Abstract
Study Objectives:
Slow wave sleep (SWS) plays a pivotal role in consolidating memories. Tiagabine has been shown to increase SWS in favor of REM sleep without impacting subjective sleep. However, it is unknown whether this effect is paralleled by an improved sleep-dependent consolidation of memory.
Design:
This double-blind within-subject crossover study tested sensitivity of overnight retention of declarative neutral and emotional materials (word pairs, pictures) as well as a procedural memory task (sequence finger tapping) to oral administration of placebo or 10 mg tiagabine (at 22:30).
Participants:
Fourteen healthy young men aged 21.9 years (range 18-28 years).
Measurements and Results:
Tiagabine significantly increased the time spent in SWS and decreased REM sleep compared to placebo. Tiagabine also enhanced slow wave activity (0.5-4.0 Hz) and density of < 1 Hz slow oscillations during NREM sleep. Fast (12-15 Hz) and slow (9-12 Hz) spindle activity, in particular that occurring phase-locked to the slow oscillation cycle, was decreased following tiagabine. Despite signs of deeper and more SWS, overnight retention of memory tested after sleep the next evening (19:30) was generally not improved after tiagabine, but on average even lower than after placebo, with this impairing effect reaching significance for procedural sequence finger tapping.
Conclusions:
Our data show that increasing slow wave sleep with tiagabine does not improve memory consolidation. Possibly this is due to functional differences from normal slow wave sleep, i.e., the concurrent suppressive influence of tiagabine on phase-locked spindle activity.
Citation:
Feld GB; Wilhelm I; Ma Y; Groch S; Binkofski F; Mölle M; Born J. Slow wave sleep induced by GABA agonist tiagabine fails to benefit memory consolidation. SLEEP 2013;36(9):1317-1326.
Keywords: SWS, sleep spindles, memory consolidation, GABA, tiagabine, skill memory
INTRODUCTION
There exists ample evidence that memory traces acquired during wakefulness rely on strengthening during sleep.1 This consolidation process is essentially supported by slow wave sleep (SWS). SWS enhances declarative memory (for episodes and facts)2,3 as well as procedural skill memories.4 The enhancing effect on memory consolidation appears to be medi-ated in particular by the neocortical < 1 Hz slow oscillation that is the hallmark of the EEG during SWS; it synchronizes the neuronal reactivation of newly acquired memory repre-sentations occurring during SWS in distributed networks to the excitable depolarizing up-state of these slow oscillations.5 This allows the redistribution of the reactivated memory repre-sentations and their stabilization for the longer term.1 Recent evidence suggests that procedural memory can also benefit from reactivation during NREM sleep and that this effect is related to sleep spindles.6 The memory-consolidating effect of the slow oscillations appears to additionally result from the fact that— in parallel with reactivations—they also synchronize thalamo-cortical spindles (12-15 Hz) to the depolarizing slow oscillation up-state.7,8 Post-learning spindle activity has been consistently found to be associated with the retention of declarative and procedural memories, especially when occurring during the depolarizing phase of the slow oscillation.9–12 Enwrapping reactivated memory information, spindles might provoke processes, like enhancing cellular calcium influx, in neocortical networks that prime plastic processes underlying the longer-term storage of the reactivated information in these networks.13
GABAergic mechanisms in the preoptic region of the hypo-thalamus contribute to the generation of NREM sleep and SWS.14 Time spent in sleep is proportional to the activity of GABA-producing neurons in the ventrolateral preoptic region of the hypothalamus.15 GABAA agonists generally enhance SWS and also slow wave activity (0.5-4.0 Hz, including < 1 Hz slow oscillations) during NREM sleep, although this enhancement can be accompanied by a reduction in spindle activity.16 Notably, these effects are opposite to those of benzodiazepines (and zolpidem) that are considered positive modulators of the GABAA receptor and increase spindle activity but reduce SWS or slow wave activity.16 While these discrepant effects are difficult to reconcile, they speak in favor of the use of agents nonspecifically increasing extracellular GABA for investigating the role of GABAergic tone in the regulation of sleep and memory, rather than specific GABA receptor agonists. Given this background, we tested the effect of the GABA reuptake inhibitor tiagabine on sleep and associated memory consolidation in healthy young volunteers. Tiagabine acts by selectively blocking the GABA-transporter GAT 117,18; it has been shown to improve sleep efficacy in healthy elderly, as it strongly increased SWS without affecting other sleep stages or subjective sleep parameters.19 With higher doses, it also decreases time in REM sleep.20 We expected that SWS-promoting effects of tiagabine would be associated with an enhanced overnight consolidation of memory, particularly declarative materials, which have proved to be highly sensitive to SWS in previous studies.3,21 We expected no benefits from tiagabine for overnight consolidation of procedural skills, which in previous studies have proved more sensitive to spindles than slow wave activity.11,22,23 As a control, we examined effects on the retention of emotional materials, which is known to profit from REM sleep.24–26
METHODS
Participants
Fourteen healthy young men aged 21.9 years (range 18-28 years) completed the study. Participants were nonsmoking and native German speakers. Only males were included to reduce variance, as cycling estradiol and progesterone levels in women can influence plasticity and GABAA receptors.27 They under-went a routine health examination prior to participation to exclude any mental or physical disease, did not take any medication at the time of the experiments, and reported a normal sleep-wake cycle. One additionally recruited participant did not complete the study due to adverse side effects. The participants were instructed to get up at 07:00 on experimental days, and during these days not to take any naps, not to ingest alcohol, and not to ingest caffeine-containing drinks (after 13:00). Before the experiment proper, participants took part in an adaption night under conditions of the experiment (including the placement of electrodes for polysomnographic recordings). The experiments were approved by the ethics committee of the University of Luebeck. Written informed consent was obtained from all participants prior to participation.
Design and Procedure
The study followed a randomized, double-blind, placebo-controlled within-subject crossover design. Participants took part in 2 experimental sessions scheduled ≥ 14 days apart. Both sessions were identical but for the oral administration of placebo or tiagabine (Gabitril 10 mg, Teva GmbH, Germany [plasma half-life 7-9 h, plasma maximum: 1-2.5 h after intake]).
On experimental nights, participants arrived at the laboratory at 19:30. Following preparation for EEG and polysomnographic recordings, the participants learned (between 21:00 and 22:30, always in the same order) neutral and emotional pictures, a declarative word-pair association task, and a procedural sequence finger-tapping task, with a 10-min break between each of the tasks. Although this protocol introduces potential order effects, this approach was chosen to increase standardization. Also, consolidation of declarative and procedural tasks can influence each other if performed back to back. However, this retroactive interference does not eliminate the beneficial effect of sleep on declarative or procedural memory28; to further minimize such effects, we introduced longer breaks between the tasks. After the learning phase and 30 min before lights were turned off (at 23:00) to enable sleep, the participants were orally administered a capsule containing either tiagabine or placebo. They were woken at 07:00 and left the lab. During the following day, participants engaged in their usual activities. They were instructed to refrain from any stressful mental or physical activities, and to keep a record of their activities during this day. In the evening they returned at 19:30, and retrieval of the memory tasks was tested—in reverse order of learning. At learning and recall tests of vigilance, mood and subjective sleepiness were performed to control these effects.
Polysomnography, Sleep Analysis, and EEG Power Analysis
The EEG was recorded continuously from electrodes (Ag-AgCl) placed according to the 10-20 System, referenced to 2 coupled electrodes attached to the mastoids. EEG signals were filtered between 0.16 and 35 Hz and sampled at a rate of 200 Hz using a BrainAmp DC (BrainProducts GmbH, Munich, Germany). Additionally, horizontal and vertical eye movements (HEOG, VEOG) and the electromyogram (chin) were recorded for standard polysomnography. Sleep architecture was deter-mined according to standard polysomnographic criteria using EEG recordings from C3 and C4.29 Scoring was carried out independently by 2 experienced technicians who were blind to the assigned treatment. Differences in scoring between the scorers were resolved by consulting a third experienced technician. For each night, total sleep time (TST), and time spent in the different sleep stages (wake; sleep stages 1, 2, 3, 4; SWS, i.e., sum of sleep stage 3 and 4; REM sleep) was calculated in minutes; the percentage of TST spent in these sleep stages was also calculated.
Average power spectra were calculated in Fz and Cz for all NREM sleep epochs of the whole night. Power spectra were calculated by Fast Fourier Transformations with a Hanning window on subsequent blocks of 2,048 data points (∼10.24 sec, 3 blocks per 30-sec epoch). Spectra were filtered by a 5-point moving average. In the averaged spectra, mean power was determined for the frequency bands of interest, i.e., the 0.5-1 Hz slow oscillation band, 1-4 Hz delta, 4-8 Hz theta, 9-12 Hz slow spindle and 12-15 Hz fast spindle frequency bands.
Spindles
Semiautomatic spindle detection was performed on an in-house program running in Matlab 2011a, which detects spindles by applying a standard algorithm,30 and calculates absolute spindle count and spindle density. In brief, first the peak frequency of fast spindle activity was assessed for each participant individually in the power spectra of sleep stage 2 (during placebo nights) as the frequency of the power maximum between 12-15 Hz. The signal was then band pass filtered ± 1.5 Hz around this peak frequency. A spindle was detected if the root mean square (RMS) of the filtered EEG signal was above the absolute spindle threshold for 0.5-3 sec. The absolute threshold for spindle detection was set for each participant individually at 1.5 standard deviations of the filtered RMS signal determined for sleep stage 2 of the placebo night, and was on average: 5.65 ± 0.44 μV.
Slow Oscillations
Detection of slow oscillations in NREM sleep was based on a standard algorithm described elsewhere in detail,30 and was performed for Fz and Cz. In a first step, the EEG was low-pass filtered at 30 Hz and down-sampled to 100 Hz. For the identification of large slow oscillations, a low-pass filter of 3.5 Hz was applied, and time points of positive to negative zero crossings were computed in the resulting signal. Then, the lowest and highest value between these time points were detected (i.e., one negative and one positive peak) for all intervals of positive to negative zero crossings with a length of 0.9 to 2 sec. The means of these values were calculated across the participant's 2 experimental nights, and those intervals were marked as slow oscillation epochs whose negative peak amplitude was lower than 1.25 times the mean negative peak and whose amplitude difference (positive peak minus negative peak) was larger than 1.25 times the mean amplitude difference. Averages of original EEG potentials were calculated for a ± 1.3-sec window around the peak of the negative half-wave of all detected slow oscillations. To analyze spindle activity that was phase -locked to slow oscillations, the average RMS activity in the slow (9-12 Hz) and fast (12-15 Hz) spindle bands was also calculated for the ± 1.3-sec windows around the negative slow oscillation peak.
Memory Tasks
The declarative verbal paired association task required learning a list of 40 pairs of semantically related words (e.g., clock-church). Different word lists were used on the participant's 2 experimental nights. During the learning phase, the word pairs were presented sequentially on a computer screen, each for 4 sec, separated by inter-stimulus intervals of 1 sec. After presentation of the entire list, performance was tested using a cued recall procedure, i.e., the first word (cue) of each pair was presented and the participant had to name the associated second word (response). The correct response word was then displayed for 2 sec, regardless of whether the response was correct, to allow re-encoding of the correct word pair. The cued-recall procedure was repeated until the participant reached a criterion of 60% correct responses. Retrieval at the end of the experimental session was tested using the same cued recall procedure as during the learning phase, except that no feed-back of the correct response word was given. Absolute differences between word pairs recalled at retrieval testing and on the criterion trial during learning served as a measure of over-night retention. Several studies have shown that consolidation of word pairs profits particularly from SWS.21,31
One hundred emotional and neutral pictures (taken from the International Affective Picture System)32 were used to measure emotional memory consolidation. 50 pictures of high arousal and negative valence were chosen for the emotional category, and 50 pictures of low arousal and medium valence were chosen for the neutral category. Sufficiently distinct pictures were chosen to enable unambiguous free recall. Twenty-five pictures of each category were presented to the participants on a computer screen for 4 sec each with an inter-stimulus interval of 1 sec. During recall, participants were asked to recall the pictures they had seen as accurately as possible and to record this in a written description that permitted assigning a single picture and to indicate each individual description with a number. These descriptions were then compared to the pictures and correct answers were used as score for emotional memory performance. Free recall was used as a dependent measure, as it appears to be more sensitive to the memory effects of sleep than recognition.33–35
The finger sequence tapping task was adopted from earlier studies, indicating very robust sleep-dependent improvements in this task.36 It requires the participant to press repeatedly one of two 5-element sequences (e.g., 4-1-3-2-4 or 4-2-3-1-4) with the fingers of the non-dominant hand on a keyboard as fast and as accurately as possible for 30-sec epochs interrupted by 30-sec breaks. The numeric sequence was displayed on the screen at all times to keep working memory demands at a minimum. A key press resulted in a white asterisk appearing underneath the current element of the sequence. Each 30-sec trial was scored for speed (number of correctly completed sequences) and errors. After each 30-sec trial, feedback was given about the number of correctly completed sequences and error rate. At learning, participants trained on twelve 30-sec trials. The average score for the last 3 of these trials was used to indicate learning performance. At retrieval after sleep, participants were tested on another 3 trials. Overnight changes in performance were calculated as absolute differences in speed and error rate between the 3 trials at retrieval and the last 3 trials at learning.
Effects unspecific to the actually learned sequence (e.g., general increases in reaction time) were measured by assessing performance on 3 blocks of a new sequence after recall of the trained sequence.
Reaction Times, Mood, and Sleepiness
Mean reaction times were assessed as a measure of vigilance in a 5-min version of the psychomotor vigilance task (PVT) 37 that required pressing a button as fast as possible whenever a bright millisecond clock presented on a dark computer screen started counting upward. After the button press, this clock displayed the reaction time. Mood was measured using the 10 positive and 10 negative items of the positive and negative affective schedule (PANAS): participants responded to items (e.g., “Do you momentarily feel scared?”) on a 5-point Likert scale ranging from 1 = “not at all” to 5 = “very much.” Subjective sleepiness was assessed with the 1-item Stanford Sleepi-ness Scale (SSS) ranging from 1 = “Feeling active, vital, alert, or wide awake” to 8 = “Asleep.” At the end of the experiment, participants were asked if they believed they had received an active agent or placebo.
Data Reduction and Statistical Analysis
Data from one participant were discarded because of poor sleep during the placebo night. For the finger-tapping task, data from one further participant were discarded because of low performance (< 2 SD from the mean) during the placebo session (including these data increased the reported effect). Sleep data from one participant could not be evaluated due to data loss in the tiagabine night (recorder malfunction). In one participant, spindles and slow oscillations could not be reliably evaluated due to EEG artifacts. Statistical analyses generally relied on analyses of variance (ANOVA; SPSS version 20.0.0 for Windows) including a repeated measures factor “Treatment” (tiagabine vs placebo), and for analysis of pictures an additional “Emotionality” factor, representing recall of neutral vs emotional pictures. Analyses of EEG measures included additional factors for “Topography” (representing the recording sites) and “Sleep stage” (stage 2 sleep, SWS). Significant ANOVA interactions were specified by post hoc t-tests. Degrees of freedom were corrected according to Greenhouse-Geisser where appropriate. The level of significance was set to P ≤ 0.05.
RESULTS
Memory
For the word-pair association task, retention of word pairs as indicated by the difference in recall at the retrieval phase minus immediate recall performance after the learning phase did not differ between the tiagabine and placebo condition (mean difference of recalled word pairs [± SEM] tiagabine: -2.17 [1.18], placebo: -1.58 [1.01], F < 0.14, P > 0.72, for respective effects of Treatment; Figure 1A). Also, numbers of recalled word pairs at retrieval testing did not differ between the treatment conditions (t11 = -0.30, P = 0.77). There were also no hints at any difference between the conditions during the learning phase (number of trials to criterion tiagabine: 1.92 [0.26], placebo: 2.33 [0.43], t11 = -1.33, P = 0.21, number of recalled words at criterion trials tiagabine: 27.50 [1.03], placebo: 27.50 [1.11], t11 = 0.00, P = 1).
Figure 1.
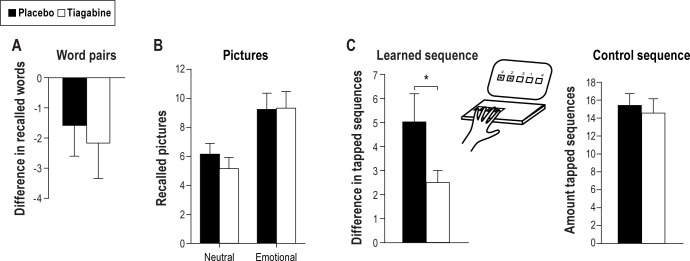
Mean (± SEM) of overnight retention of memories (A) for word pair associates, (B) for neutral and emotional pictures, and (C) for sequence finger tapping skills in the tiagabine (empty bars) and placebo condition (black bars). Retention of word pairs is indicated by the difference in the number of word pairs recalled at retrieval testing after sleep minus recall performance on the criterion trial at learning before sleep. Recall of pictures is indicated by the total number of pictures recalled during retrieval testing after sleep. Overnight gains in sequence finger tapping (C, left panel) are indicated by the difference in performance (number of correctly tapped sequences per 30-sec trial) at retrieval testing after sleep minus average performance on the last trials during training before sleep. Right panel indicates performance after sleep on a control sequence not trained before sleep. *P ≤ 0.05, for pairwise comparisons between the effects of the treatments (n = 12).
Memory for emotional and neutral pictures was also not significantly affected by tiagabine (number of recalled emotional pictures tiagabine: 9.33 [1.15], placebo: 9.25 [1.12] and neutral pictures tiagabine: 5.17 [0.78], placebo: 6.17 [0.74], F < 0.53, P > 0.48 for respective main and interaction effects of Treatment, Figure 1B). Emotional pictures were remembered better than neutral pictures in both conditions (F1,11 = 42.76, P ≤ 0.001).
For the sequence finger tapping task, the overnight gain expressed by the difference of correctly tapped sequences at recall minus performance at learning was significantly reduced by tiagabine (tiagabine: 2.50 [0.5] Placebo: 5.03 [1.17], F1,11 = 5.58, P ≤ 0.05, Figure 1C). At learning, the number of correctly tapped sequences did not differ significantly between the treatment conditions (tiagabine: 18.19 [1.56], placebo: 16.83 [1.13], t11 = -1.54, P = 0.15). Also, tapping on the control sequence did not reveal any difference between the tiagabine and placebo conditions (t11 = 0.47 and P = 0.65). Error rates were variable, and there was a trend toward error rates reducing more across sleep in the placebo condition, i.e., participants made fewer errors in the placebo condition (mean reduction in error rate tiagabine: -1.01% [1.25], placebo: -4.47% [3.25], t11 = 1.91, P = 0.08).
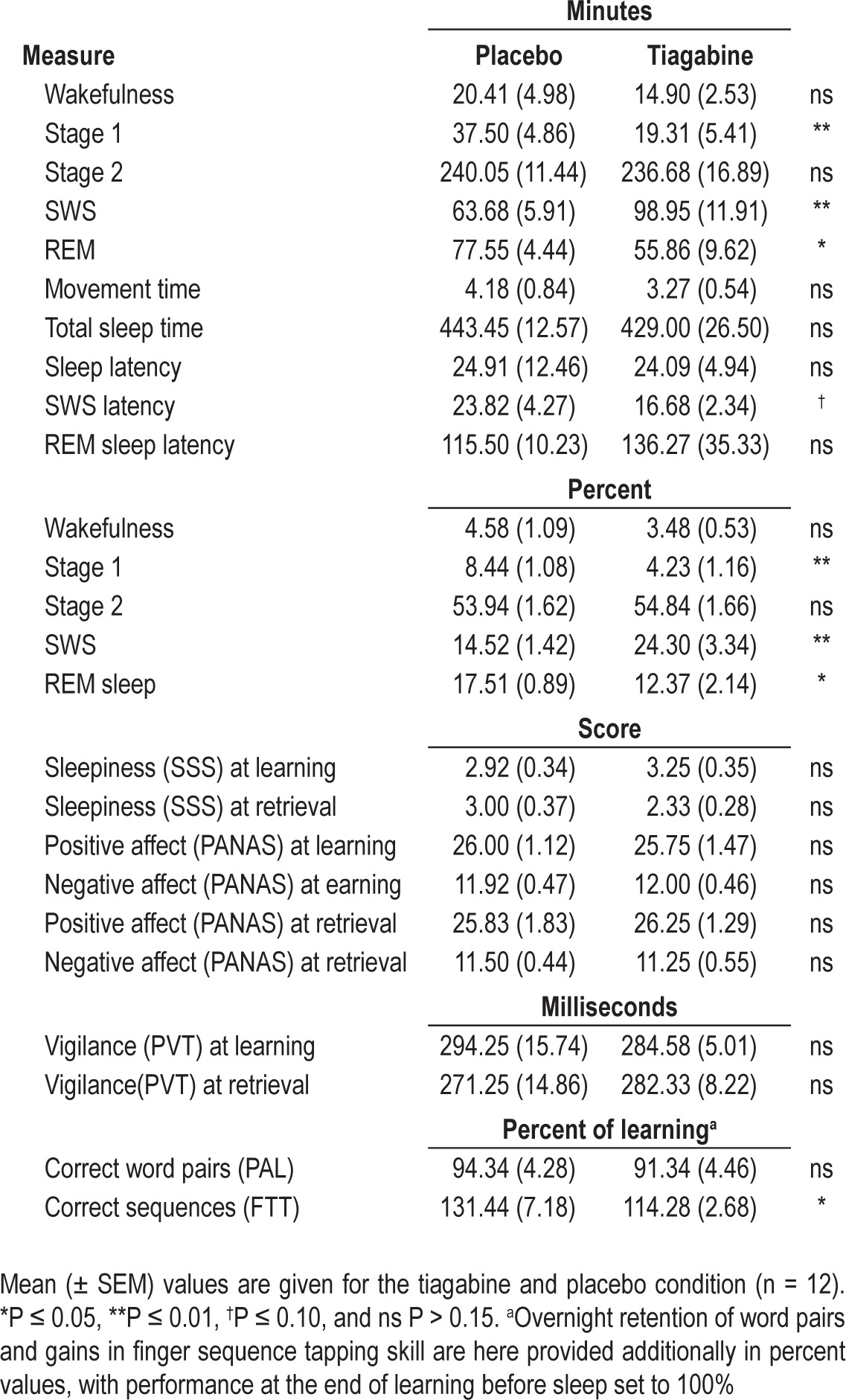
Sleep
Descriptive data for all sleep stages is provided in Table 1. During the tiagabine condition, participants spent distinctly more time in SWS (t10 = 3.10, P ≤ 0.01) but less time in stage 1 sleep (t10 = 3.46, P ≤ 0.01) than in the placebo condition. REM sleep was also reduced in the tiagabine condition (t10 = -2.54, P ≤ 0.05).
Table 1.
Sleep parameters and control measures
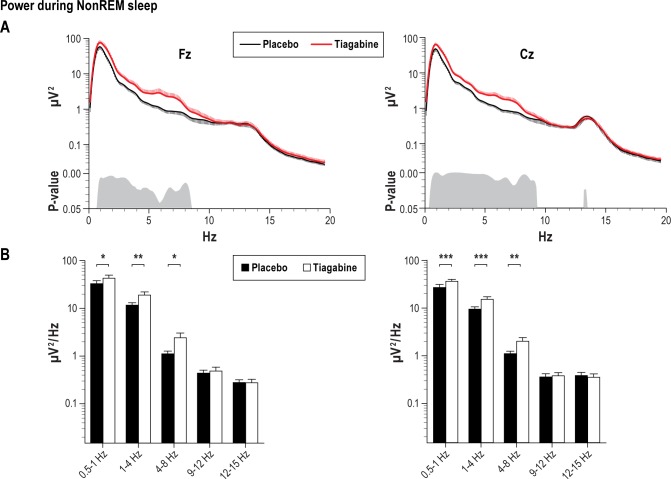
A more fine-grained analysis of EEG power during NREM sleep stage 2 and SWS indicated a significantly increased mean power density in the slow oscillation (0.5-1 Hz), delta (1-4 Hz), and theta (4-8 Hz) frequency bands during tiagabine than placebo (Figure 2A). All effects were apparent at Fz (slow oscillation: t9 = 3.01, P ≤ 0.05, delta: t9 = 11.64, P ≤ 0.01, theta: t9 = 2.35 and P ≤ 0.05, Figure 2B left) and Cz (slow oscillation: t9 = 5.22, P ≤ 0.001, delta: t9 = 4.58, P ≤ 0.001, theta: t9 = 3.76 and P ≤ 0.01, Figure 2B right). There were no significant differences between the treatment conditions for fast (12-15 Hz) and slow (9-12 Hz) spindle power (t9 < 1.69, P > 0.13).
Figure 2.
Mean (± SEM) power spectra of EEG signal during NonREM sleep at Fz (left) and Cz (right) for the tiagabine (red thick line) and placebo condition (black thin line). Bottom panels indicate significance between the effects of tiagabine and placebo. (B) Average power for frequency bands of interest: 0.5-1 Hz slow oscillation, 1-4 Hz delta, 4-8 Hz theta, 9-12 Hz slow spindle, and 12-15 Hz fast spindle bands. ***P ≤ 0.001, **P ≤ 0.01 and *P ≤ 0.05, for pairwise comparisons between the effects of the treatment (n = 10).
Analysis of discrete fast spindles, with power maxima between 13-14 Hz in this sample, showed that overall fast spindle density (spindles per 30-sec epoch) during NREM sleep was reduced in the tiagabine condition (t9 = -3.24, P ≤ 0.01; Figure 3). When differentiating sleep stage 2 and SWS, this effect was more consistent for stage 2 sleep (post hoc pairwise comparisons for stage 2 sleep: t9 = -2.71, P ≤ 0.05) than SWS (t9 = -0.74, P = 0.47, F1,9 = 12.20, P ≤ 0.01, for Treatment × Sleep stage interaction). A reducing effect of tiagabine was similarly apparent for absolute spindle counts (F1,9 = 12.64, P 0.01, for Sleep stage × Treatment, t9 = -2.00, P = 0.08, and t9 = 1.45, P = 0.18, for pairwise comparisons between the treatments for stage 2 sleep and SWS, respectively).
Figure 3.
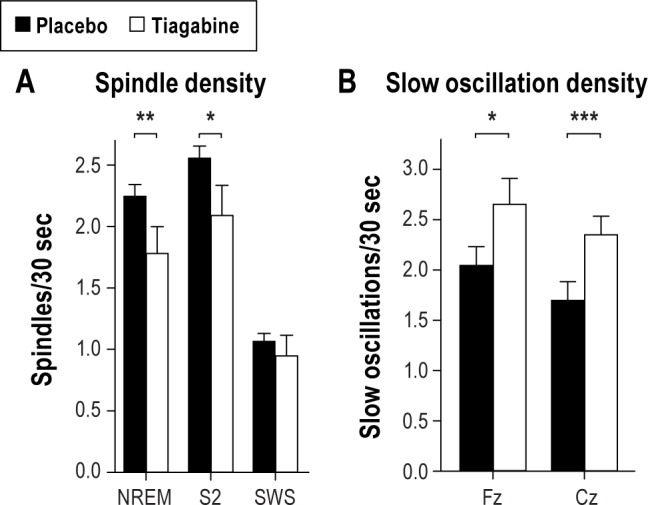
(A) Mean (± SEM) density of (fast) spindles during entire NREM sleep, and separately for sleep stage 2 and SWS, and (B) slow oscillation density during NREM sleep, separately for recordings from Fz and Cz, in the tiagabine (empty bars) and placebo condition (black bars). ***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05 for pairwise comparisons between the effects of the treatment (n = 10).
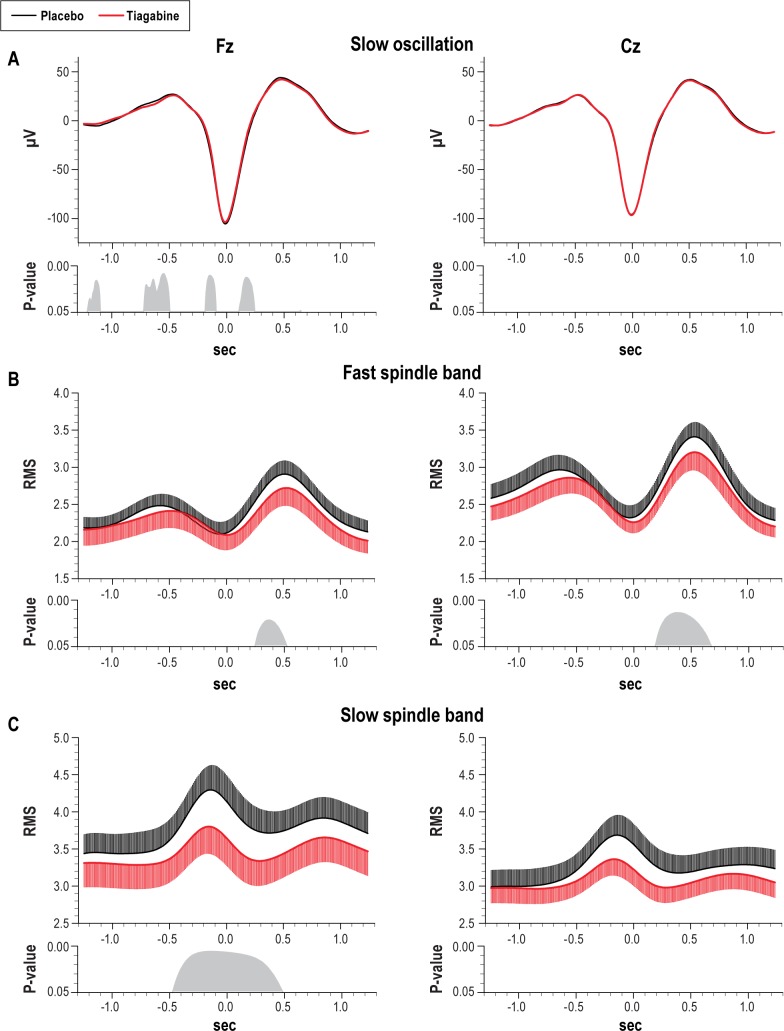
In order to further characterize the effect of tiagabine on NREM sleep, the morphology of slow oscillations as well as spindle activity occurring phase-locked during the slow oscillation cycle were analyzed. Compared with placebo, the density of slow oscillations (slow oscillations per 30-sec epoch) detected during NREM sleep under tiagabine was increased in Fz and Cz (t9 = 2.91, P = 0.05 and t9 = 9.24, P = 0.001, Figure 3B). The slow oscillation waveform detected at Cz did not differ significantly between placebo and tiagabine. At Fz, there were marginal differences occurring mainly during the increasing and decreasing flanks of the oscillation (Figure 4A for waveforms and P-values). However, peak to peak amplitude, negative half-wave amplitude, and slope of the slow oscillation did not differ between the treatment conditions (t9 < 1.41, P > 0.19). Under tiagabine, fast spindle activity (RMS) was significantly reduced during the slow oscillation up-state, i.e., 200-600 ms following the negative half-wave peak of the slow oscillation, and this effect was more pronounced at Cz than Fz (Figure 4B for data and P-values). Slow spindle activity was also reduced under tiagabine during the negative half-wave at Fz (Figure 4C).
Figure 4.
(A) Averaged EEG signal within ± 1.3 sec around the negative half-wave peak (0.0 sec) of identified slow oscillations. (B) Mean (± SEM) root mean square fast spindle (12-15 Hz) and (C) slow spindle band (9-12 Hz) activity averaged time-locked to negative half-wave peak of identified slow oscillations. Data are shown separately for recordings from Fz (left) and Cz (right), and separately for the tiagabine (red thick lines, negative going error bars indicate SEM) and placebo (black thin lines, positive going error bars indicate SEM) conditions. Respective bottom panels indicate significance between the effects of the tiagabine and placebo treatment for consecutive 5-ms bins.
Reaction Times, Mood, and Subjective Sleepiness
There were no significant differences in reaction times, mood, or subjective sleepiness between tiagabine and placebo at learning or retrieval (all t11 < 1.54, P > 0.15, Table 1 for mean and SEM). There was a trend toward participants being able to tell if they had received tiagabine or placebo, i.e., ∼50% of the sample correctly identified the active treatment and placebo in the respective conditions (χ2(1) = 3.50, P = 0.06).
DISCUSSION
A consistent finding in sleep and memory research is that memory consolidation during sleep essentially relies on SWS, especially on the synchronizing feature of the < 1 Hz slow oscillations during this sleep stage.1,3 The aim of this study was, through stimulating GABAergic neurotransmission, to enhance SWS in order to improve the consolidating effect on memory. The present data show that the administration of the GABA re-uptake inhibitor tiagabine (10 mg) has indeed the same effect on SWS in young adults as it had in previous studies in elderly,19,20 as it promoted SWS in favor of REM sleep. Although the sample size of our study was relatively small, the more detailed analyses of the EEG signal during SWS that relied on the artifactfree datasets indicated that tiagabine increases power in the lower frequency bands between 0.5-8 Hz and increased the density of slow oscillations. However, spindle activity was simultaneously reduced following tiagabine administration. Unexpectedly, analysis of the memory tasks show that the increase in SWS did not reflect in an enhancement of beneficial effect of sleep on declarative memories, which in previous studies proved to be most consistently benefited by SWS.38 There also was no influence of tiagabine on overnight retention of emotional memories. Procedural motor memory consolidation in terms of correctly tapped finger sequences was even significantly impaired by the GABA agonist, which corresponds to findings in cats of impaired sleepdependent ocular dominance plasticity after administration of the GABAA agonist zolpidem.39
The failure of tiagabine to improve overnight retention of declarative memory cannot be attributed to confounding effects of the substance on vigilance and sleepiness at the time of retrieval testing. Testing took place almost 24 hours after oral administration of tiagabine or placebo, i.e., a time when most of the substance had cleared the system (plasma half-life 7-9 h). Also, measures of vigilance (PVT), mood (PANAS), and selfreported tiredness, as well as performance on a control finger tapping sequence were comparable in both treatment conditions at retrieval testing. There were also no differences between conditions in learning performance before substance administration.
Slow wave activity, including < 1 Hz slow oscillations and 1-4 Hz delta frequency band, is a primary marker of SWS and has been consistently shown to contribute to the enhancing effect of sleep on hippocampus-dependent declarative memories as well as on procedural skill memories not relying on hippocampal function.2–4,40 Against this background, the present negative finding that tiagabine-induced increases in SWS and slow wave activity fail to enhance these memories indicates that phenotypic SWS per se is not a critical mechanism in the consolidation of these memories. Also, comparison of slow oscillations showed comparable amplitudes, slopes, and morphology for these oscillations in the tiagabine and placebo condition, which raises the question of the primary relevance of slow oscillations for memory consolidation.
However, tiagabine distinctly reduced spindle activity. Importantly, this suppressive influence was most prominent when analyzing spindle activity in synchrony with the slow oscillation cycle (Figure 5 provides a schematic illustration of slow oscillation and spindle coupling) and its presumed relationship to hippocampal memory reactivations. Consis-tent with previous studies,8,30,41 classical fast spindle activity in the 12-15 Hz range, which typically displays a more wide-spread centro-parietal cortical distribution, showed a distinct increase during the depolarizing up-phase of the slow oscillation, reflecting a driving infl uence slow oscillations exert on the thalamic generation of these spindles.42 By contrast, slow (9-12 Hz) frontal spindle activity, which is a separate kind of spindle activity whose function is less well understood, was synchronized to the up-to-down transition of the slow oscillation.8 Tiagabine profoundly suppressed both types of synchronized spindle activity during the slow oscillation cycle. In particular the classical fast spindle activity has been consistently found to be associated with overnight retention of both declarative and procedural memories.2,10,11,43 Recent studies suggest that the synchronization of fast spindle activity to the depolarizing slow oscillation up- state is critical to the consolidation effect.12,44 Specifically it has been proposed that the fast spindles represent a mechanism involved in the redistribution of memory representations that become reactivated during SWS to neocortical and striatal sites of long-term storage.1,7,45 Against this background, although density of slow oscillations was increased after tiagabine, the reduced efficacy of these slow oscillations to drive and synchronize fast spindle activity to the depolarizing upstate of these oscillations could well explain the failure of tiagabine to produce any improvement in declarative memory consolidation. An alternative explanation may be that SWS is already maximally beneficial in its unmedicated quantity. However, this is not supported by data that show increasing slow oscillations and spindles above normal physiological levels by another method, i.e., through transcranial direct current stimulation did increase declarative memory consolidation in young healthy student participants.3 Also, it could be speculated that tiagabine failed to enhance SWS-dependent memory consolidation because such enhancement was counteracted by imme-diate (retrograde) amnestic effects of the GABA agonist on hippocampal memory traces.46 Moreover, in studies of fear memory in mice, hippocampal microinjection of the GABAA receptor agonist muscimol impaired consolidation of fear context when given 4 or 6 hours following training, but not when given 1 hour after training.47 Indeed, further research seems necessary to explore immediate GABAergic effects on consolidation in hippocampal networks.
Figure 5.
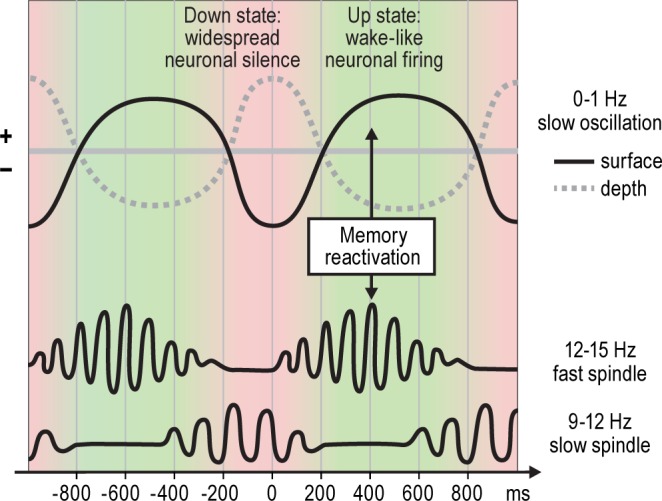
Schematic overview over the phase relationships between the slow oscillation and slow and fast sleep spindles; x-axis in milliseconds is relative to the (surface) negative slow oscillation peak. Fast spindle activity (12-15 Hz) increases during the down-to-up transition, is most pronounced during the up state of the slow oscillation and coincides with memory reactivations in the hippocampus and neocortex.8,56 Slow spindle activity (9-12 Hz), on the other hand, has its maximum during the up-to-down state transition.8 The surface EEG negative half-wave of the slow oscillation (down state), which is related to reduced neuronal fi ring, corresponds to a positive fi eld potential wave in deeper cortical layers, while the surface EEG positive half-wave (up state), which is related to increased neuronal fi ring, corresponds to a depth negative wave.30,57,58
Considering the particular robust association that has been revealed for overnight gains in procedural skills and spindle activity during retention sleep, the suppressing effect of tiagabine can also account for the significantly diminished sleep-associated increases in sequence fi nger-tapping speed in this condition. Alternatively, the diminished gains in sequence fi nger tapping could be a consequence of tiagabine reducing REM sleep, as REM sleep has been assumed to contribute to motor memory consolidation,48 and reducing cholinergic tone during REM sleep can impair motor memory consolidation.49 However, another study showed that benefi ts in performance on the same sequence motor task as used in the present study can occur in conditions of strongly suppressed REM sleep after administration of antidepressants,23 (see ref. 50 for related results in rats). While REM sleep was suppressed, overnight gains in fi nger tapping were closely associated with fast spindle activity during retention sleep, which is well in line with motor memory consolidation relying on sleep spindles rather than on REM sleep for consolidation.11,22
Based on the present findings and the available literature, we can only speculate about the neurophysiological mechanisms mediating the effects of tiagabine, particularly those that enhance slow wave activity and simultaneously reduce fast spindle activity. The generation of slow oscillations comprises a complex interplay of intrinsic voltage-dependent membrane currents, with miniature EPSPs and low threshold Ca2+ potential mediated bursts considered as initializing events within neocortical and thalamic networks, respectively.51,52 Its relatively stereotypical waveform remaining largely unaffected by tiagabine argues against an immediate effect of the GABA agonist on the slow oscillation. Rather, the general increase in slow oscillation density and power in lower EEG frequencies might originate from indirect GABAergic effects in brainstem and hypothalamic areas reducing cholinergic and histamin-ergic tone in the cortico-thalamic system, thereby reducing inhibition of the nucleus reticularis and depolarization of thalamocortical and cortical neurons.53 Decreased brainstem cholinergic tone is a major factor shifting the thalamo-cortical system toward increased slow wave activity.42 However, with regard to the suppression of spindle activity, a direct effect of tiagabine on the generating thalamic mechanisms cannot be excluded. Fast spindles (12-15 Hz) are locally generated in the thalamic reticular nucleus, which is composed entirely of GABAergic cells.54 Importantly, the thalamic GABAergic effects show a specific temporal dynamic, as tonic activation of GABAA receptors reduces the occurrence of spindles, whereas the action of agonistic modulators, which amplify the phasic response of the GABAA receptor, increases spindle occur-rence.16 Thus, the present data provide evidence that, although enhancing phenotypic SWS, tiagabine severely disturbs fast spindle activity during NREM sleep, possibly due to its action as a reuptake inhibitor, which increases GABAergic tone rather than increasing phasic GABAergic signaling.55
In conclusion, this study demonstrates that stimulating GABAergic activity by administration of the GABA reuptake inhibitor tiagabine strongly drives slow frequencies in the sleep EEG, thus producing an increase in phenotypic SWS. However, this increase is not functionally effective, as concurrently fast spindle activity, especially that occurring phase-locked to the slow oscillation up-states, is suppressed. Consequently, despite increasing phenotypic SWS, tiagabine fails to improve declarative memory consolidation during sleep and even impairs indicators of procedural memory consolidation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was performed at University of Luebeck, Department of Neuroendocrinology. This study was supported by a grant from the Deutsche Forschungsgemeinschaft SFB 654 ‘Plasticity and Sleep.’
ABBREVIATIONS
- ANOVA
analysis of variance
- PANAS
positive and negative affective schedule
- PVT
psychomotor vigilance task
- RMS
root mean square
- SSS
Stanford Sleepiness Scale
- SWS
slow wave sleep
- TST
total sleep time
REFERENCES
- 1.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 2.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 4.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–72. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Progr Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- 6.Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–6. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann TO, Molle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59:2733–42. doi: 10.1016/j.neuroimage.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 11.Tamaki M, Matsuoka T, Nittono H, Hori T. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin Neurophysiol. 2009;120:878–86. doi: 10.1016/j.clinph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Ruch S, Markes O, Duss SB, et al. Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia. 2012;50:2389–96. doi: 10.1016/j.neuropsychologia.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro S, Shi X, Engelhard M, et al. Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front Neurosci. 2007;1:43–55. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetto L, Chase MH, Torterolo P. GABAergic processes within the median preoptic nucleus promote NREM sleep. Behav Brain Res. 2012;232:60–5. doi: 10.1016/j.bbr.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 16.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–24. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 18.Fink-Jensen A, Suzdak PD, Swedberg MD, Judge ME, Hansen L, Nielsen PG. The gamma-aminobutyric acid (GABA) uptake inhibitor, tiagabine, increases extracellular brain levels of GABA in awake rats. Eur J Pharmacol. 1992;220:197–201. doi: 10.1016/0014-2999(92)90748-s. [DOI] [PubMed] [Google Scholar]
- 19.Mathias S, Wetter TC, Steiger A, Lancel M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22:247–53. doi: 10.1016/s0197-4580(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JK, Randazzo AC, Frankowski S, Shannon K, Schweitzer PK, Roth T. Dose-response effects of tiagabine on the sleep of older adults. Sleep. 2005;28:673–6. doi: 10.1093/sleep/28.6.673. [DOI] [PubMed] [Google Scholar]
- 21.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12:396–7. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 24.Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–42. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–9. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RM, Robertson EM. Off-line processing: reciprocal interactions between declarative and procedural memories. J Neurosci. 2007;27:10468–75. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A. Los Angeles Brain Information Service. UCLA: Brain Information Institute; 1968. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. [Google Scholar]
- 30.Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22:10941–7. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekstrand B, Barrett T, West J, Maier W. The effect of sleep on human long-term memory. Neurobiology of sleep and memory. 1977:419–38. [Google Scholar]
- 32.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. 2008 [Google Scholar]
- 33.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 34.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Drosopoulos S, Wagner U, Born J. Sleep enhances explicit recollection in recognition memory. Learn Mem. 2005;12:44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 38.Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 2004;11:679–85. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibt J, Aton SJ, Jha SK, Coleman T, Dumoulin MC, Frank MG. The non-benzodiazepine hypnotic zolpidem impairs sleep-dependent cortical plasticity. Sleep. 2008;31:1381–91. [PMC free article] [PubMed] [Google Scholar]
- 40.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 41.Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Cox R, Hofman WF, Talamini LM. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012;19:264–7. doi: 10.1101/lm.026252.112. [DOI] [PubMed] [Google Scholar]
- 45.Clemens Z, Molle M, Eross L, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011;33:511–20. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- 46.Chang SD, Liang KC. Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study. Neurobiol Learn Mem. 2012;98:17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Misane I, Kruis A, Pieneman AW, Ogren SO, Stiedl O. GABA(A) receptor activation in the CA1 area of the dorsal hippocampus impairs consolidation of conditioned contextual fear in C57BL/6J mice. Behav Brain Res. 2013;238:160–9. doi: 10.1016/j.bbr.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 49.Rasch B, Gais S, Born J. Impaired off-line consolidation of motor memories after combined blockade of cholinergic receptors during REM sleep-rich sleep. Neuropsychopharmacology. 2009;34:1843–53. doi: 10.1038/npp.2009.6. [DOI] [PubMed] [Google Scholar]
- 50.Watts A, Gritton HJ, Sweigart J, Poe GR. Antidepressant suppression of Non-REM sleep spindles and REM sleep impairs hippocampus-dependent learning while augmenting striatum-dependent learning. J Neurosci. 2012;32:13411–20. doi: 10.1523/JNEUROSCI.0170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crunelli V, Hughes SW. The slow (< 1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timofeev I, Grenier F, Steriade M. Impact of intrinsic properties and synaptic factors on the activity of neocortical networks in vivo. J Physiol Paris. 2000;94:343–55. doi: 10.1016/s0928-4257(00)01097-4. [DOI] [PubMed] [Google Scholar]
- 53.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–99. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 54.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–41. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Lancel M, Faulhaber J, Deisz RA. Effect of the GABA uptake inhibitor tiagabine on sleep and EEG power spectra in the rat. Br J Pharmacol. 1998;123:1471–7. doi: 10.1038/sj.bjp.0701769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 57.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–22. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]