Abstract
Aims
Conflicting evidence exists regarding whether obesity is independently associated with coronary artery calcium (CAC), a measure of coronary atherosclerosis. We examined an independent association of obesity with prevalent CAC among samples of multi-ethnic groups whose background populations have varying levels of obesity and coronary heart disease (CHD).
Methods and results
We analysed a population-based sample of 1212 men, aged 40–49 years free of clinical cardiovascular disease recruited in 2002–06; 310 Japanese in Japan (JJ), 294 Koreans in South Korea (KN), 300 Japanese Americans (JA), and 308 Whites in the USA (UW). We defined prevalent CAC as an Agatston score of ≥10. Prevalent CAC was calculated by tertile of the body mass index (BMI) in each ethnic group and was plotted against the corresponding median of tertile BMI. Additionally, logistic regression was conducted to examine whether an association of the BMI was independent of conventional risk factors. The median BMI and crude prevalence of CAC for JJ, KN, JA, and UW were 23.4, 24.4, 27.4, and 27.1 (kg/m2); 12, 11, 32, and 26 (%), respectively. Despite the absolute difference in levels of BMI and CAC across groups, higher BMI was generally associated with higher prevalent CAC in each group. After adjusting for age, smoking, alcohol, hypertension, lipids, and diabetes mellitus, the BMI was positively and independently associated with prevalent CAC in JJ, KN, UW, but not in JA.
Conclusion
In this multi-ethnic study, obesity was independently associated with subclinical stage of coronary atherosclerosis among men aged 40–49 years regardless of the BMI level.
Keywords: Coronary artery calcium, Obesity, Body mass index, Multi-ethnic, Men, Risk factors
Introduction
The relationship between obesity and coronary heart disease (CHD) has been well described, and many researchers consider obesity as an independent risk factor for clinical CHD. However, others remain wary of this viewpoint as some of the well-established CHD risk factors, such as hypertension and diabetes mellitus, are jointly associated with obesity and thus may confound the true relationship between obesity and CHD.1,2 Coronary artery calcium (CAC) is a biomarker for subclinical coronary atherosclerosis.3,4 The Agatston score, which was developed to quantify the extent of CAC,5 is shown to correlate closely with the volume of coronary artery plaque measured at autopsy6 and is considered a surrogate for the overall coronary-plaque burden.7,8 However, the relationship between obesity and CAC remains to be studied in depth as the available data document conflicting results.9–18 Furthermore, most relevant studies were conducted in Western populations in which CHD and obesity are more prevalent relative to other populations, such as East Asians.19–22 Little is known, therefore, about whether obesity is independently associated with CAC among leaner populations.
We have previously reported that the prevalence of CAC among Japanese men in Japan was significantly lower than that in Japanese Americans (JA) and Whites in the USA (UW).23,24 We also found that Japanese men in Japan had lowest of body mass index (BMI) levels of the three groups studied.25 Thus, we were motivated to examine the relationship between obesity and CAC among multi-ethnic groups. The objective of this study is to examine the relationship of the BMI, a commonly used measure of obesity, to prevalent CAC among four ethnic groups of individuals free of clinical cardiovascular disease; Japanese in Japan (JJ), Koreans in South Korea (KN), JA, and UW using the standardized protocol including the measurement of CAC. As these populations have different levels of the BMI,19,21,22,26 a comparison would provide important clue about the role of obesity in subclinical stage of coronary atherosclerosis.
Methods
Study population
Detailed methods for the enrolment of the study participants were reported elsewhere.23,24,27 In brief, we randomly selected population-based samples of 1228 men aged 40–49 years between 2002 and 2006 from four centres: 313 Japanese from Kusatsu City, Shiga, JJ; 302 Koreans from Ansan City, Gyeonggi-do, KN; 303 JA in Honolulu, Hawaii, USA; and 310 UW from Allegheny County, Pennsylvania, USA. None of the participants had clinical cardiovascular disease, type I diabetes, or other severe diseases.25 Of those, we excluded 16 individuals from the present study due to missing pertinent variables, leaving 1212 for analysis (310 JJ, 295 KN, 300 JA, and 307 UW). Informed consent was obtained from all participants. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and it was approved by the Institutional Review Board of each study centre.
CAC measurement
The detailed scanning procedures with electron-beam computed tomography were described elsewhere.24 In brief, scanning was performed using a standardized protocol with a GE-Imatron C150 EBT scanner (GE Medical Systems, South San Francisco, CA, USA). Images were obtained from the level of the aortic root to the apex of the heart. We considered CAC to be present with three contiguous pixels (area = 1 mm2) ≥130 Hounsfield units. One trained reader at the Cardiovascular Institute, University of Pittsburgh, read the images using a Digital Imaging and Communications in Medicine workstation and software by the AccuImage Diagnostic Corporation (San Francisco, CA, USA), which calculates the coronary calcium score with the Agatston scoring method5 (Agatston score). To define the presence of CAC, the Agatston score ≥10 was used. We selected this cut-off point of 10 because of (i) its clinical significance,28 (ii) the possibility that scores ranging from >0 to <10 could be an imaging artefact from spurious noise,29 and (iii) our intention to keep the consistent cut-off point with our previous studies.23,24 The reader was blinded to participant's characteristics and the study centres. The reproducibility of the electron-beam computed tomography scans had an intra-class correlation of 0.98.25
Other measurements
Body weight and height were measured while the participant was wearing light clothing without shoes. Waist circumference measured twice at the umbilical level at the end of the exhalation phase while the participant was standing upright, and the mean of the two measurements was used for analysis. Blood pressure was measured twice consecutively in the right arm of the seated participant after he sat quietly for 5 min, using an automated sphygmomanometer (BP-8800; Colin Medical Technology, Komaki, Japan). The average of the two measurements was used. Venipuncture was performed early in the clinic visit after a 12-h fast. The samples from remote sites were shipped on dry ice to the University of Pittsburgh. Serum lipids were determined with standardized methods according to the Centers for Disease Control and Prevention. We estimated low-density lipoprotein cholesterol (LDLc) by the Friedewald equation for individuals with triglycerides <4.52 mmol/L (400 mg/dL). Otherwise, we directly measured LDLc using an automated spectrophotometric assay, LDL Direct Liquid Select (Equal Diagnostics, Exton, PA, USA). Serum glucose was determined using a hexokinase, glucose-6-phosphate-dehydrogenase, and enzymatic assay.
A self-administered questionnaire was used to obtain information on demography, including smoking habits (never, past, and current), alcohol drinking (never, past, and current), use of medication(s) for elevated blood pressure, lipids, and diabetes mellitus. For drinking, qualitative information was further queried as whether the participant drank beer, wine, liquor, sake (Japanese rice wine), or other alcoholic beverages. Quantity and frequency were noted. Then, ethanol consumption (gram per day) was estimated, assuming that concentrations of alcohol were 5% for beer, 12% for wine, 40% for liquor, and 16% for sake.24 Diabetes mellitus was defined as the use of anti-diabetic medication(s) or fasting glucose ≥7.0 mmol/L (126 mg/dL). Hypertension was defined as the use of anti-hypertensive medication(s) or systolic/diastolic blood pressure ≥140/90 mmHg.
Statistical analysis
Crude and adjusted estimates of prevalent CAC (%) for each group was calculated following the standard analysis of covariance techniques and binary logistic regression models.30 We first divided each ethnic group into tertiles according to the BMI (kg/m2), and then plotted the median BMI of each tertile against the corresponding CAC prevalence. Secondly, we performed logistic regression to obtain multi-variable-adjusted odds ratios (ORs) of prevalent CAC in reference to first BMI tertile for each ethnic group (‘tertile analyses’). Thirdly, we conducted separate logistic regression analyses to obtain standardized coefficients (i.e. ORs per 1SD increase) of the BMI and other continuous risk factors within, but not across, ethnic group(s). This was because we aimed to assess the magnitude of association of the BMI with CAC relative to other risk factors within each ethnic group.
We constructed the following models with regard to adjustment. In Model 1, we adjusted for age (years) only. In Model 2, we further adjusted for smoking status (never, past, and current), alcohol drinking status (never, past, current with <23 g/day of alcohol, current with ≥23 g/day of alcohol),31 serum LDLc (continuous), and use of lipid medication (yes/no) as these covariates are considered to be associated with CAC independent of obesity.9,12,31 In Model 3, we further adjusted for diabetes mellitus (yes/no) and hypertension (yes/no). In Model 4, we added serum levels (continuous) of HDLc and natural log-transformed triglycerides (lnTG). We recognized a priori that Models 3 and 4 may introduce over-adjustment because diabetes mellitus, hypertension, HDLc, and triglycerides could be intermediaries in the causal pathway between obesity and coronary atherosclerosis.1 Therefore, we considered these models as exploratory but their use as justifiable with an aim to examine the strength of association of BMI relative to these risk factors.1 We performed post hoc analyses examining the relationship of waist circumference, a measure of abdominal obesity, with CAC following the same analytical frames as described above.
We conducted a sensitivity analysis, excluding those participants who reported the use of medication(s) for elevated blood pressure, dyslipidemia, or diabetes (Models 1–4) as such treatment may influence both the exposure (obesity) and the outcome (CAC), possibly obscuring the true association between them. A P-value of <0.05 was considered significant. All statistical tests were two-sided. SAS software (ver 9.1.3; SAS Institute, Inc., Cary, North Carolina, USA) was used for all statistical analyses.
Results
Table 1 shows the characteristics of the participants according to ethnic groups. While the average height was similar (170 cm) among three groups (JJ, KN, JA), levels of both BMI and waist circumference in JA were greater than JJ or KN (Asian groups) and similar to UW. When compared with JA or UW (US groups), Asian groups tended to have more smokers, less medication users for elevated blood pressure and for blood lipids. CAC was less prevalent in the Asian groups than the US groups.
Table 1.
Characteristics of male participants aged 40–49 years
| Japanese (n = 310) |
Koreans (n = 294) |
Japanese Americans (n = 300) |
US Whites (n = 308) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, years | 45.1 | 2.8 | 44.8 | 2.8 | 46.1 | 2.8 | 45.0 | 2.8 |
| Height, cm | 170 | 6 | 170 | 5 | 170 | 6 | 180 | 6 |
| Weight, kg | 68.7 | 10.0 | 71.1 | 8.9 | 80.2 | 14.1 | 90.4 | 14.9 |
| BMI,a kg/m2 | 23.7 | 3.1 | 24.7 | 2.7 | 27.9 | 4.3 | 27.9 | 4.2 |
| Waist circumference (cm) | 85.2 | 8.1 | 83.3 | 7.0 | 93.7 | 11.0 | 98.7 | 11.6 |
| SBP, mmHg | 125 | 16 | 122 | 14 | 128 | 13 | 122 | 11 |
| Glucose, mmol/Lb | 5.93 | 1.04 | 5.71 | 1.01 | 6.22 | 1.17 | 5.64 | 0.85 |
| Total cholesterol, mmol/Lb | 5.63 | 0.93 | 5.01 | 0.88 | 5.36 | 0.95 | 5.5 | 0.97 |
| LDLc, mmol/Lb | 3.43 | 0.93 | 3.01 | 0.83 | 3.15 | 0.85 | 3.49 | 0.87 |
| HDLc, mmol/Lb | 1.4 | 0.35 | 1.19 | 0.30 | 1.31 | 0.32 | 1.24 | 0.33 |
| Triglycerides,c mmol/Lb | 1.55 | (1.18–2.06) | 1.53 | (1.10–2.28) | 1.59 | (1.07–2.55) | 1.46 | (1.05–2.10) |
| Smoking (%) | ||||||||
| Never | 17 | 26 | 66 | 73 | ||||
| Past | 34 | 36 | 22 | 19 | ||||
| Current | 49 | 38 | 13 | 7 | ||||
| Alcohol drinking (%) | ||||||||
| Never | 31 | 48 | 48 | 47 | ||||
| Past | 2 | 7 | 14 | 8 | ||||
| Current | 68 | 45 | 38 | 44 | ||||
| Medication use (%) | ||||||||
| Elevated blood pressure | 6 | 5 | 20 | 9 | ||||
| Elevated lipids | 4 | 1 | 23 | 12 | ||||
| Diabetes mellitus | 2 | 0.3 | 6 | 1 | ||||
| Agatston scorec | 0 | (0–2) | 0 | (0–2) | 0 | (0–34) | 1 | (0–12) |
| Prevalent CACd (%) | 11.7 | 10.9 | 32.0 | 26.1 | ||||
BMI, body mass index; CAC, coronary artery calcification; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation.
aBMI: weight (kg)/height2 (m).
bThe conversion factors from millimole per litre to milligrams per decilitre for glucose, total cholesterol, LDLc, HDLc, and triglycerides are 18.02, 38.61, 38.61, 38.61, and 88.50, respectively.
cValues for triglycerides and Agatston score were given in the median and inter-quartile range in parenthesis.
dPrevalent CAC was defined as Agatston ≥10.
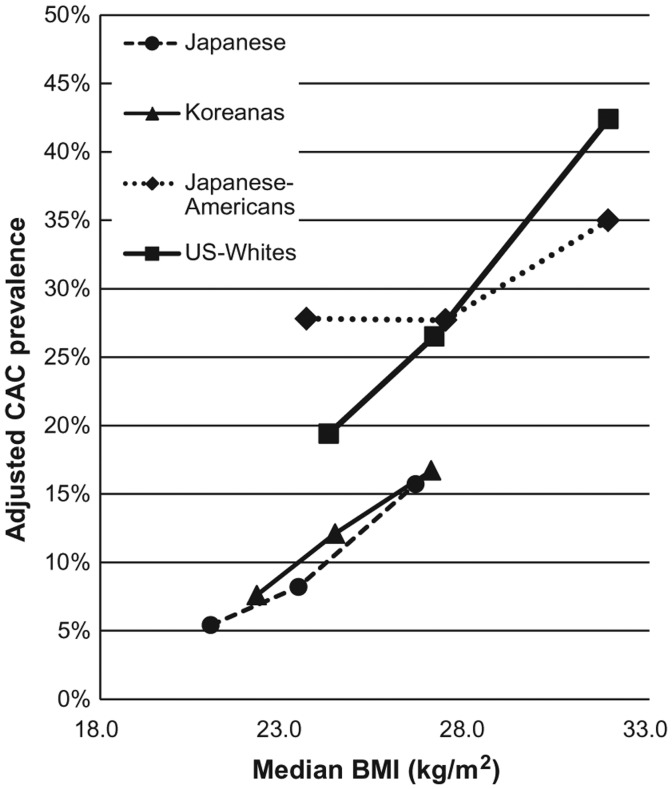
Table 2 shows the results of the tertile analyses. Despite their absolute differences in the BMI, the crude prevalence (%) of CAC increased monotonically across the BMI tertiles in all ethnic groups. The positive relation remained after adjusting for risk factors except for JA as shown in Figure 1. The logistic regression revealed the overall similar positive trend across the BMI tertiles throughout the models. Table 3 shows magnitudes of association of the BMI and other risk factors with CAC within each ethnic group after adjusting for age, smoking, alcohol, lipids, diabetes mellitus, and hypertension (Model 4). The BMI was significantly positively associated with prevalent CAC in JJ, KN, and UW, but not in JA. The magnitudes of association of the BMI were as strong as those of age or LDLc: ORs of prevalent CAC per 1 ethnic-specific SD increase in the BMI (SD 2.7–4.3 kg/m2) ranged from 1.24 to 1.87 across the four groups, while ORs per 1 SD increase in age (SD 2.8 years in all groups) ranged from 1.01 to 1.82, and ORs per 1 SD increase in LDLc (SD 0.83–0.93 mmol/L) ranged from 1.15 to 1.60. Among other risk factors, current smoking and the use of lipid medication were associated with prevalent CAC, although the associations were not always statistically significant. Results of the sensitivity analysis excluding medication users for hypertension, diabetes, or dyslipidemia (n = 1015) were similar to the main results (data not shown).
Table 2.
Crude prevalence of CAC, and adjusted OR for prevalent CAC according to BMI tertile in male participants of aged 40–49 years
| BMIa (kg/m2) |
n | Prevalent CAC (%)b | Adjustedc odds ratio for prevalent CACb |
|||||
|---|---|---|---|---|---|---|---|---|
| Median |
(IQR) | Model 1 | Model 2 | Model 3 | Model 4 | |||
| Japanese | ||||||||
| T1 | 21.0 | (19.8, 21.7) | 103 | 7 (6.8) | Ref. | Ref. | Ref. | Ref. |
| T2 | 23.4 | (23.0, 23.9) | 104 | 10 (9.6) | 1.53 | 1.58 | 1.50 | 1.70 |
| T3 | 26.6 | (25.3, 28.3) | 103 | 19 (18.4) | 3.39 | 3.45 | 3.30 | 4.01 |
| Koreans | ||||||||
| T1 | 22.3 | (21.4, 23.1) | 98 | 6 (6.1) | Ref. | Ref. | Ref. | Ref. |
| T2 | 24.4 | (24.1, 25.0) | 98 | 11 (11.2) | 1.94 | 1.60 | 1.56 | 1.48 |
| T3 | 27.1 | (26.3, 28.2) | 98 | 15 (15.3) | 2.77 | 2.38 | 2.17 | 1.92 |
| Japanese Americans | ||||||||
| T1 | 23.6 | (22.7, 24.7) | 100 | 27 (27.0) | Ref. | Ref. | Ref. | Ref. |
| T2 | 27.4 | (26.5, 28.3) | 100 | 30 (30.0) | 1.19 | 1.00 | 0.96 | 1.12 |
| T3 | 31.9 | (30.3, 34.0) | 100 | 39 (39.0) | 1.75 | 1.57 | 1.38 | 1.59 |
| US Whites | ||||||||
| T1 | 24.3 | (23.0, 24.8) | 103 | 15 (14.6) | Ref. | Ref. | Ref. | Ref. |
| T2 | 27.1 | (26.4, 28.0) | 102 | 24 (23.5) | 1.87 | 1.57 | 1.48 | 1.52 |
| T3 | 31.9 | (30.2, 33.6) | 103 | 42 (40.8) | 3.85 | 3.39 | 3.10 | 3.01 |
BMI, body mass index; CAC, coronary artery calcium; IQR, inter-quartile range; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; lnTG, natural log-transformed value of triglycerides; T1, T2, T3 denotes first, secondly, and thirdly tertile, respectively; Ref., reference.
aBMI: weight (kg)/height2 (m).
bPrevalent CAC was defined as the Agatston score ≥10.
cModel 1 adjusted for age (years). Model 2 further adjusted for smoking (never, past, and current), alcohol drinking (never, past, current <23 g/day, current ≥23 g/day), serum level of LDLc, use of lipid medication(s) (yes/no). Model 3 further adjusted for diabetes mellitus (yes/no), hypertension (yes/no). Model 4 further adjusted for serum levels of HDLc and lnTG. Diabetes mellitus was defined as the use of anti-diabetic medication(s) or fasting glucose ≥7.0 mmol/L (126 mg/dL). Hypertension was defined as the use of anti-hypertensive medication(s) or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Figure 1.
Adjusted prevalence of CAC across BMI tertiles in four ethnic groups of the male participants aged 40–49 years. Each knot represents the median BMI (kg/m2) on the x-axis and corresponding adjusted CAC prevalence (%) on the y-axis for each group-specific tertile of BMI. Prevalence of CAC was defined as the Agatston score ≥10, and adjusted for age (year), smoking status (never, past, and current), alcohol drinking status (never, past, and current with <23 g/day of alcohol, current with ≥23 g/day of alcohol), serum LDLc level (continuous) and use of lipid medication (yes/no) (Model 2). BMI, body mass index; CAC, coronary artery calcium; LDLc, low-density lipoprotein cholesterol.
Table 3.
Adjusted ORs of prevalent CACa in male participants aged 40–49 years
| Japanese (n = 310, prevalent CACa = 36) |
Koreans (n = 294, prevalent CACa = 32) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Adjusting covariates | 1 SD | ORb | (95% CI) | P-valuec | 1 SD | ORb | (95% CI) | P-valuec |
| Age per 1SD (years) | 2.8 | 1.82 | (1.16, 2.85) | <0.01 | 2.8 | 1.01 | (0.67, 1.51) | 0.97 |
| BMIa per 1SD (kg/m2) | 3.1 | 1.87 | (1.22, 2.86) | <0.01 | 2.7 | 1.56 | (1.02, 2.38) | 0.04 |
| LDLc per 1SD (mmol/L) | 0.93 | 1.60 | (1.06, 2.42) | 0.02 | 0.83 | 1.54 | (1.03, 2.31) | 0.03 |
| HDLc/1 SD (mmol/L) | 0.35 | 1.31 | (0.82, 2.08) | 0.26 | 0.30 | 1.10 | (0.68, 1.79) | 0.69 |
| lnTG (mmol/L)/1 SD | 0.46 | 0.99 | (0.65, 1.50) | 0.95 | 0.53 | 1.17 | (0.74, 1.85) | 0.50 |
| Lipid medication use | — | 2.35 | (0.46, 11.96) | 0.30 | — | 4.32 | (0.49, 37.99) | 0.19 |
| Diabetes mellitus | — | 2.15 | (0.60, 7.73) | 0.24 | — | 2.58 | (0.81, 8.20) | 0.11 |
| Hypertension | — | 0.66 | (0.25, 1.71) | 0.39 | — | 1.31 | (0.44, 3.87) | 0.63 |
| Smoking (ref. never) | ||||||||
| Past | — | 1.36 | (0.32, 5.69) | 0.68 | — | 0.74 | (0.25, 2.19) | 0.58 |
| Current | — | 2.77 | (0.71, 10.81) | 0.14 | — | 0.86 | (0.29, 2.53) | 0.78 |
| Alcohol drinking (ref. never) | ||||||||
| Past | — | 2.33 | (0.19, 28.54) | 0.51 | — | d | (–, –) | — |
| Current, <23 g/day | — | 0.60 | (0.18, 1.95) | 0.39 | — | 1.70 | (0.50, 5.78) | 0.40 |
| Current, ≥23 g/day | — | 1.28 | (0.50, 3.27) | 0.61 | — | 2.22 | (0.84, 5.90) | 0.11 |
|
Japanese Americans (n = 299,e prevalent CACa = 95) |
US Whites (n = 308, prevalent CACa = 81) |
|||||||
| Adjusting covariates | 1 SD | ORb | (95% CI) | P-valuec | 1 SD | ORb | (95% CI) | P-valuec |
| Age/1 SD (years) | 2.8 | 1.50 | (1.11, 2.01) | <0.01 | 2.8 | 1.71 | (1.26, 2.32) | <0.01 |
| BMI/1 SD (kg/m2) | 4.3 | 1.24 | (0.91, 1.69) | 0.18 | 4.2 | 1.70 | (1.23, 2.34) | <0.01 |
| LDLc/1 SD (mmol/L) | 0.85 | 1.15 | (0.86, 1.56) | 0.35 | 0.87 | 1.28 | (0.95, 1.73) | 0.11 |
| HDLc/1 SD (mmol/L) | 0.32 | 1.24 | (0.91, 1.69) | 0.18 | 0.33 | 0.77 | (0.52, 1.14) | 0.19 |
| lnTG (mmol/L)/1 SD | 0.61 | 1.02 | (0.75, 1.37) | 0.92 | 0.51 | 0.83 | (0.59, 1.18) | 0.30 |
| Lipid medication | — | 1.73 | (0.88, 3.43) | 0.11 | — | 3.26 | (1.47, 7.23) | <0.01 |
| Diabetes mellitus | — | 1.46 | (0.66, 3.26) | 0.35 | — | 1.73 | (0.41, 7.36) | 0.46 |
| Hypertension | — | 1.11 | (0.59, 2.10) | 0.74 | — | 1.16 | (0.54, 2.52) | 0.70 |
| Smoking (ref. never) | ||||||||
| Past | — | 1.14 | (0.60, 2.16) | 0.69 | — | 1.58 | (0.78, 3.21) | 0.21 |
| Current | — | 2.64 | (1.21, 5.74) | 0.01 | — | 2.08 | (0.73, 5.90) | 0.17 |
| Alcohol drinking (ref. never) | ||||||||
| Past | — | 1.08 | (0.48, 2.42) | 0.86 | — | 0.82 | (0.28, 2.41) | 0.72 |
| Current, <23 g/day | — | 0.84 | (0.32, 2.25) | 0.73 | — | 0.97 | (0.48, 1.94) | 0.93 |
| Current, ≥23 g/day | — | 1.75 | (0.90, 3.37) | 0.10 | — | 1.17 | (0.49, 2.82) | 0.72 |
BMI, body mass index; CAC, coronary artery calcium; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; lnTG, natural log-transformed value of triglycerides; OR, odds ratio; SD, standard deviation; 95% CI, 95% confidence interval.
aPrevalent CAC was defined as the Agatston score ≥10.
bFor a continuous variable, odds ratio was expressed/1 SD (ethnic group-specific SD) increase in the value.
Odds ratio was adjusted for age (years), serum levels (continuous) of LDLc, HDLc, and lnTG, use of lipid medication (yes/no), diabetes mellitus (yes/no), hypertension (yes/no), smoking (never, past, and current), and alcohol drinking (never, past, current <23 g/day, current ≥23 g/day) (Model 4). Diabetes mellitus was defined as the use of anti-diabetic medication(s) or fasting glucose ≥7.0 mmol/L (126 mg/dL). Hypertension was defined as the use of anti-hypertensive medication(s) or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
cP-values were two-sided.
dThe estimates were unable to obtain because no participants in this category had prevalent CAC.
eOne participant was excluded in this analysis due to missing HDLc.
With regard to the relation between waist circumference and CAC, the overall trend was similar but less strong and less consistent across the ethnic groups compared with those of the BMI (see Supplementary data online, Table S1 and S2). We observed a graded increase in prevalent CAC across tertiles of waist circumference only among JJ (see Supplementary data online, Figure S1). Ethnic-specific Pearson's correlation coefficients between waist circumference and BMI were 0.90 for JJ, 0.84 for KN, 0.91 for JA, and 0.89 for UW (all P-values <0.001).
Discussion
This study demonstrated that obesity, measured by the BMI, was positively and independently associated with prevalent CAC in multi-ethnic population samples of 1212 men aged 40–49-year-old asymptomatic individuals with no history of clinical cardiovascular disease, despite their absolute difference in BMI levels.
CAC is a marker for coronary atherosclerosis, shown to be related to plaque burden of the coronary artery.6,7 However, only limited numbers of studies examined the relationship between obesity and CAC with conflicting results: some reported a positive and independent association,9,10,14,17 while others showed a null,9,11–13,15 or even an inverse association.18 Furthermore, most of such studies were conducted in Western populations, in which obesity and CHD are more prevalent relative to other populations such as East Asians.19–22 Examining a dose-response of the BMI on a health outcome could be important given a recent meta-analysis questioning an increased risk of death among those overweight-to-mildly obese individuals defined by the BMI.32 Nevertheless, a dose-response of the BMI on CAC was examined only in the Rotterdam Coronary Calcification Study in the Netherlands, to our knowledge, in which a positive association between BMI and CAC was reported, and the average BMI of the male participants was 26.5 kg/m2.10 Consistent with the Rotterdam study, we observed a positive dose-response of the BMI on prevalent CAC in JJ, KN, and UW but not in JA in the tertile analyses. The overall strength of association of the BMI in all groups except for JA was almost similar to that of age, which is known to be a significant determinant of CAC among various age ranges of a population.10,14,17 Importantly, we demonstrated this association not only among a high-BMI population sample (i.e. UW: average BMI: 28 kg/m2) but also in low-BMI population samples (JJ and KN, average BMI: 24 kg/m2).
In multi-variable logistic regression, we observed a strong association of continuous BMI with CAC in most groups, even with adjustment for risk factors that are related to obesity, such as diabetes mellitus, hypertension, and lipid levels. While many researchers consider obesity as an independent risk factor for clinical CHD, others remain sceptical about this view, believing that the effects of obesity are indirect, and only mediated by established risk factors such as those mentioned above.1,2 However, given the observed independent association of BMI with CAC across the wide range of BMI in multi-ethnic groups, our results suggest that obesity plays an important role from the subclinical stage of coronary atherosclerosis independent of the conventional risk factors. Other studies have reported similar results to ours.10,12,17,33 This statistical independence may be a reflection of multiple mechanisms obesity operates in the pathogenesis of atherosclerosis, including low-grade inflammation, prothrombotic state, insulin resistance, locally released cytokines from ectopic fat tissue, and endothelial dysfunction.1,34,35
Compared with some studies reporting no significant association of obesity with CAC, we studied relatively younger adults within a narrower age range. The average age of our study sample was 44.8–46.1 (SD of 2.8 in all groups) years, whereas the corresponding average ages (SD) were 51.8 (10.6),11 56.4 (8.2),12 62.9 (10.3)9 years for population-based studies reporting a null association. The association between obesity and CAC may differ by age,18 and a role of obesity may be more important in younger adults compared with older adults.36 Therefore, analysis combining a wide age range may lead to mixed results.
It remains unclear as to why a positive association between BMI and CAC was obscured in JA compared with other groups. However, to some extent even in JA, we observed an increased trend of crude prevalence and adjusted OR (Models 1 and 4) for prevalent CAC across BMI tertiles (Table 2). We cannot reject the possibility that the obscured trend may be due to a chance.
Overall, we observed a positive association between waist circumference and CAC, which is consistent with other studies.37–39 While the observed association of waist circumference with CAC was not as strong as that of the BMI in our study, the opposite was true in another study.39 Conceptually, waist circumference is deemed to be superior to the BMI in predicting cardiometabolic risks because the former is a measure of abdominal obesity while the latter is a measure of total obesity. However, literature is inconsistent regarding the relative impact of these measures of obesity on cardiometabolic risk, atherosclerosis, and CVD, which may be attributed to differences in the population characteristics studied.1 In parallel, it remains uncertain whether BMI or waist circumference more strongly contributes to CAC.
Our data have shown that the prevalence of CAC in Asian groups (JJ and KN) were lower than in US (UW and JA) groups. The current study does not intend to explore factors responsible for the difference in absolute prevalence of CAC among ethnic groups or countries, only to analyse the association between CAC and BMI. However, we previously reported differences in prevalent CAC between JJ vs. JA23 and JJ vs. UW,24 and explored factors that may contribute to such differences including marine n-3 fatty acids25 and other factors.40
Cautions are needed in interpreting our results. First, the generalizability of the findings is limited to men aged 40–49 years. Secondly, it is known that obese individuals tend to have more image noise on electron-beam computed tomography.41 Thus, an observed prevalence of CAC may be overestimated particularly among UW and JA due to their higher BMI levels. Thirdly, cross-sectional nature of our study did not demonstrate a temporal relation of obesity resulting in increased CAC prevalence, although many studies have showed that obesity predicts future clinical CHD. Strengths of the study include standardized methods that we employed in evaluating pertinent measures including CAC, BMI, and laboratory data. Other strengths include comparison of multi-ethnic groups with different background levels of CHD and obesity. As a result, we observed a wide range of exposures (BMI) and outcomes (CAC), which is desirable in assessing a relationship between the two.
In conclusion, we found that obesity, measured by the BMI, was positively associated with prevalent CAC in multi-ethnic groups of men aged 40–49 years free of cardiovascular disease despite absolute difference in BMI and CAC levels, and that the association was independent of conventional risk factors. The finding suggests that obesity may play an important role from the subclinical stage of coronary atherosclerosis among men in this age range, regardless of ethnicity and the level of the BMI.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
Funding
This study was supported by the followings: the National Institutes of Health (grant nos R01 HL68200 and R01-HL071561); the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant nos A 13307016 and A 17209023); the Korean Centers for Disease Control and Prevention (grant no. 2004-347-6111-213); and Korea University (grant no. K0823601).
Conflict of interest: None declared.
Supplementary Material
References
- 1.Hu FB. Obesity Epidemiology. 1st ed. New York: Oxford University Press, Inc.; 2008. [Google Scholar]
- 2.Schulte H, Cullen P, Assmann G. Obesity, mortality and cardiovascular disease in the Munster Heart Study (PROCAM) Atherosclerosis. 1999;144:199–209. doi: 10.1016/s0021-9150(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 6.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 7.Bonow RO. Clinical practice. Should coronary calcium screening be used in cardiovascular prevention strategies? N Engl J Med. 2009;361:990–7. doi: 10.1056/NEJMcp0902177. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. Circulation. 2007;115:402–26. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 10.Oei HH, Vliegenthart R, Hofman A, Oudkerk M, Witteman JC. Risk factors for coronary calcification in older subjects. The Rotterdam Coronary Calcification Study. Eur Heart J. 2004;25:48–55. doi: 10.1016/j.ehj.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Dakik HA, Skouri HN, Mehio-Sibai A, Sibai T, Alam S, Sawaya J, et al. Prevalence of coronary artery calcium among asymptomatic men and women in a developing country: comparison with the USA data. Atherosclerosis. 2005;183:141–5. doi: 10.1016/j.atherosclerosis.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Schmermund A, Lehmann N, Bielak LF, Yu P, Sheedy PF, II, Cassidy-Bushrow AE, et al. Comparison of subclinical coronary atherosclerosis and risk factors in unselected populations in Germany and US-America. Atherosclerosis. 2007;195:e207–16. doi: 10.1016/j.atherosclerosis.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor AJ, Feuerstein I, Wong H, Barko W, Brazaitis M, O'Malley PG. Do conventional risk factors predict subclinical coronary artery disease? Results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141:463–8. doi: 10.1067/mhj.2001.113069. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Folsom AR, Lowe LP, Sidney S, Kiefe C, Westfall AO, et al. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–7. doi: 10.1161/01.atv.21.5.852. [DOI] [PubMed] [Google Scholar]
- 15.Maher JE, Raz JA, Bielak LF, Sheedy PF, II, Schwartz RS, Peyser PA. Potential of quantity of coronary artery calcification to identify new risk factors for asymptomatic atherosclerosis. Am J Epidemiol. 1996;144:943–53. doi: 10.1093/oxfordjournals.aje.a008864. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–84. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 17.Park HE, Kim MK, Choi SY, Lee W, Shin CS, Cho SH, et al. The prevalence and distribution of coronary artery calcium in asymptomatic Korean population. Int J Cardiovasc Imaging. 2012;28:1227–35. doi: 10.1007/s10554-011-9922-2. [DOI] [PubMed] [Google Scholar]
- 18.Kovacic JC, Lee P, Baber U, Karajgikar R, Evrard SM, Moreno P, et al. Inverse relationship between body mass index and coronary artery calcification in patients with clinically significant coronary lesions. Atherosclerosis. 2012;221:176–82. doi: 10.1016/j.atherosclerosis.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–9. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekikawa A, Horiuchi BY, Edmundowicz D, Ueshima H, Curb JD, Sutton-Tyrrell K, et al. A “natural experiment” in cardiovascular epidemiology in the early 21st century. Heart. 2003;89:255–7. doi: 10.1136/heart.89.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–87. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 22.Yatsuya H, Toyoshima H, Yamagishi K, Tamakoshi K, Taguri M, Harada A, et al. Body mass index and risk of stroke and myocardial infarction in a relatively lean population: meta-analysis of 16 Japanese cohorts using individual data. Circ Cardiovasc Qual Outcomes. 2010;3:498–505. doi: 10.1161/CIRCOUTCOMES.109.908517. [DOI] [PubMed] [Google Scholar]
- 23.Abbott RD, Ueshima H, Rodriguez BL, Kadowaki T, Masaki KH, Willcox BJ, et al. Coronary artery calcification in Japanese men in Japan and Hawaii. Am J Epidemiol. 2007;166:1280–7. doi: 10.1093/aje/kwm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T, et al. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am J Epidemiol. 2007;165:617–24. doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and White men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–24. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 27.Choo J, Ueshima H, Curb JD, Shin C, Evans RW, El-Saed A, et al. Serum n-6 fatty acids and lipoprotein subclasses in middle-aged men: the population-based cross-sectional ERA-JUMP study. Am J Clin Nutr. 2010;91:1195–203. doi: 10.3945/ajcn.2009.28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 29.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–7. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 30.Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613–21. [PubMed] [Google Scholar]
- 31.Okamura T, Kadowaki T, Sekikawa A, Murata K, Miyamatsu N, Nakamura Y, et al. Alcohol consumption and coronary artery calcium in middle-aged Japanese men. Am J Cardiol. 2006;98:141–4. doi: 10.1016/j.amjcard.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 32.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter MK, Massaro JM, Hoffmann U, O'Donnell CJ, Fox CS. Fasting glucose, obesity, and coronary artery calcification in community-based people without diabetes. Diabetes Care. 2012;35:1944–50. doi: 10.2337/dc11-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28(Suppl. 4):S58–65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 35.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Steffes MW, Gross M, Park K, Holvoet P, Kiefe CI, et al. Differential associations of weight dynamics with coronary artery calcium versus common carotid artery intima-media thickness: the CARDIA Study. Am J Epidemiol. 2010;172:180–9. doi: 10.1093/aje/kwq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bose S, Krishnamoorthy P, Varanasi A, Nair J, Schutta M, Braunstein S, et al. Measurement of waist circumference predicts coronary atherosclerosis beyond plasma adipokines. Obesity (Silver Spring) 2013;21:E118–23. doi: 10.1002/oby.20086. [DOI] [PubMed] [Google Scholar]
- 38.Lee CD, Jacobs DR, Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 39.Nasir K, Campbell CY, Santos RD, Roguin A, Braunstein JB, Carvalho JA, et al. The association of subclinical coronary atherosclerosis with abdominal and total obesity in asymptomatic men. Prev Cardiol. 2005;8:143–8. doi: 10.1111/j.1520-037x.2005.4362.x. [DOI] [PubMed] [Google Scholar]
- 40.Sekikawa A, Curb JD, Edmundowicz D, Okamura T, Choo J, Fujiyoshi A, et al. Coronary artery calcification by computed tomography in epidemiologic research and cardiovascular disease prevention. J Epidemiol. 2012;22:188–98. doi: 10.2188/jea.JE20110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sevrukov A, Pratap A, Doss C, Jelnin V, Hoff JA, Kondos GT. Electron beam tomography imaging of coronary calcium: the effect of body mass index on radiologic noise. J Comput Assist Tomogr. 2002;26:592–7. doi: 10.1097/00004728-200207000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.