Abstract
Study Objectives:
The pathological role of snoring independent of obstructive sleep apnea remains under debate. The authors hypothesized that snoring sound intensity, as assessed by mean tracheal sound energy (Leq) during sleep, is related to daytime blood pressure.
Design:
Retrospective analysis of clinical records and polysomnography data.
Setting:
Sleep laboratory at a national hospital in Japan.
Patients:
Consecutive patients who underwent diagnostic polysomnography with suspicion of sleep apnea between January 2005 and December 2009 (n = 1,118).
Interventions:
Not applicable.
Measurements and Results:
Leq was calculated from tracheal sound spectra recorded every 0.2 sec during polysomnography. Daytime high blood pressure (HBP) was defined as taking antihypertensive drugs or having a systolic blood pressure ≥ 140 mm Hg or a diastolic blood pressure ≥ 90 mmHg at the patient's first clinical visit. Patient age, sex, body mass index, apnea-hypopnea index, alcohol consumption, and smoking were considered as confounders.
Leq during sleep was associated with HBP after adjusting for all confounding factors (n = 1,074, P = 0.00019). This association was demonstrated even in nonapneic nonobese patients (n = 232, P = 0.012).
Conclusions:
The association between snoring intensity, as assessed by mean sound energy, and blood pressure suggests a pathological role for heavy snoring. Further study in a general population is warranted.
Citation:
Nakano H; Hirayama K; Sadamitsu Y; Shin S; Iwanaga T. Mean tracheal sound energy during sleep is related to daytime blood pressure. SLEEP 2013;36(9):1361-1367.
Keywords: Acoustics, hypertension, sleep apnea, snoring
INTRODUCTION
Habitual snoring is a prevalent condition that affects 22-44% of middle-aged males and 13-28% of middle-aged females.1–3 Many epidemiological studies have demonstrated that habitual snoring is a major risk factor for cardiovascular disease.4–8 Until recently, the link between snoring and cardiovascular disease was considered to be attributable to coexisting obstructive sleep apnea (OSA).9 Snoring is certainly an important marker of OSA. But it is also known that snoring itself can lead to decreased baroreceptor sensitivity/reactivity10 and can induce mechanical injury to the carotid artery endothelium,11 indicating the possibility that snoring represents its own vascular risk.12 A recent report showed that heavy snoring is associated with carotid atherosclerosis independent of apnea severity and other risk factors.13 However, a conflicting study reported no association between snoring history and the degree of carotid stenosis.14 We have reported that ambient snoring intensity is independently associated with daytime blood pressure in individuals with primary snoring or mild OSA.15 However, many conflicting results have been reported regarding this issue.16–18A recent study using objective measurement of snoring showed that snoring does not increase risk for cardiovascular events or mortality in a general population.19 Such discrepancies are not only because of the differences in subjects but also because of the differences in snoring evaluation methods. Many studies used questionnaires, which are affected by the presence or absence of a bed partner and the perception of that partner.20,21 Objective measurements also are affected by day-to-day snoring variability and a lack of standardization.9 Snoring without OSA is classified as an “apparently normal variant and unresolved issue” in the International Classification of Sleep Disorders (Second Edition). To resolve the question of whether or not snoring is innocent, it is important to use a reliable and reproducible method to quantify snoring.
Measurements of snoring as ambient sound depend on the distance from the microphone to the subject and acoustic characteristics of the test room.22 Moreover, this measurement is strongly affected by ambient noise. In contrast, tracheal sounds from the neck are far less affected by these factors.23 Not only snoring but also normal breath sounds can be detected with high intensity using this method, which allows tracheal sound measurements to be unaffected by environmental noise. There-fore, we think that tracheal sound measurement, which can be performed in a home setting, is preferable for assessing snoring.
We hypothesized that tracheal sound intensity is related to blood pressure. In this study, we used equivalent sound pressure level (Leq)24 during sleep as a tracheal sound intensity variable because Leq seems to be a good parameter to represent mean sound energy during a given time.
METHODS
Subjects
This study was conducted by retrospective review of clinical records and analysis of polysomnography (PSG) data. The subjects were consecutive patients who underwent diagnostic PSG with suspicion of sleep apnea between January 2005 and December 2009. The study sample was different from that of our previous study,15 which was conducted between September 2002 and January 2005. The main symptoms of these patients were habitual snoring (n = 985), witnessed apnea (n = 901), and daytime sleepiness (n = 607). All patients were asked to complete a questionnaire during their first hospital visit to identify their symptoms, sleep habits, alcohol consumption, cigarette smoking, and clinical history including medications.
Anthropometric and blood pressure measurements were obtained during the first visit. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters. Blood pressure was measured with a sphygmomanometer with the subject in a seated position. The average value of two or three measurements was recorded as the blood pressure value.
The initial total number of subjects was 1,220, but 49 were excluded because of a PSG record failure and an additional 53 subjects were excluded because of the lack of a clinical record about blood pressure status. The remaining 1,118 subjects were used for subsequent analyses.
This retrospective study was approved by the Institutional Review Board with a waiver of patient consent.
Polysomnography
PSG was recorded using a polygraph system (EEG7414; Nihon Kohden, Kobe, Japan). Electroencephalography (C3-A2, O2-A1), bilateral electrooculography, submental electromyography (EMG), electrocardiography, and bilateral anterior tibial EMG were recorded. Oronasal airflow was monitored using a thermocouple sensor and/or a nasal prong pressure sensor. Thoracic and abdominal respiratory movements were monitored using respiratory inductive plethysmography (RIP) (Inductotrace; Ambulatory Monitoring Inc., Brown Deer, WI, USA). Oxyhe-moglobin saturation was monitored using pulse oximetry with a finger probe at the fastest response mode (OLV-3100; Nihon Kohden). Tracheal sounds were recorded from an air-coupled microphone (RP-VC3, Panasonic, Kadoma, Japan) attached to the neck over the trachea. The tracheal sound recording system was calibrated using reference sound pressure (94 dB). All signals were digitized and stored on a personal computer (PC). Sleep stages were scored manually according to the standard criteria of Rechtschaffen and Kales.25 We calculated the sleep fragmentation index (SFI) as a parameter of sleep fragmentation, which is the total number of awakening/shifts to stage 1 from deeper nonrapid eye movement or rapid eye movement sleep divided by the total sleep time in hours.26 Apnea was defined as an episode of airflow cessation lasting ≥ 10 sec. Hypopnea was defined as a ≥ 30% decrease in the RIP sum signal amplitude or nasal pressure signal (square root transformed) amplitude lasting ≥ 10 sec with ≥ 4% oxygen desaturation.27 The apnea-hypopnea index (AHI) was calculated as the number of apnea and hypopnea events per hour of sleep. The oxygen desaturation index (ODI) was defined by the number of dips (≥ 4%) per hour in the hemoglobin-oxygen saturation level. We also calculated the duration of oxygen saturation < 90%, which was expressed as a percentage of the total examination time (CT90).
Tracheal Sounds
We recorded tracheal sounds routinely to quantify snoring and to assist in scoring respiratory events when other respiratory channel signals were insufficient. Tracheal sounds were analyzed using a PC-based compressed sound spectrograph system that we developed to detect snoring. In brief, tracheal sounds were digitized using a sound system incorporated in the PC at a sampling frequency of 11,025 Hz, from which the power spectra for 1,024 points were calculated using a fast Fourier transform and stored in the PC every 0.2 sec in real time. Thereafter, the system automatically detected snoring on the basis of the criteria in which a peak power spectral density value > 70 dB/Hz within the frequency bandwidth of 100-300 Hz was defined as snoring.28 However, we decided not to use the snoring variables (snoring time and mean snoring intensity) obtained by this method to test the a priori hypothesis in this study because such a method may not be reproducible in other laboratories with a different sound acquisition system. Instead, we used Leq during sleep. The Leq was calculated as the average of the squared ratio of the instantaneous sound pressure and a reference signal (20 μPa) over total sleep time, which was expressed in dB. The instantaneous sound pressure was recalculated from the stored power spectra data (22-1,992 Hz) using the Parseval theorem without A-weighting. The Leq represents mean sound energy during total sleep time.
Analysis
We investigated the relationship between Leq and blood pres-sure status [presence or absence of daytime high blood pressure (HBP)]. Daytime HBP was defined as taking antihypertensive drugs, systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg at the patient's first clinical visit. Multivariable logistic regression analyses were used to elucidate the independent relationship between Leq and blood pressure status after adjusting for possible confounders.
The analysis was primarily performed for all subjects. Descriptive characteristics are summarized as Leq quintiles. Continuous variables are expressed as means and standard deviations (SD) for normally distributed variables, other-wise as medians and interquartile ranges (IQR). Categorical variables are expressed as percentages. A trend analysis for each characteristic among the quintiles was performed using the Jonckheere-Terpstra test for continuous variables or the Cochran-Armitage trend test for categorical variables.
The subjects were stratified into four groups according to the presence or absence of OSA (AHI ≥ 5) and obesity (BMI ≥ 25) because both factors were considered to have a very strong confounding effect.
RESULTS
Subject Characteristics
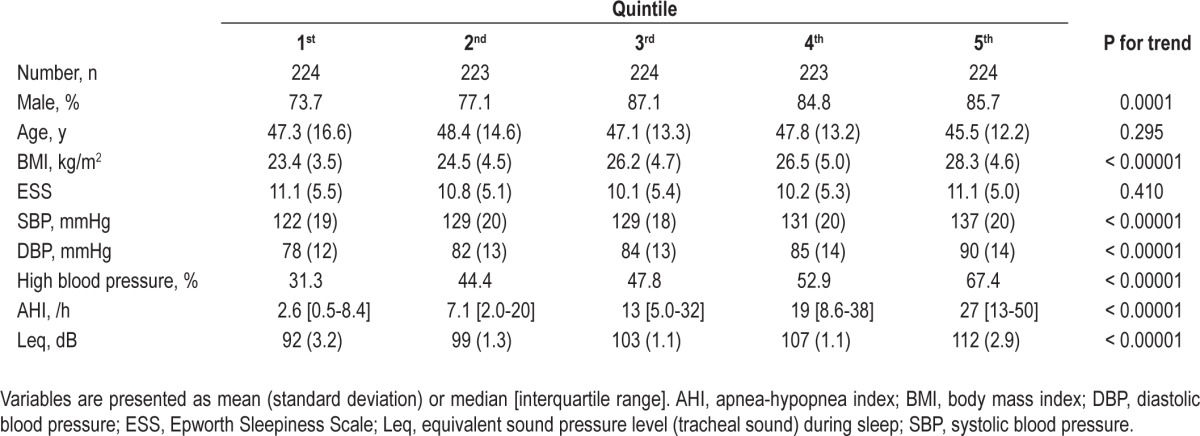
The mean age of the subjects was 47 y (SD, 14 y) and mean BMI was 25.8 kg/m2 (SD, 4.8 kg/m2). Subject sex was predomi-nantly male (82%). The median AHI was 12.0 (IQR, 3.5-30.7). The dominant type of apnea-hypopnea event was obstructive except for four central sleep apnea subjects. A total of 545 subjects (49%) were classified with HBP, and 208 of these subjects were taking antihypertensive medications. The calculated Leq value during sleep was normally distributed with a mean value of 102.5 dB (SD, 7.1 dB) (Figure 1). Descriptive characteristics according to the Leq quintiles are summarized in Table 1. Subjects in higher Leq quintiles tended to have HBP more often than those in lower Leq quintiles. The BMI and OSA-related values were also higher in the higher Leq quintiles compared with those in the lower Leq quintiles. There was a modest correlation between Leq and AHI (n = 1,118, r = 0.42).
Figure 1.
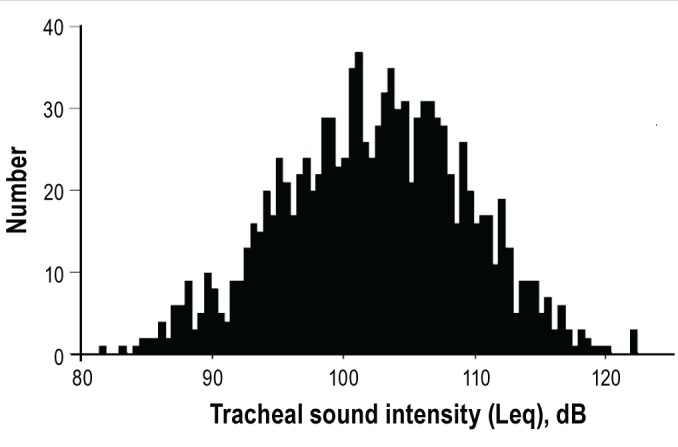
Equivalent tracheal sound pressure level (Leq) distribution in all subjects (n = 1,118).
Table 1.
Subject characteristics according to Leq quintile
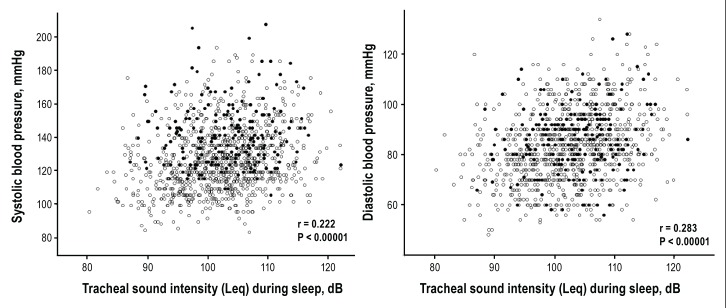
The relationship between Leq and blood pressure values is shown in Figure 2.
Figure 2.
Scatterplots showing the relationship between equivalent tracheal sound pressure level (Leq) during sleep and daytime blood pressure (n = 1,118). Closed circles and open circles represent blood pressure levels of subjects who did and did not take antihypertensive medication, respectively.
HBP Logistic Regression Analysis
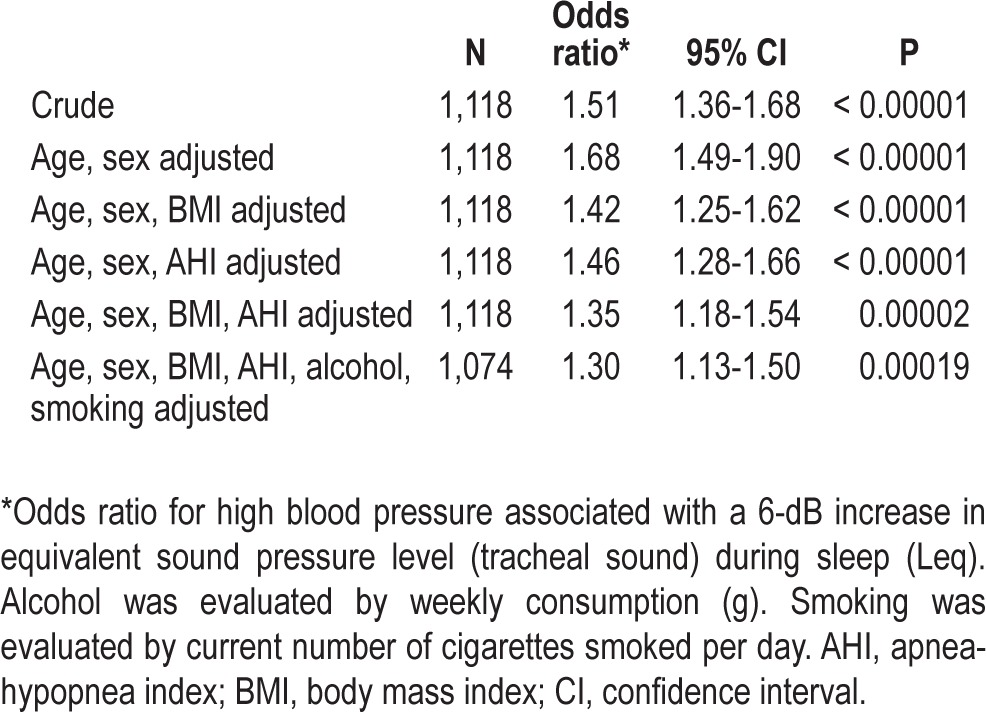
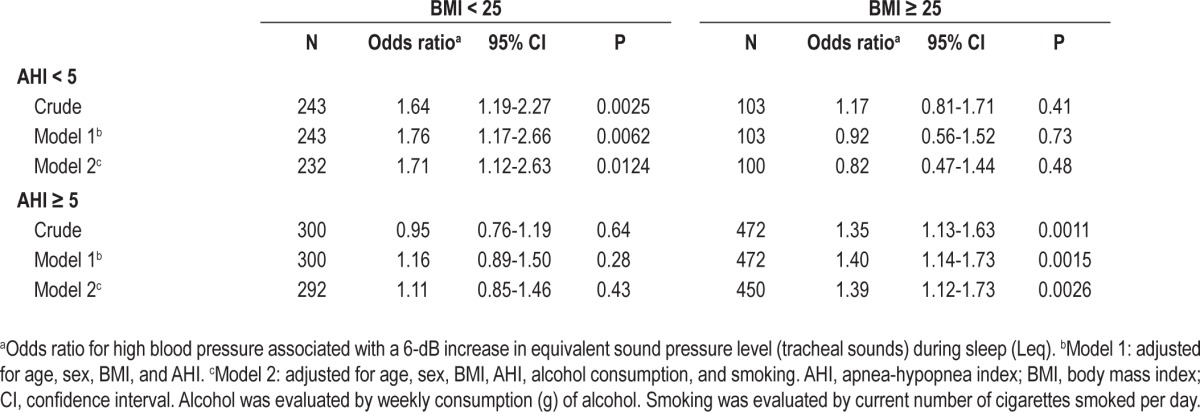
We used multiple logistic regression analysis to adjust for the confounding factors to identify the independent effect of Leq on blood pressure status. Table 2 shows the odds ratios for HBP associated with a 6-dB increase in Leq, which indicates a twofold increase in mean square root sound pressure. The crude odds ratio was 1.51, and it increased slightly after adjusting for sex and age probably because older subjects had a slightly lower Leq value than younger subjects. The odds ratio decreased considerably after adjusting for BMI and AHI. The fully adjusted odds ratio was 1.30, which was highly significant (P = 0.00019). Furthermore, the other variables related to OSA (ODI, CT90, and SFI) had almost no effect on the odds ratio when added into the statistical model. No interactions were observed between Leq and other variables including BMI, AHI, age, and sex with respect to HBP.
Table 2.
Crude and adjusted association between tracheal sound intensity during sleep and high blood pressure by logistic regression analysis
Secondary Subgroup Analysis
We considered that the previously discussed statistical analysis might raise concern about failing to exclude the effect of confounders, particularly obesity and OSA. Therefore, we performed the same analysis for the subgroups stratified according to the presence or absence of obesity and OSA. The characteristics of the subgroups are summarized in Table 3. Mean age of the nonobese apneic subgroup was markedly higher than that of the other subgroups (analysis of variance and Bonferroni post hoc test, P < 0.01). The logistic regression analysis (Table 4) showed that Leq was associated with HBP in nonobese nonapneic and in obese apneic subjects. However, Leq was not associated with HBP in nonobese apneic subjects or in obese nonapneic subjects.
Table 3.
Subgroup subject characteristics stratified according to presence or absence of obstructive sleep apnea and obesity
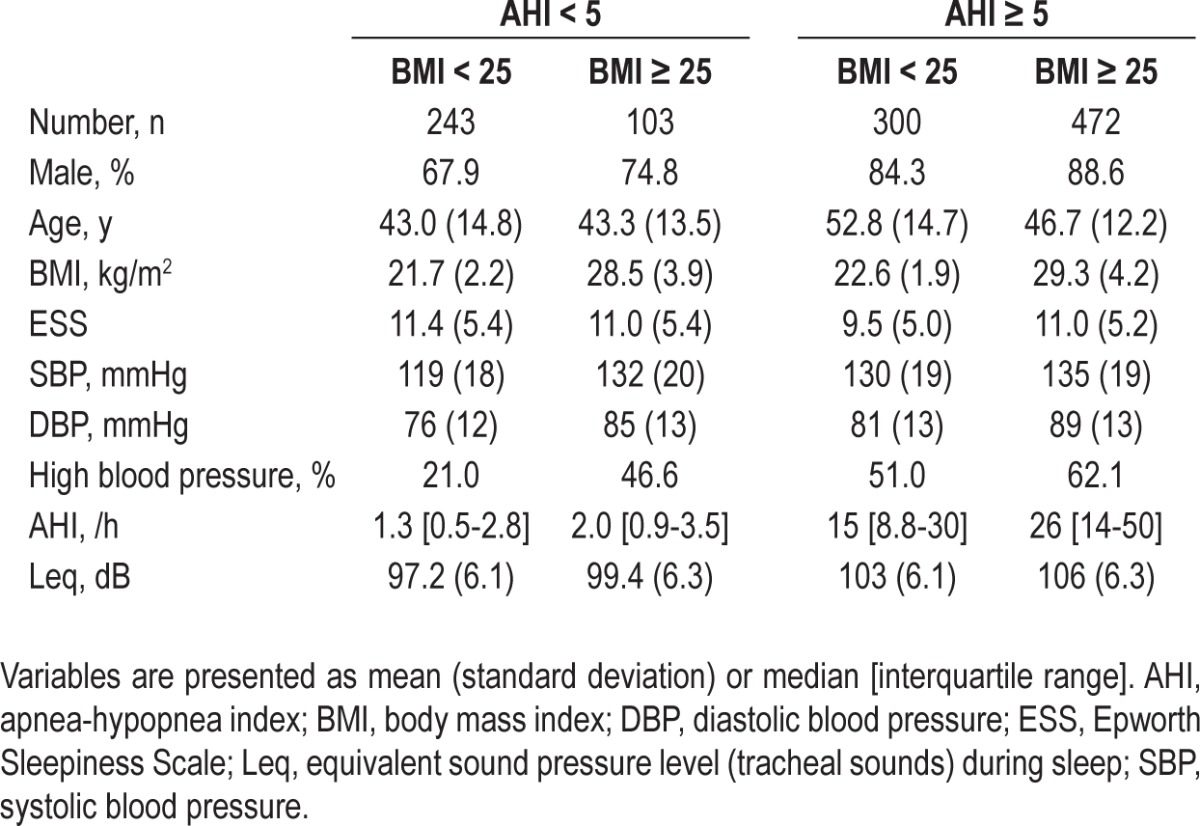
Table 4.
Crude and adjusted associations between tracheal sound intensity during sleep and high blood pressure by logistic regression analysis in the subgroups stratified according to the presence or absence of obstructive sleep apnea and obesity
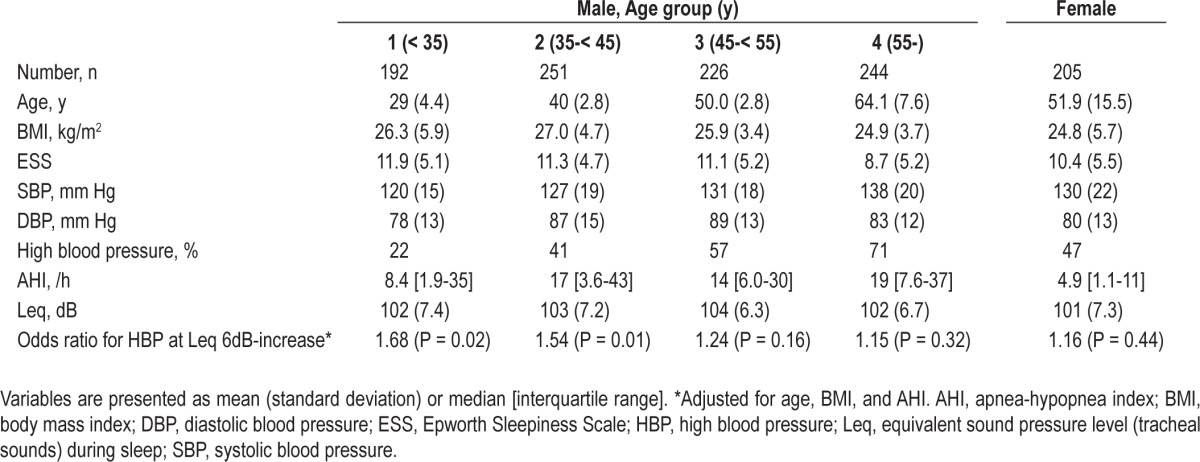
Thereafter, we tested the effect of sex and age on the relationship between Leq and blood pressure status. Consequently, the effect of snoring on blood pressure status depended on age in male subjects (Table 5). Snoring had less of an effect on blood pressure in older males and no effect in females. These results suggest the possibility that the lack of association between Leq and HBP in the nonobese apneic or obese nonapneic subgroups was due to the small number of males younger than 45 y. Therefore we attempted this analysis in these groups by excluding males age 45 y and older and females. As a result, the odds ratio (adjusted for age, BMI, and AHI) became 1.94 (n = 83, P = 0.039) and 1.19 (n = 47, P = 0.66) for the nonobese apneic and obese nonapneic subgroups, respectively.
Table 5.
Sex and age subgroup analysis
Secondary Analysis for Other Snoring Variables
We examined the other snoring variables used in our daily clinical practice for their relationship to HBP to determine whether the observed relationship between tracheal sound intensity and HBP was specific to the a priori selected variable, Leq. As a result, snoring intensity variables were strongly related with HBP after fully adjusting for confounding factors [mean snoring intensity: P = 0.00012; top 1 percentile sound intensity (L1): P = 0.00014], but snoring time was only weakly associated with HBP (P = 0.014)].
We also examined the effect of tracheal sound frequency components on the relationship to HBP. As a result, the energy of the 400-700 Hz band was more closely related to HBP than that of other bands (< 100 Hz, 100-400 Hz, 700-1,000 Hz, and 1-2 kHz). However, the strength of the association to HBP is not so different between Leq (total energy) and these band energies. Furthermore, we examined the relationship between body position-specific Leq and HBP. As a result, total Leq was more closely related to HBP than body position-specific Leqs.
DISCUSSION
We examined the relationship between Leq and daytime blood pressure status in a relatively large sample of patients who underwent diagnostic PSG. The main finding was that Leq during sleep was an independent determinant of HBP after adjusting for OSA severity and other confounding factors. This relationship was also demonstrated in nonobese nonapneic subjects.
The association between Leq and HBP was not demonstrated in the nonobese apneic or obese nonapneic subgroups. This result can be at least partially explained by the sex and age distribution of the subgroups. The current results show that the association between Leq and HBP was significant only in male subjects younger than 45 y. It seems similar to the relationship between OSA and hypertension that was reported to be prominent in subjects younger than 50 y.29 In the current study, the number of males younger than 45 y in the subgroups was 109, 47, 83, and 205, in nonobese nonapneic, obese nonapneic, nonobese apneic, and obese apneic subgroups, respectively. A significant association existed in the nonobese apneic subgroup when the subjects were confined to males younger than 45 y. The obese nonapneic subgroup of younger male subjects (n = 47) was considered too small in number to obtain any conclusion.
No standardized method is available to quantify snoring.9 We have been detecting snoring from tracheal sounds using a power-spectral density threshold. Many laboratories have detected snoring using a certain threshold value. Such a method could be easily affected by the acoustic characteristics of the microphone and its attachment,30 which can lead to different results from different sound acquisition systems. An appropriate threshold value needs to be determined according to the laboratory sound system. If the threshold is set at a relatively low value, total snoring time will be estimated greater and mean snoring intensity will be estimated lower, and vice versa. These effects may distort the estimated relationship between snoring and the outcome. Sound pressure level distribution parameters (L1, L5, and L10) are also uncertain because selecting the percentile value is arbitrary. To avoid such problems, we chose Leq as the tracheal sound intensity parameter in this study. Measuring Leq does not require a threshold value, which makes it easily applicable to many laboratories. Moreover, if there is a linear relationship between Leq (x) and blood pressure (y), the relationship is not expected to be so different across different laboratories, except for the x-intercept. Tracheal sounds can be affected by the filtering effect of neck fat tissue. However, the relationship between tracheal sound intensity and ambient sound intensity was not different among normal weight, over-weight, and obese subjects in this study (data not shown).
Leq reflects mean sound energy during a given time. Tracheal sounds during sleep, which are recorded at the surface of the neck, include breath sounds, snoring sounds, cardiovascular sounds, and muscle sounds. Of these, snoring sounds are usually 10-100 times (20-40 dB) greater in amplitude compared with that of normal breath sounds.31 Sound energy is proportional to the square of sound amplitude. Therefore, the main constituent of Leq is snoring sound, indicating that Leq reflects snoring sound intensity. Most of the subjects in this study were snorers, but “nonsnorers” also snored more or less during the sleep study.
However, it is possible that high amplitude breath sounds are the main constituent of Leq in subjects with very little snoring.
Although this study was not designed to investigate the mechanism of the link between snoring and hypertension, some considerations can be addressed. First, the association between snoring and HBP was partially dependent on and partially independent of obesity and OSA. Specifically, it was not dependent on obesity or OSA in nonapneic nonobese snorers. Second, intensity rather than snoring time is thought to be important for this association. This finding is interesting because snoring sound intensity has been reported to be correlated with pleural pressure swing amplitude.32 Stradling et al. showed that respiratory effort during sleep decreases the normal overnight fall in blood pressure in a community population.33 Mateika et al. reported that nonapneic snoring leads to a decreased baroreceptor response,10,34 and suggested that negative pleural pressure during snoring is important for this phenomenon. The current findings agree with these studies if snoring intensity is translated to respiratory effort or heightened negative pleural pressure. Moreover, a recent experimental study in rabbits demonstrated that exposure to vibrations induces carotid artery endothelial dysfunction in a vibration energy dose-dependent manner,11 suggesting the importance of snoring intensity. Finally, SFI had no effect on the association. Therefore, we think the effect of snoring on daytime blood pres-sure status is not attributable to sleep fragmentation.
This study had several limitations that should be addressed. First, the study design was a retrospective analysis of past clinical data. However, we can say that the study was hypothesis-driven and the results were not obtained by chance. Second, we used an office blood pressure value to determine blood pressure status. It would be desirable to use 24-h ambulatory blood pressure monitoring to understand the exact role of snoring intensity on blood pressure.35 Third, we did not evaluate visceral obesity. Therefore, the effect of visceral obesity as a confounding factor was not eliminated in this study. Finally, the most important limitation is that this study was conducted using a clinic sample; therefore, the results cannot be extended to the general population. Thus, it will be necessary to perform such a general population study to understand the exact significance of snoring intensity in asymptomatic subjects.
In conclusion, we showed that tracheal sound intensity, as assessed by Leq, was independently related to daytime HBP in patients with snoring or OSA, and the relationship was stronger in younger male subjects. Further study is warranted to under-stand the applicability of this finding to the general population.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
This study was supported by a grant from a Health and Labor Sciences Research Grant (22110201), Ministry of Health, Welfare and Labor.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 3.Cano-Pumarega I, Dura'n-Cantolla J, Aizpuru F, et al. Obstructive sleep apnea and systemic hypertension. Am J Respir Crit Care Med. 2011;184:1299–304. doi: 10.1164/rccm.201101-0130OC. [DOI] [PubMed] [Google Scholar]
- 4.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkilä K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J. 1987;294:16–9. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150:806–16. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 6.Zamarron C, Gude F, Otero Y, Rodriguez-Suarez JR. Snoring and myocardial infarction: a 4-year follow-up study. Respir Med. 1999;93:108–12. doi: 10.1016/s0954-6111(99)90299-8. [DOI] [PubMed] [Google Scholar]
- 7.Leineweber C, Kecklund G, Janszky I, Åkerstedt T, Orth-Gomér K. Snoring and progression of coronary artery disease: the Stockholm female coronary angiography study. Sleep. 2004;27:1344–9. doi: 10.1093/sleep/27.7.1344. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GN, Jiang CQ, Lao XQ, et al. Snoring and vascular risk factors and disease in a low-risk Chinese population: the Guangzhou Biobank Cohort Study. Sleep. 2006;29:896–900. doi: 10.1093/sleep/29.7.896. [DOI] [PubMed] [Google Scholar]
- 9.Hoffstein V. Snoring. Chest. 1996;109:201–22. doi: 10.1378/chest.109.1.201. [DOI] [PubMed] [Google Scholar]
- 10.Mateika JH, Kavey NB, Mitru G. Spontaneous baroreflex analysis in non-apneic snoring individuals during NREM sleep. Sleep. 1999;22:461–8. doi: 10.1093/sleep/22.4.461. [DOI] [PubMed] [Google Scholar]
- 11.Cho JG, Witting PK, Verma M, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34:751–7. doi: 10.5665/SLEEP.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TB, Strollo PJ., Jr A nuisance or nemesis: the adverse effects of snoring. Sleep. 2011;34:693–4. doi: 10.5665/SLEEP.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Mason RH, Mehta Z, Fonseca AC, Stradling JR, Rothwell PM. Snoring and severity of symptomatic and asymptomatic carotid stenosis: a population-based study. Sleep. 2012;35:1147–51. doi: 10.5665/sleep.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa T, Nakano H, Hirayama K, et al. Relationship between snoring sound intensity and daytime blood pressure. Sleep Biol Rhythms. 2010;8:245–53. [Google Scholar]
- 16.Stradling JR, Crosby JH. Relation between systemic hypertension and sleep hypoxaemia or snoring: analysis in 748 men drawn from general practice. Br Med J. 1990;300:75–8. doi: 10.1136/bmj.300.6717.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffstein V. Blood pressure, snoring, obesity, and nocturnal hypoxaemia. Lancet. 1994;344:643–5. doi: 10.1016/s0140-6736(94)92084-2. [DOI] [PubMed] [Google Scholar]
- 18.Sjöström C, Lindberg E, Elmasry A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57:602–7. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall NS, Wong KKH, Cullen SRJ, Knuiman MW, Grunstein RR. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the Busselton Health Study. Sleep. 2012;35:1235–40. doi: 10.5665/sleep.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffstein V, Mateika J, Anderson D. Snoring: is it in the ear of the beholder? Sleep. 1994;17:522–6. doi: 10.1093/sleep/17.6.522. [DOI] [PubMed] [Google Scholar]
- 21.Hoffstein V, Mateika S, Nash S. Comparing perceptions and measurement of snoring. Sleep. 1996;19:783–9. [PubMed] [Google Scholar]
- 22.Pevernagie D, Aarts RM, De Meyer M. The acoustics of snoring. Sleep Med Rev. 2010;14:131–44. doi: 10.1016/j.smrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Nakano H, Suzuki T, Yamauchi M, Ohnishi Y, Maekawa J. Relationship between snoring measured at the anterior neck and ambient noise. Clin Phamacol Ther. 2003;13:333–7. (in Japanese) [Google Scholar]
- 24.Dalmasso F, Prota R. Snoring: analysis, measurement, clinical implications and applications. Eur Respir J. 1996;9:146–59. doi: 10.1183/09031936.96.09010146. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. doi: 10.1046/j.1440-1819.2001.00810.x. NIH, Pub #204,1968. [DOI] [PubMed] [Google Scholar]
- 26.Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med. 2000;162:2091–6. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 27.AASM Clinical Practice Review Committee. Hypopnea in sleep disordered breathing in adults. Sleep. 2001;24:469–70. [PubMed] [Google Scholar]
- 28.Nakano H, Ikeda T, Hayashi M, Ohshima E, Onizuka A. Effects of body position on snoring in apneic and nonapneic snorers. Sleep. 2003;26:169–72. doi: 10.1093/sleep/26.2.169. [DOI] [PubMed] [Google Scholar]
- 29.Grote L, Ploch T, Heitmann J, Knaack L, Penzel T, Peter JH. Sleep-related breathing disorder is an independent risk factor for systemic hypertension. Am J Respir Crit Care Med. 1999;160:1875–82. doi: 10.1164/ajrccm.160.6.9811054. [DOI] [PubMed] [Google Scholar]
- 30.Kraman SS, Wodicka GR, Pressler GA, Pasterkamp H. Comparison of lung sound transducers using a bioacoustics transducer testing system. J Appl Physiol. 2006;101:469–76. doi: 10.1152/japplphysiol.00273.2006. [DOI] [PubMed] [Google Scholar]
- 31.Nakano H, Matsuzawa K, Ohnishi Y, Maekawa J, Narita N. Discriminative acoustic parameters for detection of snoring. Ther Res. 1997;18:2994–8. (in Japanese) [Google Scholar]
- 32.Miyazaki S, Itasaka Y, Ishikawa K, Togawa K. Acoustic analysis of snoring and the site of airway obstruction in sleep related respiratory disorders. Acta Otolaryngol Suppl. 1998;537:47–51. [PubMed] [Google Scholar]
- 33.Stradling JR, Barbour C, Glennon J, Langford BA, Crosby JH. Which aspects of breathing during sleep influence the overnight fall of blood pressure in a community population? Thorax. 2000;55:393–8. doi: 10.1136/thorax.55.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates GJ, Mateika SE, Basner RC, Mateika JH. Baroreflex sensitivity in nonapneic snorers and control subjects before and after nasal continuous positive airway pressure. Chest. 2004;126:801–7. doi: 10.1378/chest.126.3.801. [DOI] [PubMed] [Google Scholar]
- 35.Roche F, Pe'pin JL, Achour-Crawford E, et al. At 68 years, unrecognised sleep apnoea is associated with elevated ambulatory blood pressure. Eur Respir J. 2012;40:649–56. doi: 10.1183/09031936.00162710. [DOI] [PubMed] [Google Scholar]