Abstract
Background:
Hypertrophic tonsillar tissue in children with obstructive sleep apnea (OSA) has enhanced expression of glucocorticoid receptors, which may reflect low endogenous cortisol levels. We have evaluated the effect of the interaction between tonsillar hypertrophy and OSA severity on morning serum cortisol levels.
Methods:
Children with and without snoring underwent polysomnography, tonsillar size grading, and measurement of morning serum cortisol.
Results:
Seventy children (2-13 years old) were recruited: 30 with moderate-to-severe OSA (apnea-hypopnea index [AHI] > 5 episodes/h), 26 with mild OSA (AHI > 1 and ≤ 5), and 14 controls (no snoring; AHI ≤ 1). Tonsillar hypertrophy was present in 56.7%, 53.8%, and 42.9% of participants in each group, respectively. Application of a general linear model demonstrated a significant effect of the interaction between severity of OSA and tonsillar hypertrophy on cortisol levels (P = 0.04), after adjustment for obesity, gender, and age. Among children with tonsillar hypertrophy, subjects with moderate-to-severe OSA (n = 17; AHI 14.7 ± 10.6), mild OSA (n = 14; AHI 2.3 ± 1.2), and control participants (n = 6; AHI 0.7 ± 0.2) were significantly different regarding cortisol levels (P = 0.02). Subjects with moderate-to-severe OSA had lower cortisol (16.9 ± 8.7 mcg/dL) than those with mild OSA (23.3 ± 4.2; P = 0.01) and those without OSA (controls) (23.6 ± 5.3 mcg/dL; P = 0.04). In contrast, children with normal-size tonsils and moderate-to-severe OSA, mild OSA, and controls did not differ in cortisol levels.
Conclusions:
Children with moderate-to-severe obstructive sleep apnea and the phenotype of hypertrophic tonsils have reduced morning serum cortisol levels and potentially decreased glucocorticoid inhibitory effects on tonsillar growth.
Citation:
Malakasioti G; Alexopoulos EI; Varlami V; Chaidas K; Liakos N; Gourgoulianis K; Kaditis AG. Low morning serum cortisol levels in children with tonsillar hypertrophy and moderate-to-severe OSA. SLEEP 2013;36(9):1349-1354.
Keywords: Children, cortisol, sleep apnea, tonsillar hypertrophy
INTRODUCTION
Adenotonsillar hypertrophy is an important risk factor for obstructive sleep disordered breathing (SDB), but its pathogenesis remains obscure.1 Hypertrophic pharyngeal lymphoid tissue in children with obstructive sleep apnea (OSA), the most severe form of SDB, has enhanced expression of glucocorticoid receptors.2 Although this finding suggests a favor-able response profile for topical steroid therapy in children with OSA, its pathogenetic role in adenotonsillar hypertrophy is unknown. It is possible that overexpression of glucocorticoid receptors in adenoid and tonsils reflects low endogenous cortisol levels that cannot restrict lymphoid tissue growth.3,4 Addition of dexamethasone in tonsillar cell cultures from sleep apneic children induces cellular apoptosis and inhibits cellular growth rate.5
Furthermore, the effect of intermittent upper airway obstruction during sleep on the hypothalamic-pituitary-adrenal axis activity has not been clarified. Nocturnal awakenings are associated with increased pulsatile cortisol release.6 Several case-control studies have compared cortisol levels in adult subjects with and without OSA and in sleep apneic patients after treatment with continuous positive airway pressure.7 However, the majority of studies in adults have provided conflicting evidence regarding the influence of sleep apnea on the release of cortisol, and no pediatric reports have been published.
In an old report,3 low baseline and after stimulation serum cortisol levels were measured in children with tonsillar hyper-trophy and SDB, and they remained low after adenotonsillectomy. Thus, in the current study, it was hypothesized that children with OSA and tonsillar hypertrophy have lower morning serum cortisol levels relative to healthy control subjects, whereas such a relationship is not identified in subjects with OSA but without tonsillar hypertrophy. To test this hypothesis, we have evaluated the effect of the interaction between tonsillar hypertrophy and OSA severity on morning serum cortisol levels.
METHODS
Participants
Consecutive children (2-13 years) with snoring who were referred to the sleep disorders laboratory for polysomnography and had an AHI > 1/h were enrolled in the study. Subjects who underwent polysomnography for various sleep problems other than SDB and had AHI ≤ 1/h were recruited as controls. Exclusion criteria for both patients and controls were: (i) symptoms or signs of acute or chronic inflammatory disease; (ii) treatment with systemic, intranasal or inhaled corticosteroids over the past 6 months; and (iii) genetic syndromes, neuromuscular disorders, or craniofacial abnormalities. The study was approved by the institutional review board of the Larissa University Hospital, and informed consent was provided by the caregivers.
Clinical Evaluation and Polysomnography
Α detailed history was received from parents, and all participants underwent physical examination in the evening prior to polysomnography. Weight and standing height were recorded, and body mass index z-score was calculated.8 Obesity was defined as BMI z -score > 1.645. After direct inspection of the oropharynx by a single examiner (E.I.A.), tonsillar size was graded from 1+ to 4+, and tonsillar hypertrophy was diagnosed if the size was greater than 2+.9 In 10 subjects, examination of the oropharynx was repeated by the same investigator one week after polysomnography, and tonsillar size grading did not change.
Overnight polysomnography was carried out in the sleep disorders laboratory, and recordings of the following signals were obtained: electroencephalogram; right and left oculogram; submental and tibial electromyogram; body position; electrocardiogram; thoracic and abdominal wall motion; oronasal airflow (3-pronged thermistor and nasal pressure transducer); and oxygen saturation of hemoglobin (SpO2). Sleep stages and respiratory events were scored according to the American Academy of Sleep Medicine (AASM) recent recommendations.10 Respiratory arousals were defined as those occurring within 3 sec following an apnea, hypopnea, or snore.11 Apnea-hypopnea index (AHI) was the mean number of obstructive, central, and mixed apneas and hypopneas/h of total sleep time in agreement with the AASM Manual for the Scoring of Sleep and Associated Events.10 Obstructive AHI was also calculated as the mean number of obstructive and mixed apneas and hypopneas/h of total sleep time.
Measurement of Morning Serum Cortisol
Children were woken up and polysomnography was terminated around 08:30; 30 min later, a venous blood sample was collected in a test tube without anticoagulant.12 After centrifugation, serum was obtained and stored immediately at −70°C until assayed with electrochemiluminescence (ECLIA, Roche Diagnostics GmbH; Manheim, Germany), using the analyzer Modular Analytics E170 (Roche Diagnostics, Germany).
Lower and upper detection limits of the method were 0.018 and 63.4 mcg/dL, respectively.
Data Analysis
For data analysis, recruited subjects were distributed in 3 groups: (i) participants with moderate-to-severe OSA (AHI > 5/h); (ii) children with mild OSA (AHI > 1 and ≤ 5/h); and (iii) control subjects without snoring (AHI ≤ 1/h). The 3 groups were compared regarding age, gender, BMI z-score, frequency of obesity and tonsillar hypertrophy, polysomnography indices, and morning serum cortisol concentration using one-way analysis of variance followed by post hoc tests (continuous variables) or χ2 (categorical characteristics). Student t-test was also applied to compare OSA patients to control subjects and to compare participants with OSA and tonsillar hypertrophy to participants with OSA and without tonsillar hypertrophy in terms of cortisol levels. Pearson correlation was carried out to assess the association of cortisol levels with polysomnography indices and BMI z-score.
A general linear model was used to evaluate the possible effect of: (i) tonsillar hypertrophy, (ii) OSA severity, and (iii) the interaction of tonsillar hypertrophy with OSA severity on cortisol concentration. Obesity, gender, and age were included as covariates in the model. In addition, one-way analysis of variance followed by post hoc tests was carried out to compare the subgroups of children with tonsillar hypertrophy and (i) moderate-to-severe OSA, (ii) mild OSA, or (iii) controls, in terms of serum cortisol levels. The same subgroup analysis was repeated for participants without tonsillar hypertrophy.
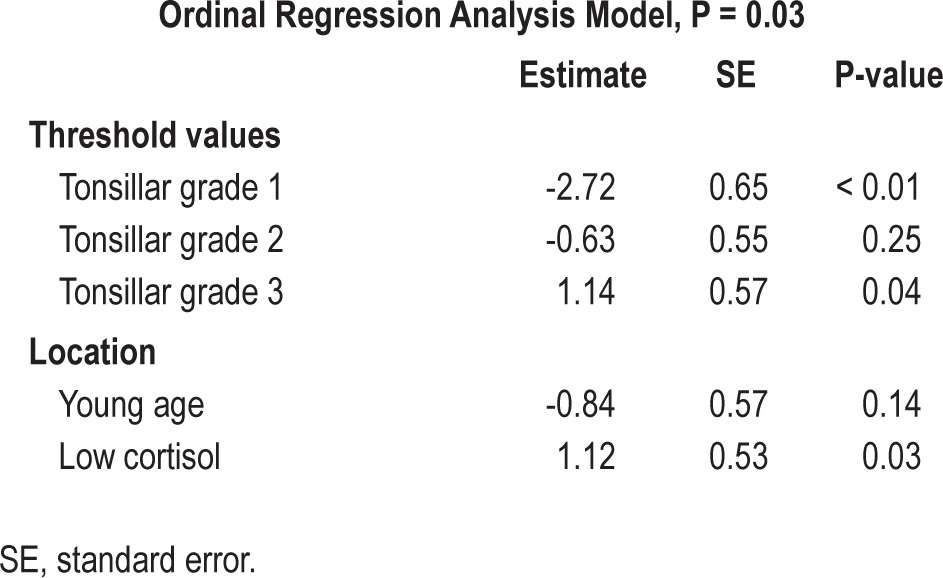
Linear regression analysis was applied in children with or without tonsillar hypertrophy to assess whether AHI or oxygen saturation of hemoglobin nadir (independent variable) were significant predictors of morning serum cortisol levels (dependent variable). Age, gender, and BMI z-score were included in the same regression analysis model. Subsequently, ordinal regression analysis was performed to evaluate low morning cortisol level (< 25th percentile of cortisol concentrations in all participants) as predictor of tonsillar size (tonsillar grade 1, 2, 3, or 4) adjusted by age (≤ 8 years vs. > 8 years).13,14
RESULTS
Subject Characteristics and Polysomnography Findings
A total of 70 children were recruited in the study, of whom 56 had OSA (AHI > 1/h) and 14 were controls without snoring (AHI ≤ 1/h). Sleep problems in the control group included nocturnal enuresis (n = 11), somniloquy (n = 1), and nocturnal bruxism (n = 2). Twenty-six of the participants with OSA had mild disease; the remaining had moderate-to-severe disease. Use of the obstructive AHI—instead of the AHI—did not change the classification of subjects in groups with mild or moderate-to-severe OSA.
The 3 study groups were similar regarding age, frequency of female gender or obesity, and prevalence of tonsillar hyper-trophy (Table 1). Children with moderate-to-severe OSA had significantly higher BMI z-scores than those with mild OSA (P < 0.05). As expected, polysomnography indices were substantially worse in children with moderate-to-severe OSA when compared to those with mild OSA or to control participants (Table 1). The 3 study groups did not differ in percentages of sleep stages (Table 1).
Table 1.
Summary statistics and significance of comparisons regarding characteristics, polysomnography indices, and morning serum cortisol levels in study groups

Morning Cortisol Serum Levels in Children with and without Tonsillar Hypertrophy
The 3 study groups were similar in terms of morning serum cortisol levels (Table 1). Patients with OSA and control subjects did not differ in serum cortisol concentrations (21.6 ± 8.4 mcg/dL vs. 22.3 ± 5.3 mcg/dL, respectively; P > 0.05). Subjects with OSA and tonsillar hypertrophy (n = 31) tended to have lower cortisol levels than those with OSA and without tonsillar hypertrophy (n = 25): 19.8 ± 7.6 mcg/dL vs. 23.8 ± 8.9 mcg/dL, respectively; P = 0.07. When both patients with OSA and control participants were considered, there were no significant correlations of morning serum cortisol concentrations with BMI z-score, AHI, obstructive AHI, respiratory arousal index, oxygen desaturation of hemoglobin (≥ 3%) index, or SpO2 nadir (P > 0.05).
Application of the general linear model did not reveal significant effects of severity of OSA (P = 0.56) or presence of tonsillar hypertrophy (P = 0.43) adjusted by obesity (P = 0.87), gender (P = 0.86), and age (P = 0.09) on the morning serum cortisol levels. However, there was a significant effect of the interaction between presence of tonsillar hypertrophy and severity of OSA on cortisol levels (P = 0.04) (Figure 1).
Figure 1.
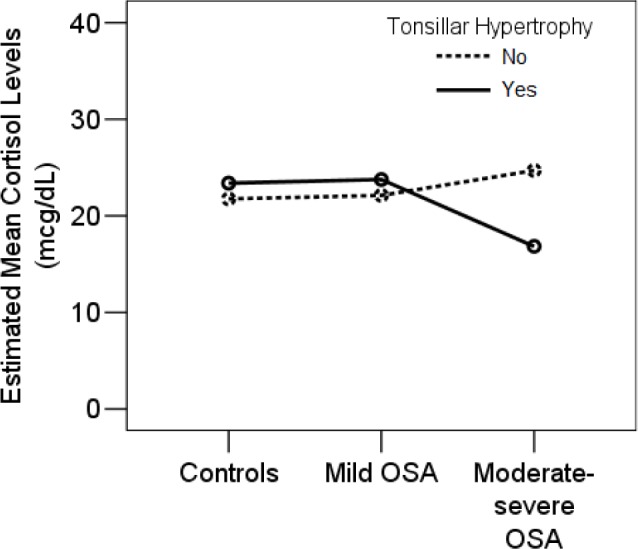
A significant effect of the interaction between presence of tonsillar hypertrophy (tonsillar size > 2+) and severity of OSA on morning serum cortisol levels was demonstrated (P = 0.04). Among children with hypertrophic tonsils, those with moderate-to-severe OSA had lower cortisol levels than subjects with mild OSA (P = 0.01) or controls (P = 0.04). No significant differences were identified in subjects without tonsillar hypertrophy.
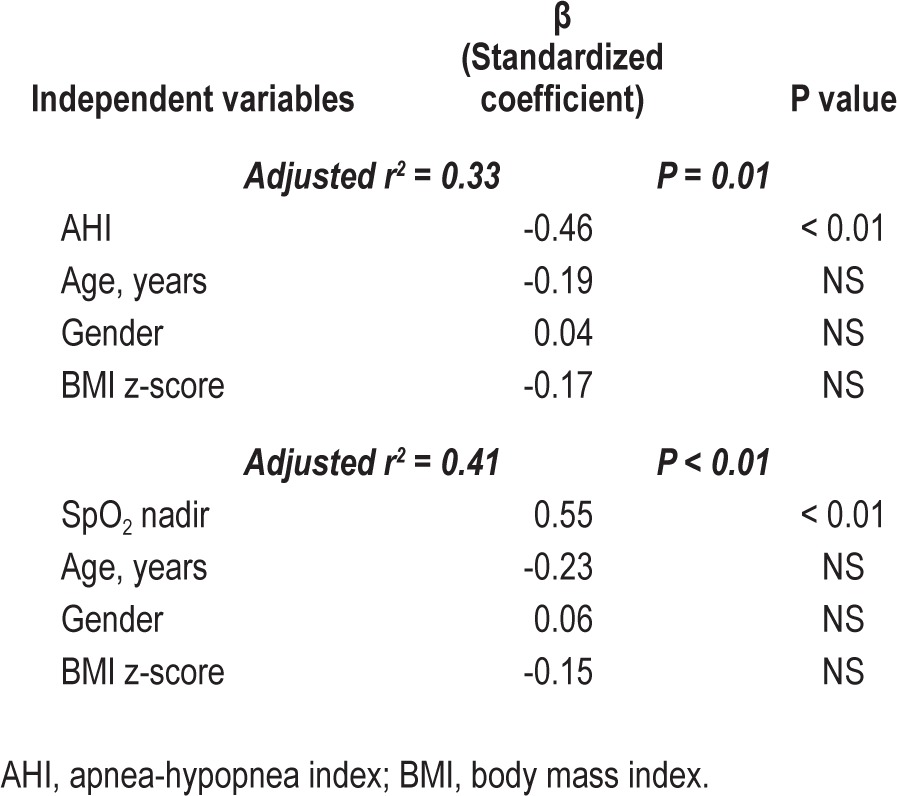
More specifically, when only subjects with tonsillar hyper-trophy were considered, children with moderate-to-severe OSA (n = 17; 6.1 ± 2.2 years; AHI 14.7 ± 10.6/h), children with mild OSA (n = 14; 6.8 ± 2.3 years; AHI 2.3 ± 1.2 episodes/h), and control participants (n = 6; 6 ± 2.1 years; AHI 0.7 ± 0.2/h) were significantly different regarding cortisol levels (P = 0.02). Participants with moderate-to-severe OSA had lower cortisol concentrations (16.9 ± 8.7 mcg/dL) than those with mild OSA (23.3 ± 4.2; P = 0.01) or control subjects (23.6 ± 5.3 mcg/dL; P = 0.04; Figure 2). Linear regression analysis revealed that in children with hypertrophic tonsils, AHI (or obstructive AHI) was negatively associated and oxygen saturation of hemoglobin nadir was positively associated with morning serum cortisol levels (r2 = 0.33, P = 0.01; r2 = 0.33, P = 0.01; and r2 = 0.41, P < 0.01, respectively; Table 2).
Figure 2.
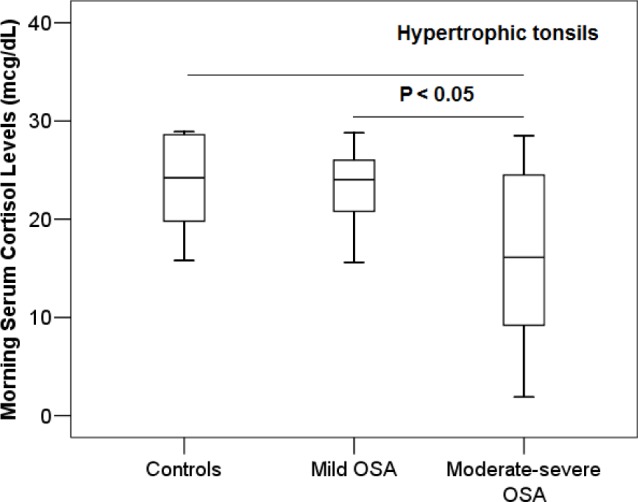
Among children with hypertrophic tonsils (tonsillar size > 2+), subjects with moderate-to-severe OSA had significantly lower morning serum cortisol levels than participants with mild OSA (P = 0.01) or controls (P = 0.04).
Table 2.
Multiple linear regression analysis models assessing the independent effect of variables on morning serum cortisol levels in children with tonsillar hypertrophy
In contrast, subjects with normal-size tonsils and moderate-to-severe OSA (n = 13; 5.1 ± 1.1 years; AHI 11.1 ± 5.6/h) had similar cortisol levels relative to subjects with normal-size tonsils and mild OSA (n = 12; 6.7 ± 2.5 years; AHI 2.4 ± 1.2/h) and relative to control participants with normal-size tonsils and without snoring (n = 8; 6.8 ± 2.8 years; AHI 0.7 ± 0.2/h): 25.6 ± 8.1 vs. 21.8 ± 9.6 and 21.3 ± 5.4 mcg/dL (P > 0.05; Figure 3). AHI (or obstructive AHI) and oxygen saturation of hemoglobin nadir were not significant predictors of morning serum cortisol concentrations (P > 0.05).
Figure 3.
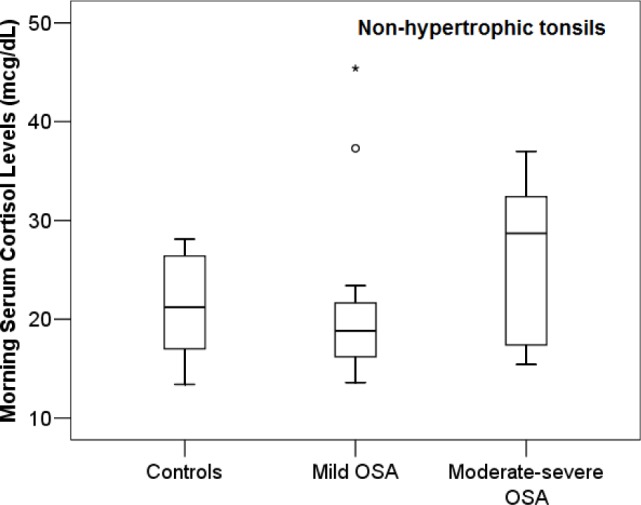
Among children without tonsillar hypertrophy (tonsillar size ≤ 2+), subjects with moderate-to-severe OSA, mild OSA, and controls did not differ in terms of morning serum cortisol levels (P > 0.05).
Low Morning Cortisol Serum Level as Predictor of Tonsillar Hypertrophy
The 25th percentile for cortisol levels in all participants was 16.5 mcg/dL. Of all participants, 81.4% were 8 years or younger. When ordinal regression analysis was performed, it was demonstrated that low morning cortisol levels (< 25th percentile) was significantly associated with greater tonsillar size (Figure 4) after adjustment for age (Table 3).
Figure 4.
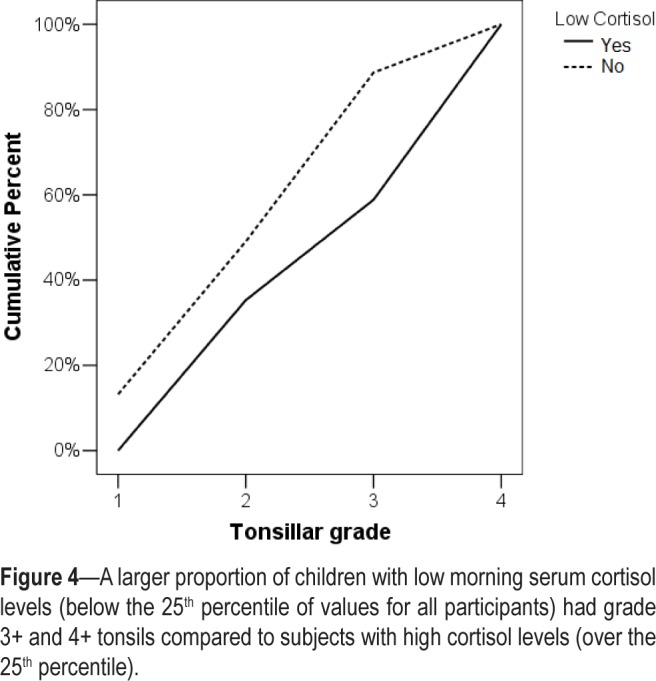
A larger proportion of children with low morning serum cortisol levels (below the 25th percentile of values for all participants) had grade 3+ and 4+ tonsils compared to subjects with high cortisol levels (over the 25th percentile).
Table 3.
Ordinal regression analysis model assessing the effect of low cortisol levels (< 25th percentile) on tonsillar size adjusted for young age
DISCUSSION
In the present study, a negative association was demonstrated between severity of OSA and morning serum cortisol levels among sleep apneic children with tonsillar hypertrophy, but not among those without enlarged tonsils. Results of the present report may indicate that children with OSA and pharyngeal lymphoid tissue hypertrophy represent a distinct subgroup as compared to sleep apneic subjects without hypertrophic palatine tonsils.15
In an old report, Soboczyński et al. studied the basal and after ACTH stimulation serum cortisol levels in children with tonsillar hypertrophy and SDB or recurrent angina and in healthy controls.3 Children with upper airway lymphoid tissue proliferation and sleep-related respiratory difficulties had lower serum cortisol levels than healthy controls, at baseline and after ACTH stimulation. Of interest, reduced levels persisted after adenotonsillectomy, implying an inherent defect in the HPA axis of children with SDB and hypertrophic tonsils. This pattern was not identified in subjects with recurrent angina.
Ordinal regression analysis indicated that participants with cortisol levels lower than the 25th percentile for all subjects were at greater risk to have large tonsils relative to subjects with high serum cortisol concentrations (over the 25th percentile). We propose that low serum cortisol concentrations in children with sleep apnea and adenotonsillar hypertrophy contribute to the uninhibited growth of pharyngeal lymphoid tissue and to persistence of OSA.
Results of the present study and of the report by Soboczynski et al.3 are consistent with the enhanced expression of α- and β-glucocorticoid receptors in tonsillar tissue excised from children with OSA relative to subjects with recurrent tonsil-litis.2 This increased expression may represent upregulation of the glucocorticoid receptors in response to low circulating cortisol.16 The active role of glucocorticoids on pharyngeal lymphoid tissue proliferation was delineated by Kheirandish-Gozal et al.5 with their work on tonsillar cell cultures. Incubation with corticosteroids abrogated the high proliferative rate of tonsillar cells, induced cellular apoptosis, and suppressed synthesis of proinflammatory cytokines.
The present study takes a step further into the understanding of the chain of events linking sleep apnea, tonsillar proliferation, and glucocorticoids. Children with moderate-to-severe SDB who manifest the clinical phenotype of hypertrophic tonsils seem to have reduced morning serum cortisol levels, and potentially decreased glucocorticoid inhibitory effects on tonsillar cell growth such as those reported by Kheirandish-Gozal et al.5
Endogenous cortisol contributes to the tight control of the immune system by suppressing inflammatory reactions and immune processes and by triggering lymphocyte apoptosis.4,16 Hypocortisolism and enhanced expression of glucocorticoid receptors follows chronic stress conditions, as, for example, posttraumatic stress disorder or Gulf War Illness.16 It has been proposed that increased concentration of glucocorticoid receptors in the pituitary cells after a period of chronic stress potentiates the negative feedback effect of cortisol on ACTH synthesis that maintains a persistent low-cortisol state.16
Intranasal administration of corticosteroids such as fluticasone for 6 weeks to children with adenoidal hypertrophy and OSA of moderate severity is accompanied by significant improvement in polysomnography indices.17 Similar beneficial effects have been demonstrated in subjects with mild OSA after treatment with intranasal budesonide.18 Clinical improvement is maintained for at least nine months after completion of the treatment course.19 These effects of intranasal corticosteroids are due to a decrease in adenoidal size and reduction in upper airway resistance.20
Release of endogenous cortisol follows a circadian rhythm with the lowest serum concentration at midnight and the highest 30 minutes after awakening.12 Hence, the low-cortisol state in children with moderate-to-severe OSA and tonsillar hyper-trophy should be confirmed in future studies using nocturnal and diurnal cortisol profile.7 Moreover, evaluation of adenoidal tissue size was not possible in control subjects since lateral neck x-rays could not be performed for ethical reasons, and parents did not consent to performance of nasopharyngoscopy by flexible endoscope. Thus, adenoidal hypertrophy was a potential confounding factor not addressed in this study.
In conclusion, the present study is the first to demonstrate a significant negative correlation between endogenous cortisol levels and OSA severity, concerning only those children with pharyngeal lymphoid tissue hypertrophy. A low- cortisol state might promote persistence of adenotonsillar hypertrophy and OSA in children.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by intramural funding (University of Thessaly Research Committee). There is no off-label or investigational use.
REFERENCES
- 1.Tsaoussoglou M, Lianou L, Maragozidis P, et al. Cysteinyl leukotriene receptors in tonsillar B- and T-lymphocytes from children with obstructive sleep apnea. Sleep Med. 2012;13:879–85. doi: 10.1016/j.sleep.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Goldbart AD, Veling MC, Goldman JL, Li RC, Brittian KR, Gozal D. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res. 2005;57:232–6. doi: 10.1203/01.PDR.0000150722.34561.E6. [DOI] [PubMed] [Google Scholar]
- 3.Soboczynski A, Grzegorowski M, Ryglewicz M, Wesolowski Z. Blood serum cortisol level in children with palatine and pharyngeal tonsil hypertrophy. Endokrynologia Polska. 1979;30:557–63. [PubMed] [Google Scholar]
- 4.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 5.Kheirandish-Gozal L, Serpero LD, Dayyat E, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33:1077–84. doi: 10.1183/09031936.00130608. [DOI] [PubMed] [Google Scholar]
- 6.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 7.Tomfohr LM, Edwards KM, Dimsdale JE. Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med Rev. 2012;16:243–9. doi: 10.1016/j.smrv.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–69. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 11.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 12.Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 13.Kaditis AG, Lianou L, Hatzinikolaou S, et al. Tonsillar size in 2- to 14-year-old children with and without snoring. Pediatric pulmonology. 2009;44:1216–22. doi: 10.1002/ppul.21126. [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou G, Kambas I, Tsaoussoglou M, Panaghiotopoulou-Gartagani P, Chrousos G, Kaditis AG. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr. 2013;162:269–74. doi: 10.1016/j.jpeds.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin. 2007;2:433–44. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Aslakson E, Gurbaxani BM, Vernon SD. Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor Biol Med Model. 2007;4:8. doi: 10.1186/1742-4682-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138:838–44. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 18.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122:e149–55. doi: 10.1542/peds.2007-3398. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos EI, Kaditis AG, Kalampouka E, et al. Nasal corticosteroids for children with snoring. Pediatr Pulmonol. 2004;38:161–7. doi: 10.1002/ppul.20079. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Mendoza-Sassi RA, Cesar JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008:CD006286. doi: 10.1002/14651858.CD006286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


