Abstract
Background:
We investigated whether body mass index (BMI) can be used as a predictive parameter indicating patients who benefit from extended aromatase inhibitor (AI) treatment.
Methods:
The ABCSG-6a trial re-randomised event-free postmenopausal hormone receptor-positive patients from the ABCSG-6 trial to receive either 3 additional years of endocrine therapy using anastrozole vs nil. In this retrospective analysis, we investigated the prognostic and predictive impact of BMI on disease outcome and safety.
Results:
In all, 634 patients (177 normal weight, 307 overweight, and 150 obese) patients were included in this analysis. Normal weight patients with additional 3 years of anastrozole halved their risk of disease recurrence (disease-free survival (DFS) HR 0.48; P=0.02) and death (HR 0.45; P=0.06) and had only a fifth of the risk of distant metastases (HR 0.22; P=0.05) compared with normal weight patients without any further treatment. In contrast, overweight+obese patients derived no benefit from additional 3 years of anastrozole (DFS HR 0.93; P=0.68; distant recurrence-free survival HR 0.91; P=0.78; and OS HR 0.9; P=0.68). The possible predictive impact of BMI on extended endocrine treatment could be strengthened by a Cox regression interaction model between BMI and treatment (P=0.07).
Conclusion:
Body mass index may be used to predict outcome benefit of extended AI treatment in patients with receptor-positive breast cancer.
Keywords: breast cancer, endocrine therapy, BMI, aromatase inhibitor, extended therapy, obesity
Aromatase inhibitors (AIs) are standard endocrine treatment for postmenopausal patients with hormone receptor-positive breast cancer. According to the ASCO guidelines 2010, AIs should be offered upfront for 5 years or after 2–3 years of tamoxifen (switch therapy) in the postmenopausal situation (Burstein et al, 2010).
However, optimal duration of endocrine therapy is still under intensive investigation. Even after 5 years of endocrine treatment a substantial risk of recurrence has to be taken into account in the follow-up of hormone receptor-positive disease (Saphner et al, 1996; EBCTCG, 2005). The MA 17 trial was the first to demonstrate a distinct disease-free survival (DFS) benefit for additional 5 years of letrozole vs placebo after 5 years of tamoxifen (Goss et al, 2003). The ABCSG-6a trial reported an advantage of additional 3 years of endocrine treatment using anastrozole after 5 years of adjuvant endocrine therapy (Jakesz et al, 2007). According to these trials, extended endocrine treatment by an AI can be recommended after 5 years of tamoxifen. The MA17R (NCT00754845), NSABP-B42 (NCT00382070), IDEAL (Netherlands Trial Registry 3077), and ABCSG-16 (SALSA; NCT 00295620) trials will hopefully show whether extended endocrine therapy after 5 years of AI improves disease outcome as well. Despite these encouraging prospects, the accumulation of side effects and perhaps an even worse treatment compliance often complicate this issue in clinical practice (Perez, 2007; Fontein et al, 2012). Therefore, the decision to use extended endocrine treatment by AI can not only be driven by prognosis. Predictive parameters indicating patients who will have a distinctive benefit from long-term endocrine therapy should be included in the decision process to outweigh the risk of side effects.
Obesity has an independent prognostic value regarding breast cancer. It has been demonstrated that overweight and obese patients experience a worse outcome regarding distant recurrences and overall survival (OS) (Ewertz et al, 2011).
Recently, the predictive value of body mass index (BMI) regarding endocrine therapy has been investigated. Overweight patients seem to derive less benefit from AIs compared with normal weight patients (Sestak et al, 2010; Pfeiler et al, 2011). It was hypothesised that normal dosages of AIs are not able to fully suppress increased oestrogen serum levels in overweight patients, which impacts on outcome. Taking this into account, BMI might be a predictive parameter whether to use AI-based or tamoxifen-based endocrine treatment. As up to two thirds of the population in developed countries like the United States are overweight and obese, BMI as a prognostic and possibly predictive parameter should be taken into consideration for treatment decision (Flegal et al, 2010).
To investigate whether BMI can be used as a predictive parameter regarding long-term endocrine treatment with an AI we re-analysed the ABCSG-6a trial, which investigated additional 3 years of anastrozole vs no further treatment in a randomised manner.
Patients and methods
The ABCSG-6a trial (NCT00300508) re-randomised event-free patients from the ABCSG-6 trial to receive either 3 additional years of endocrine therapy using anastrozole vs nil (Jakesz et al, 2007). In the ABCSG-6 trial postmenopausal, hormone-receptor positive patients with breast cancer were randomised to receive either tamoxifen for 5 years (40 mg for 2 years and 20 mg for 3 years) or tamoxifen for 5 years (40 mg for 2 years and 20 mg for 3 years) together with aminoglutethimide for the first 2 years (Schmid et al, 2003). Patients with primary unilateral stage I or II breast cancer with or without lymph-node involvement were included. At the end of ABCSG-6 trial, 1135 patients were event free (according to chest X-rays, abdominal ultrasound, mammography, and other investigations if clinically indicated) and eligible for participation in the ABCSG-6a trial. In all, 854 postmenopausal, hormone receptor-positive patients with breast cancer were randomised in ABCSG-6a (anastrozole for 3 years vs nil). In the anastrozole arm, 210 (54.3%) patients had received 5 years of tamoxifen only and 177 (45.7%) patients had received tamoxifen+aminoglutethimide, which is comparable to the group of patients without extended endocrine treatment. Extended endocrine therapy was initiated within 6 weeks after completing 5 years of adjuvant endocrine therapy in the ABCSG-6 trial. The primary end point of ABCSG-6a was recurrence-free survival and secondary end points were OS and tolerability. Details of the protocol have been reported elsewhere (Jakesz et al, 2007). This study has been approved by regulatory and ethics committees for all participating centres. Written informed consent was signed by all participating patients.
This retrospective analysis aimed to test for a prognostic and predictive effect of BMI on the end point DFS, distant recurrence-free survival (DRFS), OS, and safety. The primary hypothesis of this reanalysis was that patients with a BMI above the normal range derive less benefit from additional 3 years of anastrozole than normal weight patients. Data on height and weight at baseline of ABCSG-6a were used to calculate BMI classes according to WHO criteria with BMI values ranging from 18.5 to 24.9 kg m−2 for normal weight, from 25 to 29.9 kg m−2 for overweight, and with BMI values ⩾30 kg m−2 for obese patients. Patients without information on height or weight as well as underweight patients with a BMI of <18.5 kg m−2 were excluded from these analyses.
Disease-free survival was defined as the time from randomisation to the first occurrence of any of the following events: locoregional recurrence, distant metastasis, cancer in contralateral breast, second primary cancer, or death from any cause. For DRFS, subjects who have died without distant metastases were censored at the time of death. Disease-free survival, DRFS, and OS were analysed according to the BMI subgroups as well as the two study arms (anastrozole vs nihil).
Statistical analyses
Hazard ratios with confidence intervals and test statistics for the group comparisons were obtained from Cox proportional hazards regression models. Kaplan–Meier plots with log-rank tests were used for selected comparisons. To adjust for effects of demographic and additional prognostic factors on DFS, distant recurrence survival and OS, tumour stage, nodal stage, grade, ER, PR, and age were included in multivariate Cox regression models for the comparison of overweight/obese vs normal weight patients. A Cox regression interaction model was used to describe any interaction between BMI and treatment regarding disease outcome. Demographic data and side effects were compared using Fisher's exact test and Kruskal–Wallis test when appropriate. All analyses were performed at a two-sided significance level of 0.05.
Results
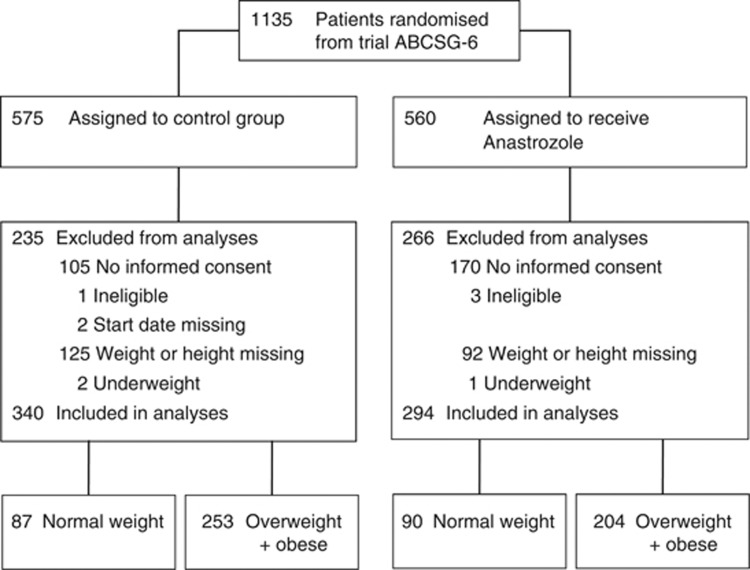
In all, 854 postmenopausal patients with breast cancer without disease recurrence after 5 years of endocrine treatment participated in the ABCSG-6a trial. For this analysis, 217 patients (92 patients from the anastrozole arm and 125 patients from the control arm) were excluded due to unavailable data on height, weight or both. Complete patient information was available in 637 patients (75%). Furthermore, three underweight patients (one from the anastrozole arm and two from the control arm) were excluded due to small numbers and for biological reasons. Therefore, 634 patients (294 patients in the anastrozole arm and 340 patients in the control arm) were included in this analysis (Figure 1).
Figure 1.
Consort diagram.
Less than one third of these patients (28%, 177 patients) were normal weight, and more than two thirds were overweight (48%, 307 patients) or obese (24%, 150 patients). Patient and tumour characteristics of the anastrozole and the control arm according to BMI category are shown in Table 1. Patient and tumour characteristics were well balanced between the four groups.
Table 1. Patient demographics and tumour characteristics.
| |
Normal weight |
Overweight+obese |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Control |
Anastrozole |
|
Control |
Anastrozole |
|
||||
| Characteristics | N | % | N | % | P-value | N | % | N | % | P-value |
| No. of patients |
87 |
|
90 |
|
|
253 |
|
204 |
|
|
| BMI values |
|
|
|
|
0.6226 |
|
|
|
|
0.4307 |
| Median | 23.2 | 23.4 | 28.3 | 28.4 | ||||||
| Range |
19.4–25.0 |
|
18.6–25.0 |
|
|
25.0–50.6 |
|
25.0–46.8 |
|
|
| Age at start of trial-6a |
|
|
|
|
0.1009 |
|
|
|
|
0.1414 |
| Median | 69 | 63 | 66 | 67.5 | ||||||
| Range |
51–83 |
|
52–82 |
|
|
52–85 |
|
51–82 |
|
|
| Cancer stage |
|
|
|
|
0.9994 |
|
|
|
|
0.9888 |
| pT1 | 55 | 63.2 | 57 | 63.3 | 150 | 59.3 | 120 | 58.8 | ||
| pT2 | 30 | 34.5 | 31 | 34.5 | 99 | 39.1 | 81 | 39.7 | ||
| pT3 |
2 |
2.3 |
2 |
2.2 |
|
4 |
1.6 |
3 |
1.5 |
|
| Nodal status |
|
|
|
|
0.8727 |
|
|
|
|
0.3244 |
| N0 | 60 | 69.0 | 65 | 72.2 | 161 | 63.6 | 124 | 60.8 | ||
| N1 | 22 | 25.3 | 19 | 21.1 | 65 | 25.7 | 65 | 31.8 | ||
| N2 | 4 | 4.6 | 4 | 4.5 | 21 | 8.3 | 13 | 6.4 | ||
| N3 |
1 |
1.1 |
2 |
2.2 |
|
6 |
2.4 |
2 |
1.0 |
|
| Tumour grading |
|
|
|
|
0.3564 |
|
|
|
|
0.7276 |
| G1 | 19 | 21.9 | 17 | 18.9 | 43 | 17.0 | 34 | 16.7 | ||
| G2 | 37 | 42.5 | 49 | 54.4 | 146 | 57.7 | 119 | 58.3 | ||
| G3 | 27 | 31.0 | 19 | 21.1 | 47 | 18.6 | 42 | 20.6 | ||
| Gx |
4 |
4.6 |
5 |
5.6 |
|
17 |
6.7 |
9 |
4.4 |
|
| Hormone-receptor status (biochemical) |
|
|
|
|
0.5415 |
|
|
|
|
0.245 |
| ER+ PgR+ | 11 | 12.6 | 14 | 15.6 | 51 | 20.2 | 54 | 26.4 | ||
| ER+ PgR− | 5 | 5.8 | 3 | 3.3 | 18 | 7.1 | 9 | 4.4 | ||
| ER− PgR+ | 0 | 0.0 | 1 | 1.1 | 3 | 1.2 | 0 | 0.0 | ||
| ER+ PgR? | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | ||
| ER? PgR+ | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| ER− PgR− | 0 | 0.0 | 1 | 1.1 | 0 | 0.0 | 1 | 0.5 | ||
| Not determ. | 0 | 0.0 | 2 | 2.2 | 5 | 2.0 | 2 | 1.0 | ||
| Unknown | 3 | 3.4 | 2 | 2.2 | 17 | 6.7 | 14 | 6.9 | ||
| Missing |
68 |
78.2 |
67 |
74.5 |
|
159 |
62.8 |
123 |
60.3 |
|
| Oestrogen-receptor status |
|
|
|
|
0.6208 |
|
|
|
|
0.0719 |
| ER− | 3 | 3.4 | 5 | 5.5 | 6 | 2.4 | 4 | 2.0 | ||
| ER+ | 17 | 19.6 | 15 | 16.7 | 41 | 16.2 | 25 | 12.2 | ||
| ER++ | 36 | 41.4 | 29 | 32.2 | 75 | 29.6 | 77 | 37.7 | ||
| ER+++ | 20 | 23.0 | 30 | 33.3 | 93 | 36.7 | 56 | 27.5 | ||
| Unknown | 5 | 5.7 | 4 | 4.5 | 26 | 10.3 | 23 | 11.3 | ||
| Missing |
6 |
6.9 |
7 |
7.8 |
|
12 |
4.7 |
19 |
9.3 |
|
| Progesterone-receptor status |
|
|
|
|
0.6318 |
|
|
|
|
0.1593 |
| PgR− | 18 | 20.7 | 11 | 12.2 | 32 | 12.7 | 18 | 8.8 | ||
| PgR+ | 15 | 17.3 | 22 | 24.4 | 50 | 19.8 | 32 | 15.7 | ||
| PgR++ | 23 | 26.4 | 25 | 27.8 | 59 | 23.3 | 59 | 28.9 | ||
| PgR+++ | 20 | 23.0 | 20 | 22.2 | 74 | 29.2 | 53 | 26.0 | ||
| Unknown | 5 | 5.7 | 4 | 4.5 | 26 | 10.3 | 23 | 11.3 | ||
| Missing |
6 |
6.9 |
8 |
8.9 |
|
12 |
4.7 |
19 |
9.3 |
|
| Type of surgery |
|
|
|
|
0.4830 |
|
|
|
|
0.6877 |
| Breast conserving | 50 | 57.5 | 47 | 52.2 | 135 | 53.4 | 105 | 51.5 | ||
| Radically modified |
37 |
42.5 |
43 |
47.8 |
|
118 |
46.6 |
99 |
48.5 |
|
| Histology |
|
|
|
|
0.0424 |
|
|
|
|
0.9632 |
| Lobular invasive | 15 | 17.2 | 24 | 26.7 | 39 | 15.4 | 35 | 17.2 | ||
| Ductal invasive | 62 | 71.3 | 63 | 70.0 | 190 | 75.1 | 151 | 74.0 | ||
| Other | 4 | 4.6 | 3 | 3.3 | 16 | 6.3 | 12 | 5.9 | ||
| Unknown | 6 | 6.9 | 0 | 0.0 | 8 | 3.2 | 6 | 2.9 | ||
Abbreviation: BMI=body mass index.
Efficacy
This analysis reports on a median follow-up of 73.2 months. In all, 218 events including 94 deaths are included in this analysis (Table 2). Comparing the whole group of overweight+obese patients (n=457) with the whole group of normal weight patients (n=177), no difference in DFS (hazard ratio 1.05; 95% CI, 0.75–1.49, P=0.76) and OS (hazard ratio 1.05; 95% CI, 0.67–1.64, P=0.83) could be observed. This missing impact of BMI on disease outcome was also true with regard to distant metastases. Overweight+obese patients had the same risk for distant metastases compared with normal weight patients in the univariate (hazard ratio 1.45; 95% CI, 0.74–2.84, P=0.27) as well as in the multivariate analysis, which included age, tumour stage, nodal stage, tumour grade, ER and PR expression (hazard ratio 1.29; 95% CI, 0.61–2.76, P=0.49). Additional multivariate analyses are shown in Tables 3 and 4.
Table 2. Events of normal weight and overweight+obese patients treated with anastrozole vs nihil.
| |
Normal weight |
Overweight+obese |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Control |
Anastrozole |
Control |
Anastrozole |
||||
| N | % | N | % | N | % | N | % | |
| Number of patients |
87 |
|
90 |
|
253 |
|
204 |
|
| All events |
42 |
48.3 |
18 |
20.0 |
95 |
37.5 |
63 |
30.9 |
| Locoregional | 3 | 3.5 | 2 | 2.2 | 11 | 4.3 | 4 | 2.0 |
| Distant | 9 | 10.4 | 2 | 2.2 | 22 | 8.7 | 16 | 7.8 |
| Contralateral | 5 | 5.7 | 2 | 2.2 | 6 | 2.4 | 4 | 2.0 |
| Secondary malignant conditions | 6 | 6.9 | 4 | 4.4 | 16 | 6.3 | 12 | 5.9 |
|
Death | ||||||||
| All | 19 | 21.8 | 8 | 8.9 | 40 | 15.8 | 27 | 13.2 |
Table 3. Overweight+obese vs normal weight: multivariant analyses including age, tumour stage, nodal stage, tumour grade, and ER and PR expression.
|
All |
3 years Anastrozole |
No treatment |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
|
DFS | ||||||
| Overweight+obese vs Normal weight |
0.89 |
0.55–1.12 |
1.27 |
0.65–2.46 |
0.68 |
0.42–1.10 |
|
Distant recurrence-free survival | ||||||
| Overweight+obese vs Normal weight |
1.29 |
0.61–2.76 |
3.41 |
0.74–15.75 |
0.79 |
0.33–1.97 |
|
Overall survival | ||||||
| Overweight+obese vs Normal weight | 0.77 | 0.49–1.28 | 0.71 | 0.27–1.86 | 0.67 | 0.34–1.35 |
Abbreviations: CI=confidence interval; DFS=disease-free survival; HR=hazard ratio.
Table 4. Anastrozole vs Control: multivariant analyses including age, tumour stage, nodal stage, tumour grade, and ER and PR expression.
|
Overweight+Obese |
Normal weight |
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
|
DFS | ||||
| Anastrozole vs Control |
0.94 |
0.62–1.45 |
0.51 |
0.26–1.00 |
|
Distant recurrence-free survival | ||||
| Anastrozole vs Control |
0.97 |
0.47–1.99 |
0.28 |
0.06–1.46 |
|
Overall survival | ||||
| Anastrozole vs Control | 0.90 | 0.51–1.61 | 0.52 | 0.20–1.31 |
Abbreviations: CI=confidence interval; DFS=disease-free survival; HR=hazard ratio.
Overweight vs normal weight according to treatment arm
Analysing patients only with no further adjuvant treatment after 5 years of endocrine therapy (control group), no difference between overweight+obese and normal weight patients with regard to DFS (hazard ratio 0.79; 95% CI, 0.52–1.23, P=0.3), DRFS (hazard ratio 0.91; 95% CI, 0.42–1.98, P=0.81) and OS (hazard ratio 0.81; 95% CI, 0.47–1.4, P=0.45) could be observed.
In contrast, in the group of patients with additional 3 years of anastrozole, overweight+obese patients had a non-significant worse DFS compared with normal weight patients (hazard ratio 1.55; 95% CI, 0.87–2.77, P=0.14). Regarding DRFS in this group of patients, overweight+obese patients had a nearly four-fold non-significant increased risk of distant metastases compared with normal weight patients (hazard ratio 3.85; 95% CI, 0.88–16.75, P=0.07). This non-significant worse DRFS of overweight+obese compared with normal weight patients could also be shown in the multivariate analysis (hazard ratio 3.41; 95% CI, 0.74–15.75, P=0.12). Only a moderate, non-significant worse OS of overweight+obese compared with normal weight patients could be identified in the group of patients with 3 additional years of anastrozole (hazard ratio 1.58; 95% CI, 0.72–3.49, P=0.25).
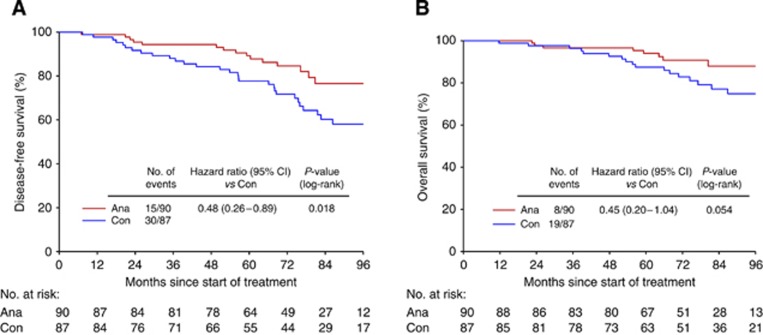
Anastrozole vs Control according to BMI
Comparison of the efficacy of additional 3 years of anastrozole with no further treatment in the group of normal weight patients revealed a significant benefit for the treatment group (Figure 2). Normal weight patients with additional 3 years of anastrozole halved their risk of disease recurrence (DFS hazard ratio 0.48; 95% CI, 0.26–0.89, P=0.02) and had only a fifth of the risk of distant metastases (hazard ratio 0.22; 95% CI, 0.05–1.0, P=0.05) compared with normal weight patients without any further treatment. This could be confirmed in the multivariate analyses, which included age, tumour stage, nodal stage, tumour grade, and ER and PR expression. The significantly decreased risk of disease recurrence in normal weight patients treated with additional 3 years of anastrozole translated into a strong trend for a better OS compared with normal weight patients without further treatment. Normal weight patients with additional 3 years of anastrozole halved their risk of death compared with normal weight patients with no further treatment (hazard ratio 0.45; 95% CI, 0.19–1.04, P=0.06). Again, this could be confirmed in the multivariate analysis, data not shown.
Figure 2.
(A) DFS: Anastrozole vs Control, normal weight patients and (B) OS: Anastrozole vs Control, normal weight patients.
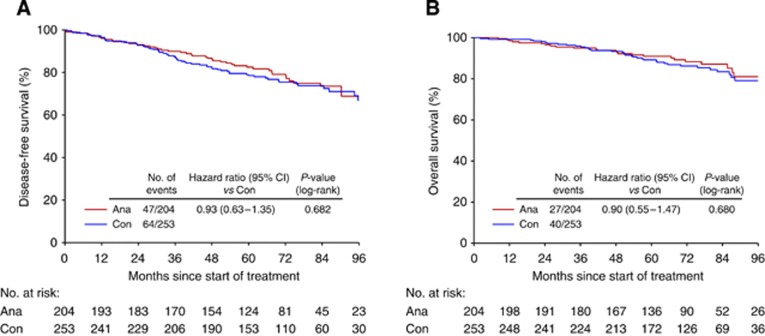
In strong contrast, overweight+obese patients did not benefit from additional 3 years of endocrine treatment with anastrozole (Figure 3). When comparing overweight+obese patients with additional 3 years of anastrozole to overweight+obese patients with no further treatment, no difference regarding DFS (hazard ratio 0.93; 95% CI, 0.63–1.35, P=0.68), DRFS (hazard ratio 0.91; 95% CI, 0.48–1.74, P=0.78), and OS (hazard ratio 0.9; 95% CI, 0.55–1.47, P=0.68) could be observed.
Figure 3.
(A) DFS: Anastrozole vs Control, overweight+obese patients and (B) OS: Anastrozole vs Control, overweight+obese patients.
Interaction between BMI and treatment
To concrete the possible impact of BMI on extended endocrine treatment with anastrozole, a Cox regression interaction model between BMI and treatment regarding DFS and OS was performed. The model showed a strong trend for interaction between BMI and treatment regarding DFS (0.07) although this did not reach statistical significance. However, hardly any interaction could be shown between BMI and treatment regarding OS (P=0.17).
Safety and tolerability
Table 5 shows side effects of both treatment arms according to BMI. As shown, using a Cochran–Mantel–Haenszel test stratified by treatment, BMI had no impact on the frequency of side effects. However, normal weight as well as overweight patients with additional 3 years of anastrozole had significant more side effects compared with patients without any further treatment (control group).
Table 5. Comparison of adverse events between BMI categories stratified by treatment.
|
Control |
Anastrozole |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Norm (n=87) |
Over (n=253) |
Norm (n=90) |
Over (n=204) |
|
||||
| Adverse event | N | % | N | % | N | % | N | % | P-value |
| Allergy |
1 |
1.1 |
1 |
0.4 |
3 |
3.3 |
5 |
2.5 |
0.467 |
| Amenorrhoea |
85 |
97.7 |
245 |
96.8 |
84 |
93.3 |
192 |
94.1 |
0.976 |
| Arrhythmia |
3 |
3.4 |
3 |
1.2 |
4 |
4.4 |
8 |
3.9 |
0.344 |
| Asthenia |
3 |
3.4 |
2 |
0.8 |
5 |
5.6 |
9 |
4.4 |
0.211 |
| Bleeding |
1 |
1.1 |
1 |
0.4 |
2 |
2.2 |
1 |
0.5 |
0.119 |
| Bone aches |
25 |
28.7 |
50 |
19.8 |
26 |
28.9 |
57 |
27.9 |
0.190 |
| Card disorders |
5 |
5.7 |
17 |
6.7 |
2 |
2.2 |
13 |
6.4 |
0.222 |
| Cutaneous toxicity |
0 |
0.0 |
1 |
0.4 |
6 |
6.7 |
10 |
4.9 |
0.647 |
| Depressions |
10 |
11.5 |
21 |
8.3 |
17 |
18.9 |
33 |
16.2 |
0.319 |
| Diarrhoea |
3 |
3.4 |
7 |
2.8 |
5 |
5.6 |
12 |
5.9 |
0.918 |
| Eczema |
1 |
1.1 |
6 |
2.4 |
1 |
1.1 |
12 |
5.9 |
0.058 |
| Fever |
1 |
1.1 |
4 |
1.6 |
3 |
3.3 |
5 |
2.5 |
0.866 |
| Headache |
10 |
11.5 |
30 |
11.9 |
20 |
22.2 |
44 |
21.6 |
0.967 |
| Haematuria |
0 |
0.0 |
2 |
0.8 |
1 |
1.1 |
3 |
1.5 |
0.505 |
| Hair loss |
1 |
1.1 |
8 |
3.2 |
12 |
13.3 |
20 |
9.8 |
0.746 |
| Hot flushes |
18 |
20.7 |
61 |
24.1 |
42 |
46.7 |
82 |
40.2 |
0.725 |
| Infections |
2 |
2.3 |
6 |
2.4 |
5 |
5.6 |
9 |
4.4 |
0.749 |
| Nausea |
2 |
2.3 |
6 |
2.4 |
9 |
10.0 |
13 |
6.4 |
0.358 |
| Obstipation |
2 |
2.3 |
9 |
3.6 |
8 |
8.9 |
14 |
6.9 |
0.858 |
| Pericarditis |
0 |
0.0 |
0 |
0.0 |
1 |
1.1 |
0 |
0.0 |
0.132 |
| Proteinuria |
0 |
0.0 |
1 |
0.4 |
1 |
1.1 |
1 |
0.5 |
0.866 |
| Somnolence |
4 |
4.6 |
12 |
4.7 |
9 |
10.0 |
26 |
12.7 |
0.557 |
| Vaginal discharge |
6 |
6.9 |
5 |
2.0 |
5 |
5.6 |
16 |
7.8 |
0.480 |
| Vaginal dryness |
11 |
12.6 |
20 |
7.9 |
16 |
17.8 |
23 |
11.3 |
0.045 |
| Vomiting |
2 |
2.3 |
5 |
2.0 |
3 |
3.3 |
5 |
2.5 |
0.659 |
| Other | 28 | 32.2 | 82 | 32.4 | 42 | 46.7 | 78 | 38.2 | 0.345 |
Abbreviation: BMI=body mass index.
Discussion
The ABCSG-6a trial was one of the first to demonstrate an advantage of additional 3 years of endocrine treatment using anastrozole compared with nihil after 5 years of endocrine treatment. The MA 17 trial and the NSABP B-33 reported improvement of disease outcome by long-term endocrine treatment with an AI as well (Goss et al, 2003; Mamounas et al, 2008). Taken these trials into consideration, extended endocrine treatment with an AI after 5 years of tamoxifen can be recommended and long-term endocrine treatment with an AI for 10 years is reasonable and under intensive investigation. Though patients with long-term endocrine treatment experience a reduction in the risk of recurrence, this goes along with a significant increase in side effects (Goss et al, 2008).
Not all patients did benefit from long-term endocrine therapy in the three mentioned prospective randomised trials. Predicitve parameters are needed to distinguish between patients who will and patients who will not benefit from a certain therapy. Predicitve parameters could help to increase the relative efficacy of a treatment and to outweigh the risk of side effects as patients likely to be non-responders can be identified and excluded from treatment.
In this reanalysis of the ABCSG-6a trial, we demonstrate that BMI is a predictive parameter regarding extended endocrine treatment with an AI. Normal weight patients experienced substantial benefit from additional 3 years of anastrozole, which halved their risk of disease recurrence and death from any cause. Overweight and obese patients did not derive benefit from long-term endocrine treatment with anastrozole.
Recently, it has been suggested that overweight patients derive not the same benefit from AIs as normal weight patients possibly due to increased aromatisation in the fat tissue. The reanalysis of the ABCSG-12 trial demonstrated that overweight premenopausal patients treated with goserelin+anastrozole have a worse outcome compared with overweight premenopausal patients treated with goserelin+tamoxifen (Pfeiler et al, 2011). Sestak et al (2010) reported that BMI impacts on the efficacy of anastrozole but not tamoxifen in postmenopausal patients with breast cancer. These two publications demonstrate an impact of BMI on the efficacy of AI and are in line with our results reported here. However, in the ABCSG-12 trial as well as in the ATAC trial, all patients received a kind of endocrine treatment (either anastrozole based or tamoxifen based), which allows to report on the relative impact of BMI on endocrine therapy only. In contrast, in ABCSG-6a halve of the patients received no treatment. Therefore, the comparison of anastrozole vs nil in normal weight and overweight patients may even better reflect the biological interaction of AI treatment and body weight.
In normal weight patients, 3 additional years of anastrozole halved the risk of disease recurrence and death compared with patients without any further treatment. It is remarkable that a relatively limited duration of endocrine treatment extension leads to a significant reduction in relapse rates (particularly distant metastases).
In contrast, overweight patients derived no benefit from 3 additional years of anastrozole when compared with overweight patients without any further treatment. This indicates that overweight and obese postmenopausal patients with breast cancer are non-responders to long-term endocrine treatment with anastrozole.
A limitation of this study is that because of its retrospective nature, patient and event numbers are somewhat limited. Further, we cannot rule out that healthier lifestyle including more physical activity in leaner patients contributes to our results. For the analyses, we combined overweight and obese patients. As previously shown, the prognostic and predictive impact of BMI is more distinct in obese than in overweight (Sestak et al, 2010; Kwan et al, 2012; Pfeiler et al, 2013). We can therefore not completely exclude that the data presented here are mainly driven by the group of obese patients, and further differentiation between limited weight excess and severe obesity is beyond numerical reason in our trial.
In contrast to other reports, we did not observe a prognostic impact of BMI in these long-term treated patients with breast cancer. Ewertz et al (2011) demonstrated a significant impact of BMI on disease outcome especially after 5 years of follow-up. Wolters et al (2012) confirmed this long-term prognostic impact of BMI ). Recently, Goodwin et al (2012) reported on a constant impact of obesity on distant recurrences over time. The missing prognostic impact of BMI in our reanalysis might be due to the fact that we do not report on events after breast cancer diagnosis but after 5 years of endocrine treatment.
In this reanalysis, we did not observe any impact of BMI on the frequency of side effects, which is in line with previous reports (Pfeiler et al, 2011, 2013). Overweight patients had the same frequency of side effects as normal weight patients regarding long-term endocrine therapy with anastrozole. This is of particular importance since overweight patients did not benefit from 3 additional years of anastrozole but significantly more side effects compared with patients without any further treatment.
This observation can be interpreted as the result of a ‘minor' reduction in estradiol serum levels in overweight and obese patients by anastrozole, which on the one hand causes noticeable side effects, but on the other hand does not lower estradiol serum levels enough to impact on clinical outcome. Recently, Folkerd et al (2012) showed a retrospective analysis of the ALIQUOT study that indeed oestrogen serum levels are lowered in overweight and obese patients but not to the same low level when compared with normal weight patients.
In conclusion, we report that normal weight patients with additional 3 years of anastrozole halve their risk of disease recurrence and death compared with normal weight patients without any further treatment. In contrast, overweight patients derive no benefit from these additional 3 years of endocrine treatment with anastrozole. However, overweight patients treated with anastrozole have the same increased rate of side effects as normal weight patients when compared with patients without any further treatment. According to our reanalysis of the ABCSG-6a, the beneficial effect of extended AI treatment seems to be more pronounced in patients with normal weight compared with patients with increased BMI.
Acknowledgments
We thank our patients who contributed to this and other ABCSG trials; ABCSG investigators, study nurses, and data management associates, both in the individual trial centres and in the ABCSG centre, who provided ongoing support; Hannes Fohler, and the statistical team.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Presented in part at the San Antonio Breast Cancer Symposium 2010 – Obesity and Breast Cancer (Poster Discussion Session).
References
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemo- therapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Jensen MB, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fontein DB, Nortier JW, Liefers GJ, Putter H, Meershoek-Klein Kranenbarg E, van den Bosch J, Maartense E, Rutgers EJ, van de Velde CJ. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol. 2012;38:110–117. doi: 10.1016/j.ejso.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Folkerd EJ, Dixon JM, Renshaw L, A'Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30:2977–2980. doi: 10.1200/JCO.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmeno- pausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- Goss PE, Muss HB, Ingle JN, Whelan TJ, Wu M. Extended adjuvant endocrine therapy in breast cancer: current status and future directions. Clin Breast Cancer. 2008;8:411–417. doi: 10.3816/CBC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, Mlineritsch B, Tausch C, Stierer M, Hofbauer F, Renner K, Dadak C, Rücklinger E, Samonigg H. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–1853. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Chen WY, Kroenke CH, Weltzien EK, Beasley JM, Nechuta SJ, Poole EM, Lu W, Holmes MD, Quesenberry CP, Jr, Pierce JP, Shu XO, Caan BJ. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132:729–739. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, Eisen A, Fehrenbacher L, Farrar WB, Atkins JN, Pajon ER, Vogel VG, Kroener JF, Hutchins LF, Robidoux A, Hoehn JL, Ingle JN, Geyer CE, Jr, Costantino JP, Wolmark N. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–1971. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- Netherlands Trial Register Investigation on the Duration of Extended Adjuvant Letrozole Treatment http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3077 .
- Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18 (suppl 8:S26–S35. doi: 10.1093/annonc/mdm263. [DOI] [PubMed] [Google Scholar]
- Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, Pöstlberger S, Steger GG, Seifert M, Dubsky P, Taucher S, Samonigg H, Bjelic-Radisic V, Greil R, Marth C, Gnant M. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 Trial. J Clin Oncol. 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- Pfeiler G, Stoeger H, Dubsky P, Mlineritsch B, Singer C, Balic M, Fitzal F, Moik M, Kwasny W, Selim U, Renner K, Ploner F, Steger GG, Seifert M, Hofbauer F, Sandbichler P, Samonigg H, Jakesz R, Greil R, Fesl C, Gnant M. Efficacy of tamoxifen±aminoglutethimide in normal weight and overweight postmenopausal patients with hormone-receptor positive breast cancer—an analysis of 1509 patients of the ABCSG-06 trial. Br J Cancer. 2013;108:1408–1414. doi: 10.1038/bjc.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- Schmid M, Jakesz R, Samonigg H, Kubista E, Gnant M, Menzel C, Seifert M, Haider K, Taucher S, Mlineritsch B, Steindorfer P, Kwasny W. Randomized trial of tamoxifen versus tamoxifen plus aminoglutethimide as adjuvant treatment in postmenopausal breast cancer patients with hormone receptor-positive disease: Austrian breast and colorectal cancer study group trial 6. J Clin Oncol. 2003;21:984–990. doi: 10.1200/JCO.2003.01.138. [DOI] [PubMed] [Google Scholar]
- Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wöckel A. Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res Treat. 2012;131:925–931. doi: 10.1007/s10549-011-1874-7. [DOI] [PubMed] [Google Scholar]