Abstract
Background:
Family history of cancer is associated with developing nasopharyngeal carcinoma (NPC); however, the impact of it on survival among established NPC patients remains unknown.
Methods:
We retrospectively analysed 1773 southern Chinese patients. Associations between a first-degree family history of NPC and overall survival (OS), locoregional relapse-free survival (LRFS) and distant metastasis-free survival (DMFS) were estimated by Cox regression.
Results:
Among 1773 patients, 207 (11.7%) reported a first-degree family history of NPC. Compared with patients without a family history, the adjusted hazard ratios among those with it were 0.60 (95% confidence interval (CI), 0.37–0.98; P=0.040) for OS, 0.52 (95% CI, 0.24–1.12; P=0.096) for LRFS and 0.51 (95% CI, 0.27–0.97; P=0.040) for DMFS. There were trends for improving OS, LRFS and DMFS with increasing number of affected relatives (Ptrend: 0.050, 0.114 and 0.044, respectively). But no significant benefits of family history in second- or third-degree relatives were observed. In subgroup analysis, we observed the effects of family history with restriction to male patients and those of advanced stage and treated with conventional radiotherapy and addition of chemotherapy.
Conclusion:
A first-degree family history of NPC is associated with improved survival of patients.
Keywords: family history, nasopharyngeal carcinoma, prognosis, survival
Nasopharyngeal carcinoma (NPC) is a squamous cell carcinoma that is especially prevalent in southern China. Despite improvements in the locoregional control rate due to the development of more precise imaging and radiotherapy (RT) and eradication of potential metastasis by chemotherapy, the survival of patients with advanced NPC remains unsatisfactory. Therefore, it is necessary to identify novel prognostic factors to recognise patients at high risk. As we know, Epstein–Barr virus (EBV) (Chien et al, 2001), southern Chinese tradition of consuming salted fish (Yu et al, 1986; Chang and Adami, 2006) and cigarette smoking (Xu et al, 2012) all play important roles in the aetiology. There is also substantial evidence for a hereditable component in developing NPC based on segregation studies, linkage analysis and candidate gene/genome-wide association studies (Feng et al, 2002; Hu et al, 2008; Bei et al, 2010). Importantly, family history of NPC has consistently been associated with an increased risk of developing the disease (Jia et al, 2004; Friborg et al, 2005; Jia et al, 2005; Yu et al, 2009). A large case–control study (Ren et al, 2010) even provided clear evidence that the risk of NPC was associated with a first-degree family history of cancers, including NPC and cancers of the head and neck, lung and breast. So it arouses a question – whether family history of NPC is associated with survival among patients with established NPC. Recently, the associations between family history and cancer survival have been studied in other cancers. Colon cancer (Chan et al, 2008; Zell et al, 2008) and gastric cancer (Han et al, 2012) patients with a first-degree family history showed a significant reduction in recurrence and death. Breast cancer patients with family history of breast cancer had a better prognosis as well (Malone et al, 2011). However, the association of family history and NPC survival has not been explored up to now. Therefore, we performed this study to evaluate the effect of a first-degree family history of NPC on the clinicopathologic characteristics and survival of patients with NPC.
Materials and methods
Patient characteristics
The study was reviewed and approved by the Human Ethics Approval Committee at Sun Yat-sen University Cancer Center. Between January 2005 and May 2007, 2103 NPC patients who were hospitalised at the Sun Yat-sen University Cancer Center were potentially eligible for inclusion in this retrospective study. A total of 1773 patients were eventually included who (1) were newly diagnosed, biopsy-proven, non-metastatic NPC patients without previous anticancer treatment, (2) were at the age of 20 or >20 years and born in southern China, (3) had a complete pretreatment evaluation including patient history, physical examination, haematology and biochemistry profiles, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography and a whole-body bone scan using single-photon emission computed tomography and (4) had a complete interview about family history, education and lifestyle behaviour.
Medical records were reviewed to extract data on basic characteristics including age, gender, education degree, cigarette smoking status at diagnosis (never/current/former smoker), alcohol drinking status at diagnosis (never/current/former drinker), pretreatment titre of serum immunoglobulin A against EBV viral capsid antigen (VCA-IgA), pathology (Shanmugaratnam and Sobin, 1991) and family history. Current smokers/drinkers were defined as patients who smoked/drank at diagnosis or had stopped for <1 year. Former smokers/drinkers were patients who had stopped smoking or drinking for at least 1 year before treatment. All patients were restaged according to the seventh edition of the UICC/AJCC staging system (Edge et al, 2009, 2010).
Treatment
All patients were treated by definitive RT. Details of the radiation techniques have been described previously (Ma et al, 2007; Chen et al, 2012). In addition, institutional guidelines recommended no chemotherapy for patients in stages I and II and induction, concurrent and adjuvant chemotherapy or combination treatment for those in stages III and IV. Induction or adjuvant chemotherapy consisted of cisplatin with 5-fluorouracil, cisplatin with taxoids or triplet of cisplatin and 5-fluorouracil plus taxoids every 3 weeks for two to three cycles. Concurrent chemotherapy consisted of cisplatin given on weeks 1, 4 and 7 of RT or cisplatin given weekly.
Family history assessment
Family history of cancer was ascertained by interviewing patients themselves and/or their family members at the time of case diagnosis. A positive family history was classified according to first degree, second degree or third degree. If the subjects had family history in several degrees of relatives, they were regarded as having a family history of closer degree in blood. We recorded the number of first-degree family members with NPC.
Follow-up
Patients were followed up every 3 months during the first 2 years and every 6 months thereafter until death. The assessments were performed by history and physical examination and nasopharyngoscopy at each follow-up visit. Local relapses were confirmed by biopsy, MRI scan or both. Regional relapses were diagnosed by clinical examination of the neck and, in doubtful cases, by fine-needle aspiration or an MRI scan of the neck. Distant metastases were diagnosed by clinical symptoms, physical examinations and imaging methods including chest radiography, bone scan, MRI and abdominal sonography. Patients with relapse, distant metastasis or in persistent disease were delivered with salvage treatment including reirradiation, chemotherapy and surgery. The follow-up duration was calculated from the first day of therapy to either the day of death or the day of the last examination.
Study end points
Our primary end point was overall survival (OS), defined as the time from treatment to death resulting from any cause. Secondary end points were locoregional relapse-free survival (LRFS) and distant metastasis-free survival (DMFS), defined as the time from treatment to the first locoregional relapse and distant metastasis, respectively.
Statistical methods
All end points were examined using Kaplan–Meier methods and the log-rank test (Kaplan and Meier, 1958). Univariate stratified survival analyses were performed with hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariate analyses were performed using the Cox proportional hazards model (Cox, 1972), adjusting for important prognostic factors. Comparisons of demographic, clinical and pathologic variables were performed using χ2 statistic or Fisher's exact test for nominal variables as appropriate. Two-sided P-values <0·05 were considered to be significant. All tests were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinicopathologic characteristics
A total of 1773 NPC patients were entered into this study (inclusion flow chart was presented in Supplementary Figure 1). Within a median follow-up duration of 71 months (range: 1–94 months) for the entire population and 74 months (range: 7–94 months) and 70 months (range: 1–94 months) for patients with and without a first-degree family history of NPC, 184/1773 (10.4%), 20/207 (9.7%) and 164/1566 (10.5%) patients were lost to follow up, respectively. Of these, 207/1773 (11.7%) patients had a family history of NPC in first-degree relatives and 52/1773 (2.9%) in second- and third-degree relatives. Fifteen of the 207 patients had both first- and second-/third-degree family history of NPC. Furthermore, 173/1773 (9.8%) patients had only one first-degree relative affected by NPC and 34/1773 (1.9%) had at least two affected first-degree family members.
The demographic and clinicopathologic characteristics of patients were compared according to with or without a first-degree family history of NPC (Table 1). There were no differences in the distributions of age group, gender, education degree, cigarette smoking status (never/current/former smoker), alcohol drinking status (never/current/former drinker), pretreatment titre of VCA-IgA (⩽1 : 160 vs >1 : 160), pathology (I/II/III) and radiation technique (all P-values ⩾0.171).
Table 1. Demographic and clinicopathologic characteristics by with or without an FD-FH-NPC.
| |
With FD-FH-NPC (n=207) |
Without FD-FH-NPC (n=1566) |
|
||
|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | P-value |
|
Age group (years) |
|
|
|
|
0.723 |
| 20–30 | 8 | 3.9 | 94 | 6.0 | |
| 31–40 | 62 | 30.0 | 424 | 27.1 | |
| 41–50 | 67 | 32.4 | 503 | 32.1 | |
| 51–60 | 47 | 22.7 | 363 | 23.2 | |
| ⩾61 |
23 |
11.1 |
182 |
11.6 |
|
|
Gender |
|
|
|
|
0.171 |
| Male | 148 | 71.5 | 1188 | 75.9 | |
| Female |
59 |
28.5 |
378 |
24.1 |
|
|
Education degree |
|
|
|
|
0.237 |
| Illiterate | 11 | 5.3 | 84 | 5.4 | |
| Primary | 56 | 27.1 | 516 | 33.0 | |
| Secondary | 105 | 50.7 | 763 | 48.7 | |
| Higher |
35 |
16.9 |
203 |
13.0 |
|
|
Smoking statusa |
|
|
|
|
0.806 |
| Current smoker | 77 | 37.2 | 619 | 39.5 | |
| Former smoker | 21 | 10.1 | 149 | 9.5 | |
| Never smoker |
109 |
52.7 |
798 |
51.0 |
|
|
Drinking statusa |
|
|
|
|
0.766 |
| Current drinker | 24 | 11.6 | 207 | 13.2 | |
| Former drinker | 4 | 1.9 | 25 | 1.6 | |
| Never drinker |
179 |
86.5 |
1334 |
85.2 |
|
|
VCA-IgA |
|
|
|
|
0.495 |
| ⩽1 : 160 | 107 | 51.7 | 770 | 49.2 | |
| >1 : 160 |
100 |
48.3 |
796 |
50.8 |
|
|
Pathologyb |
|
|
|
|
0.315c |
| I | 0 | 0 | 6 | 0.4 | |
| II | 7 | 3.4 | 86 | 5.5 | |
| III |
200 |
96.6 |
1474 |
94.1 |
|
|
T stage |
|
|
|
|
0.001 |
| T1 | 36 | 17.4 | 205 | 13.1 | |
| T2 | 72 | 34.8 | 398 | 25.4 | |
| T3 | 70 | 33.8 | 589 | 37.6 | |
| T4 |
29 |
14.0 |
374 |
23.9 |
|
|
N stage |
|
|
|
|
0.002 |
| N0 | 50 | 24.2 | 263 | 16.8 | |
| N1 | 124 | 59.9 | 883 | 56.4 | |
| N2 | 29 | 14.0 | 377 | 24.1 | |
| N3 |
4 |
1.9 |
43 |
2.7 |
|
|
Clinical stage |
|
|
|
|
<0.001 |
| I | 16 | 7.7 | 71 | 4.5 | |
| II | 76 | 36.7 | 396 | 25.3 | |
| III | 82 | 39.6 | 690 | 44.1 | |
| IVa | 29 | 14.0 | 366 | 23.4 | |
| IVb |
4 |
1.9 |
43 |
2.7 |
|
|
Chemotherapy regimens |
|
|
|
|
<0.001c |
| None | 70 | 33.8 | 357 | 22.8 | |
| IC | 27 | 13.0 | 412 | 26.3 | |
| CCRT | 69 | 33.3 | 429 | 27.4 | |
| IC+CCRT | 33 | 15.9 | 292 | 18.6 | |
| CCRT+AC | 5 | 2.4 | 58 | 3.7 | |
| IC+CCRT+AC |
3 |
1.4 |
18 |
1.1 |
|
|
Radiation technique |
|
|
|
|
0.943c |
| IMRT | 49 | 23.7 | 375 | 23.9 | |
| 3DCRT | 5 | 2.4 | 31 | 2.0 | |
| Conventional | 153 | 73.9 | 1160 | 74.1 | |
Abbreviations: AC=adjuvant chemotherapy; CCRT=concurrent chemoradiotherapy; FD-FH-NPC=first-degree family history of nasopharyngeal carcinoma; IC=induction chemotherapy; IMRT=intensity-modulated radiotherapy; VCA-IgA=immunoglobulin A against Epstein–Barr virus viral capsid antigen; WHO=World Health Organization; 3DCRT=three-dimensional conformal radiotherapy.
Current smokers/drinkers are defined as patients who smoke/drink at diagnosis or have stopped for <1 year. Former smokers/drinkers are patients who have stopped smoking or drinking for at least 1 year before treatment.
Based on the criteria of WHO histological type (1991): I=squamous cell carcinomas; II=differentiated non-keratinising carcinoma; III=undifferentiated non-keratinising carcinoma.
P-values by Fisher's exact probability test and all others by Pearson's χ2 test.
Significant differences were observed in T stage, N stage, clinical stage and chemotherapy regimens. For patients with a first-degree family history of NPC, the proportion of advanced T stage, N stage and clinical stage was lower, and accordingly, the proportion of adding chemotherapy to RT was also lower that than for those without a family history of NPC.
Effect of family history on survival
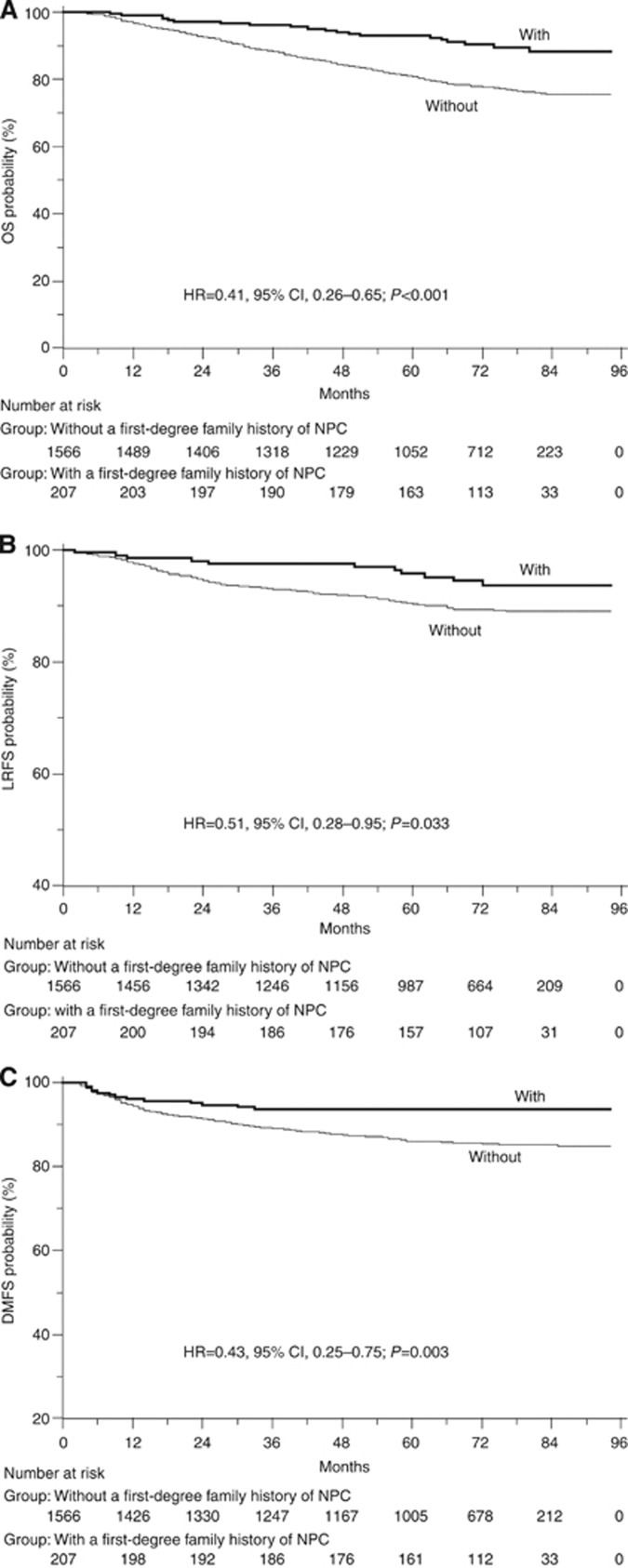
A first-degree family history of NPC was associated with a significant reduction in the risk of death (Figure 1). The 5-year OS rate was 92.6% for patients with a first-degree family history compared with 80.7% for those without such a profile (HR=0.41, 95% CI, 0.26–0.65; P<0.001). This remained unchanged after accounting for other important prognostic factors, including age group (categorical), gender, education degree (categorical), smoking status (never/current/former smoker), drinking status (never/current/former drinker), titre of VCA-IgA (⩽1 : 160 vs >1 : 160), pathology (I/II/III), T stage (T1/T2/T3/T4), N stage (N0/N1/N2/N3), chemotherapy regimens and radiation technique. Compared with patients without a first-degree family history, those with a family history of NPC had a multivariate HR of 0.60 (95% CI, 0.37–0.98; P=0.040) for death (Table 2). Furthermore, the results for the risk of locoregional relapse and distant metastasis were quite similar to those for death. In univariate analysis, a first-degree family history of NPC was associated with improved LRFS (unadjusted HR=0.51, 95% CI, 0.28–0.95; P=0.033) and DMFS (unadjusted HR=0.43, 95% CI, 0.25–0.75; P=0.003) (Figure 1). In multivariate analyses, adjusted HRs for LRFS and DMFS were 0.52 (95% CI, 0.24–1.12; P=0.096) and 0.51 (95% CI, 0.27–0.97; P=0.040) (Table 2).
Figure 1.
(A) OS, (B) LRFS and (C) DMFS curves of patients with and without a first-degree family history of NPC. .
Table 2. Multivariate analysis for OS, LRFS and DMFSa.
| |
|
OS |
LRFS |
DMFS |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. at risk | No. of events | aHR (95% CI) | P-value | No. of events | aHR (95% CI) | P-value | No. of events | aHR (95% CI) | P-value | |
|
FD-FH-NPC | ||||||||||
| Without | 1566 | 338 | 1.00 | 148 | 1.00 | 213 | 1.00 | |||
| With |
207 |
20 |
0.60 (0.37–0.98) |
0.040 |
11 |
0.52 (0.24–1.12) |
0.096 |
13 |
0.51 (0.27–0.97) |
0.040 |
|
A second- or third-degree family history of NPC | ||||||||||
| No | 1721 | 352 | 1.00 | 157 | 1.00 | 224 | 1.00 | |||
| Yes |
52 |
6 |
0.92 (0.41–2.08) |
0.841 |
2 |
0.61 (0.15–2.47) |
0.486 |
2 |
0.39 (0.10–1.58) |
0.186 |
|
Number of first-degree relatives with NPC | ||||||||||
| 0 | 1566 | 338 | 1.00 | 148 | 1.00 | 213 | 1.00 | |||
| 1 | 173 | 17 | 0.61 (0.36–1.02) | 0.059 | 9 | 0.52 (0.23–1.18) | 0.118 | 11 | 0.53 (0.27–1.03) | 0.062 |
| ⩾2 |
34 |
3 |
0.57 (0.14–2.28) |
0.423 |
2 |
0.54 (0.07–3.88) |
0.538 |
2 |
0.41 (0.06–2.91) |
0.369 |
|
FD-FH-NPC in stage I and II patients | ||||||||||
| Without | 467 | 43 | 1.00 | 31 | 1.00 | 22 | 1.00 | |||
| With |
92 |
3 |
0.31 (0.07–1.34) |
0.117 |
4 |
0.39 (0.05–2.99) |
0.364 |
2 |
0.31 (0.04–2.35) |
0.257 |
|
FD-FH-NPC in stage III and IV patients | ||||||||||
| Without | 1099 | 295 | 1.00 | 117 | 1.00 | 191 | 1.00 | |||
| With | 115 | 17 | 0.57 (0.34–0.95) | 0.033 | 7 | 0.54 (0.24–1.23) | 0.143 | 11 | 0.49 (0.25–0.96) | 0.036 |
Abbreviations: aHR=adjusted hazard ratio; CI=confidence interval; DMFS=distant metastasis-free survival; FD-FH-NPC=first-degree family history of nasopharyngeal carcinoma; LRFS=locoregional relapse-free survival; NPC=nasopharyngeal carcinoma; OS=overall survival.
Multivariate HRs and 95% CIs are adjusted for age group (categorical), gender, education degree (categorical), smoking status (never/current/former smoker), drinking status (never/current/former drinker), pathology (I/II/III), T stage (T1/T2/T3/T4), N stage (N0/N1/N2/N3), titre of VCA-IgA (⩽1 : 160 vs >1 : 160), chemotherapy regimens and radiation technique.
To specify the influence of family history on survival, we conducted second analyses (Table 2). First, compared with patients without a first-degree family history of NPC, those with one affected relative had a multivariate HR of 0.61 (95% CI, 0.36–1.02; P=0.059) for OS, 0.52 (95% CI, 0.23–1.18; P=0.118) for LRFS and 0.53 (95% CI, 0.27–1.03; P=0.062) for DMFS. For participants with two or more affected first-degree relatives, we observed a multivariate HR of 0.57 (95% CI, 0.14–2.28; P=0.423) for OS, 0.54 (95% CI, 0.07–3.88; P=0.538) for LRFS and 0.41 (95% CI, 0.06–2.91; P=0.369) for DMFS. So there was a trend for reduction of overall mortality, locoregional relapse and distant metastasis with an increasing number of affected family members (P-values for trend were 0.050, 0.114 and 0.044, respectively). Second, compared with patients without a second- or third-degree family history of NPC, those with such a profile had a multivariate HR of 0.92 (95% CI, 0.41–2.08; P=0.841) for OS, 0.61 (95% CI, 0.15–2.47; P=0.486) for LRFS and 0.39 (95% CI, 0.10–1.58; P=0.186) for DMFS. Hence, second- and third-degree family history of NPC was not significantly associated with survival. Finally, we further addressed the concern that patients with a family history of NPC might have a different prognosis related to earlier detection of their cancers. Among stage III and IV patients, we observed a multivariate HR of 0.57 (95% CI, 0.34–0.95; P=0.033) for OS, 0.54 (95% CI, 0.24–1.23; P=0.143) for LRFS and 0.49 (95% CI, 0.25–0.96; P=0.036) for DMFS when comparing patients with or without a first-degree family history of NPC. These results were quite similar to those for the entire population. Among stage I and II patients, the adjusted HRs for OS, LRFS and DMFS were 0.31 (95% CI, 0.07–1.34; P=0.117), 0.39 (95% CI, 0.05–2.99; P=0.364) and 0.31 (95% CI, 0.04–2.35; P=0.257), respectively. The OS curves of patients in stages I plus II, III and IV with or without a first-degree family history of NPC were presented in Supplementary Figure 2.
In addition, we also assessed the association between a first-degree family history of NPC and OS across strata of other potential predictors of patient outcome (Table 3). The effect of family history on the risk of death was not significantly modified by age, education degree, smoking and drinking status or titre of VCA-IgA. However, the benefit of family history in decreasing risk of death was restricted to male patients (adjusted HR=0.55, 95% CI, 0.30–0.98; P=0.043). Moreover, a family history of NPC had significant association with OS among patients treated with addition of chemotherapy to RT and with conventional radiation technique (adjusted HR=0.57, 95% CI, 0.34–0.95, P=0.033 and adjusted HR=0.55, 95% CI, 0.31–0.96, P=0.037, respectively).
Table 3. Subgroup analysis of OS by patients' characteristics in terms of with or without an FD-FH-NPC.
| |
|
|
No. of events/No. at risk |
|
|
|
|---|---|---|---|---|---|---|
| Factor | Five-year OS rate (%) | P for OS by each factor | Without FD-FH-NPC | With FD-FH-NPC | aHR (95% CI)a | P for OS between with and without FD-FH-NPC |
| Overall |
82.2 |
|
338/1566 |
20/207 |
0.60 (0.37–0.98) |
0.040 |
|
Age (years) |
|
<0.001 |
|
|
|
|
| ⩽53 | 86.3 | 197/1159 | 12/158 | 0.67 (0.37–1.21) | 0.187 | |
| >53 |
70.1 |
|
141/407 |
8/49 |
0.50 (0.20–1.23) |
0.131 |
|
Gender |
|
0.005 |
|
|
|
|
| Male | 80.5 | 276/1188 | 12/148 | 0.55 (0.30–0.98) | 0.043 | |
| Female |
87.2 |
|
62/378 |
8/59 |
0.83 (0.33–2.10) |
0.690 |
|
Education |
|
<0.001 |
|
|
|
|
| Illiterate and primary | 77.3 | 163/600 | 9/67 | 0.48 (0.21–1.09) | 0.079 | |
| Secondary and higher |
85.1 |
|
175/966 |
11/140 |
0.72 (0.39–1.35) |
0.306 |
|
Smoking status |
|
<0.001 |
|
|
|
|
| Yes | 76.2 | 219/768 | 15/98 | 0.74 (0.41–1.34) | 0.324 | |
| No |
87.9 |
|
119/798 |
5/109 |
0.41 (0.17–1.02) |
0.056 |
|
Drinking status |
|
0.056 |
|
|
|
|
| Yes | 78.5 | 61/232 | 3/28 | 0.53 (0.13–2.26) | 0.393 | |
| No |
82.8 |
|
277/1334 |
17/179 |
0.65 (0.39–1.11) |
0.112 |
|
VCA-IgA |
|
0.011 |
|
|
|
|
| ⩽1 : 160 | 84.5 | 148/770 | 8/107 | 0.45 (0.18–1.10) | 0.081 | |
| >1 : 160 |
79.9 |
|
190/796 |
12/100 |
0.73 (0.40–1.32) |
0.294 |
|
Clinical stage |
|
<0.001 |
|
|
|
|
| I+II | 92.9 | 43/467 | 3/92 | 0.31 (0.07–1.34) | 0.117 | |
| III+IV |
77.1 |
|
295/1099 |
17/115 |
0.57 (0.34–0.95) |
0.033 |
|
Chemotherapy |
|
<0.001 |
|
|
|
|
| Yes | 80.1 | 280/1209 | 16/136 | 0.57 (0.34–0.95) | 0.030 | |
| No |
88.6 |
|
58/357 |
4/71 |
0.51 (0.18–1.45) |
0.203 |
|
Radiation technique |
|
0.003 |
|
|
|
|
| IMRT/3DCRT | 86.9 | 63/406 | 5/54 | 0.79 (0.28–2.23) | 0.656 | |
| Conventional | 80.5 | 275/1160 | 15/153 | 0.55 (0.31–0.96) | 0.037 | |
Abbreviations: aHR=adjusted hazard ratio; CI=confidence interval; FD-FH-NPC=first-degree family history of nasopharyngeal carcinoma; IMRT=intensity-modulated radiotherapy; OS=overall survival; VCA-IgA=immunoglobulin A against Epstein–Barr virus viral capsid antigen; 3DCRT=three-dimensional conformal radiotherapy.
Multivariate HRs and 95% CIs are adjusted for age group (categorical), gender, education degree (categorical), smoking status (never/current/former smoker), drinking status (never/current/former drinker), pathology (I/II/III), T stage (T1/T2/T3/T4), N stage (N0/N1/N2/N3), titre of VCA-IgA (⩽1 : 160 vs >1 : 160), chemotherapy regimens and radiation technique.
Discussion
Several studies have demonstrated that family history of NPC increased the risk of developing NPC (Jia et al, 2004; Friborg et al, 2005; Jia et al, 2005; Yu et al, 2009). However, to date, no studies have examined the influence of family history of NPC on subsequent outcomes in patients with established cancer. This study included 1773 NPC patients, among which 207 (11.7%) had a family history of NPC in first-degree relatives. We demonstrated that a first-degree family history of NPC was associated with a significant reduction in risk of death, locoregional relapse and distant metastasis, and it remained unchanged after adjusting for known prognostic factors. Our findings were quite similar to previous studies in colorectal cancer (Zell et al, 2008; Morris et al, 2013), colon cancer (Chan et al, 2008), gastric cancer (Han et al, 2012) and breast cancer (Malone et al, 2011).
We then examined the effects according to the number of affected first-degree family members; resultantly, a significant trend for improvement in DMFS and OS with an increasing number of first-degree family members was observed. Whereas in second- or third-degree relatives, the effects of family history were not observed, which were consistent with reports in gastric cancer (Han et al, 2012) and breast cancer (Malone et al, 1996, 2011). Further investigations are required because this nonsignificant differential survival seemed to be driven by the small sample size of cases with a second- or third-degree family history of NPC. Additionally, we performed stratified analysis according to clinical stage and found significant effects of family history only in locoregionally advanced stage patients. This may mainly result from a large number of patients and high event rates in the advanced clinical stage stratum; the interactions between clinical stage and a first-degree family history of NPC (P<0.001) may also contribute to it. Individuals with a family history of the disease in first-degree relatives might be more likely to undergo regular NPC surveillance, such as Epstein–Barr virus antibodies and DNA copies, and had relatively early clinical stage as shown in Table 1. Despite of adjusting for smoking status and other prognostic factors, we found that the effects of family history were restricted to male patients. A recent study (Lu et al, 2013) revealed that the female sex was positively associated with an early T stage, N stage and clinical stage; reduced disease progression and cancer-related deaths and was a favourable independent prognostic factor. This suggested that some intrinsic features of the female sex should interact and confound the effects of family history. But we detected no interactions between gender and a first-degree family history of NPC (P=0.366). Therefore, the significant differences of the effects of a first-degree family history in male and female patients seem to be true if the influence of small sample size in male stratum is excluded. Moreover, we found that the effect of family history was restricted in patients treated with addition of chemotherapy to RT. Unfortunately, there were significant interactions between chemotherapy (yes/no) and a first-degree family history of NPC (P=0.046). As shown in Table 1, patients with a family history of NPC had an obvious higher proportion of RT alone treatment than patients without a family history of NPC. So it remains uncertain whether the favourable prognostic value of family history is applicable to patients with RT alone. Finally, the effect of family history was observed only in patients treated with conventional radiation technique, and no interaction between radiation technique and a first-degree family history of NPC was showed (P=0.553) when we include an interaction term in the model. This differentiation was mainly due to a higher proportion of advanced stage patients among this group than those treated with intensity-modulated radiotherapy (IMRT) and three-dimensional conformal radiotherapy (3DCRT). Meanwhile, there were low proportion of patients treated with IMRT/3DCRT and a few events in the group of positive family history. This may partially explain no effects of family history in patients treated with IMRT/3DCRT.
A major limitation of our study is that we relied on self-reported family history and might possibly misclassify family history status, especially under-reporting the second-degree family history. But prior studies have demonstrated such data to be reliable (Aitken et al, 1995; Kerber and Slattery, 1997). Another limitation is that we could not collect information on the age at which the first-degree relative was diagnosed with NPC, as the effect of family history might be different according to it. The third concern was that we failed to include data regarding pretreatment plasma EBV DNA concentrations, which had been demonstrated to strongly predict survival of NPC (Lo et al, 2000; Lin et al, 2004). However, we performed stratified analysis according to pretreatment serum EBV VCA-IgA antibody, another significant prognostic factor for survival (Ling et al, 2009), albeit DNA concentrations seemed to be superior to it in prognostic predictions for NPC (Twu et al, 2007). Studies based on EBV DNA concentrations are being planned. Moreover, the chemotherapy regimens were not totally identified with the latest NCCN guideline for different criteria of staging systems and development of treatment regimen. During the period when patients were treated, many patients were encouraged to participate in randomised trials, which also resulted in heterogeneous treatment strategies. But we conducted multivariate analyses accounting for RT technique and chemotherapy regimens. Additionally, the smoking and drinking status questionnaire was not administered during follow-up; we were unable to fully adjust for the confounding effects by smoking and drinking status at diagnosis alone. But to date, no studies have demonstrated the role of cigarette smoking or alcohol intake during or after treatment in affecting survival of NPC. In this study, we accounted for socioeconomic status by education degree alone; this limitation cannot be neglected as well. We are planning to detect the prognostic value of pretreatment body mass index – another factor partially representing socioeconomic status.
More importantly, it is not clear why a first-degree family history of NPC affects survival of patients. Our study showed more early-stage patients among those with a family history of NPC, which strongly supports an assumption of surveillance monitoring for individuals with a family history of NPC. Health-related behaviour may also explain our observations, as found in gastric cancer (Han et al, 2012). Yet we here did not observe significant differences in the proportion of smokers or drinkers in patients with or without a family history of NPC. Underlying molecular and pathogenic differences might play an intrinsic role in the effect of family history, although we observed no significantly different distribution of VCA-IgA and pathology in patients with and without a family history of NPC. Further basic researches into genetic differences were needed to fully elucidate the potential mechanisms.
To summarise, this study, as the first one, showed better survival for patients who had a first-degree family history of NPC than for patients without this profile. Further studies are needed to explore the biological, genetic or behavioural differences in NPC according to the presence of family history.
Acknowledgments
The authors received no external funding for this study. We greatly thank Jin-Xin Zhang and Xing-Hua Ma (Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University) for their assistance in statistical analysis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc).
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aitken J, Bain C, Ward M, Siskind V, MacLennan R. How accurate is self-reported family history of colorectal cancer. Am J Epidemiol. 1995;141 (9:863–871. doi: 10.1093/oxfordjournals.aje.a117522. [DOI] [PubMed] [Google Scholar]
- Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, Feng QS, Low HQ, Zhang H, He F, Tai ES, Kang T, Liu ET, Liu J, Zeng YX. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42 (7:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- Chan JA, Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Schaefer P, Whittom R, Hantel A, Goldberg RM, Warren RS, Bertagnolli M, Fuchs CS. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. JAMA. 2008;299 (21:2515–2523. doi: 10.1001/jama.299.21.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15 (10:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- Chen L, Mao Y-P, Xie F-Y, Liu L-Z, Sun Y, Tian L, Tang L-L, Lin A-H, Li L, Ma J. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol. 2012;104 (3:331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS. Serologic markers of Epstein–Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345 (26:1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A.2009AJCC Cancer Staging Manual7th edn.Springer: New York [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A.2010AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual7th edn.Springer: New York [Google Scholar]
- Feng BJ, Huang W, Shugart YY, Lee MK, Zhang F, Xia JC, Wang HY, Huang TB, Jian SW, Huang P, Feng QS, Huang LX, Yu XJ, Li D, Chen LZ, Jia WH, Fang Y, Huang HM, Zhu JL, Liu XM, Zhao Y, Liu WQ, Deng MQ, Hu WH, Wu SX, Mo HY, Hong MF, King MC, Chen Z, Zeng YX. Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet. 2002;31 (4:395–399. doi: 10.1038/ng932. [DOI] [PubMed] [Google Scholar]
- Friborg J, Wohlfahrt J, Koch A, Storm H, Olsen OR, Melbye M. Cancer susceptibility in nasopharyngeal carcinoma families – a population-based cohort study. Cancer Res. 2005;65 (18:8567–8572. doi: 10.1158/0008-5472.CAN-04-4208. [DOI] [PubMed] [Google Scholar]
- Han MA, Oh MG, Choi IJ, Park SR, Ryu KW, Nam BH, Cho SJ, Kim CG, Lee JH, Kim YW. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol. 2012;30 (7:701–708. doi: 10.1200/JCO.2011.35.3078. [DOI] [PubMed] [Google Scholar]
- Hu LF, Qiu QH, Fu SM, Sun D, Magnusson K, He B, Lindblom A, Ernberg I. A genome-wide scan suggests a susceptibility locus on 5p 13 for nasopharyngeal carcinoma. Eur J Hum Genet. 2008;16 (3:343–349. doi: 10.1038/sj.ejhg.5201951. [DOI] [PubMed] [Google Scholar]
- Jia WH, Collins A, Zeng YX, Feng BJ, Yu XJ, Huang LX, Feng QS, Huang P, Yao MH, Shugart YY. Complex segregation analysis of nasopharyngeal carcinoma in Guangdong, China: evidence for a multifactorial mode of inheritance (complex segregation analysis of NPC in China) Eur J Hum Genet. 2005;13 (2:248–252. doi: 10.1038/sj.ejhg.5201305. [DOI] [PubMed] [Google Scholar]
- Jia WH, Feng BJ, Xu ZL, Zhang XS, Huang P, Huang LX, Yu XJ, Feng QS, Yao MH, Shugart YY, Zeng YX. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer. 2004;101 (2:363–369. doi: 10.1002/cncr.20372. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case–control study. Am J Epidemiol. 1997;146 (3:244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350 (24:2461–2470. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- Ling W, Cao SM, Huang QH, Li YH, Deng MQ. Prognostic implication of pretreatment titer of serum immunoglobulin A against Epstein–Barr virus capsid antigen in nasopharyngeal carcinoma patients in Sihui, Guangdong. Ai Zheng. 2009;28 (1:57–59. [PubMed] [Google Scholar]
- Lo YM, Chan AT, Chan LY, Leung SF, Lam CW, Huang DP, Johnson PJ. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein–Barr virus DNA. Cancer Res. 2000;60 (24:6878–6881. [PubMed] [Google Scholar]
- Lu X, Wang FL, Guo X, Wang L, Zhang HB, Xia WX, Li SW, Li NW, Qian CN, Xiang YQ. Favorable prognosis of female patients with nasopharyngeal carcinoma. Chin J Cancer. 2013;32 (5:283–288. doi: 10.5732/cjc.012.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, Cui N, Cui C, Li L. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res. 2007;13 (5:1445–1452. doi: 10.1158/1078-0432.CCR-06-2059. [DOI] [PubMed] [Google Scholar]
- Malone KE, Daling JR, Doody DR, O'Brien C, Resler A, Ostrander EA, Porter PL. Family history of breast cancer in relation to tumor characteristics and mortality in a population-based study of young women with invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20 (12:2560–2571. doi: 10.1158/1055-9965.EPI-11-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone KE, Daling JR, Weiss NS, McKnight B, White E, Voigt LF. Family history and survival of young women with invasive breast carcinoma. Cancer. 1996;78 (7:1417–1425. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1417::AID-CNCR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Penegar S, Whitehouse LE, Quirke P, Finan P, Bishop DT, Wilkinson J, Houlston RS. A retrospective observational study of the relationship between family history and survival from colorectal cancer. Br J Cancer. 2013;108 (7:1502–1507. doi: 10.1038/bjc.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z-F, Liu W-S, Qin H-D, Xu Y-F, Yu D-D, Feng Q-S, Chen L-Z, Shu X-O, Zeng Y-X, Jia W-H. Effect of family history of cancers and environmental factors on risk of nasopharyngeal carcinoma in Guangdong, China. Cancer Epidemiol. 2010;34 (4:419–424. doi: 10.1016/j.canep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam K, Sobin LH.1991International Histological Classification of Tumours2nd ednWHO [Google Scholar]
- Twu CW, Wang WY, Liang WM, Jan JS, Jiang RS, Chao J, Jin YT, Lin JC. Comparison of the prognostic impact of serum anti-EBV antibody and plasma EBV DNA assays in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;67 (1:130–137. doi: 10.1016/j.ijrobp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Xu FH, Xiong D, Xu YF, Cao SM, Xue WQ, Qin HD, Liu WS, Cao JY, Zhang Y, Feng QS, Chen LZ, Li MZ, Liu ZW, Liu Q, Hong MH, Shugart YY, Zeng YX, Zeng MS, Jia WH. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein–Barr virus activation. J Natl Cancer Inst. 2012;104 (18:1396–1410. doi: 10.1093/jnci/djs320. [DOI] [PubMed] [Google Scholar]
- Yu KJ, Hsu WL, Chiang CJ, Cheng YJ, Pfeiffer RM, Diehl SR, Goldstein AM, Gravitt PE, Chen CJ, Hildesheim A. Cancer patterns in nasopharyngeal carcinoma multiplex families in Taiwan. Int J Cancer. 2009;124 (7:1622–1625. doi: 10.1002/ijc.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC, Ho JH, Lai SH, Henderson BE. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case–control study in Hong Kong. Cancer Res. 1986;46 (2:956–961. [PubMed] [Google Scholar]
- Zell JA, Honda J, Ziogas A, Anton-Culver H. Survival after colorectal cancer diagnosis is associated with colorectal cancer family history. Cancer Epidemiol Biomarkers Prev. 2008;17 (11:3134–3140. doi: 10.1158/1055-9965.EPI-08-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.