Abstract
Background
Standard doses of HSV suppressive therapy reduce plasma HIV-1 RNA levels (0.25-0.53 log10 copies/mL) among HIV-1/HSV-2 co-infected persons. Postulated mechanisms for this effect include direct inhibition of HIV-1 by acyclovir or indirect reduction by decreasing HSV-associated inflammation. We hypothesized that high-dose valacyclovir would further reduce plasma HIV-1 RNA, and that the effect would be mediated by greater suppression of HSV shedding.
Methods
34 participants with HIV-1 and HSV-2 not on antiretroviral therapy were enrolled into a randomized, open-label cross-over trial of valacyclovir 1000 mg twice daily or acyclovir 400 mg twice daily for 12 weeks, followed by a two week washout, and then the alternate treatment arm for 12 weeks. HSV DNA was measured from daily self-collected genital swabs for the initial 4 weeks of each arm and HIV-1 RNA was quantified from weekly plasma samples.
Results
28 participants provided plasma samples and genital swabs on both acyclovir and valacyclovir. The genital HSV-2 shedding rate was the same on valacyclovir and acyclovir (7.8% vs. 8.2% of days; RR, 0.95; 95% CI, 0.66-1.37; p=0.78). Plasma HIV-1 RNA was 0.27 log10 copies/mL lower on valacyclovir compared with acyclovir (95% CI, −0.41 to −0.14 log10 copies/mL; p<0.001); this was unchanged after adjustment for genital HSV-2 shedding.
Conclusions
High-dose valacyclovir reduces plasma HIV-1 RNA levels more than standard-dose acyclovir in HIV-1/HSV-2 seropositive persons not receiving antiretroviral therapy. The incremental reduction in plasma HIV-1 RNA achieved is not mediated by greater genital HSV-2 suppression.
Keywords: HIV, viral load, HSV, valacyclovir, acyclovir
Introduction
Decreasing plasma HIV-1 RNA in infected persons can prevent transmission to susceptible partners.1-6 However, at the end of 2010, it is estimated that less than half of those who met WHO guidelines for initiation of antiretroviral therapy (ART) were receiving antiretroviral medications.7 While the gap between the goal of universal access and the realities of the current levels of antiretroviral coverage exists, alternative strategies to decrease plasma HIV-1 RNA- with the goal of reducing transmission and delaying HIV-1 disease progression- will remain important.
Standard-dose HSV suppressive therapy (acyclovir 400 mg or valacyclovir 500 mg twice daily) among persons coinfected with HIV-1 and HSV-2 reduced plasma HIV-1 RNA levels by 0.25-0.53 log10 copies/mL in several randomized controlled trials8-15 and acyclovir modestly slows HIV-1 progression in patients not receiving ART.16,17 Postulated mechanisms by which anti-HSV therapy lowers plasma HIV-1 RNA levels include decreasing pro-inflammatory cytokines,18 decreasing HIV-1 transcriptional activation by HSV-encoded proteins,18,19 and direct inhibition of HIV-1 reverse transcriptase.20,21 In one study, high-dose valacyclovir (1.5 g twice daily) was associated with a decline in plasma HIV-1 RNA by 1.23 log10 copies/mL,22 suggesting a dose-response relationship. To investigate whether the reduction in plasma HIV-1 RNA with increasing doses of anti-HSV therapy is mediated by greater HSV suppression, we conducted a randomized, open-label cross-over trial of high-dose valacyclovir compared with standard-dose acyclovir in HSV-2/HIV-1 coinfected persons in which we measured the effect of therapy on both plasma HIV-1 RNA and genital HSV shedding.
Methods
Study Population
We recruited HIV-1/HSV-2 coinfected patients in Seattle, WA, between January 2008 and June 2010. Inclusion criteria were an age ≥18 years, detectable plasma HIV-1 RNA, the intention to remain in the area, and no current or planned initiation of ART during the study. Exclusion criteria were prior adverse reaction to acyclovir, valacyclovir, or famciclovir, current or planned open-label use of antivirals with anti-HSV activity, including ganciclovir, foscarnet, and cidofovir, active or prior CMV disease, history of seizures, creatinine >1.5 mg/dL, serum transaminases >3 times the upper limit of normal, hematocrit <30%, absolute neutrophil count (ANC) <1000/μL, platelet count <75,000/μL, history of thrombotic microangiopathy, and pregnancy.
Study Design
Participants underwent randomization in a 1:1 ratio to receive open-label acyclovir 400 mg twice daily or valacyclovir 1000 mg twice daily. The randomization sequence, developed by the study statistician (AM), was implemented using sealed study kits. After 12 weeks on therapy, participants underwent a two week washout period and then crossed over to the alternative treatment arm for 12 weeks.
Clinical and Laboratory Procedures
At the screening visit, HSV-2 seropositivity was determined by Western blot.23 HIV-1 Western blot, plasma HIV-1 RNA PCR, CD4 count, and routine clinical safety labs were also performed. Testing for Neisseria gonorrhoeae and Chlamydia trachomatis was performed by culture of rectal swabs from men and by nucleic acid amplification testing of urine samples from men and cervical swabs from women; participants who tested positive were treated and became eligible for enrollment 14 days after completing therapy.
Participants provided daily self-collected genital and perianal swabs for HSV PCR using previously described methods,24,25 and maintained a diary of genital symptoms for the first four weeks of each treatment period.26 DNA from 200 μl of PCR buffer surrounding each swab was extracted with a QIAamp 96 DNA Blood Kit (Qiagen) following the manufacturer’s recommendations. HSV gB specific Taqman quantitative real-time PCR was performed using QuantiTect multiplex PCR master mix (Qiagen) on a 7900HT sequencing detection system. The sequences of HSV gB primers and probe were previously described.27 An internal control was included in each reaction to monitor for false negative results due to inhibition of the PCR assay.28 We reported as positive those samples in which ≥150 copies of HSV DNA/mL were detected.29 Weekly plasma samples were collected for HIV-1 RNA quantification by a TaqMan RT-PCR assay.30 The lower limit of quantification was 50 copies of HIV-1 RNA/mL. Participants who, on screening, had detectable plasma HIV-1 RNA below the limit of quantification were retained in the analysis. Plasma CMV DNA was also quantified by a CMV-specific TaqMan PCR assay; we reported samples in which ≥50 copies of CMV DNA/mL were detected as positive.28 Laboratory staff were not aware of treatment assignments. At each visit we assessed symptoms of genital herpes reactivation, medication tolerance, and adherence by pill counts. At the end of each study arm, participants’ creatinine, CD4 count, and complete blood count were measured. Throughout the study, condoms, risk reduction, and adherence counseling were provided.
To more precisely assess the kinetics of plasma HIV-1 RNA decline after initiation of valacyclovir, beginning in April 2010, we invited participants, including those who already completed the study, to participate in a substudy which measured plasma HIV-1 RNA one day prior to, at initiation, and at 6, 24, 48, and 72 hours after initiating valacyclovir.
Sample Size Calculation and Statistical Analysis
We estimated that a sample size of 29 participants would be required to detect a 0.25 log10 copies/mL difference10,11 in plasma HIV-1 RNA between the study arms and a sample size of 38 participants, with four weeks of daily genital swabs per treatment arm, would be required to detect a decrease in the HSV shedding rate from 4%, the rate expected on standard suppressive therapy,13 to 1%, with 80% power, at a two-sided type I error rate of 5%. We have recently validated a more accurate method of sample size computation for crossover shedding studies.31 This rigorously validated calculation, which accounts for person-to-person variability in shedding over time, required 26 participants to detect a 50% reduction in genital HSV shedding.
The co-primary endpoints of the study were the plasma HIV-1 RNA level and genital HSV shedding rate during high-dose valacyclovir compared with standard-dose acyclovir administration. Secondary endpoints included days with genital herpes lesions and the quantity of HSV-2 DNA from genital swabs during shedding episodes on each arm. Plasma HIV-1 RNA and genital HSV DNA levels were log10 transformed to stabilize variance. The genital shedding rate was defined as the number of swabs with HSV divided by the number of swabs collected. A genital shedding episode was defined as a consecutive series of positive genital swabs separated by at least 2 negative swabs. Analyses were modified intent-to-treat, including those participants who contributed at least one specimen on both arms. The first day of treatment and all washout days were excluded from analysis. Participants who initiated ART were censored at that time.
Linear mixed models were used to compare plasma HIV-1 RNA and HSV-2 DNA quantity from genital swabs by treatment arm. We explored whether the effect of valacyclovir compared with acyclovir on plasma HIV-1 RNA levels was modified, in separate models, by baseline plasma HIV-1 RNA level, CD4 count, or detection of CMV viremia by testing the significance of interaction terms. Genital HSV-2 shedding rates were compared by treatment arm using random effects poisson regression. The Wilcoxon signed-rank test was used to compare the total number of genital shedding episodes, days with genital HSV-2 lesions, and drug adherence rate by treatment arm. Cox regression analysis, accounting for multiple episodes per participant,32 was used to compare episode duration by treatment arm. Period effects on plasma HIV-1 RNA and genital HSV-2 shedding rates were assessed by testing the significance of session adjusted for treatment arm. Carryover effects were evaluated by testing the interaction of treatment and session. Correlation of drug adherence on each treatment arm with change in mean plasma HIV-1 RNA levels from baseline and genital HSV-2 shedding rates was evaluated with Spearman correlation coefficients.
First-phase viral decay slopes were determined for substudy participants, for whom plasma HIV-1 RNA levels were assessed more frequently over the first three days of valacyclovir treatment. Previous studies of HIV-1 viral decay after antiretroviral administration have observed an initial static phase prior to the exponential decline in the first phase of viral decay.33-35 Viral decay slopes, determined by linear regression using robust variance estimates in clusters, consequently included data points beginning at 24 hours.
The protocol was approved by the University of Washington Human Subjects Review Committee and participants gave written informed consent. The study was funded by a grant to the University of Washington from GlaxoSmithKline; additional funding came from grants from the National Institutes of Health. The University of Washington investigators wrote the protocol, conducted the study, analyzed the data, and prepared the manuscript for publication.
Results
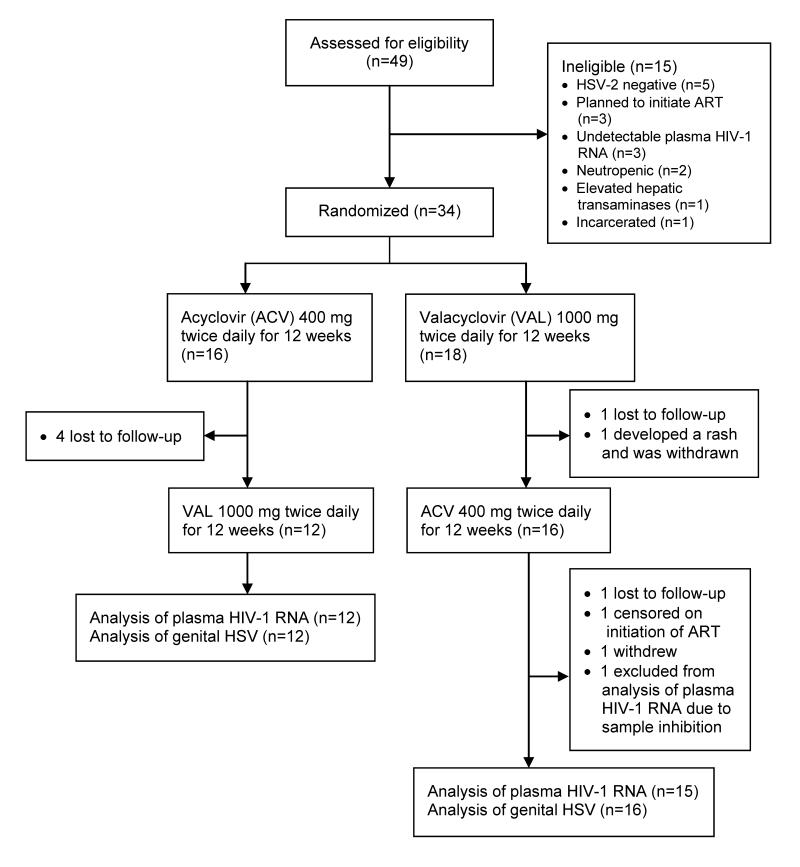
Of forty-nine persons screened, fifteen were found to be ineligible for reasons including lack of HSV-2 infection (n=5), plans to initiate ART (n=3), and undetectable plasma HIV-1 RNA (n=3). Of the thirty-four persons enrolled, three (8.8%) participants were treated for Chlamydia, including one participant who was treated for both Chlamydia and Gonorrhea, prior to enrollment. Six participants did not contribute to both arms of the study. Thus, the final analysis set included twenty-eight participants, of whom twelve (43%) initiated acyclovir and sixteen (57%) initiated valacyclovir first (Figure 1). The mean age of participants was 44 (range, 24-46); eighteen (64%) were Caucasian and twenty-three (82%) were men (Table 1). Overall, from participants during treatment with acyclovir or valacyclovir, we obtained and analyzed 594 plasma samples for HIV-1 RNA quantification, for 570 of which we were also able to measure CMV DNA, and 1425 genital swabs for HSV DNA quantification.
Figure 1.
Enrollment and follow-up of participants. ART: Antiretroviral therapy.
Table 1.
Demographic and clinical characteristics of the study population.
| Characteristic | Acyclovir, followed by valacyclovir (N=12) |
Valacyclovir, followed by acyclovir (N=16) |
Total (N=28) |
|---|---|---|---|
| Age, mean (range), years | 43 (24-66) | 45 (29-66) | 44 (24-66) |
| Sex, N (%) | |||
| Male | 8 (67) | 15 (94) | 23 (82) |
| Female | 4 (33) | 1 (6) | 5 (18) |
| Race, N (%) | |||
| Caucasian | 8 (67) | 10 (63) | 18 (64) |
| African-American | 3 (25) | 5 (31) | 8 (29) |
| Other | 1 (8) | 1 (6) | 2 (7) |
| HSV Serology, N (%) | |||
| HSV-2+ | 3 (25) | 6 (37) | 9 (32) |
| HSV-1/2+ | 9 (75) | 10 (63) | 19 (68) |
| CD4 Count at Screening, Mean (Range), cells/μL | 627 (359-894) |
424 (199-1055) |
511 (199-1055) |
| Plasma HIV-1 RNA at Screening, Mean (Range), log10 copies/mL |
3.3 (1.2-5.5) |
4.3 (3.5-5.1) |
3.9 (1.2-5.5) |
Effect of Study Drug on Plasma HIV-1 RNA
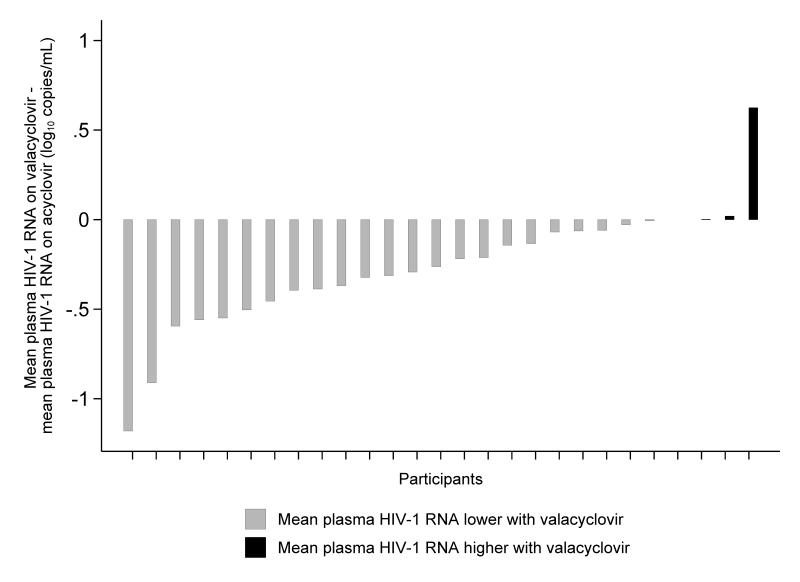
Twenty-seven participants had plasma HIV-1 RNA levels available for analysis, as samples for one participant were persistently inhibited. Of these participants, twenty-three (85%) had a mean plasma HIV-1 RNA level lower on valacyclovir than acyclovir (Figure 2 and 3). Plasma HIV-1 RNA during treatment was 0.27 log10 copies/mL lower (95% CI, −0.41 to −0.14 log10 copies/mL; p<0.001) on valacyclovir compared with acyclovir (Table 2). Sixteen (59%) of twenty-seven participants on valacyclovir and five (20%) of twenty-five participants on acyclovir achieved a decrease in mean plasma HIV-1 RNA by ≥0.25 log10 copies/mL relative to baseline. No association between genital HSV shedding and plasma HIV-1 RNA was detected. When the genital HSV shedding rate on drug was included in the model, there was no change in the results: plasma HIV-1 RNA during treatment was 0.27 log10 copies/mL lower on valacyclovir compared with acyclovir (95% CI, −0.41 to −0.14 log10 copies/mL; p<0.001). We found no evidence that the baseline HIV-1 RNA level, CD4 count, or detection of CMV viremia modified the effect of valacyclovir compared with acyclovir on plasma HIV-1 RNA (Table 2). Because the mean plasma HIV-1 RNA was higher in participants who started in the valacyclovir arm, evaluation for period effect was adjusted for baseline plasma HIV-1 RNA on each drug arm; no period (p=0.27) or carry-over (p=0.39) effects were found. One participant received interferon and ribavirin during the acyclovir arm for Hepatitis C treatment. When this participant was excluded from the analysis, plasma HIV-1 RNA during treatment was 0.31 log10 copies/mL lower (95% CI, −0.43 to −0.19 log10 copies/mL; p<0.001) on valacyclovir compared with acyclovir. Two participants in the analysis had plasma HIV-1 RNA below the limit of quantification (<50 copies/ml); when these participants were excluded the results were similar.
Figure 2.
Difference in mean plasma HIV-1 RNA level between valacyclovir and acyclovir per participant, ordered by the magnitude of difference.
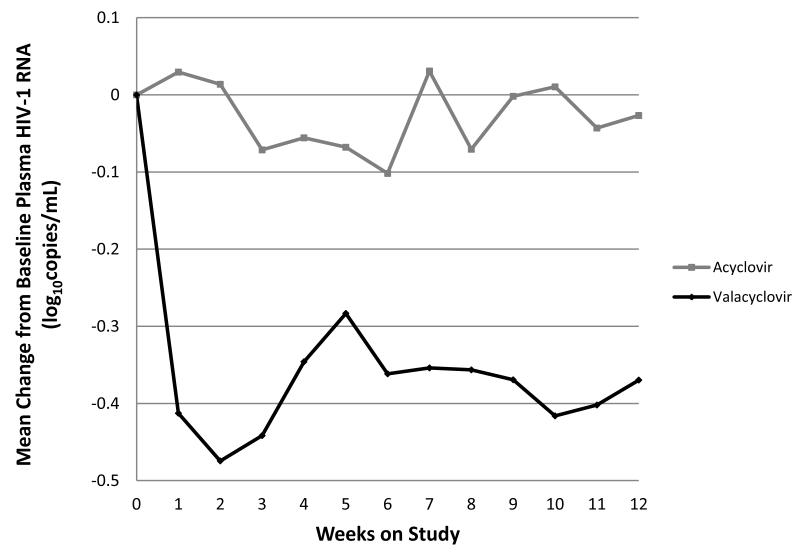
Figure 3.
Mean change from baseline plasma HIV-1 RNA over time on valacyclovir compared with acyclovir.
Table 2.
Difference in plasma HIV-1 RNA during treatment with valacyclovir and acyclovir.
| N | Plasma HIV-1 RNA during treatment with valacyclovir – plasma HIV-1 RNA during treatment with acyclovir (log10 copies/mL) |
P- value |
|
|---|---|---|---|
| All participants | 27 | −0.27 (−0.41, −0.14) | <0.001 |
| CD4 count (cells/μL) | 0.75 | ||
| <350 | 5 | −0.24 (−0.56, 0.08) | |
| 350-499 | 11 | −0.23 (−0.44, −0.01) | |
| ≥500 | 11 | −0.35 (−0.79, 0.10) | |
| Baseline HIV-1 RNA (copies/mL) |
0.80 | ||
| <50,000 | 21 | −0.26 (−0.42, −0.10) | |
| ≥50,000 | 6 | −0.30 (−0.57, −0.03) | |
| Any CMV viremia during study |
0.34 | ||
| No | 22 | −0.24 (−0.39, −0.09) | |
| Yes | 5 | −0.41 (−0.72, −0.09) |
Effect of Study Drug on Genital HSV Shedding
Twenty-eight participants collected swabs of genital skin and mucosa during both study arms. The HSV shedding rate did not differ between study arms: HSV was detected on 7.8% of days (55 of 707) on valacyclovir and 8.2% of days (59 of 718) on acyclovir (RR, 0.95; 95% CI, 0.66-1.37; p=0.78). There were 19 HSV shedding episodes on acyclovir and 18 on valacyclovir. No significant difference was seen in episode duration by treatment arm (p=0.68). The median quantity of HSV-2 DNA for positive samples was 3.0 log10 copies/mL on both arms (range, acyclovir, 2.2 to 6.4, valacyclovir, 2.2 to 6.8 log10 copies/mL; p=0.67). Genital lesions were present on 7 (1.0%) of 772 days, occurring in only one participant during the valacyclovir arm, compared with 27 (4.0%) of 750 days, occurring in two participants during the acyclovir arm (p=0.16). 93% of participants did not report any genital lesion throughout the study and 94% of all shedding episodes were asymptomatic. There was a significant period effect such that rates of HSV-2 shedding were 76% (range, 60 - 85%) lower during the second treatment arm compared to the first treatment arm regardless of treatment given (p<0.001). There was no significant drug carry-over (p=0.58). When the participant receiving interferon and ribavirin was excluded from analysis, we still found no significant difference in the rate of HSV detection on valacyclovir compared with acyclovir (RR: 0.82; 95% CI, 0.55-1.23; p=0.33).
Effect of Study Drug on Plasma CMV DNA
CMV DNA was detected in six plasma samples from five participants during treatment with acyclovir and six samples from two participants during treatment with valacyclovir. The mean quantity of CMV DNA for positive samples was 2.00 log10 copies/mL during acyclovir and 1.99 log10 copies/mL during valacyclovir treatment.
Valacyclovir Substudy
Ten participants completed the substudy, during which plasma was collected for HIV-1 RNA more frequently for the first 72 hours after valacyclovir initiation. Two participants had plasma HIV-1 RNA <50 copies/mL at the time of valacyclovir initiation and were excluded from analysis. The remaining eight participants were all men. The mean age was 47 years (range, 29-66 years). At enrollment, the mean CD4 count was 410 cells/μL (range, 242-714 cells/μL) and the mean plasma HIV-1 RNA level was 4.10 log10 copies/mL (range, 3.57 to 4.97 log10 copies/mL). Seven of the eight participants experienced a decline in plasma HIV-1 RNA between 24 and 72 hours (Figure 4; see Supplemental Digital Content). On average, plasma HIV-1 RNA decreased by −0.20 log10 copies/mL/day (95% CI, −0.38 to −0.19 log10 copies/mL/day).
Adherence to Study Drug
The median adherence rate was 93% on valacyclovir (range, 34-100%) and 96% on acyclovir (range, 75-100%; p=0.23). We did not find a correlation between drug adherence and genital HSV shedding on valacyclovir (r=0.02) or acyclovir (r=-0.16), nor between adherence and the change in mean plasma HIV-1 RNA from baseline on valacyclovir (r = −0.10) or acyclovir (r = 0.06). One participant took additional doses of acyclovir for three days during the acyclovir arm. Two participants used valacyclovir during the acyclovir arm, one for twelve days and the other for nine days. Both acyclovir and valacyclovir were taken by one participant during the washout period, stopping the day before starting the valacyclovir arm. All participants were included in the analysis according to their assigned treatment group.
Adverse Reactions
One participant developed neutropenia during the acyclovir arm, with a nadir ANC of 570 cells/μL, thought to be related to concomitant interferon use. With cessation of interferon therapy, the ANC normalized and remained normal during 12 weeks of high-dose valacyclovir. One participant developed urticaria after 2.5 weeks of valacyclovir and was discontinued from the study; this participant was not included in the analysis since he did not participate in the acyclovir arm. Two participants with depression developed exacerbations during the valacyclovir arm, one of whom was hospitalized for two weeks and did not follow-up during this period. Both participants were included in the analysis.
Discussion
In our study, valacyclovir, dosed at 1000 mg twice daily, reduced plasma HIV-1 RNA levels by 0.27 log10 copies/mL more than standard-dose acyclovir (400 mg twice daily) in HIV-1/HSV-2 seropositive persons not on ART. We did not, however, find a difference in the rate of genital HSV shedding between the two arms and the decrease in plasma HIV-1 RNA was unchanged after adjusting for genital HSV reactivation, suggesting that the differential effect of high-dose valacyclovir on HIV-1 RNA is not mediated by greater HSV-2 suppression.
The absence of differential effect of high-dose valacyclovir compared with standard-dose acyclovir on genital HSV shedding contrasts with the suggestion of greater efficacy of valacyclovir 500 mg twice daily compared with standard-dose acyclovir in another study of HIV-1 infected patients.36 It is consistent, however, with a prior study of immunocompetent hosts that showed no difference in HSV suppression by valacyclovir 500 mg twice daily compared with standard-dose acyclovir.26
The median adherence rate was 93% during the valacyclovir arm and 96% during the acyclovir arm. Imperfect adherence would be expected to attenuate the effect size; the genital HSV shedding rate found in this study, however, was comparable to the rates of 4-14% found in prior studies of HIV-1/HSV-2 coinfected persons treated with HSV suppressive therapy.13,37,38 No correlation was found between adherence and HSV shedding or reduction in plasma HIV-1 RNA.
Recent studies have raised the question of whether acyclovir directly inhibits HIV-1 replication. After phosphorylation of acyclovir by kinases of human herpesviruses (HHV)20 and possibly non-HHV kinases,39 and subsequent phosphorylation by cellular kinases, acyclovir triphosphate may competitively inhibit HIV-1 reverse transcriptase as a guanosine-5′triphosphate analog chain terminator.20,21 Previous studies of HSV suppressive therapy with standard-dose acyclovir and valacyclovir have demonstrated a similar decrease in plasma HIV-1 RNA (0.25-0.53 log10 copies/mL) compared with the response seen with 24 weeks of AZT monotherapy.40 If acyclovir is a reverse transcriptase inhibitor, the development of reverse transcriptase resistance mutations with acyclovir monotherapy would be expected. Such resistance has been seen in vitro with the development of V75I, T69N, and M184I reverse transcriptase mutations.21 These mutations have not been recognized in patients treated with prolonged administration of suppressive doses of acyclovir and valacyclovir in the absence of ART.41,42 The decrease in plasma HIV-1 RNA as a result of suppressive acyclovir therapy has also been sustained over 24 months,41 which would be unexpected with acyclovir monotherapy, or any other reverse transcriptase inhibitor when given alone. A potential explanation for this discrepancy is that, in vivo, the fitness cost associated with these resistance mutations may outweigh the survival advantage of acyclovir resistance when acyclovir is provided at standard suppressive doses.41,42
The rate of decline of plasma HIV-1 RNA in the first week of treatment with high-dose valacyclovir is smaller than for a potent reverse transcriptase inhibitor such as tenofovir, where the first phase viral decay slope is 0.32-0.39 log10 copies/mL/day when provided as monotherapy.33 We also found considerable variability among participants in the decline in plasma HIV-1 RNA achieved with valacyclovir and acyclovir, in contrast to patients treated with antiretrovirals. Consequently, the mechanism of in vivo anti-HIV-1 effect has yet to be clarified.
A recent study found that HIV progression was delayed preferentially among patients receiving suppressive acyclovir with plasma HIV-1 RNA ≥50,000 copies/mL.17 Although we did not find that baseline plasma HIV-1 RNA ≥50,000 vs. <50,000 log10 copies/mL modified the effect of valacyclovir compared with acyclovir on plasma HIV-1 RNA, inference is limited by the small number of participants in our study with plasma HIV-1 RNA ≥50,000 log10 copies/mL. If persons with a high plasma HIV-1 viral load do experience a greater decline in plasma HIV-1 RNA with anti-HSV therapy, it is possible that the relatively low mean baseline plasma HIV-1 RNA in this study (3.89 log10 copies/mL compared with 4.0 to 4.6 log10 copies/mL in other studies of the effect of anti-HSV therapy on plasma HIV-1 RNA)8-13,22,43 may have contributed to the decreased effect size observed in comparison to previous studies.8-13 As most previous trials occurred in developing countries, however, many other differences between the populations may impact the effect of anti-HSV therapy on plasma HIV-1 RNA.
Despite decreasing plasma HIV-1 RNA by 0.25 log10 copies/mL, standard-dose acyclovir was not found to decrease HIV-1 transmission.10 We do not know the optimal dose of anti-HSV therapy to decrease plasma HIV-1 RNA, but it appears that there is a dose-response based on this study and the recent publication by Mugwanya et al.,22 where a 50% higher dose of valacyclovir (1.5 g twice daily) was associated with a 0.62 log10 copies/mL reduction in plasma HIV-1 RNA compared with standard-dose acyclovir. The higher dose of valacyclovir may be required for it to act as a more potent antiretroviral21 or for it to adequately suppress the effect of other herpesviruses on up-regulating HIV-1 transcription. We explored whether the difference in plasma HIV-1 RNA achieved with high-dose valacyclovir vs. acyclovir was greater among the participants in whom CMV viremia was detected compared to participants in whom it was not; the difference was not statistically significant. Inference is limited by the small number of participants with CMV viremia during the study and reliance on CMV viremia as a surrogate for CMV reactivation. To further investigate the potential role of CMV, plasma HIV-1 RNA on acyclovir and high-dose valacyclovir could be compared in CMV seronegative and CMV seropositive persons; but we acknowledge that enrollment into such a study would be challenging.
Whether the greater decline in HIV-1 RNA achieved by high-dose valacyclovir would translate into a more substantial delay of HIV progression than was observed with acyclovir,16,17 or would provide sufficiently potent reduction that could reduce HIV-1 transmission, is not known. Mathematical modeling has suggested that a decrease in mean plasma HIV-1 RNA by 0.74 log10 copies/mL could decrease heterosexual HIV-1 transmission by 50%.44 While the provision of ART to all HIV-positive persons irrespective of CD4 count is the ideal approach to reduce HIV-1 transmission since treatment decreases transmission by at least 96%,1 at the end of 2010, it is estimated that less than half of those who met WHO guidelines for initiation of ART (CD4<350 cells/μL) were receiving antiretroviral medications7 and few national HIV treatment guidelines currently recommend universal ART.45 Until we can successfully expand ART access, high-dose valacyclovir, if demonstrated to decrease HIV transmission by persons not yet eligible for ART, could offer a provisional means to decrease the number of new HIV infections. Future studies, in persons on ART with suppressed plasma HIV-1 RNA, could also assess whether high-dose anti-HSV therapy could reduce genital HIV-1 RNA shedding, which may be a potential transmission risk in persons with suppressed plasma viral load.46
Our findings are limited by high loss to follow-up, which is especially problematic in cross-over studies and decreases power. HIV-1 infected patients with detectable plasma HIV-1 RNA are becoming increasingly difficult to enroll in studies in developed countries where antiretrovirals are available and prescribed early in the course of HIV-1 disease to decrease associated morbidity and mortality, prevent transmission to sexual partners, and reduce community viral load.47 Since persons who remain viremic and not on treatment are often those with psychosocial comorbidities, such as serious mental illness, substance abuse, and poverty,48-50 maintaining high adherence to study protocol has become challenging in this population. As we collected swabs on a daily basis, we may have underestimated the frequency of shedding by missing episodes <24 hours in duration,25 although this would be expected to similarly affect both arms. The cross-over design of this trial, with each participant serving as their own control, increased efficiency and decreased potential confounding. This design also mitigated potential ramifications of the period effect that we found for HSV-2 shedding rates, since in the second treatment period, each group received a different drug.
By simultaneous measurement of daily genital HSV DNA in each participant, this study demonstrates that the incremental reduction in plasma HIV-1 RNA achieved by high-dose valacyclovir compared with standard-dose acyclovir is not mediated by greater genital HSV suppression. Further research is needed to determine the characteristics that modify the effect of anti-HSV therapy on HIV-1 RNA and to determine the potential population-level impact of high-dose valacyclovir on HIV-1.
Supplementary Material
Figure 4. Plasma HIV-1 RNA decline during the first 72 hours of valacyclovir treatment for substudy participants.
Acknowledgments
Sources of Support: This work was supported by a grant to the University of Washington from GlaxoSmithKline. Additional support was provided by the following grants from the National Institutes of Health: P01 AI30731 (A.W., L.C.), K24 AI071113 (A.W.), and T32 AI07140 (T.P.).
Footnotes
ClinicalTrials.gov number, NCT00527618
Presented in part: July 2011 at the 19th Meeting of the International Society for Sexually Transmitted Diseases Research (ISSTDR), Quebec City, Canada.
COI Statement: TP, MS, JB, KD, NO, MH, SS: No conflicts.
CJ is a research investigator for AiCuris GmbH.
AM is a consultant for Immune Design Corp.
LC is a consultant for AiCuris GmbH, is the head of the scientific advisory board for and holds stock (<1% of company) in Immune Design Corp, and is listed as a co-inventor in several patents describing antigens and epitopes to which T-cell responses to HSV-2 are directed.
AW is a consultant for AiCuris GmbH and has received research funding from GSK, Agenus, Gilead Sciences and Genocea Biosciences.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Bunnell R, Ekwaru JP, Solberg P, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20(1):85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 4.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Romero J, Castilla J, Hernando V, et al. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access, Progress Report 2011. WHO; 2011. [Google Scholar]
- 8.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356(8):790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198(12):1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23(4):461–469. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49(1):77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196(10):1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 14.Ludema C, Cole SR, Poole C, et al. Meta-analysis of randomized trials on the association of prophylactic acyclovir and HIV-1 viral load in individuals coinfected with herpes simplex virus-2. AIDS. 2011;25(10):1265–1269. doi: 10.1097/QAD.0b013e328347fa37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnabas RV, Webb EL, Weiss HA, et al. The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS. 2011;25(13):1559–1573. doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375(9717):824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12(6):441–8. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriuchi M, Moriuchi H, Williams R, et al. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. Virology. 2000;278(2):534–540. doi: 10.1006/viro.2000.0667. [DOI] [PubMed] [Google Scholar]
- 19.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8(8):490–497. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 20.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4(3):260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283(46):31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugwanya K, Baeten JM, Mugo NR, et al. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis. 2011;204(12):1912–1917. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley RL, Militoni J, Lee F, et al. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26(4):662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305(14):1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J Acquir Immune Defic Syndr. 2010;54(5):482–488. doi: 10.1097/QAI.0b013e3181d91322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 27.Jerome KR, Huang ML, Wald A, et al. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40(7):2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42(3):1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magaret AS, Wald A, Huang ML, et al. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45(5):1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coombs RW, Lockhart D, Ross SO, et al. Lower genitourinary tract sources of seminal HIV. J Acquir Immune Defic Syndr. 2006;41(4):430–438. doi: 10.1097/01.qai.0000209895.82255.08. [DOI] [PubMed] [Google Scholar]
- 31.Magaret A, Stanaway J. Sample size for a binomial rate with autocorrelation. Stat Comm Infect Dis. 2011;3(1):1–20. doi: 10.2202/1948-4690.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei L, Lin D, Weissfeld L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 33.Louie M, Hogan C, Hurley A, et al. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS. 2003;17(8):1151–1156. doi: 10.1097/00002030-200305230-00006. [DOI] [PubMed] [Google Scholar]
- 34.Ho DD, Neumann AU, Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 35.Perelson A, Neumann A, Markowitz M, et al. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 36.Conant MA, Schacker TW, Murphy RL, et al. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13(1):12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 37.Cowan FM, Pascoe SJ, Barlow KL, et al. A randomised placebo-controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV-1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sex Transm Infect. 2008;84(7):548–553. doi: 10.1136/sti.2008.031153. [DOI] [PubMed] [Google Scholar]
- 38.Tanton C, Weiss HA, LeGoff J, et al. Patterns of herpes simplex virus shedding over 1 month and the impact of acyclovir and HIV in HSV-2-seropositive women in Tanzania. Sex Transm Infect. 2011;87(5):406–411. doi: 10.1136/sti.2010.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon MA, Parsons TL, Shen L, et al. Consistent inhibition of HIV-1 replication in CD4+ T cells by acyclovir without detection of human herpesviruses. J Virol. 2011;85(9):4618–4622. doi: 10.1128/JVI.02423-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Engl J Med. 1995;333(25):1662–1669. doi: 10.1056/NEJM199512213332502. [DOI] [PubMed] [Google Scholar]
- 41.Baeten JM, Lingappa J, Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011;203(1):117–121. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HN, Wang J, Hughes J, et al. Effect of acyclovir on HIV-1 set point among herpes simplex virus type 2-seropositive persons during early HIV-1 infection. J Infect Dis. 2010;202(5):734–738. doi: 10.1086/655662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacker T, Zeh J, Hu H, et al. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186(12):1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 44.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of HIV-1 plasma RNA reductions on heterosexual HIV-1 transmission risk. PLOS ONE. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antiretroviral treatment as prevention of HIV and TB: 2012 update. WHO; 2012. [Google Scholar]
- 46.Sheth PM, Yi TJ, Kovacs C, et al. Mucosal correlates of isolated HIV semen shedding during effective antiretroviral therapy. Mucosal Immunol. 2012;5(3):248–257. doi: 10.1038/mi.2012.1. [DOI] [PubMed] [Google Scholar]
- 47.Panel on Antiretroviral Guidelines for Adults and Adolescents [March 27, 2012];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 48.Andersen R, Bozzette S, Shapiro M, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Serv Res. 2000;35(2):389–416. [PMC free article] [PubMed] [Google Scholar]
- 49.Himelhoch S, Chander G, Fleishman JA, et al. Access to HAART and utilization of inpatient medical hospital services among HIV-infected patients with co-occurring serious mental illness and injection drug use. Gen Hosp Psychiatry. 2007;29(6):518–525. doi: 10.1016/j.genhosppsych.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng EH, Hare CB, Kahn JO, et al. The Effect of a “Universal Antiretroviral Therapy” Recommendation on HIV RNA Levels Among HIV-Infected Patients Entering Care With a CD4 Count Greater Than 500/μL in a Public Health Setting. Clin Infect Dis. 2012;55(12):1690–1697. doi: 10.1093/cid/cis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 4. Plasma HIV-1 RNA decline during the first 72 hours of valacyclovir treatment for substudy participants.