Abstract
Study Objectives:
Growing evidence suggests that sleep disturbances precede by years the clinical onset of Alzheimer disease (AD). The goal of the current study is to determine whether changes in polysomnographic (PSG) sleep patterns accompany subjective sleep complaints in patients with mild cognitive impairment (MCI). We further examine whether meaningful changes in objective sleep physiology are predicted by self-reported sleep measures in MCI patients, and whether incipient neurodegeneration contributes to exacerbate sleep misperception.
Design, Setting, and Participants:
Overnight PSG recordings and self-reported sleep measures were obtained from 25 healthy elderly (HE) subjects and 25 patients with MCI at the sleep laboratory.
Results:
Both PSG and self-reported sleep measures confirmed that sleep is altered in patients with MCI. Whereas subjective sleep responses predicted fragmentation of slow wave sleep (SWS) in HE individuals, this relationship was not evident in MCI patients. Furthermore, patients with MCI showed significant discrepancies in the estimation of sleep onset latency when compared with HE subjects.
Conclusions:
Sleep is significantly impaired in patients with mild cognitive impairment at both the objective and subjective level, which may be used as a surrogate marker of preclinical Alzheimer disease. Taken together, these findings aid in the development of novel therapeutic strategies devoted to improve sleep in the elderly population at risk of developing Alzheimer disease.
Citation:
Hita-Yañez E; Atienza M; Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. SLEEP 2013;36(9):1327-1334.
Keywords: Aging, Alzheimer disease, ApoE, mild cognitive impairment, polysomnography, self-reports, sleep, sleep misperception
INTRODUCTION
A large body of evidence suggests that sleep disturbances are common in the elderly.1,2 These age-related sleep changes have been attributed to damage of neuronal circuits involved in the circadian and homeostatic regulation of sleep,3 resulting in reduced sleep duration, problems initiating and maintaining sleep, and decreased slow wave sleep (SWS) and rapid eye movement (REM) sleep.4 Poor sleep in older adults has also been frequently associated with chronic diseases and mental health,5,6 and correlate with age-related cognitive decline.7,8 However, it is unknown to date whether sleep problems may themselves trigger or exacerbate chronic diseases in older adults.
Aging is the major risk factor for the development of AD,9 a particularly debilitating condition that appears as the most common cause of long-term institutionalization in persons older than 65 y, with annual costs ranging from $21 billion (US healthcare system) to €189 billion (European Union).10,11 Sleep disorders have a significant effect on patients with AD and their caregivers, being one of the most troubling symptoms during the progression of disease.12,13 Recent polysomnographic (PSG) evidence shows that disturbed sleep patterns emerge years before clinical diagnosis, during the prodromal stage known as mild cognitive impairment (MCI).14 More specifically, patients with MCI exhibited smaller amounts of REM sleep and increased SWS fragmentations than HE individuals.14 The reduction of REM sleep was found to be more remarkable in those patients with MCI carrying the apolipoprotein E (ApoE) ε4 allele,14 which is considered the major genetic risk factor for AD.15
Previous longitudinal and cross-sectional studies carried out with either PSG recordings16 or subjective sleep reports17 concurred that cognitive aging is significantly related to sleep integrity. However, sleep disturbances in preclinical stages of AD remain underrecognized by many healthcare professionals. One of the reasons might be the common belief that sleep problems are normal signs of aging regardless of the patient's predementia condition. An abbreviated sleep history containing relevant sleep questions might assist in discriminating between memory decline caused by normal aging or by AD pathophysiology. This could lead to targeted educational and effective clinical programs to improving sleep quality of patients with MCI, and to enhancing the quality of life of this population at risk of developing AD. Furthermore, determining subjective sleep predictors of physiological sleep in patients with MCI would lead to better cost-effectiveness strategies for the use of PSG techniques in healthcare systems devoted to elderly people.
In the current study, we first assessed if subjective sleep differs between HE subjects and patients with MCI. We next studied whether meaningful group differences in sleep architecture between HE individuals and MCI patients14 could be predicted by responses to five questions relevant to the sleep of patients with MCI relevant to the sleep of patients with MCI/AD, and whether the ApoE ε4 genotype contributes to enhancing this prediction. Finally, we evaluated whether sleep misperception gains relevance in prodromal stages of AD or if it accompanies cognitive aging regardless of incipient neurodegeneration.
METHODS
Patients
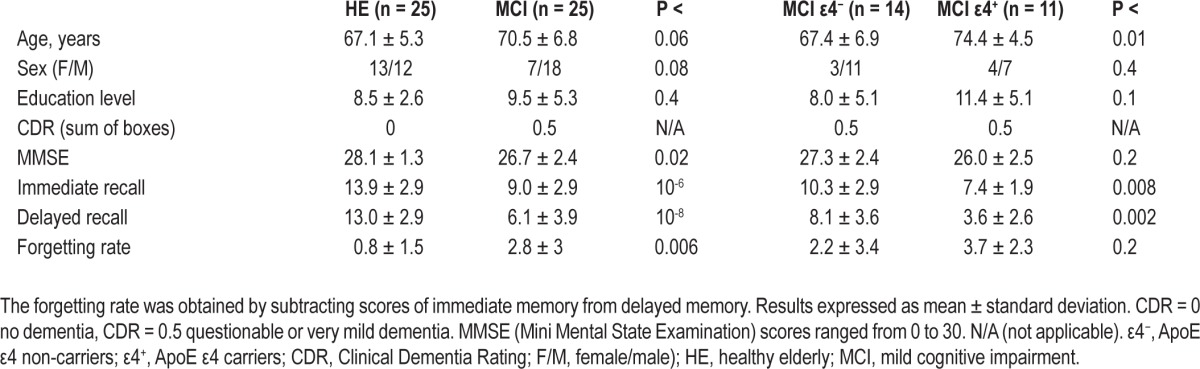
Twenty-five patients with MCI (7 females, mean age: 70.5 ± 6.8 y) and 25 HE subjects (13 females, mean age: 67.1 ± 5.3 y) were enrolled in the study, after they signed an informed consent. Experimental procedures were previously approved by the Ethical Committee for Human Research at the University Pablo de Olavide. Demographic and cognitive profiles of the participant groups are shown in Table 1.
Table 1.
Demographic characteristics and cognitive profile
Both HE subjects and patients with MCI underwent a neurological examination to exclude potential neurological diseases. Cerebral magnetic resonance imaging (MRI) was also performed on all candidates to rule out lesions such as territorial cerebral infarction, brain tumor, hippocampal sclerosis, and/or vascular malformations. Those candidates who showed periventricular and/or deep white matter damage, derived from scorings ≥ 2 on the Fazekas ischemic scale,18 were not included in the study. Other exclusion criteria were a history of neurological conditions, psychiatric disorders, and/or major medical illness (chronic renal, hepatic, pulmonary, or endocrine), the use of medication affecting the sleep-wake cycle (benzodiazepines, tricyclic and/or sero-tonin reuptake inhibitors), the presence of depressive symptoms (assessed with the abbreviated version of the Geriatric Depression Scale, using 5 as a cutoff score), and/or having complaints of sleep disordered breathing, movement disorders during sleep or unusual sleep schedules (i.e., shift work), which was corroborated by bed partners and/or caregivers. Patients with MCI were not taking cholinesterase inhibitors at the time of recruiting.
The diagnosis of MCI was based on consensus criteria:19 (1) subjective memory complaints corroborated by the informant, (2) objective memory loss substantiated by neuropsychological tests (scorings 1.5 standard deviations below the age-appropriate mean; immediate and delayed recall were assessed by the Spanish version of the Logical Memory subtest contained in the Wechsler Memory Scale-Third Edition), (3) global score of 0.5 (questionable dementia) in the clinical dementia rating (CDR), (4) normal independence function, and (5) not meeting the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) criteria for dementia. Global cognitive status was assessed with the Mini Mental State Examination (MMSE). Inclusion criteria for HE subjects were absence of objective memory deterioration as revealed by the same neuropsychological tests used with MCI patients, CDR global score of 0 (no dementia), and normal independent function.
ApoE Genotyping
Genomic DNA was isolated from 3 mL human whole blood using a standard salting-out protocol.20 ApoE polymorphisms were determined with polymerase chain reaction (Step-One Plus, Applied Biosystems, USA) using predesigned TaqMan single nucleotide polymorphisms genotyping assays (Applied Biosystems, USA).
PSG Recordings
The PSG protocol included simultaneous recordings of electroencephalography (EEG), vertical and horizontal electrooculography, and electromyography of submental muscles. Electrophysiological recordings were performed with gold cup, 10 mm- diameter electrodes (Grass, USA) filled with electrolytic cream, and attached with surgical tape (face placements) and collodion (scalp placements). Overnight PSG recordings were performed in a sound-attenuated bedroom with infrared video-controlled supervision. Respiratory measures were not included in the protocol because none of the participants reported complaints of sleep disordered breathing, corroborated by their bed partners. Furthermore, scores of the Epworth Sleepiness Scale (ESS) were below the cutoff for suspected sleep disorders associated with excessive daytime sleepiness.21
Electrophysiological recordings were amplified (BrainAmp MR, Brain Products, Germany), filtered (0.1-100 Hz bandpass), digitized (250 Hz, 16-bit resolution), and stored in digital format for off-line analysis. A trained technician, blind to the study purpose, conducted scoring of sleep stages following standard criteria.22 Criteria for scoring EEG arousals were taken from the American Sleep Disorders Association report,23 and the level of sleep fragmentation was determined by the arousal index in each sleep stage. This index resulted from dividing the number of arousals appearing in a sleep stage by the time (in hours) spent in that sleep stage. For the purpose of the current study, only those PSG parameters that showed significant group differences between HE subjects and patients with MCI (i.e., REM percentage and density of SWS arousals14) were correlated with self-reported sleep data.
Subjective Sleep Data
Subjective sleep measures were collected the same day from the PSG recording through direct interviewing. All participants were asked to answer five questions focused on sleep parameters, sleep symptoms, and sleep quality over the past few months (Table 2). These five questions were included in the current study on the basis of sleep disturbances previously reported in patients with AD or MCI: longer latencies to sleep onset24 (item 1), shortened sleep duration25 (item 2), increased sleep arousals and/or wake ups after sleep onset14,26 (items 3 and 4), and poorer sleep quality12 (item 5).
Table 2.
Self-reported sleep questions

Statistical Analysis
Statistical analyses were conducted using SPSS v.15 (SPSS Inc., Chicago, IL). A logarithmic transformation was applied to non-normally distributed dependent variables to approach normal distribution. Group differences in sex (HE versus MCI; MCI ε4 noncarriers versus MCI ε4 carriers) were evaluated by applying the chi-square test, whereas group differences in the remaining demographic and cognitive variables were assessed with the Student t-test.
Group differences in PSG and subjective sleep measures were separately assessed by analyses of covariance (ANCOVA). Next, multivariate regression (MVAR) analyses were applied to examine if self-reported sleep predicted group differences in PSG parameters. If statistical significance was reached in at least one group, we then assessed group differences between regression slopes. Both ANCOVAs and MVAR analyses (within and between groups) included age and sex as covariates.
Given the relationship between memory consolidation and sleep continuity,27 we further evaluated whether any sleep parameter, derived from either overnight PSG recordings or self-reports, predicted memory performance (imme-diate, delayed memory, and forgetting rate) in each group separately (HE, MCI, MCI ε4 noncarriers, MCI ε4 carriers). If significance was reached in at least one group, we then assessed group differences between regression slopes. MVAR analyses (within and between groups) included age and sex as covariates.
We finally evaluated whether sleep misperception gains relevance during prodromal AD stages. To achieve this goal, two mixed ANCOVAs were performed with either sleep onset latency (SOL) or sleep duration (subjective versus objective) as the within-subject factor, group (either HE versus MCI or MCI ε4 noncarriers versus MCI ε4 carriers) as the between-subject factor, and age and sex as covariates.
RESULTS
Demographic and Cognitive Profile
HE subjects and patients with MCI showed similar demo-graphic profiles (Table 1). Age was comparable in the two groups but differed between MCI ε4 carriers and MCI ε4 noncarriers (P < 0.01). Although sex did not differ between HE and MCI, this variable showed a trend toward significance (P < 0.08). Eleven patients with MCI exhibited the genotype ApoE ε4 and the remaining 14 patients were ε4 noncarriers. Overall, the prevalence of the allele ε4 in the ApoE was 3.6-fold greater in MCI than in HE, being present in 44% of MCI patients in contrast with 12% of HE subjects. The presence of the ApoE ε4 allele was not used as selection criterion during the recruiting process, its distribution in our MCI sample resulted entirely from chance.
Patients with MCI showed impairments in immediate (P < 10-6) and delayed recall (P < 10-8), as well as in the forget-ting rate (immediate minus delayed recall; P < 0.006) when compared with HE subjects (Table 1). In addition, memory, but not the forgetting rate, was significantly more affected in MCI ε4 carriers than in noncarriers (immediate, P < 0.008; delayed, P < 0.002). The same pattern of results (HE > MCI-ε4− > MCI-ε4+) was corroborated when memory performance was compared among the three groups (Pillai trace, F4,94 = 10.6, P < 10-6; post hoc analyses, P < 0.03).
PSG Sleep
Group differences in PSG sleep variables were reported else-where.14 Here, statistical analyses were repeated introducing age and sex as covariates into the general linear model, although results remained unchanged. Briefly, SWS was significantly disrupted in patients with MCI as revealed by the higher density of arousals occurring during this cerebral state (HE = 0.09 ± 0.11; MCI = 0.19 ± 0.10; P < 0.01). REM sleep was also significantly shortened in patients with MCI (HE = 14.7 ± 3.7; MCI = 10.1 ± 5.4; P < 0.007). This effect was especially evident in MCI ε4 carriers (7.4 ± 5.5; P < 0.04) when compared with MCI ε4 noncarriers (12.3 ± 4.3).
Self-Reported Sleep
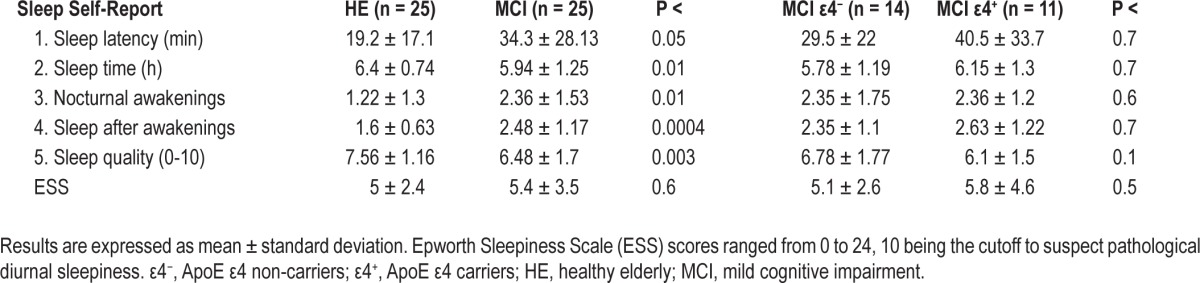
Overall, self-perception of sleep was worse in patients with MCI than in HE individulas (Pillai trace, F5,42 = 5.37, P < 0.001). Post hoc analyses showed that MCI patients reported longer SOL (F1,46 = 3.92, P < 0.05), shorter sleep time (F1,46 = 6.02, P < 0.01), increased nocturnal awakenings (F1,46 = 6.97, P < 0.01), more difficulty in sleeping after nocturnal awakenings (F1,46 = 14.34, P < 0.0004), and poorer sleep quality (F1,46 = 10.08, P < 0.003). No differences in subjective sleep were found when compared responses between MCI ε4 carriers and noncarriers. Table 3 summarizes averaged group responses to the sleep questions employed in this study. Subjective levels of daytime sleepiness did not differ between groups, as revealed by ESS scores (Table 3).
Table 3.
Self-reported sleep in HE subjects and patients with MCI
Relationships Between Sleep Physiology and Self-Reports of Sleep
We further investigated whether significant group differences in meaningful PSG sleep parameters (SWS arousals and REM percentage)14 correlated with responses to the five sleep items in HE subjects and patients with MCI, separately. The regression analysis yielded a positive relationship between SWS arousals and self-reported sleep in HE subjects (F7,24 = 3.1, P < 0.02, adjusted R squared = 0.38), but no significant associations between objective and subjective sleep were found in MCI patients. Post hoc analyses revealed that two sleep items mainly accounted for significant correlations between SWS arousals and self-estimation of sleep disturbances in HE subjects: “difficulty in sleeping after nocturnal awakenings” (P < 0.03, r = 0.4) and “number of nocturnal awakenings” (P < 0.04, r = 0.37). However, only correlations performed with “difficulty in sleeping after awakenings” significantly distinguished normal from pathological aging (F5,49 = 4.32, P < 0.003, adjusted R squared = 0.25; beta for the interaction term = 0.64, P < 0.03), although correlations with “number of nocturnal awakenings” also showed a trend toward significance (P < 0.07). Figure 1 illustrates between-group regression analyses for the abovementioned relationships. Neither responses to sleep questions nor the ApoE ε4 polymorphism predicted the amount of REM sleep in HE subjects and patients with MCI.
Figure 1.
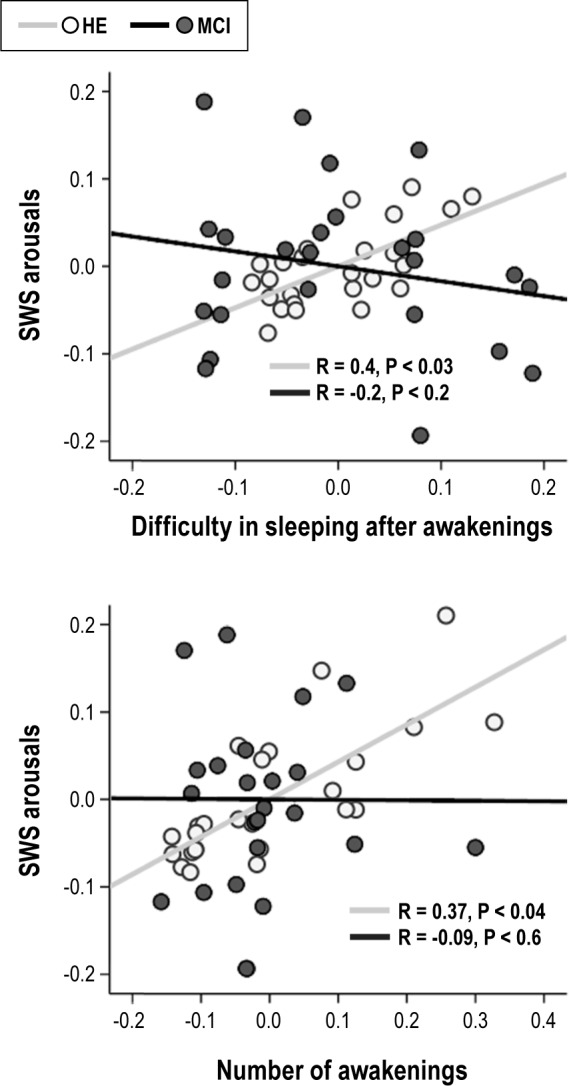
Scatter plots displaying relationships between density of slow wave sleep (SWS) arousals and either self-reported difficulty in sleeping after awakenings (top panel) or number of awakenings (bottom panel) after controlling for the effects of age and sex. Note that only HE subjects showed significant relationships between objective and subjective sleep.
Relationships Between Sleep and Memory Performance
Regression analyses revealed no significant relationships between significant sleep parameters (derived from either PSG data or self-reports) and memory-forgetting indices in any group. Therefore, comparisons between regression slopes were not performed.
Sleep Misperception in Healthy Aging and MCI
Figure 2 illustrates group differences between PSG-objective and subjective estimation of the SOL and sleep duration. We found that only the SOL was significantly overestimated (F1,46 = 9.3, P < 0.004), showing a trend toward significance for the interaction effect (F1,46 = 3.5, P < 0.07). In agreement with this trend, post hoc analyses revealed that patients with MCI overestimated the SOL (P < 10-4; self-reports = 34.3 ± 4.8; PSG = 14.7 ± 1.5) compared with HE subjects (self-reports = 19.2 ± 4.7; PSG = 11.3 ± 1.5). The presence of the ε4 allele in patients with MCI did not influence sleep perception.
Figure 2.
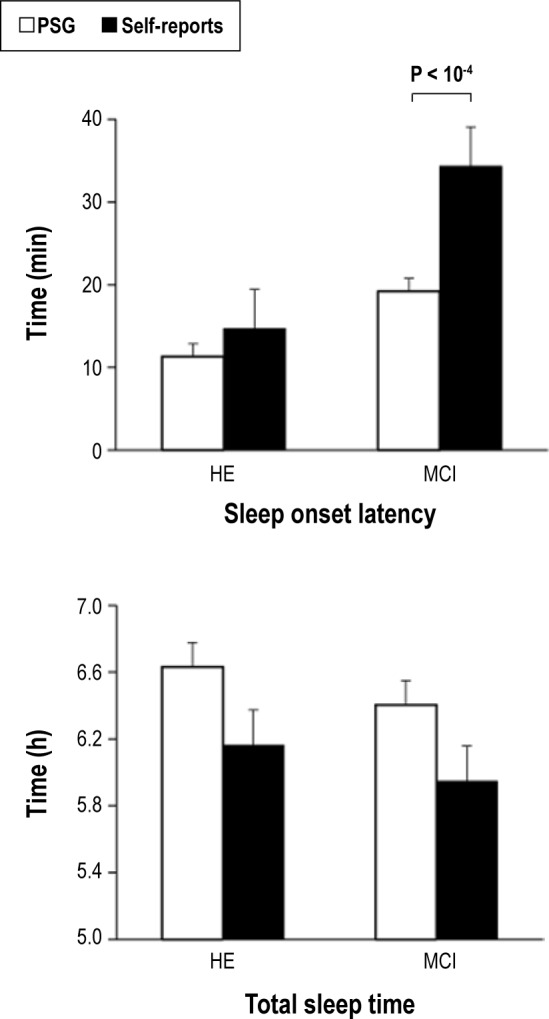
Sleep misperception in prodromal stages of Alzheimer disease. PSG and subjective estimation of sleep onset latency (top panel) and total sleep time (bottom panel) in HE subjects and MCI patients. HE, healthy elderly; MCI, mild cognitive impairment; PSG, polysomnography.
DISCUSSION
The current study provides compelling evidence of objective and subjective sleep disturbances in patients with MCI, suggesting that sleep problems precede in years the clinical onset of AD, and therefore adding support to a positive feed-back loop between impaired sleep and Aβ levels previously reported in animal models of AD.28 Our results further indicated a poor correspondence between objective sleep physiology and subjective estimates of sleep in this preclinical population. In particular, patients with MCI were unable to establish associations between SWS fragmentation and self-reports related to sleep quality, and they also exhibited significant discrepancies in the estimation of SOL when compared with HE subjects.
Sleep Disturbances in MCI: From Objective Physiology to Self-Reported Measures
Evidence suggesting that aging-related cognitive decline could be exacerbated by a loss of sleep integrity7,8 has fed the hypothesis that sleep disturbances in older adults might anticipate AD,29 which has been recently supported by studies using mouse models of β-amyloidosis.28,30 These studies showed that sleep disruptions appeared after plaque formation, and reversed after elimination of Aβ deposits.28 Given that Aβ aggregation become evident years before the clinical onset of AD,31,32 examining sleep disturbances during preclinical stages of AD might have important implications for early diagnosis and disease progression.
The belief that MCI status is the preclinical stage of AD has received strong support from neuropathological,31,32 biochemical,33 neuroimaging,34,35 and neurophysiological findings.36 Although overnight PSG studies are rare in patients with MCI,14,26 previous research using subjective measures concur that sleep problems are more frequent in patients with MCI than in HE subjects.37–39 The current study corroborates these findings by using five sleep questions focused on aspects of sleep affected in AD and patients with MCI.12,14,24–26 Adding this short questionnaire into the routine medical history of elderly people with memory deficits might help to identify those at risk of developing AD in primary care practice.
To the best of our knowledge, this is the first study addressing the issue of sleep misperception in preclinical stages of AD. Our results showed that patients with MCI overestimate the SOL, although temporal skills are preserved in AD.40 Two lines of research support that SOL misperception in MCI patients might result from memory deficits caused by early neuro-degeneration. First, it is well established that perception of retrospective timing relies more strongly on memory than on attention, especially when time estimations are in the range of minutes.41 Second, this process requires from the integrity of the medial temporal lobe,42 which is devastated by neurofi-brillary pathology and cell loss early in the course of AD.43–46 Accordingly, neuroimaging studies conducted in patients with MCI have consistently showed significant atrophies of medial temporal lobe able to predict conversion from MCI to AD,47 as well as decreased volume of the perforant pathway that plays an important role in memory function.48,49
The SOL is also overestimated in patients with suspected hypersomnolence.50 One might speculate that excessive diurnal somnolence, caused by sleep related breathing and/ or sleep movement disorders, underlies sleep onset misperception in our MCI population. Although we did not objectively exclude the presence of sleep apnea and/or periodic limb movements in our sample, subjective levels of daytime sleepiness did not differ between groups, and ESS scores were in all cases below the threshold for suspecting sleep disorders associated with excessive daytime sleepiness. Furthermore, neither study participants nor their bed partners reported symptoms associated with these sleep disorders. We there-fore believe that sleep onset misperception is intensified by memory deficits in patients with MCI, providing novel insight into the interaction between early neurodegeneration, and sleep perception in preclinical stages of AD.
Possession of one or two copies of the ε4 allele in the ApoE gene has been suggested as the major genetic risk factor for developing AD in patients with MCI.51 Whereas REM deficits in patients with MCI are aggravated by the presence of the ApoE ε4 genotype,14 this condition was neither associated with higher prevalence of self-reported sleep disturbances nor predicted significant relationships between objective and subjective sleep alterations. Recent studies have found that human ApoE4-targeted replacement mice, but not wild-type control mice, showed significant reduction of SWS and REM sleep during acute exposure to intermittent hypoxia and sleep fragmentation.52 Building on these findings, we suggest that physiological but not subjective sleep is more vulnerable to the presence of the ε4 allele in patients with MCI. However, further longitudinal studies are needed to establish whether MCI ApoE ε4 carriers showing altered PSG sleep patterns convert faster to AD than those only showing subjective sleep complaints.
This study has several potential sources of bias that should be noted. First, we compared PSG sleep data recorded in 1 night with subjective sleep quality over the past few months to establish relationships between objective and subjective sleep in HE subjects and patients with MCI. This approach implicitly assumes that PSG data obtained from one single night is representative of a typical night in the past few months. A more reliable approach would have been to correlate overnight PSG recordings with self-reports of sleep referring to that particular night. Second, PSG sleep studies were performed without previous adaptation of participants to the sleep laboratory. As a consequence, our results may be affected by the first-night effect (i.e., differences observed on the first PSG sleep recording in comparison with consecutive ones),53,54 which has previously been demonstrated to affect older patients more than younger ones.55 This hypothesis is, however, less plausible because the effect of the first-night effects on sleep structure would be expected to be similar in both HE subjects and patients with MCI.
Basal Forebrain: Where AD Meets Sleep and Cognition
Different regions of basal forebrain (BF) are involved in nonrapid eye movement (NREM) sleep regulation, as revealed by lesion56 and stimulation studies.57 In addition, several studies have shown that NREM sleep is significantly reduced in patients with AD.13,58 Convergent evidence further suggested that BF cholinergic neurons are selectively vulnerable to AD neurodegeneration,59,60 adding support to the hypothesis that cholinergic dysfunctions are partially responsible for the cognitive deficits observed in patients with AD.61 We have recently extended this hypothesis to preclinical stages of AD, showing that patients with MCI exhibited significant volume reductions of the nucleus basalis of Meynert that in turn correlated with impaired cognition in this preclinical population.34 Therefore, damage of BF nuclei together with altered SWS might exacerbate cognitive dysfunctions in patients with MCI.
Convergent evidence also supports relationships between impaired sleep and lesions of BF nuclei in patients with MCI. First, BF regions involved in SWS regulation are damaged in patients with MCI.34,56,57 Second, Aβ plaques appear years before the clinical onset of AD,31,32 likely triggering a positive feedback loop between Aβ concentrations and sleep-wake irregularities during preclinical AD stages.28 In line with the second hypothesis, increased disruption of SWS might lead to impaired cognitive integrity due to higher Aβ concentrations, which in turn might contribute to sleep misperception observed in patients with MCI. Recent evidence has shown that sleep disturbances increase proinflammatory cytokine levels and they further induce microglia activation in the mouse hippocampus, leading to deficits in hippocampal-dependent learning and memory consolidation.62 Collectively, these findings suggest that SWS disruptions reported in patients with MCI could both activate the amyloid cascade and induce neuroinflammation in the hippocampus. Both complementary conditions pave the way to AD progression.
No significant associations were found between SWS fragmentation and memory performance/forgetting rate, neither in HE subjects nor in patients with MCI. However, this lack of significance does not allow us to fully discard associations between sleep integrity and memory performance in prodromal stages of AD.26 Future investigations should include memory indices more sensitive to AD neuropathology. For instance, patients with MCI have more difficulties in remembering relations among items or between an item and its context (associative memory) rather than individual items.63 In support of this hypothesis, we recently found in patients with MCI significant correlations between gray matter volume of the entorhinal cortex and associative memory deficits, but not with imme-diate or delayed recall.64 Therefore, indices of associative memory might be more appropriate to investigating potential relationships between memory performance and sleep integrity in MCI patients.
Only one study has previously evaluated potential relation-ships between objective and subjective measures of sleep in early to moderate stages of AD, although objective sleep parameters were obtained with actigraphic recordings.24 Authors found significant discrepancies between objective and subjective sleep in patients with AD, but not in control subjects.24 Our results confirm these findings and allow us to extend sleep misperception to years before the clinical onset of AD, which might result from complex interactions between sleep disruptions and high levels of Aβ.28 Much more research is needed to fully understand relationships between sleep disturbances and Aβ levels during the continuum healthy aging to severe AD, and to establish whether improving sleep in preclinical stages of AD is a beneficial strategy in slowing the progression of this neurodegenerative condition.
CONCLUSIONS
Sleep complaints are commonly underdiagnosed in the geriatric population although they constitute a significant source of concern in patients with dementia. The current study confirms that sleep disturbances in patients with MCI can be determined on the basis of both overnight PSG recordings and self-reports. Our results further revealed that patients with MCI not only are unable to establish coherent relationships between objective and subjective sleep but they also have significant difficulties in correctly estimating the SOL, which might result from memory deficits intrinsic to this preclinical condition. Taken together, these results add support to reciprocal relationships between impaired sleep and Aβ levels,28 suggesting that this positive feedback loop begins years before the clinical onset of AD. Results of the current study have also important implications for early diagnosis of AD, and might aid in the development of novel therapeutic strategies devoted to improve sleep in elderly patients with impaired cognition.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by research grants from the Spanish Ministry of Economy and Competitiveness (SAF2011-25463, to Dr. Cantero), and from the Regional Ministry of Innovation, Science and Enterprise, Junta de Andalucia (P09-CTS-4604, to Dr. Cantero).
Footnotes
A commentary on this article appears in this issue on page 1275.
REFERENCES
- 1.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinz PN, Vitiello MV, Raskind MA, Thorpy MJ. Geriatrics: sleep disorders and aging. N Engl J Med. 1990;323:520–6. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 3.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–42. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 4.Espiritu JR. Aging-related sleep changes. Clin Geriatr Med. 2008;24:1–14. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–6. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 6.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–7. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer's disease in a community population. JAMA. 1995;273:1354–9. [PubMed] [Google Scholar]
- 10.Luengo-Fernandez R, Leal J, Gray AM. Cost of dementia in the pre-enlargement countries of the European Union. J Alzheimers Dis. 2011;27:187–96. doi: 10.3233/JAD-2011-102019. [DOI] [PubMed] [Google Scholar]
- 11.Arrighi HM, McLaughlin T, Leibman C. Prevalence and impact of dementia-related functional limitations in the United States, 2001 to 2005. Alzheimer Dis Assoc Disord. 2010;24:72–8. doi: 10.1097/WAD.0b013e3181a1a87d. [DOI] [PubMed] [Google Scholar]
- 12.Vitiello MV, Prinz PN. Alzheimer's disease. Sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99. [PubMed] [Google Scholar]
- 13.Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging. 1982;3:371–7. doi: 10.1016/0197-4580(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 14.Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE ε4 genotype. Curr Alzheimer Res. 2012;9:290–7. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel R, Herzog A, Köberle S. Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol Psychiatry. 1999;45:435–42. doi: 10.1016/s0006-3223(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 17.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 18.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 19.Petersen PR, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. Los Angeles, California: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 23.American Sleep Disorder Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 24.Most EI, Aboudan S, Scheltens P, Van Someren EJ. Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer disease. Am J Geriatr Psychiatry. 2012;20:460–7. doi: 10.1097/JGP.0b013e318252e3ff. [DOI] [PubMed] [Google Scholar]
- 25.Prinz PN, Peskind ER, Vitaliano PP, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 26.Westerberg CE, Mander BA, Florczak SM, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolls A, Colas D, Adamantidis A, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA. 2011;108:13305–10. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osorio RS, Pirraglia E, Agüera-Ortiz LF, et al. Greater risk of Alzheimer's disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–62. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 32.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 33.Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9:705–10. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 34.Grothe M, Zaborszky L, Atienza M, et al. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cereb Cortex. 2010;20:1685–95. doi: 10.1093/cercor/bhp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantero JL, Atienza M, Gomez-Herrero G, et al. Functional integrity of thalamo-cortical circuits differentiates normal aging from mild cognitive impairment. Hum Brain Mapp. 2009;30:3944–57. doi: 10.1002/hbm.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21:654–66. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Cho HS, Hong CH, Kim DG, Oh BH. Differences in neuropsychiatric symptoms according to mild cognitive impairment subtypes in the community. Dement Geriatr Cogn Disord. 2008;26:212–7. doi: 10.1159/000153431. [DOI] [PubMed] [Google Scholar]
- 39.Geda YE, Smith GE, Knopman DS, et al. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004;16:51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 40.Levy B, Dreier T. Preservation of temporal skills in Alzheimer's disease. Perc Mot Skills. 1997;85:83–96. doi: 10.2466/pms.1997.85.1.83. [DOI] [PubMed] [Google Scholar]
- 41.Ornstein RE, editor. On the experience of time. Baltimore: Penguin Books; 1970. [Google Scholar]
- 42.Noulhiane M, Pouthas V, Hasboun D, Baulac M, Samson S. Role of the medial temporal lobe in time estimation in the range of minutes. Neuroreport. 2007;18:1035–8. doi: 10.1097/WNR.0b013e3281668be1. [DOI] [PubMed] [Google Scholar]
- 43.Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–13. [PubMed] [Google Scholar]
- 44.Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer's disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–74. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 47.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 48.Rogalski EJ, Murphy CM, deToledo-Morrell L, et al. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: a diffusion tensor imaging study. Behav Neurol. 2009;21:51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:10041–5. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chervin RD, Guilleminault C. Overestimation of sleep latency by patients with suspected hypersomnolence. Sleep. 1996;19:94–100. doi: 10.1093/sleep/19.2.94. [DOI] [PubMed] [Google Scholar]
- 51.Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–84. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 52.Kaushal N, Ramesh V, Gozal D. Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2012;303:R19–29. doi: 10.1152/ajpregu.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Browman CP, Cartwright RD. The first-night effect on sleep and dreams. Biol Psychiatry. 1980;15:809–12. [PubMed] [Google Scholar]
- 54.Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 55.Webb WB, Campbell SS. The first night effect revisited with age as a variable. Waking Sleeping. 1979;3:319–24. [PubMed] [Google Scholar]
- 56.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–5. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 57.Sterman MB, Clemente CD. Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp Neurol. 1962;6:103–17. doi: 10.1016/0014-4886(62)90081-x. [DOI] [PubMed] [Google Scholar]
- 58.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer's disease. J Gerontol. 1990;45:131–8. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 59.Arendt T, Bigl V, Tennstedt A, Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985;14:1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- 60.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 61.Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–90. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 62.Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–55. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Troyer AK, Murphy KJ, Anderson ND, Hayman-Abello BA, Craik FI, Moscovitch M. Item and associative memory in amnestic mild cognitive impairment: performance on standardized memory tests. Neuropsychology. 2008;22:10–6. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- 64.Atienza M, Atalaia-Silva KC, Gonzalez-Escamilla G, Gil-Neciga E, Suarez-Gonzalez A, Cantero JL. Associative memory deficits in mild cognitive impairment: the role of hippocampal formation. Neuroimage. 2011;57:1331–42. doi: 10.1016/j.neuroimage.2011.05.047. [DOI] [PubMed] [Google Scholar]


