Abstract
Background
The Occluded Artery Trial (OAT) was a large, randomized controlled trial published in 2006 that demonstrated no benefit to routine percutaneous coronary intervention (PCI) of persistently totally occluded infarct-related arteries (IRA) identified a minimum of 24 hours (on calendar days 3–28) after myocardial infarction (MI). The purpose of this study was to determine the impact of OAT results and consequent change in guideline recommendations for PCI for treatment of persistently occluded IRAs.
Methods
We identified all patients enrolled in the CathPCI Registry, from 2005 to 2008, undergoing catheterization more than 24 hours after MI with a totally occluded native coronary artery and no major OAT exclusion criteria. We examined trends in monthly rates of PCI for occlusions after OAT publication and after guideline revisions. Because reporting of diagnostic catheterizations was not mandatory, we examined trends among hospitals in the highest quartile for reporting of diagnostic procedures.
Results
A total of 28 780 patient visits from 896 hospitals were included. Overall, we found no significant decline in the adjusted monthly rate of PCI of occlusions after publication of OAT (odds ratio [OR], 0.997; 95% confidence interval [CI], 0.989–1.006) or after guideline revisions (OR, 1.007; 95% CI, 0.992–1.022). Among hospitals consistently reporting diagnostic catheterizations, there was no significant decline after OAT publication (OR, 1.018; 95% CI, 0.995–1.042), and there was a trend toward decline after guideline revisions (OR, 0.963; 95% CI, 0.920–1.000).
Conclusion
These findings suggest that the results of OAT and consequent guideline revisions have not, to date, been fully incorporated into clinical practice in a large cross-section of hospitals in the United States.
The purpose of major clinical trials is to establish a scientific basis for clinical practice. However, few trials are subjected to rigorous impact analyses. The Occluded Artery Trial(OAT) was a large, randomized controlled trial funded by the National Heart, Lung, and Blood Institute testing routine percutaneous recanalization of persistently totally occluded infarct-related arteries (IRAs) identified a minimum of 24 hours(on calendar days 3–28) after myocardial infarction (MI).1 No reduction in death, reinfarction, or class IV heart failure was observed. These primary results informed 2007 updates of 3 American College of Cardiology(ACC)/American Heart Association (AHA) guidelines (unstable angina and non–ST elevation MI [NSTEMI], ST elevation MI [STEMI], and percutaneous coronary intervention [PCI]).2–4
A bias favoring PCI for persistent IRA occlusion drove practice prior to the OAT and was supported by experimental and observational data.5,6 The OAT results provided objective evidence that the use of PCI did not lead to a reduction in clinical events and that the beneficial effect on angina and quality of life was small and not durable. Percutaneous coronary intervention was more costly than optimal medical therapy alone; hence, these findings should have discouraged routine PCI in this setting.
The National Cardiovascular Data Registry (NCDR), Washington, DC, and its CathPCI Registry (version 3.0) offers an opportunity to measure the degree to which clinical trials affect US cardiology practice.7 The registry collected data on all PCI procedures at participating centers in 2004. We used these data to examine whether publication of OAT in December 2006, and the resultant change in the STEMI and NSTEMI guideline updates (published from August through December 2007) reduced the application of PCI for treatment of occluded IRAs identified at least 24 hours after MI.
METHODS
STUDY POPULATION AND DATA SOURCES
The cohort for this study was identified using the CathPCI Registry, version 3.0, data from 1042 hospitals across the US. Patients undergoing cardiac catheterization following an admission for STEMI or NSTEMI from January 1, 2005, through December 31, 2008, were eligible. We applied additional clinical criteria to define a population that reflected OAT eligibility.8 Patients were included if the interval from symptom onset to admission was greater than 24 hours or at least 2 calendar days separated admission from catheterization. The following exclusion criteria were applied: (1) congestive heart failure (CHF) on presentation, (2) cardiogenic shock or intra-aortic balloon pump placement prior to catheterization, (3) emergent or salvage catheterization, (4) facilitated or rescue PCI for STEMI, (5) coronary artery bypass grafting (CABG) during the catheterization admission, and (6) at least 50% left main stenosis or severe 3-vessel disease (≥70% stenosis in the left anterior descending, right, and either ramus or circumflex arteries).
Details of the CathPCI registry have been described previously.9 Systematic data entry, quality assurance, and auditing programs are used to ensure that only data meeting predetermined criteria for completeness and accuracy are entered into the database. The overall rate of missing data was less than 0.5% across all collected dataelements, except for baseline creatinine (approximately 10% missing) and lesion length (approximately 2% missing).
The CathPCI Registry does not code specifically for the IRA. To identify all persistently occluded IRAs, all patients with a 100% native stenosis were initially captured. To minimize the capture of occlusions unrelated to the admission MI, we excluded those with prior CABG. To estimate the remaining number of included patients with occlusions unrelated to the acute MI, we examined the proportion of patients undergoing PCI of nonoccluded targets as well as temporal changes in the rate of nonoccluded PCI. The registry also does not specifically code for angina at rest or severe ischemia on noninvasive testing. We attempted to capture these OAT exclusion criteria by excluding patients who underwent emergent or salvage catheterization. The proportion of remaining patients with severe ischemia would not be expected to change dramatically over the time course of this study.
PRIMARY ANALYSIS
We examined trends in the monthly rate of PCI for occlusions after MI within and among all participating hospitals across the following time periods of interest: (1) prior to publication of the OAT (January 1, 2005, to November 30, 2006), (2) after simultaneous presentation and publication of the OAT but before revision of practice guidelines (December 1, 2006, to November 30, 2007), and (3) after revision of guidelines reflecting the OAT (December 1, 2007, to December 31, 2008). We adjusted for baseline differences between the time periods using covariates adapted from the validated mortality risk factors for the CathPCI database, which were also predictors of undergoing PCI for an occlusion.
PRINCIPAL SECONDARY ANALYSIS
Reporting of diagnostic-only cardiac catheterizations (ie, without PCI) is not mandatory in the CathPCI Registry, and incomplete reporting would lead to overestimation of PCI rates. In a prespecified secondary analysis, we examined trends in the monthly rate of PCI for occlusions among hospitals that consistently reported at least 3 times as many diagnostic catheterizations as catheterizations leading to PCI, corresponding to the top quartile among participating sites.
ADDITIONAL SECONDARY ANALYSES
Most patients enrolled in the OAT had STEMI/Q wave MI, and results may have been incorporated into practice differentially for patients with STEMI vs NSTEMI. Incorporation of the OAT results may also have been influenced by the type of hospital (university, government, or private) or insurance provider (government, commercial, health maintenance organization, or other). We examined trends in the rate of PCI for persistent occlusions among these additional subgroups.
The OAT excluded patients with severe heart failure (New York Heart Association [NYHA] Functional classifications 3 and 4).8 Since the registry does not reliably code for severity of heart failure on presentation, our primary analysis excluded patients presenting with any heart failure symptoms. In a supplementary analysis, however, we examined trends in the rate of PCI for occlusions among patients with CHF at presentation. All secondary analyses were prespecified.
STATISTICAL METHODS
Baseline characteristics across the time periods were compared using the χ2 rank–based group means score test for categorical variables and the χ2 rank correlation test for continuous or ordinal variables.
The crude proportion of PCI for occlusions identified after MI was compared across the 3 time periods of interest using the χ2 rank–based group means score test. The additive spline transformation was used to fit the rate of occluded PCI over the procedure date based on the logistic model. Four knots were selected according to the 5%, 35%, 65%, and 95% quantiles. Evaluation of the trends in the monthly rate of PCI for occlusion within the time periods of interest and comparisons of trends between time periods was performed using a generalized estimating equation model that adjusted for differences in patient characteristics across time as well as for within-hospital clustering. Wald χ2 tests were used to test the significance of time trends. The final model was adjusted for age, sex, insurance payer, prior MI, prior CHF, prior renal failure, cerebrovascular disease, prior PCI, peripheral vascular disease, chronic lung disease, STEMI at presentation, time from symptom onset to presentation, and number of diseased vessels. All statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc, Cary, North Carolina).
RESULTS
From January 1, 2005, through December 31, 2008, the CathPCI Registry captured 670 043 laboratory visits, from 996 sites, of patients undergoing angiography at least 24 hours after MI. Of these, 28 780 patient visits from 896 hospitals met all inclusion and exclusion criteria (eFigure; http://www.archinternmed.com). Among the 896 hospitals, 631 (70.4%) contributed patients over the entire study period. Cardiac catheterization was performed prior to the OAT publication in 11 083 patients, after publication of the OAT but before the guideline revisions in 7838 patients, and after the guideline revisions in 9859 patients.
Comparison of the demographic and clinical characteristics of patients across the 3 time periods is presented in Table 1. The mean age of patients (range, 61.5–61.7 years) and the proportion of women (range, 31.9%–32.6%) were similar across the 3 time periods. Most patients presented with NSTEMI (90.7% overall), and this proportion increased over time (P<.001). Most patients had either 1-vessel disease (43.9%) or 2-vessel disease (49.9%).
Table 1.
Baseline Characteristicsa
| No. (%) |
|||||
|---|---|---|---|---|---|
| Characteristic | Total Cohort (n=28 780) | Catheterization Prior to OAT (n=11 083) | Catheterization Between OAT and Guidelines (n=7838) | Catheterization After Guidelines (n=9859) | P Value |
| Baseline Characteristic | |||||
| Demographic | |||||
| Age, mean (SD), y | 61.64 (13.34) | 61.66 (13.26) | 61.50 (13.43) | 61.73 (13.37) | .96 |
| Female sex | 9268 (32.20) | 3615 (32.62) | 2503 (31.93) | 3150 (31.95) | .29 |
| BMI, mean (SD) | 29.90 (6.64) | 29.82 (6.60) | 29.90 (6.66) | 29.99 (6.66) | .04 |
| Insurance payer | |||||
| Government | 12 704 (44.14) | 4964 (44.79) | 3374 (43.05) | 4366 (44.28) | <.001 |
| Commercial | 8649 (30.05) | 3333 (30.07) | 2407 (30.71) | 2909 (29.51) | |
| HMO | 3692 (12.83) | 1495 (13.49) | 990 (12.63) | 1207 (12.24) | |
| Other | 2696 (9.37) | 933 (8.41) | 782 (9.97) | 981 (9.95) | |
| History | |||||
| MI | 6719 (23.35) | 2663 (24.03) | 1787 (22.80) | 2269 (23.01) | .17 |
| PCI | 6589 (22.89) | 2311 (20.85) | 1827 (23.31) | 2451 (24.86) | <.001 |
| CHF | 1817 (6.31) | 702 (6.33) | 484 (6.18) | 631 (6.40) | .71 |
| Diabetes mellitus | 8151 (28.32) | 3117 (28.12) | 2187 (27.90) | 2847 (28.88) | .18 |
|
| |||||
| Presenting Characteristics | |||||
| ACS type | |||||
| NSTEMI | 26 096 (90.67) | 9854 (88.91) | 7112 (90.74) | 9130 (92.61) | <.001 |
| STEMI | 2684 (9.33) | 1229 (11.09) | 726 (9.26) | 729 (7.39) | |
| STEMI receiving fibrinolysis | 561 (1.95) | 278 (2.51) | 136 (1.74) | 147 (1.49) | <.001 |
| Ejection fraction, mean (SD) | 49.21 (11.85) | 49.25 (11.91) | 49.25 (11.90) | 49.14 (11.76) | .81 |
| Positive results from noninvasive testing | 15 731 (54.66) | 6132 (55.33) | 4250 (54.22) | 5349 (54.25) | .11 |
|
| |||||
| Angiography | |||||
| Diseased vessels, No. | |||||
| 1 | 12 621 (43.85) | 4850 (43.76) | 3466 (44.22) | 4305 (43.67) | .19 |
| 2 | 14 351 (49.86) | 5574 (50.29) | 3865 (49.31) | 4912 (49.82) | |
| 3 | 1803 (6.26) | 656 (5.92) | 505 (6.44) | 642 (6.51) | |
| Site of occlusion | |||||
| Proximal LAD | 3159 (10.98) | 1251 (11.29) | 834 (10.64) | 1074 (10.89) | .90 |
| Mid/distal LAD | 4647 (16.15) | 1780 (16.06) | 1289 (16.45) | 1578 (16.01) | .50 |
| Circumflex | 8310 (28.87) | 3155 (28.47) | 2253 (28.74) | 2902 (29.44) | .46 |
| RCA | 14372 (49.94) | 5570 (50.26) | 3930 (50.14) | 4872 (49.42) | .12 |
| Ramus | 555 (1.93) | 201 (1.81) | 154 (1.96) | 200 (2.03) | <.001 |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHF, congestive heart failure; HMO, health maintenance organization; LAD, left anterior descending; MI, myocardial infarction; NSTEMI, non–ST elevation Ml; OAT, occluded artery trial; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST elevation MI.
Patients with missing data were excluded from this table; therefore, column numbers may not sum up to the total group number.
The procedural characteristics among those patients undergoing PCI for total occlusion are presented in Table 2. All patients undergoing PCI had thrombolysis in MI flows of 0 or 1. Multivessel PCI was performed in 12.5% of patients, and this rate decreased steadily over time (P=.002). Almost half the patients (48.9%) received 1 stent only, and 64.2% of patients received at least 1 drug-eluting stent (DES). The proportion of patients receiving DES decreased markedly over time, corresponding to a widely recognized reduction in DES use generally that followed reports of late and very late DES thrombosis.10 There were less procedural complications in the latter time periods, driven by decreased general and bleeding complications.
Table 2.
Procedural Characteristics of Percutaneous Coronary Intervention (PCI) for Persistent Total Occlusion After Myocardial Infarction (MI)
| No. (%) |
|||||
|---|---|---|---|---|---|
| Procedural Characteristic | Total Cohort (n=6231) | Catheterization Prior to OAT (n=2438) | Catheterization After OAT (n=1673) | Catheterization After Guidelines (n=2120) | P Value |
| PCI characteristics | |||||
| Multivessel PCI | 1903 (12.47) | 800 (13.32) | 525 (12.69) | 578 (11.29) | .002 |
| Stents, No. | |||||
| 0 | 1806 (11.83) | 634 (10.55) | 503 (12.16) | 669 (13.07) | <.001 |
| 1 | 7470 (48.94) | 2900 (48.27) | 2006 (48.50) | 2564 (50.09) | |
| ≥2 | 5987 (39.23) | 2474 (41.18) | 1627 (39.34) | 1886 (36.84) | |
| Any DES implanted | 9805 (64.24) | 4778 (79.53) | 2244 (54.26) | 2783 (54.37) | <.001 |
| Site of occlusion PCI | |||||
| Proximal LAD | 1460 (9.57) | 561 (9.34) | 423 (10.23) | 476 (9.30) | .43 |
| Other LAD | 2306 (14.67) | 884 (14.71) | 621 (15.01) | 801 (15.65) | |
| RCA | 6692 (42.83) | 2707 (45.06) | 1796 (43.42) | 2189 (42.76) | |
| Left circumflex | 4560 (29.19) | 1766 (29.39) | 1230 (29.74) | 1564 (30.55) | |
| Ramus | 245 (1.57) | 90 (1.50) | 66 (1.60) | 89 (1.74) | |
| Lesion risk higha | 8484 (55.59) | 3338 (55.56) | 2356 (56.96) | 2790 (54.50) | .31 |
| Postprocedural TIMI 3 flow | 13 728 (89.94) | 5376 (89.48) | 3728 (90.14) | 4624 (90.33) | .43 |
| PCI complications | |||||
| General complicationb | 823 (2.86) | 341 (3.08) | 245 (3.13) | 237 (2.41) | .005 |
| Bleeding complication | 594 (2.07) | 262 (2.37) | 161 (2.06) | 171 (1.74) | .001 |
| Vascular complication | 160 (0.56) | 68 (0.61) | 46 (0.59) | 46 (0.47) | .16 |
| Any complication | 1423 (4.95) | 616 (5.97) | 398 (5.08) | 409 (4.15) | <.001 |
| Death same day as procedure | 31 (0.15) | 15 (0.19) | 7 (0.12) | 9 (0.13) | .36 |
Abbreviations: DES, drug-eluting stent; LAD, left anterior descending; OAT, occluded artery trial; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
High-risk lesions had at least 1 of the following characteristics: length greater than 20 mm, excessive tortuosity of the proximal segment, extremely angulated segments, and inability to protect major side branches.
General complications included periprocedural myocardial infarction, cardiogenic shock, congestive heart failure, cerebrovascular accident, tamponade, thrombocytopenia, contrast reaction, or renal failure.
PRIMARY ANALYSIS
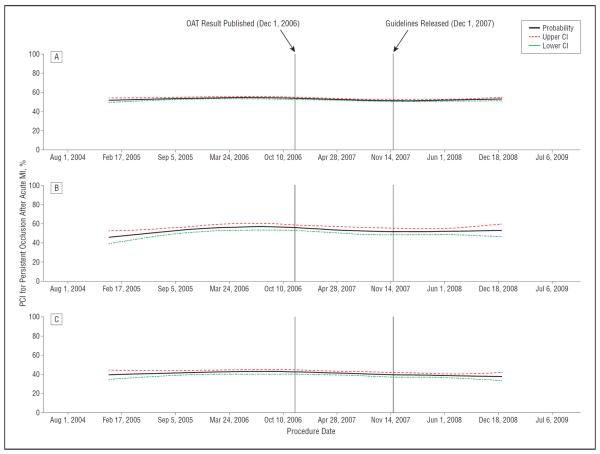
Overall, just over half of all patients with qualifying coronary occlusions (53.0%) underwent PCI targeting a total occlusion identified after MI. Overall, 25.3% of patients did not undergo PCI while 21.7% underwent PCI of nonoccluded targets only. There was no change in the rate of PCI for nonoccluded targets over the study period (P=.18). Changes in the unadjusted rate of PCI for occlusions identified after MI over time are depicted in Figure 1. The time from symptom onset to admission was less than 24 hours in 47.6% and greater than 24 hours in 47.5%. Those presenting within 24 hours had an interval between admission to angiography of at least 2 days, as per the inclusion criteria.
Figure 1.
Unadjusted rates of percutaneous coronary intervention (PCI) for occlusions identified after myocardial infarction (MI) over time. The unadjusted rate of PCI for persistent total coronary artery occlusions after acute MI over the study period is shown, along with 95% confidence intervals, for the overall study population (A), for patients presenting with an ST elevation MI (B), and only those patients treated in hospitals in the highest quartile for reporting of diagnostic catheterizations (C). CI indicates confidence interval; OAT, the Occluded Artery Trial.
The crude rate of PCI for total occlusions was slightly but significantly lower after the publication of the OAT (52.8% vs 54.2% before publication) and dropped again after the guideline revisions (51.9%; P<.001 for comparison across all 3 groups). However, there was an unexpected peak in the rate of PCI for occlusions in March, 2006, 8 months prior to the presentation and publication of the OAT results. This peak substantially accounted for the observed decline in the crude rate of PCI in subsequent time periods.
To account for this peak in the examination of trends in PCI within the time periods, the first time period was divided into 2 at the peak rate of PCI for occlusions (January 2005 to March 2006 and April 2006 to November 2006). There was a significant decline in the adjusted rate of PCI for occlusions from the peak in March 2006 to the OAT publication (odds ratio [OR] for occluded PCI per 30-day increase in time, 0.976; 95% confidence interval [CI], 0.964–0.987). However, there was no significant further decline after publication of the OAT (OR, 0.997; 95% CI, 0.989–1.006) or after the guideline revisions (OR, 1.007; 95% CI, 0.992–1.022). There was no difference in the adjusted monthly trends of occluded PCI between the time period after publication of the OAT and the time period after the guideline revisions (P=.40 for comparison of slopes) (Table 3).
Table 3.
Odds Ratios (ORs) for Change in the Rate of Percutaneous Coronary Intervention (PCI) for Persistent Occlusions After Myocardial Infarction, per 30-Day Period
| Adjusted Analysisa |
|||
|---|---|---|---|
| Time Period | Total, No. | OR (95% CI) | P Value |
| Overall study population | |||
| Before the OAT publication (Mar–Dec 2006) | 4893 | 0.976 (0.964–0.987) | <.001 |
| After the OAT publication (Dec 2006–Nov 2007) | 7812 | 0.997 (0.988–1.006) | .54 |
| After guideline revisions (Dec 2007–Mar 2009) | 9859 | 1.007 (0.992–1.022) | .34 |
| Hospitals reporting a ratio of ≥ 3:1 for diagnostic to PCI procedures | |||
| Before OAT publication (Mar–Dec 2006) | 981 | 0.977 (0.954–1.001) | .06 |
| After the OAT publication | 1467 | 1.018 (0.9957–1.042) | .12 |
| After guideline revisions | 1906 | 0.963 (0.928–1.000) | .047 |
| STEMI at presentation | |||
| Before OAT publication (Mar–Dec 2006) | 533 | 0.980 (0.949–1.012) | .21 |
| After the OAT publication | 725 | 0.999 (0.972–1.027) | .96 |
| After guideline revisions | 729 | 0.997 (0.950–1.046) | .91 |
Abbreviations: CI, confidence interval; OAT, occluded artery trial; STEMI, ST elevation myocardial infarction.
The final model was adjusted for age, sex, insurance payer, prior myocardial infarction, prior congestive heart failure, prior renal failure, cerebrovascular disease, prior PCI, peripheral vascular disease, chronic lung disease, STEMI at presentation, time from symptom onset to presentation, and number of diseased vessels.
PRINCIPAL SECONDARY ANALYSIS
Among hospitals with the highest quartile for reporting of diagnostic catheterizations (a ratio of diagnostic to PCI catheterizations of ≥3:1), a total of 5542 patients with qualifying occlusions were included, of whom 41.9% did not receive PCI, 17.7% underwent PCI of a nonoccluded target only, and the remaining 40.4% underwent PCI of an occlusion. The crude rate of PCI for total occlusions declined significantly from 42.4% prior to the OAT to 39.9% after the OAT but before the guideline revisions, and to 38.5% after the guideline revisions (P=.01). After adjustment using the generalized estimating equation, there was no difference in the monthly rate of PCI for occlusions after publication of the OAT (OR, 1.018; 95% CI, 0.995–1.042). There was, however, a trend towards decline in the adjusted monthly rate of occluded PCI after the guideline revisions (OR, 0.963; 95% CI, 0.928–1.000).
ADDITIONAL SECONDARY ANALYSES
Overall, patients presenting with STEMI and NSTEMI were equally likely to undergo PCI for an occlusion (53.5% vs 53.0%, respectively; P=.61). Among the subgroup of patients presenting with STEMI (2684 patients), there was no difference in the adjusted monthly rate of PCI for occlusions after publication of the OAT (OR, 0.999; 95% CI, 0.972–1.027) or after the guideline revisions (OR, 0.997; 95% CI, 0.950–1.046). These findings are summarized in Table 3.
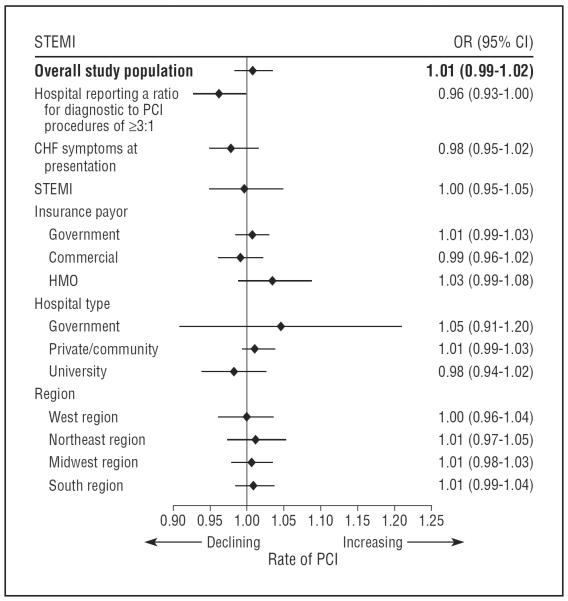
Patients with symptoms of CHF at presentation were less likely to receive PCI for occlusions identified after MI than those without (34.0% vs 55.4%, respectively; P<.001). There was no change in the rate of PCI for occlusions after publication of the OAT or after guideline publication when subgroups were analyzed on the basis of heart failure symptoms at presentation, insurance payer, geographical region, or hospital type (Figure 2).
Figure 2.
Adjusted trends in the rate of percutaneous coronary intervention (PCI) for occlusions identified after myocardial infarction among selected subgroups following guideline revisions. The odds ratios presented here refer to the adjusted odds of receiving PCI for a persistent occlusion, per 30-day increase in time, during the specified time period. They reflect overall trends in the use of PCI for total occlusions during the period from December 2007 to December 2008. CHF indicates congestive heart failure; CI, confidence interval; HMO, health maintenance organization; OR, odds ratio; STEMI, ST elevation myocardial infarction.
COMMENT
Overall, we found no change in the adjusted rate of PCI for total occlusions identified at least 24 hours after MI following the publication of the OAT or the revision of the major guidelines. Although there was a trend toward a decrease in the PCI rate in the subset of hospitals in the highest quartile of diagnostic catheterization reporting, the magnitude of the decline was small, especially in comparison with reported rates of decline in PCI following the COURAGE trial.11 Despite more than 2 years of follow-up since the publication of the OAT and over 1 year of follow-up since the guideline revisions, PCI for total occlusions identified after MI among patients similar to those enrolled in the OAT continues to be performed in a considerable proportion of patients. These findings suggest that the evidence provided by the OAT and other small studies12,13 and the resultant class III guideline recommendations2–4 (“should not be performed”) for PCI in clinically stable patients with persistently occluded IRAs more than 24 hours after STEMI or NSTEMI have not, to date, been widely incorporated into clinical practice in a large cross-section of hospitals in the United States.
The reasons for the lack of impact of the ACC/AHA guideline recommendations on clinical practice in the United States are likely multifactorial. The OAT was a negative trial overall, but it did not demonstrate excessive harm from PCI apart from a trend toward increased reinfarction.1 Cardiologists and interventionalists have been quick to incorporate the results of positive clinical device trials and related guideline recommendations in the past14 and to respond to trials reporting clinically significant safety concerns.15,16 However, there has been substantial lag in the impact of recommendations regarding medical therapy such as β blockers and angiotensin-converting enzyme inhibitor use in acute coronary syndrome.15–18 To our knowledge, the impact of anegative trial demonstrating lack of efficacy and excess cost of a procedure and subsequent guideline revisions has not been previously assessed. Physicians may be less likely to alter their practice based on negative results, especially when there are important competing factors. Barriers preventing physician adoption of clinical practice guidelines are incompletely understood.19,20 Analysis of physician behaviors suggest a wide spectrum of factors contributing to this clinical inertia, including lack of agreement regarding interpretation of data, especially when it contradicts long-held beliefs and external in fluences, such as conflicting patient expectations and financial incentives to perform the unindicated procedure and fear of litigation.21,22 In addition,the incentives to move clinicians toward evidence-based practice are unclear, although enhanced payment through the pay-for-performance initiatives will test the effect of financial incentives.
Most of the patients in our cohort presented with NSTEMI. Aggregate clinical trial data and guidelines currently support routine invasive management following NSTEMI,2 and very early post-NSTEMI angiography is now commonplace. In the post-NSTEMI setting where persistent occlusion of the infarct-related artery is identified, this apparent incongruity of evidence may lead to continued equipoise among clinicians. Physicians may elect to perform PCI at that sitting, even in the absence of functional testing demonstrating severe ischemia. Nonetheless, the findings of the OAT were consistent across the STEMI and NSTEMI subgroups, and this is reflected in the current guidelines.2
There were important limitations to our study. While we strived to define a population within the CathPCI registry that reflected the OAT inclusion and exclusion criteria, there are considerable differences between the cohort in this study and that of the OAT. The population in this study consisted predominantly of NSTEMI; however, the rate of PCI for occlusions was not different from that seen in patients with STEMI. The OAT also excluded patients with angina at rest and those with severe ischemia on noninvasive testing. The CathPCI Registry does not collect information on either of these variables; however, we did attempt to capture these patients by excluding those undergoing emergency or salvage PCI.
The absolute rates of PCI for persistent occlusions reported in this study should be interpreted with caution. The CathPCI Registry does not code for the IRA, and our angiographic inclusion criteria may have resulted in the inclusion of some patients with coronary occlusions unrelated to their recent MI, leading to underestimation of the actual rate of PCI for persistent total occlusions. Conversely, nonmandatory reporting of diagnostic catheterizations in the registry may have led to overestimation of the rate of PCI for total occlusions. Nonetheless, these limitations would have had no influence on the ability to detect trends in use of PCI for total occlusions identified after MI.
We were not able to assess whether the new guideline recommendations resulted in a change in the rate of referral for cardiac catheterization among patients presenting at least 24 hours after MI. However, the OAT did not test the need for angiography in these patients, and the class III recommendation in the guidelines address only patients with total occlusion identified at coronary angiography. However, most patients with NSTEMI or STEMI treated with thrombolysis in the United States eventually undergo cardiac catheterization.6 The proportion of patients who did not undergo angiography is likely small and would not influence the overall findings of this study.
Finally, the generalizability of the CathPCI registry population to that of the entire United States has not been formally evaluated. However, the registry is the single largest clinical catheterization database in the country, with participating centers from every geographic region and includes both academic and community practices. The results of this study cannot be generalized to practice outside the United States. The impact of the OAT and major society guideline recommendations in countries with different models of health care delivery and practice environments has not been assessed.
In conclusion, among this large cross-section of hospitals in the United States we found only modest evidence that the results of the OAT and its incorporation into major guideline revisions have influenced cardiology and interventional cardiology practice over the subsequent 1 to 2 years. Percutaneous coronary intervention of total occlusions identified greater than 24 hours after MI remains commonplace despite little evidence to support its use in stable patients and new clinical practice guidelines recommending against it. The results of this study are a cause for concern on 2 levels. First, they imply that many stable patients with recent MI and persistent infarct artery occlusion continue to undergo a costly and ineffective procedure. Second, a large public, scientific, and human patient investment in the generation of robust clinical evidence has yet to broadly influence US practice. The factors accounting for this incomplete knowledge transfer over this time period remain uncertain.
Supplementary Material
Acknowledgments
Funding/Support: The CathPCI Registry is an initiative of the American College of Cardiology Foundation and the Society for Cardiovascular Angiography and Interventions. This research was supported by the American College of Cardiology Foundation's NCDR. The project described was also supported by awards U01 HL062509 and U01 HL062511 from the National Heart, Lung, and Blood Institute.
Role of the Sponsors: The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Wang and Mr Dai had access to the original data. Study concept and design: Deyell, Buller, Lamas, and Hochman. Acquisition of data: Buller, Miller, and Srinivas. Analysis and interpretation of data: Deyell, Buller, Miller, Wang, Dai, Lamas, Srinivas, and Hochman. Drafting of the manuscript: Deyell, Buller, Lamas, and Hochman. Critical revision of the manuscript for important intellectual content: Deyell, Buller, Miller, Wang, Dai, Srinivas, and Hochman. Statistical analysis: Wang and Dai. Obtained funding: Deyell and Lamas. Administrative, technical, and material support: Deyell, Wang, and Hochman. Study supervision: Wang, Lamas, Srinivas, and Hochman.
Financial Disclosure: None reported.
Publisher's Disclaimer: Disclaimer: The views expressed in this manuscript represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com, nor do they represent official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Online-Only Material: The eFigure is available at http://www.archinternmed.com.
REFERENCES
- 1.Hochman JS, Lamas GA, Buller CE, et al. Occluded Artery Trial Investigators Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355(23):2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) American College of Emergency Physicians. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons. American Association of Cardiovascular and Pulmonary Rehabilitation. Society for Academic Emergency Medicine ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Hand M, Armstrong PW, et al. 2004 Writing Committee Members 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117(2):296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 4.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2005 Writing Committee Members 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117(2):261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 5.Fox KA, Goodman SG, Anderson FA, Jr, et al. GRACE Investigators From guidelines to clinical practice: the impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24(15):1414–1424. doi: 10.1016/s0195-668x(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 6.Spencer FA, Goldberg RJ, Frederick PD, Malmgren J, Becker RC, Gore JM. Age and the utilization of cardiac catheterization following uncomplicated first acute myocardial infarction treated with thrombolytic therapy (the Second National Registry of Myocardial Infarction [NRMI-2]) Am J Cardiol. 2001;88(2):107–111. doi: 10.1016/s0002-9149(01)01602-2. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub WS, McKay CR, Riner RN, et al. American College of Cardiology Database Committee The American College of Cardiology National Database: progress and challenges. J Am Coll Cardiol. 1997;29(2):459–465. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Lamas GA, Knatterud GL, et al. Occluded Artery Trial Research Group Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150(4):627–642. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37(8):2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 10.Roe MT, Chen AY, Cannon CP, et al. CRUSADE and ACTION-GWTG Registry Participants Temporal changes in the use of drug-eluting stents for patients with non-ST-Segment-elevation myocardial infarction undergoing percutaneous coronary intervention from 2006 to 2008: results from the can rapid risk stratification of unstable angina patients supress ADverse outcomes with early implementation of the ACC/AHA guidelines (CRUSADE) and acute coronary treatment and intervention outcomes network-get with the guidelines (ACTION-GWTG) registries. Circ Cardiovasc Qual Outcomes. 2009;2(5):414–420. doi: 10.1161/CIRCOUTCOMES.109.850248. [DOI] [PubMed] [Google Scholar]
- 11.Dauerman HL, Piper WD, Ahmed B, et al. Recent changes in the practice of elective PCI for stable angina [abstract 4296] Circulation. 2009;120(suppl 931):4296. [Google Scholar]
- 12.Ioannidis JP, Katritsis DG. Percutaneous coronary intervention for late reperfusion after myocardial infarction in stable patients. Am Heart J. 2007;154(6):1065–1071. doi: 10.1016/j.ahj.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Steg PG, Thuaire C, Himbert D, et al. DECOPI Investigators DECOPI (DEsobstruction COronaire en Post-Infarctus): a randomized multi-centre trial of occluded artery angioplasty after acute myocardial infarction. Eur Heart J. 2004;25(24):2187–2194. doi: 10.1016/j.ehj.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Kandzari DE, Roe MT, Ohman EM, et al. Frequency, predictors, and outcomes of drug-eluting stent utilization in patients with high-risk non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2005;96(6):750–755. doi: 10.1016/j.amjcard.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JP, Cohen DJ, Jones PG, Bach RG, Spertus JA, Krumholz HM. Impact of the Food and Drug Administration's Public Health Notification on the adoption of drug-eluting stents. Am J Cardiol. 2007;99(9):1227–1229. doi: 10.1016/j.amjcard.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rogers WJ, Canto JG, Lambrew CT, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000;36(7):2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 18.Fox KA, Steg PG, Eagle KA, et al. GRACE Investigators Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297(17):1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 19.Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? Milbank Q. 1998;76(4):517–563. 509. doi: 10.1111/1468-0009.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(suppl 2):II46–II54. doi: 10.1097/00005650-200108002-00003. 8. [DOI] [PubMed] [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? a framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 22.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.