dysfunction present in untreated OSA (n = 21), and (2) CPAP treatment efficacy studies were used to establish whether executive impairments were permanent in OSA (n = 19): 5 studies met criteria for inclusion in both groups.
Categorization of Executive Function
Table 1 presents the subdomains of executive function and the tests ascribed to these subdomains.
Table 1.
The five subdomains of executive function and the tests that measure these facets that were utilised in the studies included in the present meta-analysis
Data Extraction and Analysis
Data extracted and coded from the final articles included author/s, whether published or not, journal and year of publication (if applicable), study design, sample size and participant details when available (gender, years of formal education, body mass index [BMI], age, diagnostic criteria, AHI or RDI, oxygen desaturation indices [time spent below 90%: CT90] and sleep fragmentation indices [Arousal Index: ArI]), source of OSA sample (clinical vs. community) and neuropsychological assessments employed (see Appendix 1 for further details). Means, standard deviations and sample size were extracted to examine the relationships between the variables of interest.
In the instance that participants had been assessed at multiple time points after CPAP treatment we chose the most distant time point, as we wanted to examine the effect of continuous treatment on executive dysfunction. Furthermore, if participants were divided into compliant (> 4 h for 80% of nights) and noncompliant users, only the compliant user information was included. These 2 decisions were made so as best to evaluate the benefit to individuals who utilize their CPAP devices as recommended over the long term. These choices led us to lose only 1 subsample from a paper, but no whole papers.
Data Processing
The program Comprehensive Meta-Analysis version 2.2.06441 was used to synthesize data, calculate effect sizes, and create forest plots.
RESULTS
Description of Studies
From the articles identified (N = 35), 21 studies compared people with untreated OSA to a control sample, 19 compared people with OSA before and after treatment; 5 studies had both a comparison to controls participants and neurocognitive testing performance before and after treatment. These studies represent 40 samples. Only 1 study was recruited from a community setting,42 thereby making 98% of studies from clinical settings.
In total, there were 551 healthy controls (74% male; mean age 49.46 ± 8.96 years; mean ESS 5.52 ± 2.41; mean BMI 25.42 ± 2.50) and 1,010 participants with OSA (81% male; mean age 50.40 ± 7.43 years; mean AHI/RDI 47.58 ± 15.98; mean arousal index (ArI) 36.15 ± 17.66; mean cumulative time below 90% oxygen saturation (CT90) 40.07 ± 28.55; mean education 13.73 ± 1.48 years; mean months of CPAP treatment 2.89 ± 2.22 months; mean hours CPAP use per night 5.34 ± 1.01; mean Epworth Sleepiness Scale (ESS) score 12.02 ± 2.38; mean body mass index (BMI) 33.12 ± 2.76. Individual study details for sample size, publication source, age, indices of disease severity (AHI or RDI), oxygen desaturation (CT90), and sleep fragmentation (ArI), years of education, and length of CPAP treatment are given in Appendix 1.
In the present meta-analysis, only studies with matched control and OSA participant variables were chosen, except that as expected, the control and OSA groups differed significantly in BMI, t(1228) = -56.03, P < 0.001, and ESS, t(1118) = -51.15, P < 0.001 scores.
Calculation of Effect Sizes
Random effect sizes were calculated. The random effects model assumes that each study has a different underlying “true” effect size due to differing sample demographic variables.43 In the present meta-analysis, samples differed on such variables as disease severity, age, gender, screening measures, oxygen saturation, and sleep fragmentation (for full descriptive details of each study see Appendix 1). A random effects model accounts for these between-studies differences, as well as within-study participant differences.
An effect was calculated for each sample across each of the 5 domains. For comparisons between the OSA group and healthy controls, Cohen's d was calculated according to the following formula:
 |
where effect sizes of d ≤ 0.20 are considered small, d = 0.50 medium, d ≥ 0.80 large and d ≥ 1.00 very large.44 Larger, positive effects indicate poorer performance for the OSA group.
In OSA group pre-treatment compared to post-treatment, Cohen's d effect sizes were calculated with the following formula:
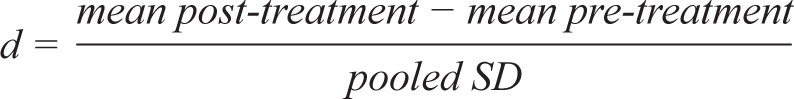 |
Higher scores indicate greater improvement post CPAP treatment.
The random effect size estimates between OSA and control groups for each domain are displayed in Table 2. All 5 subdomains of executive function were impaired, compared to controls. A very large effect was noted for Inhibition, a large effect was present for Updating and Fluid Reasoning, and medium effect sizes were present for Shifting and Generativity. These results indicate medium to very large deficits in executive function performance across all 5 domains in individuals with OSA, when compared to control.
Table 2.
Mean effect sizes for the differences between OSA to control groups, and pre-treatment to post-treatment groups
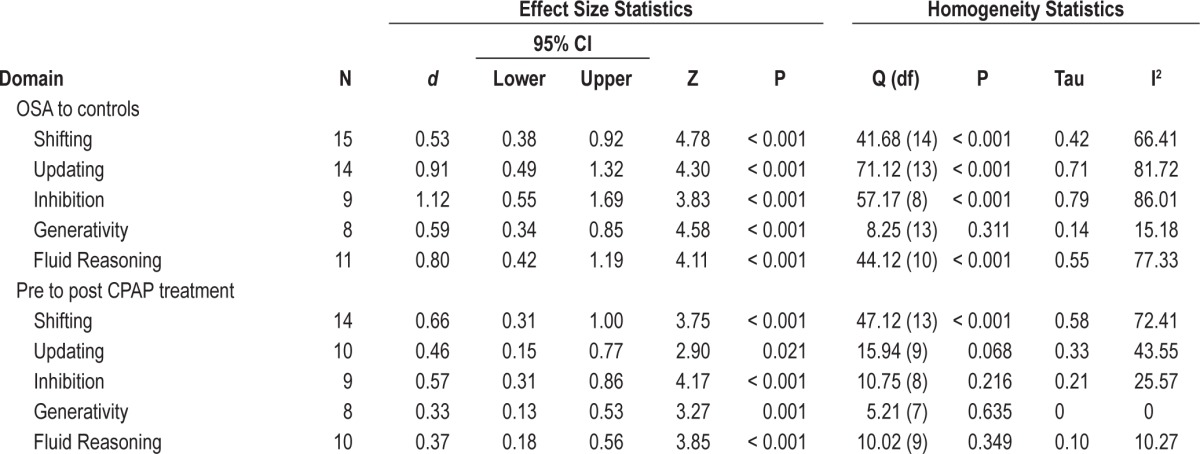
The random effect size estimates between OSA pre-treatment and post-treatment are displayed in Table 2. All 5 subdomains of executive function demonstrated improvement after CPAP treatment. Medium effect sizes were found in Shifting and Inhibition, and small effect sizes were noted in Updating, Fluid Reasoning, and Generativity. These results indicate small to medium size improvements across all 5 domains of executive function with CPAP treatment.
Forest plots for individual studies are available in Appendices 2 and 3.
Heterogeneity
Heterogeneity of effect sizes is a measure of difference between a study's true effect size and the observed effect size. The true effect size is the actual effect size in the underlying population, while the observed effect size is the effect measured in the sample.45 Heterogeneity was investigated visually with forest plots (Appendices 2 and 3), and statistically using Cochrane's Q statistic, the T2 and I2 statistics (Table 2). When the Q statistic is significant, this suggests there is a significant difference between the observed and true effect. However the Q statistic is vulnerable to small sample size, hence T2 and I2 can provide an estimate of the proportion of real variance caused by extraneous study variables such as age or test used.45 In any instance that Q was significant, I2 was examined to quantify the degree of heterogeneity.
For the comparisons between controls and individuals with OSA, significant heterogeneity was present in all domains. However, the I2 ranged between 77.33 and 86.01, suggesting at least 77% of the variance was generated from real, between-group differences.
For the comparisons of pre- and post-treatment, significant heterogeneity was present in the domain, Shifting. However, the I2 was 72.41 suggesting at least 72% of the variance was generated from real, between-time differences.
Moderator analysis, using a random effects model, was conducted to explore potential, between-study differences that may explain this heterogeneity.
Moderator Analysis
Pre-treatment OSA to Controls
Moderators investigated were age (Group 1 < 0, Group 2 ≥ 50 years), stringent inclusion/exclusion criteria (Group 1 = no, Group 2 = yes), control group selection criteria (Group 1 = PSG, Group 2 = Questionnaire), and publication status (Group 1 = Published, Group 2 = Not published). Papers were considered to have stringent inclusion/exclusion criteria if they excluded participants with factors that could potentially affect cognition, such as a history of traumatic brain injury, certain medications or diseases. None of these moderators changed the effect significantly.
We were unable to examine the impact of disease severity as measured by AHI as there were insufficient studies reporting effects of moderate OSA (AHI 15-29) in each of the 5 subdomains. A reviewer suggested dividing only those with AHI ≥ 30 into 2 groups and rerunning analyses. Accordingly, we divided the samples into severe OSA (AHI 30-50, N = 15) and very severe OSA (AHI 51+, N = 17). Where there were sufficient samples (i.e., 2 or more) for each executive domain, all effects remained significant and severity did not moderate the findings.
Pre-Treatment to Post-Treatment Differences
Between-study differences were also investigated using moderator analysis. Moderators were age, stringent inclusion/ exclusion criteria, control selection criteria (PSG or questionnaire), length of CPAP use, and publication status. No variables significantly moderated the effect of CPAP treatment on the executive burden of OSA.
Likewise, disease severity, as measured by AHI, could not be examined as there were insufficient studies reporting effects of moderate OSA in each of the 5 subdomains. As above, as recommended by a reviewer, we explored the impact of severity within individuals with AHI ≥ 30. As before, all effects remained significant, and severity did not moderate the findings.
Furthermore, we were unable to examine the impact of CPAP compliance on effect sizes as only one study divided the participants into compliant and noncompliant users; all other studies excluded noncompliant individuals or reported only the group mean compliance which was always above 4 h.
However, and as recommended by a reviewer, we explored months on CPAP by dividing samples into short (0-5 months, N = 13) and long (≥ 5.5 months, N = 5) term CPAP use. Where there were sufficient samples (i.e., ≥ 2) for each executive domain, all effects remained significant and months on CPAP did not moderate the findings.
Risk of Publication Bias
There is evidence to suggest that studies with a significant result are more likely to be published, and that published studies are more likely to be available for meta-analysis.45 Publication bias was inspected visually using funnel plots. These were asymmetrical, indicating the presence of bias. The Egger test for asymmetry46 was used to investigate this further.
Pre-treatment OSA to Controls
For the OSA to control samples, the Egger test was nonsig-nificant for Inhibition (intercept 4.79; 95% CI: 0.04 to 9.54; P = 0.05), and Updating (intercept 5.84; 95% CI: -0.63 to 12.32; P = 0.07), but was significant for Fluid Reasoning (intercept 4.49; 95% CI: 0.84 to 8.13; P = 0.02), Generativity (intercept 4.55; 95% CI: 0.65 to 8.45; P = 0.03) and Shifting (intercept 4.48; 95% CI: 1.72 to 7.25; P = 0.002). However, Rosenthal's fail-safe N, which represents the number of studies needed to create an overall nonsignificant effect,47 was 172 nonsignificant studies for Fluid Reasoning, 49 studies for Generativity, and 232 studies for Shifting.
Duval and Tweedie's Trim and Fill procedure48 was used to determine the best estimate of an unbiased, overall effect size for the OSA (cf. control samples for the domains of Fluid Reasoning, Generativity, and Shifting). For Fluid Reasoning, the overall effect size was reduced from a large effect of 0.80 to a small effect of 0.26. For Generativity, the overall effect size shifted from a medium effect of 0.59 to a small effect of 0.45. For Shifting, the overall effect size was reduced from a medium effect of 0.53 to a small effect of 0.37. This suggests publication bias may be inflating the estimates in the domains of Fluid Reasoning, Generativity, and Shifting, but that there were still significant differences between those with OSA and controls in these domains.
Pre-Treatment to Post-Treatment Differences
For the pre- to post-treatment effects, the Egger test was nonsignificant for all 5 domains; Shifting (intercept 1.62; 95% CI: -1.39 to 4.63; P = 0.26), Generativity (intercept 0.70; 95% CI: -1.16 to 2.56; P = 0.39), Fluid Reasoning (intercept 0.17; 95% CI: -1.43 to 1.76; P = 0.82), Inhibition (intercept 8.61; 95% CI: -8.29 to 25.52; P = 0.27), and Updating (intercept 0.86; 95% CI: -12.73 to 14.45; P = 0.89). This indicates no publication bias in these domains.
DISCUSSION
The current paper builds on previous reviews by focusing on executive function within five theoretically driven subcomponents. Three questions were posed: (1) which specific executive functions are affected by the presence of untreated OSA?; (2) if executive functions are impaired, does treatment help to remediate these deficits?; and (3) are any of these effects moderated by publication status, sample source, study design, age, disease severity, treatment length or control screening?
Findings of the Present Review
The results from the present analysis indicate that executive function is impaired in OSA compared to control participants across all five subcomponents. People with OSA have difficulty Shifting between tasks or mental sets, Updating and monitoring working memory representations, Inhibiting dominant or prepotent responses, they struggle with Generating new information without external input or efficiently accessing long term memory, and they have significant problems with Fluid Reasoning or problem solving. Further to this, the present research demonstrated that if participants undertake CPAP treatment, executive function difficulties across these five subdomains are reduced.
This meta-analysis was unable to assess the impact of CPAP compliance on improvement in executive function. Articles assessed in the present review excluded individuals who were not compliant with treatment, or reported only the group mean number of hours CPAP was used, except in one instance where participants were divided into compliant or noncompliant; hence, we cannot make any concluding statement on improvements in executive function in individuals who do not follow their treatment regime optimally. However, exploration of the impact of months of CPAP use revealed no additional gain with extended use (6 months or more).
A recent review49 summarized the current understanding of cognitive function across a number of domains including executive function. The authors found that in two reviews10,17 and two meta-analyses11,50 meeting inclusion criteria, executive function was impaired by comparison with controls and norms. The present results provide further support that executive function is impaired in people with OSA. Furthermore this review49 examined improvement in executive function following CPAP treatment in one meta-analysis10 and one literature review.17 The reviews examined came to opposing conclusions; hence, the summary was inconclusive. The present meta- analysis suggests that, overall, CPAP treatment is successful in improving executive function difficulties caused by OSA, and adds to the available evidence supporting the benefits of CPAP treatment.
Past reviews have grouped executive function into one combined domain, or collapsed them by test, making it impossible to delineate the subcomponents of executive function that are impaired. The present paper views executive dysfunction within current neuropsychological understanding of executive function. Such a framework provides a possible explanation for relationship or work difficulties seen in OSA. Individuals with OSA may experience relationship51,52 or work productivity difficulties,5,53 as they may not be able to inhibit inappropriate responses to aggravating social situations, or solve novel problems in a work place with Fluid Reasoning.
Effect of Moderators
The present study was not able to examine the impact of disease severity on OSA across the full range of AHI, as there were insufficient numbers of mild (AHI 5-14) and moderate samples (AHI 15-29). However, comparison of severe (AHI 30-50) and very severe (AHI 50+) OSA samples revealed no impact of severity on the deficits found, and no impact on executive consequences of CPAP treatment. The literature is divided with regard to whether there is a relationship between disease severity as measured by AHI and cognitive dysfunction.7,10,54 In a recent meta-analysis of episodic memory function, Wallace and Bucks16 found no relationship with disease severity. In a systematic review, Aloia et al.10 found no relationship between disease severity and executive function; however, they did find a positive relationship between disease severity and global cognitive function and attention/vigilance. As yet, the link between OSA disease severity and cognition is unclear. This is most likely due to the complex picture of comorbidity, and as yet, no definitive way of measuring disease onset in OSA.55
Previous studies have demonstrated that age and OSA results in a double burden, with older individuals exhibiting poorer cognition.56,57 The present meta-analysis did not find this same relationship, as age did not significantly moderate the results. In studies comparing older and younger participants with OSA, older adults have been found to be more impaired on tests of executive functioning.56,57 The lack of effect in the current meta-analytic review may be due to assessing the effect of age using group averages, which resulted in similar age distributions across samples. Primary comparison studies which explore the interaction of age and OSA on these executive function subcom-ponents will be important for clarifying this relationship.
Furthermore, selection of controls with PSG or questionnaires did not significantly moderate the findings. This result may seem surprising, given that estimates of undiagnosed OSA are high.1,2,58 However, this finding is consistent with that recently reported by Wallace and Bucks.16 While it is still the case that some control participants in primary studies may have undiagnosed OSA, including studies which have used questionnaire screening procedures does not appear to confer a risk of failing to find an effect in meta-analyses in OSA.
Heterogeneity among Results
Many of the domains demonstrated a high level of heterogeneity in the test results, indicating that there may be other factors in each domain influencing the observed mean. However, further analysis demonstrated this heterogeneity did not obscure real differences in each subdomain of EF. The domain that exhibited the most heterogeneity was the domain with the largest number of different tests, Fluid Reasoning, in which there were thirteen different tests. Neurocognitive tests, especially executive function tests, even purportedly measuring the same domain or sub-domain, will also capture facets of other cognitive or motor skills. For example Trails B, a measure of Shifting, also requires attention and taps into psychomotor speed.13 Furthermore, few tests currently used to assess executive function were originally designed for the specific purpose of measuring executive function.18,21
Future research exploring the executive dysfunction of OSA may benefit from selecting measures more closely targeted at executive functions and designed to fractionate performance into theoretically driven and dissociable subcomponents. One such measure is the random number generation (RNG) task.59 This task takes only a few minutes, is easily administered with a laptop computer, and provides measures of Shifting and Inhibition.20,59
One other factor that might lead to heterogeneity is premorbid IQ or intelligence. This is because greater IQ and/ or education appears to provide “protection” against cognitive decline because of greater cognitive reserve.60,61 Unfortunately, not all studies provided a measure of academic achievement or premorbid intelligence (IQ). Given that age decreases reserve and IQ increases it, primary studies that stratify the sample into age and IQ groups when examining the impact of OSA on executive function are needed.
Limitations
An issue that cannot be addressed by the current review is whether the deficits evidenced in the literature are primary or secondary; i.e., whether the deficits found in OSA in executive function are due to neurological damage (primary effect) or to impairments in attention which themselves are the result of sleep fragmentation, sleep deprivation and the associated excessive daytime sleepiness.62,63 Given evidence of frontal activation in participants completing these tasks64 and the presence of struc-tural abnormalities in the frontal lobes of individuals with OSA,65 it seems likely that these executive function deficits are a primary and direct consequence of OSA (see Beebe and Gozal66 for a review; but see Durmer and Dinges67 for evidence that similar frontal changes are also seen post sleep loss). Indeed, one study,62 which controlled for attention deficits while exploring executive dysfunction differences between OSA and controls, concluded that most of the executive difficulties were secondary to attention problems. This study demonstrated that the one area with deficits remaining after controlling for attention was Shifting. More studies of this nature and the development of tasks that can tease apart the contributions of attention and executive cognitive processes to task performance are required.
CONCLUSIONS
People with OSA have difficulty with the executive facets of Shifting, Updating, inhibiting, Generativity, and with Fluid Reasoning. Further, the present research indicates that all these difficulties improve with CPAP treatment. Age and disease severity did not moderate the effects found, however, further studies are needed exploring the extent of primary and secondary effects, and the impact of age, and premorbid ability (cognitive reserve).
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Participant characteristics for each study for Controls, OSA, and Treatment samples
Forest plots of the effect size and confidence intervals for each study for pre-treatment OSA participants compared with controls studies in all 5 subdomains of executive function (A) Generativity, (B) Fluid Reasoning, (C) Inhibition, (D) Shifting and (E) Updating. SD, standard difference; SE, standard error; LL, lower limit; UL, upper limit.
Forest plots of the effect size and confidence intervals for pre-treatment to post-treatment studies in all 5 sub-domains of executive function (A) Generativity, (B) Fluid Reasoning, (C) Inhibition, (D) Shifting and (E) Updating. SD, standard difference; SE, standard error; LL, lower limit; UL, upper limit.
SUPPLEMENTAL REFERENCES
- 1.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia MS, Knoepke CE, Lee-Chiong T. The new local coverage determination criteria for adherence to positive airway pressure treatment; testing the limits? Chest. 2010;138:1–8. doi: 10.1378/chest.09-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182:413–9. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey GL. Neuropsychological functioning, sleep and vigilance in men with obstructive sleep apnea syndrome treated with continuous positive airway pressure. The Florida State University. 1993 [Google Scholar]
- 5.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Bédard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP) J Clin Exp Neuropsychol. 1993;15:330–41. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 8.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 9.Castronovo V, Canessa N, Ferini-Strambi L, et al. Brain activation changes before and after pap treatment in obstructive sleep apnea. Sleep. 2009;32:1161–72. doi: 10.1093/sleep/32.9.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daurat A, Foret J, Bret-Dibat JL, Fureix C, Tiberge M. Spatial and temporal memories are affected by sleep fragmentation in obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2008;30:91–101. doi: 10.1080/13803390701236116. [DOI] [PubMed] [Google Scholar]
- 11.Dolan DC. Cognitive dysfunction in middle-aged adults vs. older adults with obstructive sleep apnea. Texas: University of Texas; 2009. p. 104. [Google Scholar]
- 12.Engleman HM. Daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome (SAHS) Edinburgh: University of Edinburgh; 1995. [Google Scholar]
- 13.Ferini-Strambi L, Baietto C, Di Gioia MR. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 14.Findley LJ, Presty SK, Barth JT, Suratt PM. Impaired cognition and vigilance in elderly subjects with sleep apnea. In: Kuna ST, Suratt PM, Remmers JE, editors. Sleep and respiration in aging adults. New York: Elsevier; 1991. pp. 259–65. [Google Scholar]
- 15.Froehling B. Neuropsychological dysfunction in sleep apnoea syndrome: response to nasal CPAP. Chicago: University of Health Sciences; 1991. [Google Scholar]
- 16.Gale S, Hopkins RO. Effects of hypoxia on the brain: Neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 17.Gast H, Schwalen S, Ringendahl H, Jorg J, Hirshkowitz M. Sleep-related breathing disorders and continuous positive airway pressure-related changes in cognition. Sleep Med Clin. 2006;1:499–511. [Google Scholar]
- 18.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnoea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 19.Grenèche J, Kreiger J, Bertrand F, Erhardt C, Maumy M, Tassi P. Short-term memory performances during sustained wakefulness in patients with obstructive sleep apnea-hypopnea syndrome. Brain Cogn. 2011;75:39–50. doi: 10.1016/j.bandc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Disord. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 21.Lojander J, Kajaste S, Maasilta P, Partinen M. Cognitive function and treatment of obstructive sleep apnea syndrome. J Sleep Res. 1999;8:71–6. doi: 10.1046/j.1365-2869.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu A, Mazza S, Décary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9:112–20. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 23.McGovern TJ. Virginia: Norfolk State University, Old Dominion University; 2007. Clinical utility of the Alzheimers quick test: comparison of the AQT, TMT, COWA, and MMSE for distinguishing mild/moderate dementia, sleep apnea and controls. [Google Scholar]
- 24.Meurice JC, Marc I, Series F. Efficacy of Auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1996;153:794–8. doi: 10.1164/ajrccm.153.2.8564134. [DOI] [PubMed] [Google Scholar]
- 25.Naëgelé B, Thouvard V, Pepin JL, et al. Deficits of cognitive executive functions in patients with sleep apnoea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 26.Neu D, Kajosch H, Peigneux P, Verbanck P, Linkowski P, Le-Bon O. Cognitive impairment in fatigue and sleepiness associated conditions. Psychiatry Res. 2011;189:128–34. doi: 10.1016/j.psychres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 29.Rouleau I, Deary IJ, Chicoine AJ, Montplaisir J. Procedural skill learning in obstructive sleep apnea syndrome. Sleep. 2002;25:398–498. [PubMed] [Google Scholar]
- 30.Saunamäki T, Himanen S-L, Polo O, Jehkonen M. Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Eur Neurol. 2010;63:215–20. doi: 10.1159/000278301. [DOI] [PubMed] [Google Scholar]
- 31.Sharma H, Sharma SK, Kadhiravan T, et al. Pattern and correlates of neurocognitive dysfunction in Asian Indian adults with severe obstructive sleep apnoea. Indian J Med Res. 2010;132:409–14. [PubMed] [Google Scholar]
- 32.Sloan KA. Psychology. Missouri: Washington University; 1989. Neuropsychological function in obstructive sleep apnea; p. 121. [Google Scholar]
- 33.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verstraeten E, Cluydts R, Pevemagie D, et al. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep. 2004;27:685–93. [PubMed] [Google Scholar]
- 35.Walker CP. Neuropsychological changes in obstructive sleep apnoea syndrome patients following nasal CPAP treatment. Birmingham: University of Alabama; 1990. [Google Scholar]
- 36.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. 2005;5:13. doi: 10.1186/1471-2288-5-13. (Article not included in the meta-analysis.) [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Al-Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnoea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–93. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Butkov N, Lee-Chiong T. Fundamentals of sleep technology. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 3.Young T, Palta N, Dempsey J. The occurrence of sleep disordered breathing among middle-aged adults. N Eng J Med. 1993;32:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ghanim N, Comondore VR, Fleetham J, et al. The economic impact of obstructive sleep apnoea. Lung. 2008;186:7–12. doi: 10.1007/s00408-007-9055-5. [DOI] [PubMed] [Google Scholar]
- 5.Hillman DR, Murphy AS, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 6.Sassani A, Findley LJ, Kryger MD, et al. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnoea. Sleep. 2004;27:453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JC. Neurological and neurobehavioural sequelae of obstructive sleep apnea. Neurorehabilitation. 2010;26:85–94. doi: 10.3233/NRE-2010-0538. [DOI] [PubMed] [Google Scholar]
- 8.Kryger MH. Atlas of clinical sleep medicine. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 9.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aloia MS, Arnedt T, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 11.Beebe DW, Groesz L, Wells C, et al. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Adrover-Roig D, Sesé A, Barceló F, et al. A latent variable approach to executive control in healthy ageing. Brain Cogn. 2012;78:284–99. doi: 10.1016/j.bandc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 14.Elliot R. Executive functions and their disorders. Br Med Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Larner AJ. Neuropsychological neurology: the neurocognitive impairments of neurological disorders. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 16.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36:203–20. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunamäki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 18.Banich MT. Executive function: the search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. [Google Scholar]
- 19.Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–65. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 20.Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 21.Suchy Y. Executive functioning: overview, assessment, and research issues for non-neuropsychologists. Ann Behav Med. 2009;37:106–16. doi: 10.1007/s12160-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 22.Chan RC, Chen EY, Cheung EF, et al. The components of executive functioning in a cohort of patients with chronic schizophrenia: A multiple single-case study design. Schizophr Res. 2006;81:173–89. doi: 10.1016/j.schres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26:874–90. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Chan RCK, Zheng L, et al. Executive functioning in healthy elderly Chinese people. Arch Clin Neuropsychol. 2007;22:501–11. doi: 10.1016/j.acn.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery C, Fisk JE, Newcombe R, et al. The differential effects of ecstasy/polydrug use on executive components: shifting, inhibition, updating and access to semantic memory. Psychopharmacology. 2005;182:262–76. doi: 10.1007/s00213-005-0065-9. [DOI] [PubMed] [Google Scholar]
- 26.Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. Oxford: Oxford University Press; 2006. [Google Scholar]
- 27.AASM Task Force. Sleep related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 28.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan DC, Livingston E. Obstructive sleep apnoea syndrome and weight loss: review. Sleep Disord. 2012;2012:163296. doi: 10.1155/2012/163296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong CW, O'Driscoll DM, Truby H, et al. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17:123–31. doi: 10.1016/j.smrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Cheng HC, Dai ZK, Wu JR, et al. Pediatric upper airway emergencies. Taiwan Society of Pediatric Pulmonology. 2012:25–31. [Google Scholar]
- 32.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Resp Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 33.Phillips BA, Schmitt FA, Berry DT, et al. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest. 1990;98:325–30. doi: 10.1378/chest.98.2.325. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan CE, Berthon-Jones M, Issa FG, et al. Reversal of obstructive sleep apnea by continuous positive airway pressure applied through the nares. Lancet. 1981;8225:862. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan CE, Issa FG. Obstructive sleep apnea. Philadelphia: WB Saunders; 1985. [Google Scholar]
- 36.Estrada A, Kelley AM, Webb CM, et al. Modafinil as a replacement for dextroamphetamine for sustaining alertness in military helicopter pilots. Aviat Space Environ Med. 2012;83:556–67. doi: 10.3357/asem.3129.2012. [DOI] [PubMed] [Google Scholar]
- 37.Ray K, Chatterjee A, Panjwani U, et al. Modafinil improves event related potentials P300 and contingent negative variation after 24 h sleep deprivation. Life Sci. 2012:94–9. doi: 10.1016/j.lfs.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Resp Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 39.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 40.Sharwood LN, Elkington J, Stevenson M, et al. Assessing sleepiness and sleep disorders in Australian long-distance commercial vehicle drivers: self-report versus an “at home” monitoring device. Sleep. 2012;35:469–75. doi: 10.5665/sleep.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2. Biostat: Englewood NJ; 2005. [Google Scholar]
- 42.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal R. Writing meta-analytic reviews. Psychol Bull. 1995;118:183–92. [Google Scholar]
- 44.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 45.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. West Sussex, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 46.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:185–93. [Google Scholar]
- 48.Duval S, Tweedie R. Trim and fill: A simple funnel-plot based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 49.Bucks R, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 50.Fulda S, Schulz H. Cognitive dysfunction in sleep-related breathing disorders: a meta-analysis. Sleep Res Online. 2003;5:19–51. [Google Scholar]
- 51.Kales A, Caldwell AB, Cadieux RJ, et al. Severe obstructive sleep apnea—II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 52.Engleman HM, Douglas NJ. Sleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9:42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Carlson BW, Neelon VJ, Carlson JR, et al. Cerebral oxygenation in wake and during sleep and its relationship to cognitive function in community-dwelling older adults without sleep disordered breathing. J Gerontol. 2011;66A:150–6. doi: 10.1093/gerona/glq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valencia-Flores M, Bliwise DL, Guilleminault C, et al. Cognitive function in patients with sleep apnea after acute nocturnal nasal continuous positive airway pressure (CPAP) treatment: sleepiness and hypoxemia effects. J Clin Exp Neuropsychol. 1996:197–210. doi: 10.1080/01688639608408275. [DOI] [PubMed] [Google Scholar]
- 56.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182:413–9. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonelli-Incalzi R, Marra C, Salvigni BL, et al. Does cognitive dysfunction conform to a distinctive pattern in obstructive sleep apnea syndrome? J Sleep Res. 2004:79–86. doi: 10.1111/j.1365-2869.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 58.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 59.Maes JH, Eling PA, Reelick MF, et al. Assessing executive functioning: On the validity, reliability, and sensitivity of a click/point random number generation task in healthy adults and patients with cognitive decline. J Clin Exp Neuropsychol. 2011;33:366–78. doi: 10.1080/13803395.2010.524149. [DOI] [PubMed] [Google Scholar]
- 60.Alchanatis M, Zias N, Deligiorgis N, et al. Sleep apnea-related cognitive deficits and intelligence: an implication of cognitive reserve theory. J Sleep Res. 2005;14:69–75. doi: 10.1111/j.1365-2869.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 61.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- 62.Verstraeten E, Cluydts R, Pevemagie D, et al. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep. 2004;27:685–93. [PubMed] [Google Scholar]
- 63.Verstraeten E, Cluydts R. Executive control of attention in sleep apnea patients: theoretical concepts and methodological considerations. Sleep Med Rev. 2004;8:257–67. doi: 10.1016/j.smrv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Lin M, Shunwei L, et al. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnea. Sleep Med. 2011;12:335–40. doi: 10.1016/j.sleep.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–71. [PubMed] [Google Scholar]
- 66.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 67.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Participant characteristics for each study for Controls, OSA, and Treatment samples
Forest plots of the effect size and confidence intervals for each study for pre-treatment OSA participants compared with controls studies in all 5 subdomains of executive function (A) Generativity, (B) Fluid Reasoning, (C) Inhibition, (D) Shifting and (E) Updating. SD, standard difference; SE, standard error; LL, lower limit; UL, upper limit.
Forest plots of the effect size and confidence intervals for pre-treatment to post-treatment studies in all 5 sub-domains of executive function (A) Generativity, (B) Fluid Reasoning, (C) Inhibition, (D) Shifting and (E) Updating. SD, standard difference; SE, standard error; LL, lower limit; UL, upper limit.