Abstract
Background
The US Preventive Services Task Force recently concluded that the harms of existing prostate-specific antigen (PSA) screening strategies outweigh benefits.
Objective
To evaluate comparative effectiveness of alternative PSA screening strategies.
Design
Microsimulation model of prostate cancer incidence and mortality quantifying harms and lives saved for alternative PSA screening strategies.
Data Sources
National and trial data on PSA growth, screening and biopsy patterns, incidence, treatment distributions, treatment efficacy, and mortality.
Target Population
A contemporary cohort of US men.
Time Horizon
Lifetime.
Perspective
Societal.
Intervention
35 screening strategies that vary by start/stop ages, inter-screening intervals, and thresholds for biopsy referral.
Outcome Measurements
PSA tests, false positive tests, cancers detected, overdiagnoses, prostate cancer deaths, lives saved, and months of life saved.
Results of Base-Case Analysis
Without screening, the risk of prostate cancer death is 2.86%. A reference strategy that screens men aged 50–74 annually with a PSA threshold for biopsy referral of 4 μg/L reduces the risk of prostate cancer death to 2.15% with risk of overdiagnosis of 3.3%. A strategy that uses higher PSA thresholds for biopsy referral in older men achieves a similar risk of prostate cancer death (2.23%) but reduces the risk of overdiagnosis to 2.3%. A strategy that screens biennially with longer inter-screen intervals for men with low PSA levels achieves similar risks of prostate cancer death (2.27%) and overdiagnosis (2.4%) but reduces total tests by 59% and false positive tests by 50%.
Results of Sensitivity Analysis
Varying incidence inputs or reducing the survival improvement due to screening did not change conclusions.
Limitations
The model is a simplification of prostate cancer natural history, and the survival improvement due to screening is uncertain.
Conclusions
PSA screening strategies that use higher thresholds for biopsy referral for older men and that screen men with low PSA levels less frequently can reduce harms while preserving lives saved compared to standard screening.
Primary Funding Source
National Cancer Institute.
Keywords: Public health policy, decision analysis, prostate cancer screening, simulation modeling
INTRODUCTION
Prostate cancer screening is one of the most controversial topics in public health policy. Although PSA testing is ubiquitous in the US, there has always been uncertainty about its efficacy and effectiveness. Sustained declines in prostate cancer mortality since the first wave of screening in the early 1990s suggest benefit but are not conclusive, as improvements in prostate cancer treatment may also explain the decrease in prostate cancer deaths.
Results from randomized screening trials conducted in Europe and the US have only stoked the controversy. The Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial in the US showed no difference between prostate cancer mortality rates in intervention and control arms (1), while the European Randomized Study of Screening for Prostate Cancer (ERSPC) showed a significant mortality reduction but documented a high frequency of overdiagnosis per life saved (2). Updated results from both studies confirmed their original findings (3, 4), with fewer overdiagnoses per life saved in the ERSPC under additional follow-up. The trial results have been extensively debated, and it is now clear that the PLCO results reflect a comparison of organized annual screening versus opportunistic screening rather than screening versus no screening (3, 5). Still, largely on the basis of these trial results, the US Preventive Services Task Force (USPSTF) recently recommended against routine PSA-based screening (6).
Other organizations have updated or are in the process of updating their guidelines in light of the trial results. To date, no other published guideline recommends against PSA screening, with many encouraging informed decision making at an individual level. However, Welch (7) points out that such informed decision making carries an enormous burden and argues that strategies that make the harm-benefit tradeoff more favorable are urgently needed.
The USPSTF recommendation also identifies the need for additional research to “evaluate the benefits and harms of modifications of the use of existing prostate cancer screening tools” and to “optimize the benefits while minimizing the harms” (6). In this article we take up that challenge and address the following question: Can we identify strategies that reduce the harms of screening while preserving its impact on detection and survival? In other words, can we screen smarter for prostate cancer?
METHODS
There are many potential avenues to smarter screening because there are many parameters that define a screening strategy: ages to start and stop screening, the inter-screening interval, and the threshold for biopsy referral, and all but the starting age may depend on prior results. All parameters have been topics of debate, but it is unlikely that novel combinations will be explored in a prospective randomized setting (8, 9). An alternative is to model disease incidence and mortality under observed screening practices, then study model-projected outcomes under alternative screening strategies.
The Fred Hutchinson Cancer Research Center (FHCRC) microsimulation model of prostate cancer was developed as part of the Cancer Intervention and Surveillance Modeling Network (http://cisnet.cancer.gov), a consortium of investigators whose goal is to use modeling to understand the roles of different cancer interventions in explaining trends in cancer incidence and mortality.
The incidence component of the model consists of two linked parts: PSA growth and disease progression. PSA growth is based on data from the control arm of the Prostate Cancer Prevention Trial (Figure 1(a)). Disease progression consists of tumor onset, metastatic spread, and clinical diagnosis that would occur in the absence of PSA screening, with the risks of events after onset indicated by PSA levels (Figure 1(b)). To calibrate the model we superimpose PSA screening according to observed US screening patterns and obtain model-projected disease incidence. We then identify rates of onset, metastasis, and clinical diagnosis so that model-projected incidence matches observed incidence (10, 11). The calibrated model closely replicates observed age-adjusted incidence rates by stage and grade.
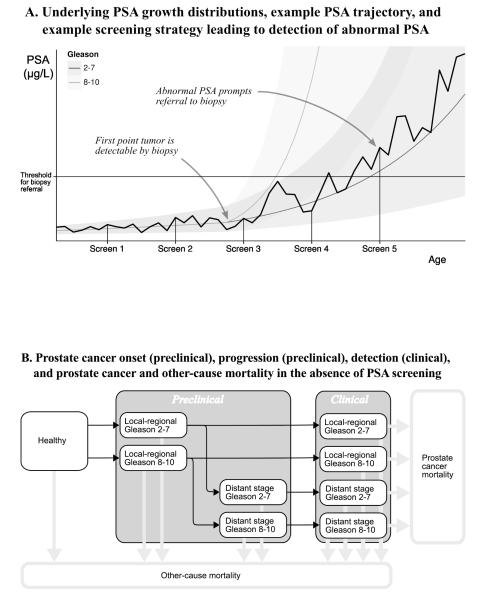
Figure 1. Fred Hutchinson Cancer Research Center prostate cancer incidence model.
(A) Underlying PSA growth before and after onset of Gleason 2–7 and 8–10 tumors. Thin gray lines are PSA trajectories by age for the two Gleason categories. Shaded bands around those lines illustrate between-individual variability in PSA values based on interquartile ranges. The dark jagged line illustrates an example PSA trajectory for a man who develops a Gleason 2–7 tumor. In this example, PSA exceeds the threshold for biopsy referral on the fifth test of a schematic screening strategy.
(B) The model’s healthy, preclinical, clinical, prostate cancer mortality, and other-cause mortality states in the absence of screening.
PSA = prostate-specific antigen
The mortality component of the model consists of disease-specific and other-cause survival. Disease-specific survival depends on age, stage (local-regional or distant), and grade (Gleason 2– 7 or 8–10) at diagnosis. For local-regional cases, disease-specific survival also depends on primary treatment (radiation or surgery), which is assumed to be administered according to patterns observed in the 9 core areas of the Surveillance, Epidemiology, and End Results (SEER) program in 2005.
Screening that identifies non-overdiagnosed disease prior to clinical diagnosis results in the identification of earlier-stage tumors than might be identified without screening, leading to a reduction in prostate cancer mortality (Appendix Figure 1). We refer to this as a “stage-shift model” for the impact of screening on prostate cancer mortality.
The candidate screening strategies we consider are all 32 combinations of: (1) two ages to start (40 or 50 years) and stop (69 or 74 years) screening, (2) two inter-screening intervals (annual or biennial), and (3) four thresholds for biopsy referral (PSA 4.0 μg/L; PSA 2.5 μg/L; PSA 4.0 μg/L or PSA velocity 0.35 μg/L/year; or PSA > 95th percentile for age (PSA 2.5 for ages 40–49, 3.5 for 50–59, 4.5 for 60–69, and 6.5 for 70–74 years)). The strategies are motivated by contemporary controversies. Some studies advocate lowering the screening start age from 50 to 40 years and others argue for lowering the PSA threshold for biopsy (12-14). Some studies suggest reducing the frequency of screening for men with low PSA levels (14); we operationalize this idea by evaluating an adaptive strategy (8) which screens biennially but increases the screening interval to 5 years if PSA is below its age-specific median (15).
We also evaluate strategies that have been recommended by guidelines groups. Based on recommendations from the American Cancer Society (ACS), we evaluate a strategy that changes the inter-screening interval from annual to biennial if PSA is below 2.5 μg/L (16). Based on recommendations from the National Comprehensive Cancer Network (NCCN) (17), we evaluate a strategy with annual screening starting at age 40 but that expands to 5-year intervals if baseline PSA is below 1.0 μg/L, changes to annual screening at age 50, and refers to biopsy if PSA exceeds 2.5 μg/L or PSA velocity exceeds 0.35 μg/L/year. We do not include a strategy from the American Urological Association as they recommend starting screening at age 40 but do not indicate a screening interval or biopsy threshold. Our reference strategy is annual screening ages 50–74 with a PSA threshold of 4.0 μg/L for biopsy referral.
For each candidate screening strategy, we project a range of negative (number of tests, false positive tests, overdiagnoses, and prostate cancer deaths) and positive (cancers detected, lives saved, and months of life saved) outcomes. Since unnecessary biopsy- and treatment-related complications represent fixed fractions of false-positive tests and overdiagnoses, we do not present these outcomes separately. Projected outcomes are presented for a contemporary man aged 40 based on a simulated cohort of 100 million men for each screening strategy. Outcomes are reported as the mean number of events or the lifetime probability of each outcome. We also calculate the additional number needed to detect (NND) to prevent one prostate cancer death, which represents overdiagnoses per life saved and has become established as a summary measure of the harm-benefit tradeoff in prostate cancer screening (18, 19).
Model Validation
We previously calibrated the FHCRC prostate model using data through the year 2000 (10, 11). To validate the incidence component of the model, we compare observed and model-projected age-adjusted incidence by stage through the year 2005. To validate the mortality component of our model, we simulate the ERSPC which, based on the protocol at most ERSPC centers, screened men every 4 years and biopsied 86% of men with a PSA above 3.0 μg/L (4). Using this framework, we calculate model-projected prostate cancer mortality rates for screened versus unscreened cohorts age 55–69 after 11 years of follow-up and compare the projected absolute and relative mortality reductions with observed.
Sensitivity Analysis
Recognizing that the incidence and mortality model inputs are subject to uncertainty, we conduct a sensitivity analysis to determine the robustness of our findings across a range of plausible values. In this analysis, we focus on the inputs that are unobservable, namely the rates of disease onset, metastasis, and clinical detection in the incidence model and the extent of screening impact in the mortality model. Our previous work calibrating the incidence model to US prostate cancer incidence trends yielded a range of values for each parameter, and we run the model 100 times under each screening strategy, each time sampling the parameters from their respective ranges to determine the variability in results that would be induced by varying these inputs. In addition, we run all screening strategies under several settings for the survival impact of screening, ranging from no impact to the impact consistent with the stage-shift model (Appendix Figure 1).
Role of the Funding Source
This study was funded by the National Cancer Institute and the Centers for Disease Control, which had no role in the design or execution of this study.
RESULTS
Under no screening, the model projects a lifetime chance of a prostate cancer diagnosis of 12.0% and a lifetime chance of dying of prostate cancer of 2.86%. The chance of diagnosis is higher than estimates from the pre-PSA era of 9% (20), but our projected probability of diagnosis assumes contemporary biopsy practices, which are more sensitive than pre-PSA protocols (21).
Model Validation
Model-projected age-adjusted incidence closely matches observed incidence through the year 2005 (Appendix Figure 2), indicating that without any parameter change the model predicts incidence reasonably well beyond its years of calibration (1975–2000). The simulation of the ERSPC projects that screening reduces mortality by 28% after 11 years of follow-up, close to the reduction of 29% estimated by trial investigators after correction for non-compliance (4); and it projects an absolute mortality reduction of 2.08 per 1000 men enrolled after 11 years of follow-up, higher than the 1.07 per 1000 men enrolled observed in the trial (4). At least part of the discrepancy is likely due to cross over to screening in the control arm of the trial (22).
Table 1 summarizes lifetime outcomes projected under all 35 screening strategies, numbered in descending order by probability of life saved; the reference strategy (annual screening for ages 50–74 with PSA threshold for biopsy referral 4.0 μg/L) ranks 8th. Appendix Figure 3 demonstrates how outcomes under that reference strategy change when any one of its parameters (screening ages, inter-screening interval, PSA level threshold, PSA velocity threshold) changes.
Table 1.
Lifetime harms and benefits projected for a 40-year-old man under alternative prostate-specific antigen screening strategies.
| Screening policy |
Projected lifetime outcomes for a 40-year-old man |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Policy no. | Screening ages |
Inter-screening interval | Criterion for biopsy referral | Mean no. of screens |
Mean no. of false positives |
Probability of ≥1 false positive |
Probability of cancer detected |
Probability of overdiagnosis |
Probability of cancer death |
Probability of life saved |
Mean months of life saved |
Number needed to detect |
| 1 (NCCN) |
40–74 | annual (quinquennial if age < 50 and PSA < 1) |
PSA > 2.5 or PSAV > 0.35 | 21.8 | 3.4 | 44% | 18.2% | 6.0% | 2.02% | 0.85% | 1.00 | 7.08 |
| 2 | 40–74 | annual | PSA > 4.0 or PSAV > 0.35 | 29.5 | 3.0 | 45% | 17.9% | 5.8% | 2.03% | 0.84% | 1.00 | 6.90 |
| 3 | 50–74 | annual | PSA > 4.0 or PSAV > 0.35 | 20.0 | 2.7 | 44% | 17.7% | 5.5% | 2.05% | 0.81% | 0.96 | 6.84 |
| 4 | 40–74 | annual | PSA > 2.5 | 29.7 | 2.8 | 32% | 17.0% | 4.9% | 2.06% | 0.81% | 0.96 | 6.08 |
| 5 | 50–74 | annual | PSA > 2.5 | 20.1 | 2.8 | 31% | 16.8% | 4.7% | 2.08% | 0.78% | 0.94 | 6.01 |
| 6 | 40–74 | annual | PSA > 4.0 | 30.0 | 1.7 | 22% | 15.5% | 3.5% | 2.13% | 0.72% | 0.88 | 4.79 |
| 7 | 40–74 | biennial | PSA > 2.5 | 15.4 | 1.5 | 29% | 16.1% | 4.0% | 2.14% | 0.71% | 0.85 | 5.58 |
| 8 | 50–74 | annual | PSA > 4.0 | 20.3 | 1.6 | 21% | 15.3% | 3.3% | 2.15% | 0.70% | 0.86 | 4.70 |
| 9 (ACS) |
50–74 | annual (biennial if PSA <2.5) |
PSA > 4.0 | 12.1 | 1.6 | 21% | 15.3% | 3.3% | 2.15% | 0.70% | 0.86 | 4.70 |
| 10 | 40–74 | biennial | PSA > 4.0 or PSAV > 0.35 | 15.4 | 1.1 | 26% | 15.6% | 3.6% | 2.16% | 0.69% | 0.84 | 5.13 |
| 11 | 50–74 | biennial | PSA > 2.5 | 10.6 | 1.4 | 29% | 15.9% | 3.8% | 2.16% | 0.69% | 0.84 | 5.51 |
| 12 | 40–69 | annual | PSA > 4.0 or PSAV > 0.35 | 26.6 | 2.1 | 41% | 16.0% | 3.9% | 2.18% | 0.67% | 0.89 | 5.77 |
| 13 | 50–74 | biennial | PSA > 4.0 or PSAV > 0.35 | 10.6 | 1.0 | 26% | 15.5% | 3.4% | 2.19% | 0.67% | 0.82 | 5.07 |
| 14 | 50–69 | annual | PSA > 4.0 or PSAV > 0.35 | 17.0 | 1.9 | 40% | 15.7% | 3.7% | 2.21% | 0.65% | 0.85 | 5.67 |
| 15 | 40–74 | annual | PSA > 95th percentile for age | 30.0 | 1.2 | 16% | 14.4% | 2.4% | 2.21% | 0.64% | 0.83 | 3.78 |
| 16 | 40–74 | biennial | PSA > 4.0 | 15.5 | 0.9 | 20% | 14.9% | 2.8% | 2.22% | 0.64% | 0.78 | 4.42 |
| 17 | 40–69 | annual | PSA > 2.5 | 26.8 | 1.9 | 27% | 15.1% | 3.1% | 2.21% | 0.63% | 0.84 | 4.85 |
| 18 | 50–74 | biennial | PSA > 4.0 | 10.6 | 0.8 | 20% | 14.7% | 2.7% | 2.23% | 0.61% | 0.77 | 4.34 |
| 19 | 50-74 | biennial | PSA > 4.0 | 10.6 | 0.8 | 20% | 14.7% | 2.7% | 2.23% | 0.61% | 0.77 | 4.34 |
| 20 | 50-74 | annual | PSA > 95th percentile for age | 20.4 | 1.2 | 15% | 14.3% | 2.3% | 2.23% | 0.61% | 0.81 | 3.71 |
| 21 | 50-69 | annual | PSA > 2.5 | 17.1 | 1.8 | 27% | 14.9% | 2.9% | 2.24% | 0.61% | 0.82 | 4.75 |
| 22 (V&L) |
45-74 | biennial (quinquennial if PSA < median for age) |
PSA > 4.0 | 8.3 | 0.8 | 19% | 14.4% | 2.4% | 2.27% | 0.58% | 0.75 | 4.09 |
| 23 | 40-69 | annual | PSA > 4.0 | 27.0 | 1.0 | 17% | 14.0% | 2.0% | 2.30% | 0.54% | 0.75 | 3.66 |
| 24 | 40-74 | biennial | PSA > 95th percentile for age | 15.5 | 0.6 | 14% | 13.8% | 1.8% | 2.31% | 0.54% | 0.73 | 3.39 |
| 25 | 40-69 | biennial | PSA > 2.5 | 13.6 | 0.9 | 24% | 14.2% | 2.2% | 2.33% | 0.52% | 0.72 | 4.20 |
| 26 | 50-69 | annual | PSA > 4.0 | 17.3 | 1.0 | 17% | 13.8% | 1.8% | 2.32% | 0.51% | 0.73 | 3.58 |
| 27 | 50-74 | biennial | PSA > 95th percentile for age | 10.6 | 0.6 | 14% | 13.7% | 1.7% | 2.33% | 0.51% | 0.70 | 3.32 |
| 28 | 40-69 | annual | PSA > 95th percentile for age | 27.0 | 0.9 | 15% | 13.7% | 1.7% | 2.33% | 0.51% | 0.73 | 3.29 |
| 29 | 40-69 | biennial | PSA > 4.0 or PSAV > 0.35 | 13.6 | 0.7 | 21% | 13.9% | 1.9% | 2.34% | 0.50% | 0.71 | 3.90 |
| 30 | 50-69 | biennial | PSA > 2.5 | 8.7 | 0.9 | 23% | 14.0% | 2.0% | 2.35% | 0.49% | 0.70 | 4.12 |
| 31 | 50-69 | annual | PSA > 95th percentile for age | 17.3 | 0.8 | 14% | 13.5% | 1.5% | 2.35% | 0.48% | 0.71 | 3.20 |
| 32 | 50-69 | biennial | PSA > 4.0 or PSAV > 0.35 | 8.7 | 0.6 | 20% | 13.8% | 1.8% | 2.37% | 0.47% | 0.67 | 3.85 |
| 33 | 40-69 | biennial | PSA > 4.0 | 13.6 | 0.5 | 15% | 13.4% | 1.4% | 2.41% | 0.43% | 0.64 | 3.18 |
| 34 | 40-69 | biennial | PSA > 95th percentile for age | 13.6 | 0.5 | 13% | 13.2% | 1.3% | 2.42% | 0.42% | 0.63 | 2.99 |
| 35 | 50-69 | biennial | PSA > 4.0 | 8.7 | 0.5 | 14% | 13.3% | 1.3% | 2.43% | 0.41% | 0.61 | 3.11 |
PSA = prostate-specific antigen level (μg/L); PSAV = prostate-specific antigen velocity (μg/L/year); NCCN = policy based on recommendations from the National Comprehensive Cancer Network; ACS = policy based on recommendations from the American Cancer Society; V&L = policy based on recommendations from Vickers and Lilja (8). Median PSA values are 0.7, 1.0, 1.4, and 2.0 and 95th percentile PSA values are 2.5, 3.5, 4.5, and 6.5 μg/L for ages 40–49, 50–59, 60–69, and 70–74 years (15). Number needed to detect by screening to save one life is overdiagnoses divided by lives saved. Probability of life saved and mean months of life saved are based on the model assumption that a patient whose stage was shifted from distant to local-regional stage by screening receives a corresponding survival benefit. Strategies are sorted by the probability of life saved. For comparison, in the absence of screening, the model projects 12.0% probability of cancer detection and 2.86% probability of cancer death.
The reference strategy yields a 15.3% lifetime chance of diagnosis, a 3.3% lifetime chance of overdiagnosis, and a 2.15% lifetime chance of prostate cancer death, a relative reduction of 24.8% compared to the 2.86% chance of prostate cancer death with no screening. Under this reference strategy, the lifetime chance of a false positive test is 21% and the NND is 4.7, which is similar to other long-term estimates (23, 24). Unless otherwise stated all results are presented relative to this strategy.
The NCCN strategy (Strategy 1) saves the most lives. However, the lifetime risks of a false positive test and of overdiagnosis are nearly doubled compared with the reference strategy. In general, lowering the PSA threshold or adding a velocity threshold generates substantial harms relative to incremental lives saved (Strategies 3 and 5).
Varying ages to start and stop screening has a substantial impact on lives saved and on overdiagnoses. Lowering the starting age to 40 (Strategy 6) increases the probability of life saved and overdiagnosis and substantially increases the number of PSA tests. Lowering the stopping age to 69 (Strategy 26) leads to a relative reduction of the probability of life saved by 27%, but the probability of overdiagnosis is nearly halved and the probability of a false positive PSA decreases by nearly 20%. The latter finding reflects the fact that a significant proportion of men diagnosed with lethal prostate cancer in the absence of screening are over 70, and these men have the potential to be detected early, but many more men in this age group have cancers that would not have affected their life expectancy, so screening this age group substantially increases the number overdiagnosed. Screening men up to age 74 but increasing the threshold for biopsy referral via an age-dependent PSA cutoff (Strategy 20) reduces overdiagnoses by one-third (to 2.3%) while only slightly altering the lives saved (to 2.23%). Therefore, one approach to preserve the impact of screening on mortality while controlling overdiagnosis may be to screen older men more conservatively (stopping at age 69 or increasing the PSA threshold for biopsy referral for ages 70–74).
The performance of the ACS strategy (Strategy 9) exactly parallels the reference strategy with no reduction in overdiagnoses and equivalent lives saved. The only impact of this strategy relative to the reference strategy is to reduce the number of tests conducted. This suggests that, holding starting age and PSA threshold fixed, if PSA is low, the interval between PSA assessments can be increased to biennial examinations without affecting other outcomes. Screening every five years rather than every two years when PSA is below the median for PSA within 10-year age groups (Strategy 22) lowers the average number of tests by one-third and overdiagnoses by one-quarter relative to a biennial strategy while only reducing the chance of life saved by a relative 17%.
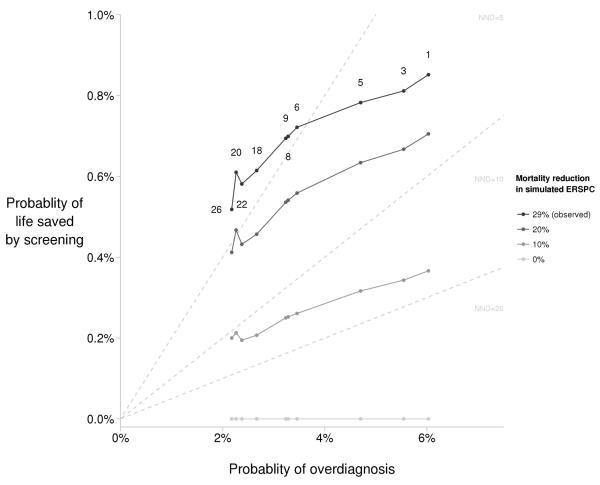
Figure 2 illustrates the tradeoffs between the probability of life saved (X value) and the probability of overdiagnosis (X value) for selected screening strategies. Projections under the base case survival impact correspond to the 29% mortality reduction observed in the ERSPC after 11 years of follow-up (corrected for non-compliance) and are connected by the darkest line at the top. The NND for each strategy is the ratio X/Y, and dashed lines originating from the origin (representing no screening) illustrate fixed NND values of 5, 10, and 20 for reference. Strategies 1, 3, and 5 have NND between 5 and 10 because they fall between the radiating lines NND=5 and NND=10; remaining strategies under the 29% mortality reduction assumption all have NND<5. The figure illustrates that relative to stopping screening at age 69 (Strategy 26), continuing screening through age 74 but with age-dependent PSA thresholds for biopsy (Strategy 20) increases probability of life saved (absolute increase 0.1%) much more than it increases overdiagnosis (absolute increase 0.05%). The figure also shows results obtained in analyses of sensitivity to the survival impact (i.e., for mortality reductions of 20%, 10%, and 0%).
Figure 2. Tradeoff between lifetime probabilities of life saved by screening and overdiagnosis for selected screening strategies.
This figure illustrates the tradeoffs for selected PSA screening strategies under a range of assumed impacts of screening on prostate cancer survival. Each point represents the tradeoff for 10 of the 35 screening strategies examined in this study: the reference strategy (Strategy 8), strategies that differ from the reference by a single screening parameter (Strategies 3, 5, 6, 9, 18, 20, and 26—see Appendix Figure 3), and strategies based on recommendations by the National Comprehensive Cancer Network (Strategy 1), the American Cancer Society (Strategy 9), and by Vickers and Lilja (Strategy 22) (8); see Table 1 for strategy details. The assumed impacts of screening on prostate cancer survival correspond to mortality reductions of 29% (the reduction observed in the ERSPC trial after correction for non-compliance), 20%, 10%, and 0% projected in a simulated version of the ERSPC after 11 years of follow-up. Probability of life saved by screening corresponding to a mortality reduction of 29% is based on the assumption that a patient whose stage was shifted from distant to local-regional stage by screening receives the survival of the earlier stage. Probability of life saved by screening corresponding to mortality reductions of 20%, 10%, and 0% in the simulated version of the ERSPC are based on a generalization of this stage-shift assumption which projects prostate cancer survival on a continuum between no impact for cases with short lead times and the full stage shift for cases with long lead times. Probability of overdiagnosis is based on model-projected competing risks of prostate cancer detection and other-cause mortality. Shaded gray lines connect projections under the same mortality reduction.
The additional number needed to detect (NND) to prevent one prostate cancer death is an established summary measure of the harm-benefit tradeoff in prostate cancer screening compared to no screening, defined as the overdiagnoses divided by lives saved by screening. The NND corresponding to any point in the figure is obtained as the ratio of the probability of overdiagnosis (X value) to the probability of life saved (Y value). For reference, dashed lines radiating from the origin (representing no screening) illustrate fixed NND values of 5, 10, and 20. For a given probability of overdiagnosis, as the probability of life saved by screening decreases, the corresponding NND increases. For the mortality reduction of 29%, NNDs range from 7.1 (Strategy 1) to 3.6 (Strategy 26), and for the mortality reduction of 10%, NNDs range from 16.5 (Strategy 1) to 9.9 (Strategy 26). A strategy that falls between two NND lines (e.g., NND=5 and NND=10) has an NND between those NND values.
Different strategies will be preferred depending on relative weighting of the probabilities of life saved and overdiagnosis. Among the strategies considered, Strategy 1 maximizes the probability of life saved and will be the preferred strategy if survival is the highest priority. Strategy 26 minimizes the probability of overdiagnosis and will be preferred if the morbidity associated with treatment is the greatest concern. For priorities between these extremes, the preferred strategy will be based on the most favorable balance between probabilities of life saved and overdiagnosis. For example, assuming the mortality reduction of 29%, a target tradeoff of 5 or fewer overdiagnoses per life saved (i.e., NND≤5) would identify strategies above and to the left of the NND=5 line. Assuming a mortality reduction of 20%, a target tradeoff of 5 or fewer overdiagnoses per life saved identifies Strategy 20 as the only option. If the target tradeoff is NND≤3, none of the strategies considered will satisfy this constraint. Assuming a mortality reduction of 10%, no strategies satisfy the constraint that NND≤10.
ERSPC = European Randomized Study of Screening for Prostate Cancer
Sensitivity Analysis
Varying the incidence model inputs produces very little variation in absolute model-projected outcomes (results not shown). Further, overall conclusions regarding tradeoffs across candidate strategies are robust to our sensitivity analysis on assumed survival impact. Less intensive strategies—i.e., those with fewer screens or higher thresholds for biopsy referral among older men—generally produce a considerably lower risk of overdiagnosis with modest impact on relative rankings of disease-specific deaths or lives saved (Figure 2).
DISCUSSION
Since the advent of PSA screening, there has been uncertainty about screening benefit and concern about screening harms. The recent USPSTF recommendation against PSA screening for prostate cancer has raised awareness of the harms of existing screening strategies. In response, we sought to identify smarter screening strategies using microsimulation modeling.
The use of modeling in policy development is becoming more accepted (25, 26). The USPSTF relied on modeling to determine strategies for breast (27) and colorectal cancer screening (28). And numerous models have been developed to study prostate cancer screening (29-32). Indeed, a recent publication considered six different strategies for prostate cancer screening (24). However, like other existing prostate screening models, it did not conceptualize the disease process in a way that permits comprehensive evaluation of all screening strategy parameters. Our model is unique in that it not only represents individual PSA over time but also explicitly links PSA growth with disease progression, which is linked with mortality. As a consequence, we can explore outcomes due to varying PSA thresholds for biopsy referral as well as variations in screening ages and intervals, which may change dynamically depending on PSA levels. By quantifying the likelihood of a false positive test, overdiagnosis, or life saved associated with a broad range of screening strategies, we can identify strategies that reduce harms but preserve the impact of early detection on prostate cancer mortality.
Our results yield several important conclusions. First, we find that aggressive screening strategies, particularly those that lower the PSA threshold for biopsy, do reduce prostate cancer mortality relative to the reference strategy. However, the harms of unnecessary biopsies, diagnoses, and treatments may be unacceptable. Quantifying the magnitude of these harms relative to potential gains in lives saved is critical for determining whether the projected harms are acceptable.
Second, we find substantial improvements in the harm-benefit tradeoff of PSA screening with less frequent testing and more conservative criteria for biopsy referral in older men. These approaches preserve the majority of the survival impact and markedly reduce screening harms compared with the reference strategy. In particular, using age-specific PSA thresholds for biopsy referral (Strategy 20) reduces false positive tests by a relative 25% and overdiagnoses by 30% while preserving 87% of lives saved under the reference strategy. Alternatively, using longer inter-screen intervals for men with low PSA levels (Strategy 22) reduces false positive tests by a relative 50% and overdiagnoses by 27% while preserving 83% of lives saved under the reference strategy. These adaptive, personalized strategies represent prototypes for a smarter approach to screening.
When smarter screening strategies achieve similar absolute probabilities of life saved, the choice between them depends on relative weighting of overdiagnosis and other harms. Using these two prototype strategies as an example, Strategy 22 reduces total tests by a relative 59% and false positive tests by 33% but increases overdiagnoses by 5% relative to Strategy 20. In general, the relative weighting of harms, like the relative weighting of benefits and harms, may depend on whether one adopts an individual or societal perspective. If an individual perspective is adopted, preferences may be variable across the population.
Other investigators have recommended personalized strategies for PSA screening as a means to reduce harms while preserving benefit. Carter et al. (14) suggested that the inter-screening interval should be lengthened in men with low PSA. The risk calculator from the Prostate Cancer Prevention Trial produces a personalized prediction of the risk of occult disease based on PSA, age, race, and family history (33). In principle we could compare an approach based on this calculator with other personalized strategies, but this would require adding race and family history to the model, recalibrating the model accordingly, and determining a reasonable risk threshold for biopsy referral. This is possible in principle but beyond the scope of the present study.
We recognize that every model is necessarily a simplification of reality and is limited by its assumptions. Our model is no exception. We allow the likelihood of developing high-grade disease to vary with age but do not model grade progression. Due to limitations in the SEER data used to calibrate the model, we are limited to two stages (SEER local-regional or distant stage) and two grades (Gleason 2–7 or 8–10). We model survival benefit via a stage-shift mechanism which is likely also a simplification. Yet, a close match between our calibrated model and observed incidence and absolute and relative mortality reductions in a simulated ERSPC give us confidence that we are producing a valid representation of the likely tradeoffs involved in screening for a complex heterogeneous disease. Our model also does not incorporate utilities and does not produce quality-adjusted estimates of the impact of screening on survival. However, existing data on utilities associated with prostate cancer screening and post-diagnosis health states are extremely limited (34) and we do not feel that they are sufficiently reliable for modeling at this time. Further versions of the model will include other elements that are missing in the present version, including utilities once adequate data become available, costs, and race-specific disease progression.
In his recent editorial (7), Welch concludes that “In the case of the prostate, for the past two decades we’ve been looking too damn hard. That’s what’s led to so many biopsies and so much overdiagnosis.” By screening smarter, we look less hard, particularly in older men at the highest risk of overdiagnosis. As demonstrated in the PLCO trial and supported by our model results across a broad range of alternative strategies, there are diminishing returns to intensive screening. If we recognize that realistic screening strategies must achieve an acceptable balance of benefits and harms as opposed to unconditionally maximizing benefits, we can improve on the effectiveness of existing PSA-based screening for prostate cancer.
Supplementary Material
Acknowledgment
The authors thank Jeffrey Katcher for developing a flexible interface for specifying candidate PSA screening strategies and Drs. Jeanne Mandelblatt and Andrew Vickers for helpful comments on an earlier draft.
Grant support: This work was supported by Award Numbers R01 CA131874 and U01 CA88160 from the National Cancer Institute and Award Number U01 CA157224 from the National Cancer Institute and the Centers for Disease Control.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Centers for Disease Control.
Contributor Information
Roman Gulati, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M2-B230, P.O. Box 19024, Seattle, WA 98109-1024. Tel: +1.206.667.7795. Fax: +1.206.667.7264. rgulati@fhcrc.org.
John L. Gore, Department of Urology, University of Washington, 1959 NE Pacific St, Box 356510, Seattle, WA 98195-6510. Tel: +1.206.221.6430. Fax: +1.206.543.3272. jlgore@u.washington.edu.
Ruth Etzioni, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M2-B230, P.O. Box 19024, Seattle, WA 98109-1024. Tel: +1.206.667.6561. Fax: +1.206.667.7264. retzioni@fhcrc.org.
References
- 1.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Prostate Cancer Screening in the Randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality Results after 13 Years of Follow-up. J Natl Cancer Inst. 2012;104:1–8. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. The New England journal of medicine. 2012;366(11):981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulati R, Tsodikov A, Wever EM, Mariotto AB, Heijnsdijk EA, Katcher J, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes and Control. 2012;23(6):827–35. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyer VA, on behalf of the USPSTF Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2012;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 7.Welch HG. A piece of my mind. Making the call. JAMA : the journal of the American Medical Association. 2011;306(24):2649–50. doi: 10.1001/jama.2011.1898. [DOI] [PubMed] [Google Scholar]
- 8.Vickers AJ, Lilja H. Prostate cancer: estimating the benefits of PSA screening. Nature reviews Urology. 2009;6(6):301–3. doi: 10.1038/nrurol.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S, Carlsson S, Braithwaite RS. Prostate cancer: Modeling the outcomes of prostate cancer screening. Nat Rev Urol. 2012;9(4):183–5. doi: 10.1038/nrurol.2012.34. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni R, Gulati R, Katcher J, Inoue L. A surveillance model of prostate cancer trends informs PSA screening policies. 2nd General Meeting of the International Microsimulation Association--Microsimulation: Bridging data and policy; Ottawa, Canada. 2009. [Google Scholar]
- 11.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11(4):707–19. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RM. Viewpoint: Limiting prostate cancer screening. Ann Intern Med. 2006;144(6):438–40. doi: 10.7326/0003-4819-144-6-200603210-00011. [DOI] [PubMed] [Google Scholar]
- 13.Catalona WJ, Loeb S, Han M. Viewpoint: Expanding prostate cancer screening. Ann Intern Med. 2006;144(6):441–3. doi: 10.7326/0003-4819-144-6-200603210-00012. [DOI] [PubMed] [Google Scholar]
- 14.Carter HB, Epstein JI, Chan DW, Fozard JL, Pearson JD. Recommended prostate-specific antigen testing intervals for the detection of curable prostate cancer. JAMA. 1997;277(18):1456–60. [PubMed] [Google Scholar]
- 15.Oesterling JE, Jacobsen SJ, Chute CG, Guess HA, Girman CJ, Panser LA, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. Journal of the American Medical Association. 1993;270(7):860–4. [PubMed] [Google Scholar]
- 16.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 17.Kawachi MH, Bahnson RR, Barry M, Busby JE, Carroll PR, Carter HB, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8(2):240–62. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 18.Crawford ED, Grubb R, 3rd, Black A, Andriole GL, Jr., Chen MH, Izmirlian G, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. Journal of Clinical Oncology. 2011;29(4):355–61. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb S, Vonesh EF, Metter EJ, Carter HB, Gann PH, Catalona WJ. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? Journal of Clinical Oncology. 2011;29(4):464–7. doi: 10.1200/JCO.2010.30.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistical Research and Applications Branch, Division of Cancer Control and Population Sciences, National Cancer Institute DevCan--Probability of developing or dying of cancer. Available at http://srab.cancer.gov/devcan/
- 21.Gore JL, Shariat SF, Miles BJ, Kadmon D, Jiang N, Wheeler TM, et al. Optimal combinations of systematic sextant and laterally directed biopsies for the detection of prostate cancer. Journal of Urology. 2001;165(5):1554–9. [PubMed] [Google Scholar]
- 22.Ciatto S, Zappa M, Villers A, Paez A, Otto SJ, Auvinen A. Contamination by opportunistic screening in the European Randomized Study of Prostate Cancer Screening. BJU International. 2003;92(Suppl 2):97–100. doi: 10.1111/j.1464-410x.2003.04407.x. [DOI] [PubMed] [Google Scholar]
- 23.Gulati R, Mariotto AB, Chen S, Gore JL, Etzioni R. Long-term projections of the harm-benefit trade-off in prostate cancer screening are more favorable than previous short-term estimates. Journal of Clinical Epidemiology. 2011;64(12):1412–7. doi: 10.1016/j.jclinepi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heijnsdijk EA, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. New England Journal of Medicine. 2012;367(7):595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glied S, Tilipman N. Simulation modeling of health care policy. Annual Review of Public Health. 2010;31:439–55. doi: 10.1146/annurev.publhealth.012809.103542. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein MC, Toy EL, Sandberg EA, Neumann PJ, Evans JS, Kuntz KM, et al. Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001;4(5):348–61. doi: 10.1046/j.1524-4733.2001.45061.x. [DOI] [PubMed] [Google Scholar]
- 27.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–47. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schröder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 30.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat in Med. 2006;25(16):2846–66. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 31.Wever EM, Draisma G, Heijnsdijk EA, de Koning HJ. How does early detection by screening affect disease progression? Modeling estimated benefits in prostate cancer screening. Med Decis Making. 2011 doi: 10.1177/0272989X10396717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GH-M, Auvinen A, Yen AM-F, Hakama M, Tammela TL, Stenman U-H, et al. The impact of interscreening interval and age on prostate cancer screening with prostate-specific antigen. European Urology. 2012;61(5):1011–8. doi: 10.1016/j.eururo.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 34.Sox HC. Quality of life and guidelines for PSA screening. New England Journal of Medicine. 2012;367(7):669–71. doi: 10.1056/NEJMe1207165. [DOI] [PubMed] [Google Scholar]
- 35.Etzioni R, Gulati R, Tsodikov A, Wever EM, Penson DF, Heijnsdijk EA, et al. The prostate cancer conundrum revisited: Treatment changes and prostate cancer mortality declines. Cancer. 2012 doi: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 37.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116(22):5226–34. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics . Vital statistics of the United States. Volume II: Mortality, part A. Government Printing Office; Washington DC: various years. [Google Scholar]
- 39.Mariotto A, Etzioni R, Krapcho M, Feuer EJ. Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109(9):1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 40.Pinsky PF, Andriole GL, Kramer BS, Hayes RB, Prorok PC, Gohagan JK. Prostate biopsy following a positive screen in the Prostate, Lung, Colorectal and Ovarian cancer screening trial. J Urol. 2005;173(3):746–50. doi: 10.1097/01.ju.0000152697.25708.71. discussion 50-51. [DOI] [PubMed] [Google Scholar]
- 41.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. Journal of Urology. 2000;163(1):152–7. [PubMed] [Google Scholar]
- 42.Presti JCJ, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: Results of a prospective clinical trial. J Urol. 2000;163(1):163–6. discussion 6-7. [PubMed] [Google Scholar]
- 43.Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: A systematic review. Journal of Urology. 2006;175(5):1605–12. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.