Abstract
Study Objectives:
In schizophrenia there is a dramatic reduction of sleep spindles that predicts deficient sleep-dependent memory consolidation. Eszopiclone (Lunesta), a non-benzodiazepine hypnotic, acts on γ-aminobutyric acid (GABA) neurons in the thalamic reticular nucleus where spindles are generated. We investigated whether eszopiclone could increase spindles and thereby improve memory consolidation in schizophrenia.
Design:
In a double-blind design, patients were randomly assigned to receive either placebo or 3 mg of eszopiclone. Patients completed Baseline and Treatment visits, each consisting of two consecutive nights of polysomnography. On the second night of each visit, patients were trained on the motor sequence task (MST) at bedtime and tested the following morning.
Setting:
Academic research center.
Participants:
Twenty-one chronic, medicated schizophrenia outpatients.
Measurements and Results:
We compared the effects of two nights of eszopiclone vs. placebo on stage 2 sleep spindles and overnight changes in MST performance. Eszopiclone increased the number and density of spindles over baseline levels significantly more than placebo, but did not significantly enhance overnight MST improvement. In the combined eszopiclone and placebo groups, spindle number and density predicted overnight MST improvement.
Conclusion:
Eszopiclone significantly increased sleep spindles, which correlated with overnight motor sequence task improvement. These findings provide partial support for the hypothesis that the spindle deficit in schizophrenia impairs sleep-dependent memory consolidation and may be ameliorated by eszopiclone. Larger samples may be needed to detect a significant effect on memory. Given the general role of sleep spindles in cognition, they offer a promising novel potential target for treating cognitive deficits in schizophrenia.
Citation:
Wamsley EJ; Shinn AK; Tucker MA; Ono KE; McKinley SK; Ely AV; Goff DC; Stickgold R; Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. SLEEP 2013;36(9):1369-1376.
Keywords: Schizophrenia, procedural learning, motor skill, sleep spindles, memory consolidation, automation
INTRODUCTION
Cognitive deficits are the strongest predictor of functional outcome in schizophrenia,1 and antipsychotic drugs (APDs) are relatively ineffective in treating them (e.g., Sergi2). Amelioration of cognitive deficits has thus become a priority of the schizophrenia research community and is the focus of large-scale studies.3 A limitation of these efforts is that cognition is measured in cross-section. While this provides a valid snapshot of function, it misses critical aspects of learning and memory that happen offline, over time and with sleep. Following encoding, memories undergo “consolidation” processes that stabilize, enhance, integrate, and reorganize memory in the brain. A wealth of evidence from molecular, cellular, neural network, regional brain activation, and behavioral studies of birds,4 rodents,5 cats,6 and humans7 demonstrates that sleep plays a critical role in memory consolidation. This work suggests an evolutionarily conserved function for sleep in memory consolidation. In schizophrenia, there is evidence of both abnormal sleep8 and impaired memory consolidation,9 yet little is known about how abnormal sleep contributes to memory consolidation deficits. Understanding this contribution may open new avenues to treatment.
Recent studies find marked impairments (reduced by 67% to 100%) of sleep -dependent consolidation of motor procedural memory10–12 and dramatically reduced sleep spindle activity11–15 in schizophrenia. Sleep spindles are a defining characteristic of stage 2 NREM sleep, evident in the EEG as brief powerful bursts of 12-15 Hz synchronous activity.16 We recently reported that the sleep spindle and memory deficits are correlated and hypothesized that the reduction in sleep spindles impairs memory consolidation in schizophrenia.15 This hypothesis is consistent with evidence from animal studies that sleep spindles are a key mechanism of synaptic plasticity that mediates memory consolidation during sleep17 and from human studies that link spindles to the sleep-dependent consolidation of both procedural and declarative memory.18 In the present study, we investigated whether eszopiclone (Lunesta), a non-benzodiazepine hypnotic agent that acts on γ-aminobutyric acid (GABA)ergic neurons in the thalamic reticular nucleus (TRN),19 where sleep spindles are generated,20 could increase sleep spindles and thereby improve memory consolidation in schizophrenia.
While the cause of the spindle deficit in schizophrenia is unknown, reduced spindle activity may reflect TRN dysfunction, consistent with evidence of TRN abnormalities in schizophrenia.21 The TRN is comprised entirely of GABAergic neurons22 that primarily project to glutamatergic thalamic neurons, which then project to the cortex. Cortical neurons, in turn, send glutamatergic inputs back to N-methyl-D-aspartate acid (NMDA) receptors on TRN neurons. Thus, spindles are mediated by a thalamocortical feedback loop that is regulated by both GABAergic and NMDA-receptor mediated glutamatergic neurotransmission.23 Eszopiclone acts on GABAA receptors,24 particularly on subunits preferentially expressed in the TRN.19 It has demonstrated safety and sustained efficacy in treating insomnia25 and is approved for long-term treatment. The potential for eszopiclone to increase spindles without causing physiologic tolerance or serious withdrawal effects,26 and its apparent lack of detrimental effects on cognition and psychomotor function the following day, make it a viable potential adjunctive agent.27,28
To test the hypothesis that eszopiclone would increase sleep spindles and thereby improve memory consolidation in schizophrenia, we conducted a double-blind, placebo-controlled study. Medically and clinically stable outpatients with schizophrenia participated in Baseline and Treatment visits, each consisting of 2 consecutive nights of polysomnography. We employed the same simple, well-characterized test of motor procedural memory as in our previous studies of schizophrenia, the finger tapping motor sequence task (MST).29,30 Improvement of MST performance occurs after sleep—but not after an equivalent period of wake and MST improvement—as well as overnight improvement on other simple motor skill tasks-correlates with the amount of stage 2 NREM sleep30 and with the number and density of sleep spindles.31–33 In schizophrenia, overnight MST improvement is markedly reduced,10,11 and in the data from the Baseline visit of the present study reported elsewhere,15 this reduction correlated with spindle number and density, which were also markedly reduced (by 36% and 38%, respectively). Here we report the findings from the Treatment visit examining the effects of eszopiclone versus placebo on stage 2 sleep spindles and overnight MST improvement in patients.
METHODS
Participants
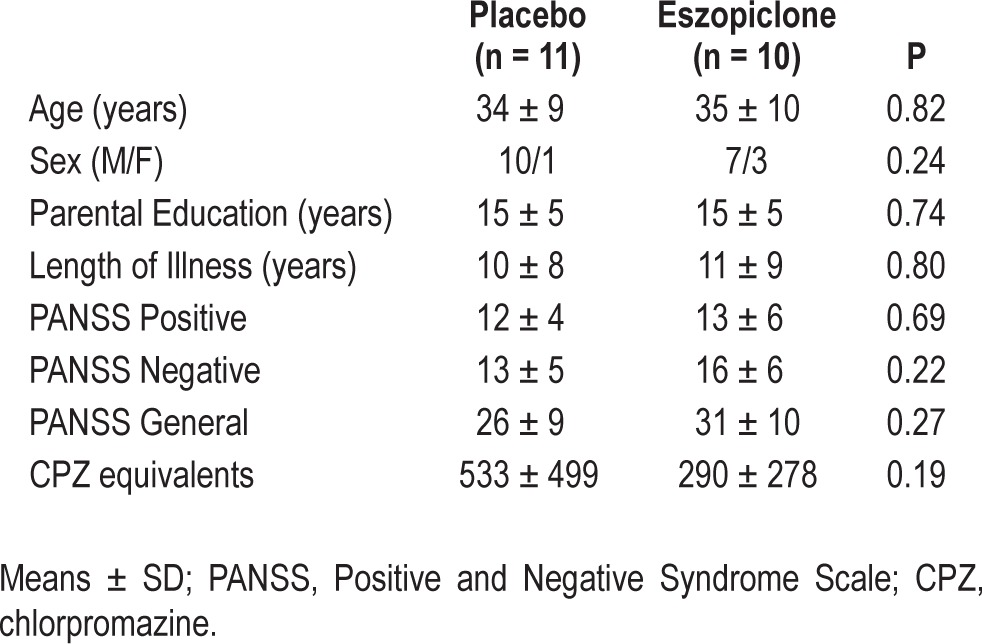
Twenty-five schizophrenia outpatients were recruited from an urban mental health center; 21 completed the study. All had been maintained on stable doses of second-generation APDs for ≥ 6 weeks, and 12 took diverse adjunctive medications for anxiety, agitation, and/or concurrent mood disturbance (see Table S1 in the supplemental material). There were no medication changes during study participation. Potential participants were screened to exclude those with diagnosed sleep disorders, treatment with sleep medications, a history of significant head injury or neurological illness, and a history of substance abuse or dependence within the past 6 months based on patient interview, chart review, and clinician report. Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV34 and symptoms were rated with the Positive and Negative Syndrome Scale.35 All participants gave written informed consent. The study was approved by the institutional review boards of Massachusetts General Hospital and the Massachusetts Department of Mental Health. Participants were randomly assigned in a double-blind fashion to either eszopiclone (3 mg; n = 10) or placebo (n = 11) groups. The groups did not differ significantly in age, sex, parental education, symptom ratings, or APD dose as measured by chlorpromazine equivalents (Table 1).
Table 1.
Demographic characteristics and description of study samples
Procedures
In the week prior to their Baseline visit to the Clinical Research Center (CRC), participants completed informed consent, demographic questionnaires, and rating scales, and toured the CRC. Baseline and Treatment visits to the CRC were separated by one week (Figure 1). Each visit involved polysomnography (PSG) on 2 consecutive weeknights, with the first night of each visit serving as the non-learning night. On the second (learning) night of each visit, participants were trained on the finger tapping motor sequence task (MST) 1 h prior to their usual bedtime, wired for PSG, and allowed to sleep for up to 10 h. They were tested on the MST 1 h after awakening. Participants engaged in their usual activities during the day. During the Treatment visit, 3 mg of eszopiclone or an identical capsule containing placebo was administered double-blind on both nights after PSG wiring and approximately 30 min prior to retiring.
Figure 1.
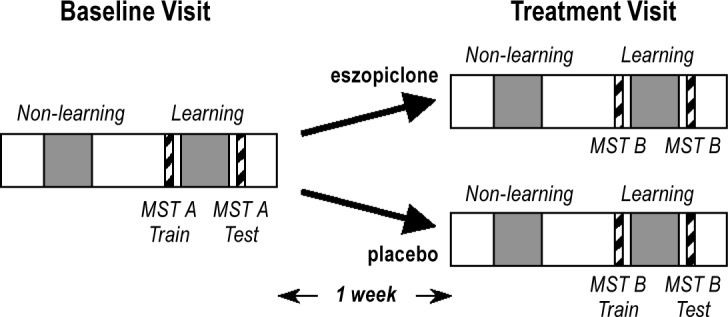
Time course of study. Twenty-one schizophrenia patients participated in Baseline and Treatment visits each consisting of 2 consecutive nights (shaded areas). The visits were separated by one week. On the second (learning) night of each visit they trained on alternate versions of the finger tapping motor sequence task (MST) prior to bedtime and were tested the following morning. For the Treatment visit, they were randomized to placebo or eszopiclone.
Polysomnography (PSG)
PSG data were acquired at 100 Hz using an Embla N7000 system (Medcare Systems, Buffalo NY), with a standard montage of EEG (electroencephalography; F3, F4, C3, Cz, C4, Pz, O1, O2) referenced to the linked mastoids, EMG (electromyography), and EOG (electrooculography).
Finger Tapping Motor Sequence Task (MST)
The MST involves pressing 4 numerically labeled keys on a standard computer keyboard with the fingers of the left hand, repeating a 5-element sequence “as quickly and accurately as possible” for 30 s. Different sequences (e.g., 4-1-3-2-4 and 1-4-2-3-1) were employed for the Baseline and Treatment visits in a counter-balanced order across subjects. There is no transfer of learning between sequences on this task.36 The numeric sequence was displayed at the top of the screen, and dots appeared beneath it with each keystroke. During both training and test sessions, participants alternated tapping and resting for periods of 30 s for a total of 12 tapping trials. The primary outcome measure was the number of correct sequences per trial, which reflects both the speed and accuracy of performance. Overnight improvement was calculated as the percent increase in correct sequences from the last 3 training trials to the first 3 test trials the following morning.30 Prior to each MST administration, participants completed the Stanford Sleepiness Scale (SSS) to measure subjective alertness.37
Data Analysis
Sleep Architecture
Each 30-s epoch of PSG sleep was visually classified into stages (Wake, 1, 2, 3, 4, and REM) according to standard criteria38 by raters blind to visit, group, and night. Sleep stages are reported as total min spent in each stage. Sleep efficiency was computed as the total sleep time (TST) divided by the total time in bed. Wake time after sleep onset (WASO) is the number of minutes spent awake between sleep onset and arising from bed. Following AASM criteria, an arousal from sleep was scored whenever there was a shift to waking EEG frequencies lasting ≥ 3 s. Total number of arousals per night, arousals per hour, and the mean duration of arousals were quantified.
Spindle and Stage 2 Spectral Power Measurements
We measured spindles during stage 2 sleep as previously described.15 PSG data were preprocessed and analyzed using BrainVision Analyzer (v. 2.0, BrainProducts, Munich Germany) and MatLab (v. R2009b, The MathWorks, Natick MA) soft-ware. Artifacts were automatically detected and removed and EEG data filtered at 0.5-35 Hz. Artifact rejection was confirmed by visual inspection.
Power spectral density (μV2/Hz) was calculated by fast Fourier transform (FFT), applying a Hanning window to successive 3-s epochs of stage 2 sleep with 50% overlap. Sigma band (12-15 Hz) spectral power, which correlates with sleep spindle activity, was divided into low (12-13.5 Hz) and high (13.5-15 Hz) sigma band power.39,40 We also examined delta (1-4 Hz), theta (4-7 Hz), alpha (8-12 Hz), and beta (15-35 Hz) band spectral power within stage 2 sleep.
Sleep spindles were automatically detected at all electrodes using a wavelet-based algorithm, previously validated against hand-counted spindles in both patients with schizophrenia and healthy individuals.15 For each spindle identified at Cz, amplitude, sigma power, frequency, and duration were calculated based on analysis of 2-s EEG epochs centered on the point of spindle detection. Spindle amplitude is the maximal voltage following 12- 15 Hz band pass filtering of the spindle; frequency is the peak spectral power of the spindle following FFT decomposition; sigma power is the mean FFT-derived power spectral density across the 12-15 Hz range; and duration is calculated as the half-height width of wavelet energy within the spindle frequency range.
Finally, we assessed the sigma coherence during spindles across EEG channels. Coherence (Coh) across EEG channels (ci) was assessed for each 2-s epoch during which a spindle was auto-detected in any channel. Coherence values range from 0-1, calculated as the cross-spectrum (CS)-to-autospectrum coherence ratio within the full 12-15 Hz sigma frequency band (f) according to the formula:
Eszopiclone Effects
We examined the effects of eszopiclone on sleep parameters and overnight improvement of MST performance using repeated measures ANOVAs with factors for Drug Group (Placebo, Eszopiclone) and Visit (Baseline, Treatment) and planned post hoc contrasts. The analysis of sleep parameters also included a factor for Night (Non-learning, Learning), but as there were no significant main effects or interactions with Night, unless otherwise indicated, we report results as the average of the 2 nights of each Visit. For both spindle parameters and MST improvement, we expected to find Drug Group by Visit interactions reflecting that, relative to baseline values, treatment with eszopiclone increased sleep spindles and memory consolidation significantly more than placebo.
To test the relationship between sleep spindles and memory consolidation, spindle number and density were correlated with overnight MST improvement. ANCOVAs were used to test for effects of Drug Group (placebo vs. eszopiclone) on the slope of these relations.
APD Effects
We examined the correlations of APD dosage measured in chlorpromazine equivalents41 with spindle parameters and overnight MST improvement. Because participants taking clozapine (n = 9) exhibited significantly lower spindle densities at Baseline,15 we examined the effects of eszopiclone on spindle parameters and overnight MST improvement in patients divided by clozapine treatment using repeated measures ANOVAs with factors for Clozapine (No, Yes), Drug Group (Placebo, Eszopi-clone), and Visit (Baseline, Treatment).
RESULTS
Sleep Quality and Architecture
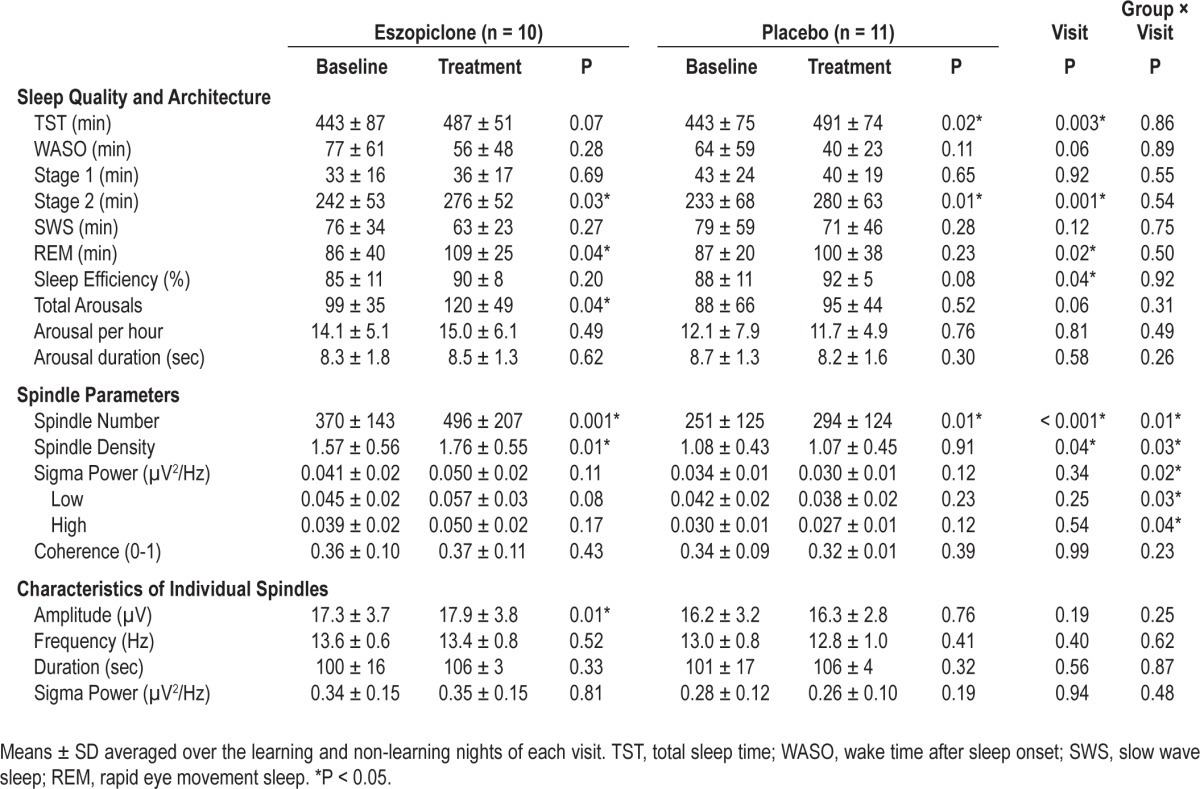
There were no significant effects of eszopiclone compared with placebo on any measure of sleep quality or architecture (Table 2). Patients generally slept better during the Treatment visit as indicated by significant increases in TST and sleep efficiency and a trend towards decreased WASO. There was a trend towards increased arousals at the Treatment visit, which likely reflected the increased TST, since the number of arousals per hour did not change. Patients also showed significant increases in the durations of both stage 2 and REM sleep at the Treatment visit.
Table 2.
Changes in sleep quality, architecture, spindle parameters, and individual spindle characteristics in the eszopiclone compared with the placebo group
Spindle Number, Density, and Coherence
Eszopiclone significantly increased sleep spindle number and density during stage 2 sleep compared to placebo (Figure 2, Table 2). Although greater in the eszopiclone group (34% increase; P = 0.001), the placebo group also showed a significant increase in spindle number (17%, P = 0.01). This likely reflects the increased duration of stage 2 sleep during the Treatment visit, since only the eszopiclone group also showed a significant increase in spindle density (eszopiclone: 11%, P = 0.01; placebo: -1%, P = 0.91).
Figure 2.
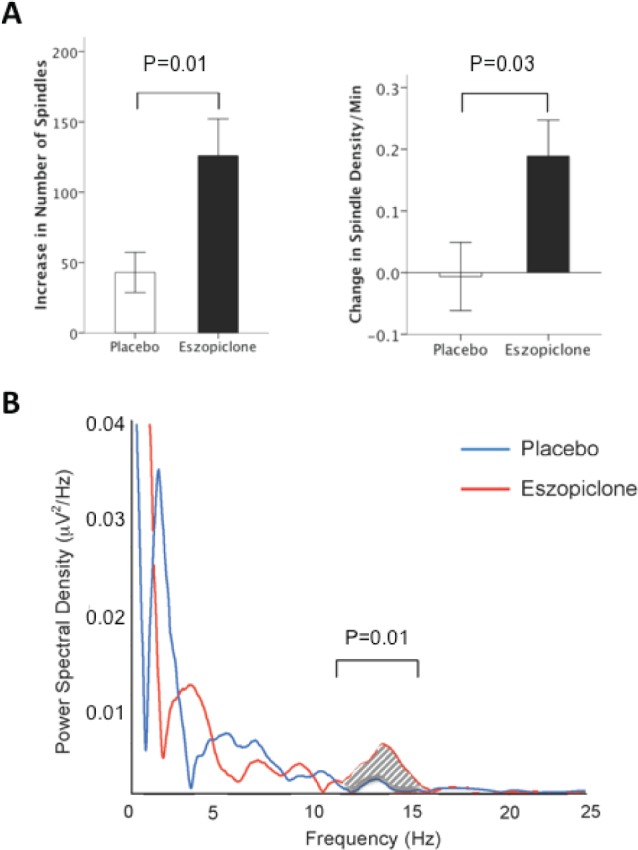
Eszopiclone effects on spindle parameters during stage 2 sleep. (A) Changes in spindle number and density from Baseline to Treatment in the placebo and eszopiclone groups; error bars = SEM. (B) Spectral power during stage 2 sleep from 0 to 25 Hz for both groups at the Treatment visit, recorded from the Cz electrode. Sigma power (12-15 Hz) was significantly greater in the eszopiclone group. There were no group differences in the delta (1-4 Hz), theta (4-7 Hz), alpha (8-12 Hz), or beta (15-35 Hz) frequency bands at the Treatment visit. Spectral profiles were similar across all recording sites.
The effects of eszopiclone on spindle number and density did not appear to be topographically specific, increasing at all electrodes from the Baseline to the Treatment visit (paired t-tests for spindle number: P < 0.02 for all electrodes; spindle density: P < 0.05 at all electrodes except F4, P = 0.14 and O2, P = 0.32). Additionally, the distribution of spindles across the scalp in the eszopiclone group was similar to placebo, with maximal spindle densities at Pz.
Eszopiclone did not significantly affect spindle coherence (Table 2).
Characteristics of Individual Sleep Spindles
Eszopiclone did not affect spindle frequency, duration, amplitude, or sigma power significantly more than placebo (Table 2); there were no significant Drug Group by Visit interactions and no significant main effects of Visit. Although spindle amplitude increased significantly from Baseline only in the eszopiclone group (3.2%, P = 0.01), this increase did not differ significantly from that in the placebo group (0.7%, P = 0.76).
Stage 2 EEG Spectral Power
Consistent with its effects on sleep spindles, eszopiclone significantly increased sigma power during stage 2 sleep compared with placebo. Significant Drug Group by Visit interactions were seen for total spectral power in the sigma-frequency band, as well as for both slow and fast sigma power (Table 2). Although eszopiclone did not significantly alter theta, alpha, or beta power during this same period, it did produce a significant decrease in delta power compared with placebo (Drug Group × Visit interaction: F1,19 = 7.97, P = 0.01; eszopiclone: t9 = -3.09, P = 0.01, placebo: t10 = -0.004, P = 0.99).
Overnight MST Improvement
The key finding was that relative to Baseline levels, treatment with eszopiclone did not increase overnight MST improvement significantly more than placebo (Figure 3, Drug Group by Visit interaction, P = 0.55). At the Treatment visit, however, patients taking eszopiclone showed significant overnight MST improvement (bedtime vs. morning: 21% ± 25% SD, t9 = 2.64, P = 0.03) that was numerically, but not significantly, greater than their own overnight improvement at the Baseline visit (8% ± 19%, t9 = 1.39, P = 0.20; Baseline vs. Treatment, P = 0.12) as well as that of healthy controls at the Baseline visit (15% ± 13%, t16 = 4.92, P = 0.0002 as previously reported15; eszopiclone patients vs. controls, P = 0.47). Patients taking placebo, in contrast, showed no signifi cant overnight improvement during either the Baseline (-5% ± 25%, t9 = 0.61, P = 0.55) or Treatment visit (2% ± 22%, t10 = 0.23, P = 0.83; Baseline vs. Treatment, P = 0.40). At the Treatment visit, the placebo group differed from the eszopiclone group at a trend level (t19 = 1.89, P = 0.07). There were no Group × Visit interactions for SSS ratings indicating that eszopiclone did not affect subjective alertness prior to MST administrations differently from placebo (bedtime: P = 0.81; morning: P = 0.80). Moreover, the eszopiclone and placebo groups did not differ in SSS alertness ratings at either the bedtime (placebo = 2.5 ± 1.4, eszopiclone = 2.5 ± 1.6, P = 0.53) or morning (placebo = 2.1 ± 0.9, eszopiclone = 2.4 ± 1.0, P = 0.89) MST sessions.
Figure 3.
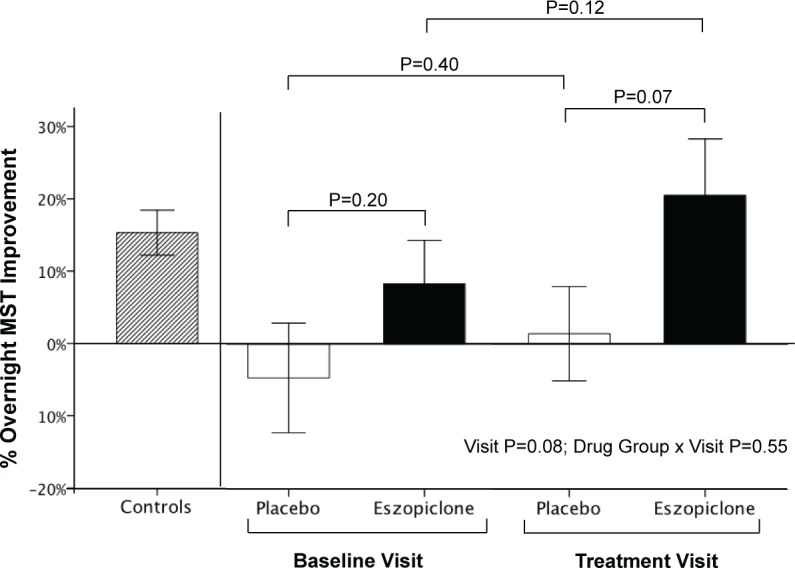
Changes in sleep-dependent memory consolidation with eszopiclone. Overnight MST improvement of demographically-matched healthy controls (n = 17) during the Baseline visit reported in15 and in schizophrenia patients divided by Drug Group (Placebo, Eszopiclone) and Visit (Baseline, Treatment); error bars = SEM.
Relations of Overnight Improvement with Spindle Parameters
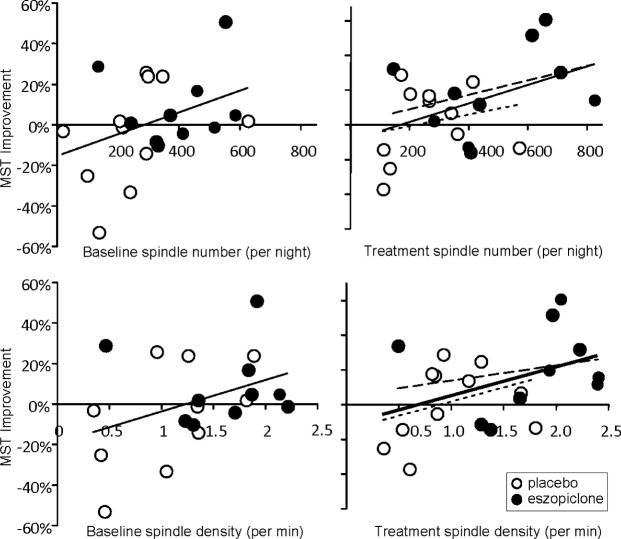
On the learning night of the Treatment visit, both spindle number (r21 = 0.45, P = 0.04) and density (r21 = 0.44, P = 0.05) predicted overnight MST improvement in the combined eszopiclone and placebo groups (Figure 4). The slopes of these relations did not differ between groups and the slopes of each group were similar to the slopes of the combined group data. These correlations are similar in magnitude to those observed on the learning night of the Baseline visit (spindle number: r21 = 0.38, P = 0.09; spindle density: r21 = 0.39, P = 0.08). Correlations were not signifi cant when spindle number and density were averaged across the 2 nights of the Treatment visit, or on the non-learning night alone.
Figure 4.
Overnight MST improvement as a function of spindle number and density on the learning night in patients divided by Drug Group (Placebo, Eszopiclone) and Visit (Baseline, Treatment). Long dashed regression lines: eszopiclone group; short dashed lines: placebo; solid lines: combined groups.
APD Effects
APD dosage did not correlate with any spindle parameter or with overnight MST improvement. Whether or not patients took clozapine however, significantly modulated the effect of eszopiclone on spindle density (Group × Visit × Clozapine interaction: F1,17 = 4.34, P = 0.05). Eszopiclone had no effect on spindle density in clozapine patients (Group × Visit interaction: P = 0.95), but had a large effect in non-clozapine patients (Group × Visit interaction: F1,10 = 11.94, P = 0.006). Clozapine status did not significantly modulate the effects of eszopiclone on overnight MST improvement (Group × Visit × Clozapine interaction, P = 0.37) and neither the clozapine patients (Group × Visit interaction, P = 0.71) nor the non-clozapine patients (Group × Visit interaction, P = 0.36) showed a signifi cant effect of eszopiclone vs. placebo on overnight MST improvement.
DISCUSSION
Patients with schizophrenia show profound reductions in sleep spindles11–14 and sleepdependent memory consolidation.10–12 Here, in a placebo-controlled, double-blind study, eszopiclone increased sleep spindles significantly more than placebo in schizophrenia. Eszopiclone, however, did not significantly enhance overnight improvement of motor procedural memory. As in our Baseline data15 and prior studies of healthy individuals,31,33 sleep spindle density predicted overnight MST improvement at the treatment visit. The strength of this relation (r = 0.44) was remarkably similar to that of a prior study of schizophrenia (r = 0.4611). Thus, the present findings provide partial support for the theory that the spindle deficit in schizophrenia impairs sleep-dependent memory consolidation and can be effectively treated. These findings, along with evidence that sleep spindles play a more general role in cognition,18 suggest that the sleep spindle deficit in schizophrenia represents a promising novel potential target for the treatment of cognitive deficits.
The observed effects of eszopiclone were limited to a marked increase in sleep spindles and sigma power accompanied by a decrease in delta power during stage 2 sleep. In contrast, there were no significant effects on sleep architecture or quality, thus excluding the possibility that the effects of eszopiclone on spindles are secondary to its more general effects on sleep quality or architecture. The lack of a significant effect on sleep quality contrasts with prior reports that eszopiclone significantly reduces WASO while increasing sleep efficiency in placebo-controlled clinical trials in primary insomnia.26 These differences may reflect both the relatively small sample size and the exclusion of individuals with insomnia in the present study. It is also possible that schizophrenia, its treatment, or other related factors interact with eszopiclone to modify its effects. While eszopiclone has been shown to increase the duration of stage 2 sleep,26 to our knowledge, this is the first report that it increases spindles. It is therefore unknown whether this effect would generalize to other populations.
Whether our findings are specific to eszopiclone or reflect a more general property of sedative-hypnotic agents also remains unknown. Benzodiazepines and other sedative-hypnotic agents that act on GABAA receptors have been shown to increase sigma-frequency activity and spindle number.42,43 One study that examined memory consolidation found that triazolam, which has been found to increase spindles,44 resulted in an overnight deterioration of MST performance in healthy individuals despite improved sleep quality, while zolpidem did not differ from placebo in its effects on sleep and memory.45 The overnight deterioration of MST performance on triazolam may reflect its effect on response time (slowing of 20 ms on a separate continuous performance task), which may have obscured any effects of increased spindles if present. It is also possible that benzodiazepine-induced increases in sleep spindles would only lead to increased memory consolidation when spindles are deficient, as is the case in schizophrenia. A third possibility is that the spindles induced by benzodiazepines differ from those produced by eszopiclone and are, for some unknown reason, ineffective in improving memory consolidation. In support of this possibility, prior studies have reported that benzodiaze-pine-induced spindles differ from typical spindles in frequency, amplitude, and duration.46,47 In contrast, eszopiclone did not alter the frequency, amplitude, duration, and topographical distribution of sleep spindles. Finally, the differential effect of eszopiclone may reflect its unique GABAA receptor subunit profile. While its subunit selectivity profile has not been established, eszopiclone has greater affinity for α3β3γ2 GABAA receptors, which are expressed on TRN neurons, than for the α1β2γ2 subtype found on thalamocortical relay neurons, where zolpidem exerts greater effects.19 As a result, eszopiclone has preferential effects on GABAergic neurons in the TRN. Further study is necessary to deter-mine whether other GABAergic hypnotics also affect sleep-dependent memory.
While the mechanism of eszopiclonemediated increases in spindles has not been investigated, the unique, voltage-dependent firing properties of TRN neurons have been well-described.48 Like most neurons, TRN cells fire in “tonic” mode at resting membrane potential. However, when TRN neurons are relatively hyperpolarized at rest (to roughly -70 mV)—due to increased inhibitory inputs, decreased excitatory post-synaptic potentials, or both—the low threshold Ca2+ conductance is de-inactivated, and the neurons fire in “burst” mode. During burst mode, a depolarizing input activates T-type Ca2+ channels, leading to a low threshold Ca2+ spike (LTS), and to rhythmic bursts of action potentials. Rhythmic bursting in TRN neurons produces powerful and prolonged inhibition in target thalamocortical relay neurons, and is critical for generating and synchronizing sleep spindles. Neurons in the TRN primarily express the α3β3γ2 subtype of GABAA receptors,49 and eszopiclone slows the decay phase of inhibitory post-synaptic potentials (IPSPs) in these neurons.19 We speculate that eszopiclone increases spindles by prolonging these IPSP's thereby increasing the time during which TRN neurons can fire in burst mode.
While these initial findings are promising, and demonstrate that eszopiclone increases spindles in schizophrenia, there are several important limitations that temper our conclusions. First, replication is necessary due to the small sample size, which likely limited our power to detect significant effects on memory consolidation. Although eszopiclone significantly increased sleep spindles, which correlated with memory consolidation, and despite the significant overnight MST improvement during treatment with eszopiclone, the increased memory consolidation seen on eszopiclone was not statistically different from placebo. If, as hypothesized, eszopiclone improves memory via its effects on spindles, the failure to reach significance may reflect that memory consolidation is a more distal, and there-fore less sensitive index of eszopiclone effects than spindles, requiring a larger sample size to reach significance. It is note-worthy, however, that the eszopiclone-treated group is the first published sample of schizophrenia patients to show significant sleep-dependent improvement of motor procedural memory.10–12
A second limitation is that despite the randomization of participants to treatment groups, the eszopiclone group had significantly greater spindle density than the placebo group at the Baseline visit (Table 2). As spindle density at Baseline did not correlate with the change in spindle density from the Base-line to the Treatment visit (P = 0.70), the greater increase in spindle density in patients treated with eszopiclone is unlikely to reflect Baseline differences.
A third limitation is that eszopiclone was added to a stable, clinically indicated medication regimen that differed across participants. All patients were treated with a variety of second-generation APDs, and twelve also took adjunctive agents, primarily antidepressants, but also benzodiazepines, stimulants, and other mood stabilizers. While APD dosage was not related to any measure of spindle activity and the effects of chronic APD treatment are unknown, a prior study reported decreased spindle activity following a single dose of olanzapine in schizophrenia.50 Benzodiazepines are known to increase spindles (as reported above) and one study suggested that selective serotonin reuptake inhibitors (SSRIs) increase spindle density,51 but data on SSRIs and other antidepressants are very limited. We lacked adequate power to fully examine the effects of these different medication classes in our sample. As all patients were maintained on a stable medication regimen during study participation, the increases in spindle density and number in the eszopiclone group were most likely due to the addition of eszopiclone. Although patients taking clozapine showed significantly fewer spindles than non-clozapine patients at both visits, the spindle reduction remained significant in non-clozapine patients, and the effects of eszopiclone on spindles were only present in non-clozapine patients. It is unclear whether the more pronounced spindle deficit and the diminished response to eszopiclone in clozapine patients reflect medication effects or that the patients taking clozapine were more severely ill and treatment refractory.
Finally, as our studies have been limited to chronic, medicated patients, it is unknown whether early course and unmedicated patients also have sleep-dependent memory and spindle deficits. Evidence of intact spindle activity in non-schizophrenia psychiatric patients taking APDs suggests that the spindle deficit in schizophrenia is unlikely to be an APD side effect.14 Further, findings of correlations between sigma power during NREM sleep and measures of attention and reasoning in APD-naïve patients with recent-onset psychosis suggest that spindle activity is also relevant to cognitive function in early course untreated individuals.52
Sleep spindles correlate with a wide range of cognitive measures including declarative and procedural memory consolidation, learning potential, and general intelligence.18 In addition, in two prior reports, reduced spindle activity predicted more severe positive symptoms in schizophrenia.14,15 These findings raise intriguing questions about whether long-term pharmacological enhancement of spindles can improve memory consolidation, cognition more generally, and positive symptoms in schizophrenia. In summary, the present findings link a specific cognitive deficit in schizophrenia (sleepdependent memory consolidation) to a particular mechanism (sleep spindles) and raise the possibility of effective intervention. The investigation of the role of sleep in memory consolidation in schizophrenia has the potential to substantially expand current models of cognitive deficits and to lead to interventions that improve quality of life.
DISCLOSURE STATEMENT
This work was funded by research Grant #30675 to Dr. Manoach from Sunovion Pharmaceuticals, Inc. (formerly Sepracor, Inc.) which markets eszopiclone; the National Institutes of Health R01-MH48832 and T32-HL07901; and the National Center for Research Resources UL1-RR025758 and M01-RR-01066, Harvard Clinical and Translational Science Center.
Dr. Goff has participated in trials funded by PamLab, Novartis, GlaxoSmithKline, and Pfizer, Inc. Dr. Shinn's salary is paid in part through grants from Merck & Co., and Pfizer, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the staff of the MGH Clinical Research Center, particularly Mary E. Sullivan, PhD, NP, and to John Vetrano at the MGH Clinical Trials Pharmacy, for their support.
SUPPLEMENTAL MATERIAL
Patient medications
REFERENCES
- 1.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 2.Sergi MJ, Green MF, Widmark C, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007;164:1585–92. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]
- 3.Marder SR, Fenton W, Youens K. Schizophrenia, IX: Cognition in schizophrenia-the MATRICS initiative. Am J Psychiatry. 2004;161:25. doi: 10.1176/appi.ajp.161.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–4. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 6.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–87. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 7.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–43. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manoach DS, Stickgold R. Does abnormal sleep impair memory consolidation in schizophrenia? Front Hum Neurosci. 2009;3:21. doi: 10.3389/neuro.09.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–6. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Manoach DS, Thakkar KN, Stroynowski E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–20. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2011;44:42–7. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Ferrarelli F, Peterson MJ, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–48. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wamsley E, Tucker MA, Shinn AK, et al. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–61. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Gennaro L, Ferrara M, Bertini M. Topographical distribution of spindles: variations between and within nrem sleep cycles. Sleep Res Online. 2000;3:155–60. [PubMed] [Google Scholar]
- 17.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogel SM, Smith CT. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328:1000–6. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- 20.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–71. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 21.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–9. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 22.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–54. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001;86:1365–75. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 24.Ye M, Garcia-Rill E. Potentiating effect of eszopiclone on GABA(A) receptor-mediated responses in pedunculopontine neurons. Sleep. 2009;32:879–87. doi: 10.1093/sleep/32.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 26.Monti JM, Pandi-Perumal SR. Eszopiclone: its use in the treatment of insomnia. Neuropsychiatr Dis Treat. 2007;3:441–53. [PMC free article] [PubMed] [Google Scholar]
- 27.Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–91. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 28.Boyle J, Trick L, Johnsen S, Roach J, Rubens R. Next-day cognition, psychomotor function, and driving-related skills following nighttime administration of eszopiclone. Hum Psychopharmacol. 2008;23:385–97. doi: 10.1002/hup.936. [DOI] [PubMed] [Google Scholar]
- 29.Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–8. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 31.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13-15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–11. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barakat M, Doyon J, Debas K, et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217:117–21. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/PW/PSY SCREEN) [Google Scholar]
- 35.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoddes E, Zarcone V, Smythe H, Philips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 39.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 40.Schabus M, Dang-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 42.Borbely AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–94. [PubMed] [Google Scholar]
- 43.Kubicki S, Herrmann WM, Holler L, Haag C. On the distribution of REM and NREM sleep under two benzodiazepines with comparable receptor affinity but different kinetic properties. Pharmacopsychiatry. 1987;20:270–7. doi: 10.1055/s-2007-1017120. [DOI] [PubMed] [Google Scholar]
- 44.Johnson LC, Spinweber CL. Effect of a short-acting benzodiazepine on brain electrical activity during sleep. Electroencephalogr Clin Neurophysiol. 1981;52:89–97. doi: 10.1016/0013-4694(81)90193-0. [DOI] [PubMed] [Google Scholar]
- 45.Morgan PT, Kehne JH, Sprenger KJ, Malison RT. Retrograde effects of triazolam and zolpidem on sleep-dependent motor learning in humans. J Sleep Res. 2010;19:157–64. doi: 10.1111/j.1365-2869.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 46.Suetsugi M, Mizuki Y, Ushijima I, Kobayashi T, Watanabe Y. The effects of diazepam on sleep spindles: a qualitative and quantitative analysis. Neuropsychobiology. 2001;43:49–53. doi: 10.1159/000054865. [DOI] [PubMed] [Google Scholar]
- 47.Feshchenko VA, Veselis RA, Reinsel RA. Comparison of the EEG effects of midazolam, thiopental, and propofol: the role of underlying oscillatory systems. Neuropsychobiology. 1997;35:211–20. doi: 10.1159/000119347. [DOI] [PubMed] [Google Scholar]
- 48.Sherman SM, Guillery RW. Exploring the thalamus and it role in cortical function. 2nd ed. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 49.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 50.Goder R, Fritzer G, Gottwald B, et al. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry. 2008;41:92–9. doi: 10.1055/s-2007-1004592. [DOI] [PubMed] [Google Scholar]
- 51.Dotan Y, Suraiya S, Pillar G. [Sleep spindles in post traumatic stress disorder: significant importance of selective serotonin reuptake inhibitors] Harefuah. 2008;147:763–7. 839–40. [PubMed] [Google Scholar]
- 52.Keshavan MS, Montrose DM, Miewald JM, Jindal RD. Sleep correlates of cognition in early course psychotic disorders. Schizophr Res. 2011;131:231–4. doi: 10.1016/j.schres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient medications