Abstract
Background:
Aside from tumour stage and treatment, little is known about potential factors that may influence survival in colorectal cancer patients. The aim of this study was to investigate the associations between physical activity, obesity and smoking and disease-specific and overall mortality after a colorectal cancer diagnosis.
Methods:
A cohort of 879 colorectal cancer patients, diagnosed in Western Australia between 2005 and 2007, were followed up to 30 June 2012. Cox's regression models were used to estimate the hazard ratios (HR) for colorectal cancer-specific and overall mortality associated with self-reported pre-diagnosis physical activity, body mass index (BMI) and smoking.
Results:
Significantly lower overall and colorectal cancer-specific mortality was seen in females who reported any level of recent physical activity than in females reporting no activity. The colorectal cancer-specific mortality HR for increasing levels of physical activity in females were 0.34 (95% CI=0.15, 0.75), 0.37 (95% CI=0.17, 0.81) and 0.41 (95% CI=0.18, 0.90). Overweight and obese women had almost twice the risk of dying from any cause or colorectal cancer compared with women of normal weight. Females who were current smokers had worse overall and colorectal cancer-specific mortality than never smokers (overall HR=2.64, 95% CI=1.18, 5.93; colorectal cancer-specific HR=2.70, 95% CI=1.16, 6.29). No significant associations were found in males.
Conclusion:
Physical activity, BMI and smoking may influence survival after a diagnosis of colorectal cancer, with more pronounced results found for females than for males.
Keywords: physical activity, obesity, smoking, colorectal cancer, survival
Although colorectal cancer survival has improved in the last few decades, due partly to earlier detection and more effective treatments (Faivre-Finn et al, 2002), colorectal cancer is still one of the major causes of cancer deaths in developed countries such as Australia (Australian Institute of Health and Welfare and Australasian Association of Cancer Registries, 2010; Ferlay et al, 2010), The main predictors of colorectal cancer prognosis are tumour stage and treatment (Zlobec and Lugli, 2008), but little is known about other potential factors influencing survival in colorectal cancer patients.
Lifestyle factors such as physical activity, obesity and cigarette smoking have been shown to be associated with the risk of colon and/or rectal cancers (World Cancer Research Fund and American Institute for Cancer Research, 2007; Boyle et al, 2012b), and in recent years the effect that these factors have on the prognosis of colorectal cancer has started to receive research attention. However, a recent review of the literature concerning the role of body mass index (BMI) and physical activity in colorectal cancer survival concluded that no firm conclusions could be drawn from the limited number of studies to date (Vrieling and Kampman, 2010). There is also limited research about the effect of smoking on colorectal cancer survival (Phipps et al, 2011).
The aim of this study was to investigate the association between modifiable lifestyle factors (pre-diagnosis physical activity, obesity and smoking) and disease-specific and overall survival after a diagnosis of colorectal cancer.
Materials and methods
The Western Australia Bowel Health Study
The participants in this cohort study were the 918 cases from a case–control study of colorectal cancer (The Western Australian Bowel Health Study; WABOHS) that was conducted in Western Australia between 2005 and 2007 (Clapin et al, 2012). The patients in WABOHS were males and females aged between 40 and 79 years and were recruited from the Western Australia Cancer Registry soon after diagnosis. The median time from diagnosis to completion of the study questionnaire was 113 days (interquartile range 94–139 days). Of the 1544 eligible patients invited to participate in the WABOHS, 918 (59.5%) participated. Notification of all cancers, excluding non-melanoma skin cancer, is mandatory in Western Australia. The participants completed self-administered questionnaires that asked about lifestyle and medical risk factors for colorectal cancer, such as recreational physical activity, smoking history, height, weight, medication use and diet. Follow-up of the 918 patients began at the date of colorectal cancer diagnosis and ended at death or 30 June 2012, whichever came first. This survival study and the WABOHS received approval from the Human Research Ethics Committees at the Western Australian Department of Health and The University of Western Australia. Informed consent was obtained from all participants in the WABOHS.
Exposure measurements
To account for possible changes in behaviour as a result of colorectal cancer symptoms and/or diagnosis, BMI and smoking status were determined 1 year before study enrolment, and physical activity performed in the 1 year before study invitation was excluded.
Information about recreational physical activity performed during three age periods (19–34 years, 35–50 years and ⩾51 years) was collected using a questionnaire based on others that have been shown to be reliable (Friedenreich et al, 1998; Chasan-Taber et al, 2002). More information about the coding of the physical activity data can be found elsewhere (Boyle et al, 2011a). In brief, data from the physical activity questionnaire were used to calculate total, moderate-intensity and vigorous-intensity metabolic-equivalent hours (MET-hours) per week for each age period. Activities were classified as moderate intensity or vigorous intensity according to their respective metabolic-equivalent value (Ainsworth et al, 2000). Physical activity performed in the age period that the participant was in at the time of study enrolment was considered to be ‘recent recreational physical activity'. Total recent recreational physical activity was categorised as 0, 0.1–11.9, 12–29.9 and ⩾30 MET-hours per week. Recent moderate-intensity activity and recent vigorous-intensity activity were both categorised as 0, 0.1–5.9, 6–17.9 and ⩾18 MET-hours per week. A ‘lifetime recreational physical activity' variable was also created for total, moderate-intensity and vigorous-intensity physical activity. This variable categorised participants as always low activity or as high activity in one-, two- or all-age periods (Boyle et al, 2011a).
We also looked at the association between resistance training and sedentary behaviour, and disease-specific and overall survival after a diagnosis of colorectal cancer. For resistance training, participants were categorised as having definitely, possibly or not performed resistance training over the lifetime, based on the name of the activities they listed on the physical activity questionnaire (Boyle et al, 2012a). Sedentary behaviour was measured in the occupational domain using a classification system based on job title (U.S. Department of Labor, 1991; Boyle et al, 2011b). This method of classifying occupational activity has been shown to have good agreement with self-reported job activity, particularly for sedentary (sitting) and light (standing) jobs (Boyle and Leong, 2012). Participants were classified as having spent 0 years, >0 years but <10 years, or ⩾10 years, in sedentary work.
Information on height and weight 1 year before study enrolment was collected, and these data were used to calculate BMI. Body mass index was classified as normal weight (BMI<25 kg m−2), overweight (25⩽BMI<30 kg m−2) or obese (BMI⩾30 kg m−2). Very few participants (n=3) were underweight (BMI<17.5 kg m−2), so these participants were included in the normal weight category.
Participants were classified as current, former or never smokers 1 year before study enrolment based on the self-reported information collected in the WABOHS.
Outcome measurement
Date and cause of deaths in the cohort were identified by linkage to the Western Australian Cancer Registry and the Western Australian Registry of Births, Deaths and Marriages. Cause of death was coded by the Australian Bureau of Statistics and/or the Western Australian Cancer Registry. Deaths that were coded as having been caused by colorectal cancer by either of these sources were classified as colorectal cancer-specific deaths in this study.
Statistical analysis
Cox's proportional hazard regression models were used to estimate the hazard ratios (HR) for colorectal cancer-specific mortality and overall mortality associated with physical activity, BMI and smoking. Age, sex, socioeconomic status, tumour stage and diabetes were considered to be potential confounders and were included as covariates in all models, and physical activity, BMI and smoking were mutually adjusted for each other. Socioeconomic status was based on the residential postcode and was classified using the Index of Relative Socio-economic Disadvantage from the Socio-Economic Indexes for Areas (Australian Bureau of Statistics, 2008). Information on tumour stage was collected from pathology or medical records and was classified as stage I, II, III or IV, or unknown. Participants were classified as having diabetes, high blood sugar levels or neither, based on self-report. Where appropriate, tests for trend were conducted by entering ordinal categorical variables into the model as continuous variables. Proportional hazards assumptions were tested using scaled Schoenfeld residuals (Schoenfeld, 1982). No violations of the proportionality assumption were observed for any of the covariates included in the colorectal cancer-specific or overall mortality models.
We repeated the analyses stratified by sex, cancer site (colon or rectum) and cancer stage (stage I–III or stage IV). We also added interaction terms to non-stratified models to identify whether sex, cancer site or cancer stage significantly modified the associations between mortality and physical activity, BMI and/or smoking. Participants with unknown cancer stage were excluded from the stage-stratified and stage-exposure interaction analyses.
Kaplan–Meier curves of colorectal cancer-specific survival across physical activity, BMI and smoking categories were generated for males and females separately.
Missing data from one or more of the exposure variables resulted in 39 participants being excluded, leaving 879 participants in this study. Stata 11.2 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
A total of 224 deaths (155 males, 69 females) occurred during follow-up, of which 187 (128 male, 59 females) were due to colorectal cancer. Median follow-up time was 5.9 years in those participants still alive at the end of follow-up, and 5.6 years among all participants.
The demographic, lifestyle and clinical characteristics of the participants are shown in Table 1. The median age of the 879 participants (542 males, 337 females) at diagnosis was 65 years. Almost 15% of the participants reported doing no recent recreational physical activity, 10% were current smokers and 50% were former smokers. Almost 40% were overweight and ∼30% were obese 1 year before study enrolment.
Table 1. Lifestyle, demographic and clinical characteristics of the 879 patients.
| Characteristic | n (%) |
|---|---|
|
Sex | |
| Female | 337 (38.3) |
| Male |
542 (61.7) |
| Age, years (median, interquartile range) |
65, 59–72 |
|
Total recent recreational physical activity (MET-hours per week; %) | |
| 0 | 130 (14.8) |
| 0.1–8.9 | 229 (26.1) |
| 9–23.9 | 264 (30.0) |
| 24–41.9 |
256 (29.1) |
|
Body mass index 1 year ago (%) | |
| <25 | 283 (32.2) |
| 25–29.9 | 338 (38.5) |
| ⩾30 |
258 (29.4) |
|
Smoking status (%) | |
| Never | 347 (39.5) |
| Former | 444 (50.5) |
| Current |
88 (10.0) |
|
Cancer site (%) | |
| Colon | 558 (63.5) |
| Rectum |
321 (36.5) |
|
Stage (%) | |
| One | 264 (30.0) |
| Two | 220 (25.0) |
| Three | 239 (27.2) |
| Four | 47 (5.3) |
| Unknown |
109 (12.4) |
|
Diabetes (%) | |
| No | 701 (79.7) |
| High blood sugar | 49 (5.6) |
| Diabetes |
129 (14.7) |
|
Socioeconomic disadvantage (%) | |
| 1 (Most disadvantaged) | 153 (17.4) |
| 2 | 179 (20.4) |
| 3 | 177 (20.1) |
| 4 | 193 (22.0) |
| 5 (Least disadvantaged) | 177 (20.1) |
Abbreviation: MET-hours=metabolic-equivalent hours.
As expected, cancer stage was strongly associated with colorectal cancer-specific mortality (Table 2). The association between stage and all-cause mortality was also strong, although slightly attenuated compared with colorectal cancer mortality. Males had a poorer overall and colorectal cancer-specific mortality than females, although the difference was not statistically significant.
Table 2. Adjusted HR for the association between lifestyle, demographic and clinical characteristics and overall and colorectal cancer-specific mortality.
| |
Overall mortality |
Colorectal cancer-specific mortality |
|||||
|---|---|---|---|---|---|---|---|
| Person-time at risk (years) | Deaths (n) | HRa | 95% CI | Deaths (n) | HRa | 95% CI | |
|
Total recent MET-hours per week | |||||||
| 0 | 624 | 48 | 1.00 | 33 | 1.00 | ||
| 0.1–8.9 | 1197 | 55 | 0.67 | 0.45, 0.99 | 44 | 0.78 | 0.49, 1.25 |
| 9–23.9 | 1324 | 63 | 0.75 | 0.51, 1.11 | 56 | 0.99 | 0.63, 1.56 |
| 23.9–41.9 | 1324 | 58 | 0.66 | 0.44, 0.98 | 54 | 0.91 | 0.58, 1.42 |
|
Ptrend |
|
|
|
0.116 |
|
|
0.940 |
|
Body mass index 1 year ago | |||||||
| <25 | 1471 | 62 | 1.00 | 47 | 1.00 | ||
| 25–29.9 | 1687 | 94 | 1.33 | 0.95, 1.85 | 82 | 1.51 | 1.04, 2.18 |
| ⩾30 | 1315 | 68 | 1.26 | 0.88, 1.81 | 58 | 1.33 | 0.89, 1.99 |
|
Ptrend |
|
|
|
0.201 |
|
|
0.170 |
|
Smoking status | |||||||
| Never | 1772 | 89 | 1.00 | 74 | 1.00 | ||
| Former | 2289 | 104 | 0.87 | 0.64, 1.18 | 87 | 0.94 | 0.67, 1.30 |
| Current |
412 |
31 |
1.31 |
0.86, 2.01 |
26 |
1.31 |
0.82, 2.09 |
|
Sex | |||||||
| Female | 1780 | 69 | 1.00 | 59 | 1.00 | ||
| Male |
2694 |
155 |
1.33 |
0.97, 1.80 |
128 |
1.24 |
0.89, 1.74 |
|
Stage | |||||||
| I | 1445 | 28 | 1.00 | 18 | 1.00 | ||
| II | 1191 | 43 | 1.91 | 1.18, 3.08 | 32 | 2.21 | 1.24, 3.94 |
| III | 1134 | 89 | 4.07 | 2.66, 6.24 | 79 | 5.41 | 3.23, 9.04 |
| IV | 142 | 38 | 12.97 | 7.85, 21.44 | 38 | 20.65 | 11.61, 36.72 |
| Unknown |
561 |
26 |
2.60 |
1.52, 4.46 |
20 |
2.97 |
1.57, 5.64 |
|
Diabetes | |||||||
| No | 3535 | 186 | 1.00 | 154 | 1.00 | ||
| High blood sugar | 264 | 11 | 0.76 | 0.41, 1.41 | 10 | 0.81 | 0.42, 1.57 |
| Diabetes |
674 |
27 |
0.68 |
0.45, 1.03 |
23 |
0.75 |
0.48, 1.19 |
|
Socioeconomic disadvantage | |||||||
| 1 (Most disadvantaged) | 754 | 48 | 1.00 | 42 | 1.00 | ||
| 2 | 919 | 38 | 0.81 | 0.53, 1.25 | 32 | 0.78 | 0.49, 1.24 |
| 3 | 917 | 42 | 0.79 | 0.52, 1.20 | 34 | 0.70 | 0.44, 1.10 |
| 4 | 1016 | 49 | 0.92 | 0.61, 1.38 | 38 | 0.81 | 0.52, 1.27 |
| 5 (Least disadvantaged) | 868 | 47 | 1.07 | 0.70, 1.63 | 41 | 0.99 | 0.63, 1.55 |
Abbreviations: CI=confidence intervals; HR=hazards ratio; MET-hours=metabolic-equivalent hours.
Adjusted for age, sex, socioeconomic status, tumour stage and diabetes. Physical activity, body mass index and smoking were all mutually adjusted for each other.
Physical activity
Participants who performed any amount of recent recreational physical activity reduced their risk of all-cause mortality compared with those who reported no recent physical activity; the HR for increasing levels of physical activity were 0.67 (95% CI=0.45, 0.99), 0.66 (95% CI=0.44, 0.98) and 0.75 (95% CI=0.51, 1.11) (Table 2). Sex-stratified analyses revealed a significant dose–response relationship between recent recreational physical activity and overall mortality in females (Ptrend=0.027), with significant risk reductions seen for women who performed any amount of recreational physical activity (Table 3). Recent recreational physical activity was not significantly associated with overall mortality in males. The inverse association between recent recreational physical activity and overall survival was not seen in participants with a stage IV colorectal cancer and appeared to be stronger in rectal cancer patients than in colon cancer patients, although there was no significant interaction between physical activity and cancer site.
Table 3. Adjusted HR for the association between lifestyle characteristics and overall and colorectal cancer-specific mortality, stratified by sex, cancer site and cancer stage.
|
Sex |
Colorectal cancer site |
Colorectal cancer stagea |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Male |
Female |
Colon |
Rectum |
Stage I–III |
Stage IV |
||||||||||||
| n | HRb | 95% CI | n | HRb | 95% CI | n | HRb | 95% CI | n | HRb | 95% CI | n | HRb | 95% CI | n | HRb | 95% CI | |
|
Overall mortality | ||||||||||||||||||
|
Total recent MET-hours per week (%) | ||||||||||||||||||
| 0 |
88 |
1.00 |
|
42 |
1.00 |
|
86 |
1.00 |
|
44 |
1.00 |
|
108 |
1.00 |
|
10 |
1.00 |
|
| 0.1–11.9 |
136 |
0.76 |
0.46, 1.25 |
93 |
0.45 |
0.22, 0.92 |
140 |
0.89 |
0.53, 1.49 |
89 |
0.38 |
0.19, 0.75 |
188 |
0.55 |
0.35, 0.86 |
13 |
0.95 |
0.30, 2.97 |
| 12–29.9 |
155 |
0.99 |
0.61, 1.58 |
109 |
0.38 |
0.19, 0.78 |
159 |
0.82 |
0.50, 1.37 |
105 |
0.63 |
0.33, 1.19 |
221 |
0.55 |
0.35, 0.85 |
10 |
3.44 |
1.01, 11.68 |
| ⩾30 |
163 |
0.83 |
0.51, 1.34 |
93 |
0.38 |
0.18, 0.80 |
173 |
0.75 |
0.45, 1.25 |
83 |
0.49 |
0.25, 0.95 |
206 |
0.49 |
0.31, 0.77 |
14 |
2.45 |
0.81, 7.39 |
|
Ptrend |
|
|
0.731 |
|
|
0.027 |
|
|
0.247 |
|
|
0.267 |
|
|
0.008 |
|
|
0.043 |
| Pinteraction | 0.158 | 0.468 | 0.007 | |||||||||||||||
|
Body mass index 1 year ago | ||||||||||||||||||
| <25 |
144 |
1.00 |
|
139 |
1.00 |
|
182 |
1.00 |
|
101 |
1.00 |
|
240 |
1.00 |
|
11 |
1.00 |
|
| 25–29.9 |
231 |
1.12 |
0.76, 1.65 |
107 |
1.95 |
1.05, 3.60 |
202 |
1.28 |
0.84, 1.95 |
136 |
1.32 |
0.75, 2.32 |
278 |
1.33 |
0.90, 1.96 |
19 |
1.37 |
0.55, 3.42 |
| ⩾30 |
167 |
1.01 |
0.65, 1.57 |
91 |
1.95 |
0.98, 3.86 |
174 |
1.05 |
0.67, 1.65 |
84 |
1.81 |
0.98, 3.34 |
205 |
1.34 |
0.88, 2.04 |
17 |
1.06 |
0.31, 3.57 |
|
Ptrend |
|
|
0.964 |
|
|
0.046 |
|
|
0.866 |
|
|
0.058 |
|
|
0.167 |
|
|
0.854 |
|
Pinteraction |
0.287 |
0.492 |
0.613 |
|||||||||||||||
|
Smoking status | ||||||||||||||||||
| Never |
150 |
1.00 |
|
197 |
1.00 |
|
227 |
1.00 |
|
120 |
1.00 |
|
287 |
1.00 |
|
19 |
1.00 |
|
| Former |
336 |
0.77 |
0.54, 1.10 |
108 |
1.04 |
0.60, 1.79 |
279 |
0.90 |
0.61, 1.32 |
165 |
0.82 |
0.50, 1.36 |
367 |
0.70 |
0.49, 1.01 |
22 |
2.85 |
1.09, 7.50 |
| Current |
56 |
1.05 |
0.62, 1.77 |
32 |
2.64 |
1.18, 5.93 |
52 |
1.42 |
0.81, 2.48 |
36 |
1.47 |
0.72, 2.98 |
69 |
1.40 |
0.84, 2.31 |
6 |
1.58 |
0.40, 6.25 |
|
Pinteraction |
0.226 |
0.653 |
0.028 |
|||||||||||||||
|
Colorectal cancer-specific mortality | ||||||||||||||||||
|
Total recent MET-hours per week (%) | ||||||||||||||||||
| 0 |
88 |
1.00 |
|
42 |
1.00 |
|
86 |
1.00 |
|
44 |
1.00 |
|
108 |
1.00 |
|
10 |
1.00 |
|
| 0.1–11.9 |
136 |
1.14 |
0.63, 2.06 |
93 |
0.34 |
0.15, 0.75 |
140 |
1.25 |
0.68, 2.31 |
89 |
0.37 |
0.17, 0.83 |
188 |
0.65 |
0.37, 1.13 |
13 |
0.95 |
0.30, 2.97 |
| 12–29.9 |
155 |
1.54 |
0.87, 2.71 |
109 |
0.37 |
0.17, 0.81 |
159 |
1.24 |
0.69, 2.24 |
105 |
0.73 |
0.35, 1.51 |
221 |
0.77 |
0.46, 1.29 |
10 |
3.44 |
1.01, 11.68 |
| ⩾30 |
163 |
1.35 |
0.77, 2.37 |
93 |
0.41 |
0.18, 0.90 |
173 |
1.17 |
0.65, 2.11 |
83 |
0.60 |
0.29, 1.24 |
206 |
0.74 |
0.44, 1.26 |
14 |
2.45 |
0.81, 7.39 |
|
Ptrend |
|
|
0.209 |
|
|
0.143 |
|
|
0.727 |
|
|
0.709 |
|
|
0.558 |
|
|
0.043 |
|
Pinteraction |
0.030 |
0.220 |
0.140 | |||||||||||||||
|
Body mass index 1 year ago | ||||||||||||||||||
| <25 |
144 |
1.00 |
|
139 |
1.00 |
|
182 |
1.00 |
|
101 |
1.00 |
|
240 |
1.00 |
|
11 |
1.00 |
|
| 25–29.9 |
231 |
1.36 |
0.87, 2.12 |
107 |
1.90 |
0.97, 3.72 |
202 |
1.41 |
0.89, 2.24 |
136 |
1.42 |
0.73, 2.77 |
278 |
1.53 |
0.98, 2.38 |
19 |
1.37 |
0.55, 3.42 |
| ⩾30 |
167 |
1.19 |
0.72, 1.96 |
91 |
1.79 |
0.84, 3.80 |
174 |
0.93 |
0.56, 1.55 |
84 |
2.40 |
1.21, 4.74 |
205 |
1.49 |
0.92, 2.40 |
17 |
1.06 |
0.31, 3.57 |
|
Ptrend |
|
|
0.524 |
|
|
0.115 |
|
|
0.722 |
|
|
0.011 |
|
|
0.103 |
|
|
0.854 |
|
Pinteraction |
0.758 |
0.114 |
0.323 |
|||||||||||||||
|
Smoking status | ||||||||||||||||||
| Never |
150 |
1.00 |
|
197 |
1.00 |
|
227 |
1.00 |
|
120 |
1.00 |
|
287 |
1.00 |
|
19 |
1.00 |
|
| Former |
336 |
0.78 |
0.52, 1.15 |
108 |
1.23 |
0.68, 2.23 |
279 |
0.95 |
0.63, 1.44 |
165 |
0.84 |
0.48, 1.49 |
367 |
0.72 |
0.49, 1.08 |
22 |
2.85 |
1.09, 7.50 |
| Current |
56 |
0.93 |
0.51, 1.68 |
32 |
2.70 |
1.16, 6.29 |
52 |
1.30 |
0.70, 2.44 |
36 |
1.63 |
0.76, 3.50 |
69 |
1.26 |
0.72, 2.20 |
6 |
1.58 |
0.40, 6.25 |
| Pinteraction | 0.078 | 0.881 | 0.047 | |||||||||||||||
Abbreviations: CI=confidence intervals; HR=hazards ratio; MET-hours=metabolic-equivalent hours.
Excludes participants for whom cancer stage was unknown.
Adjusted for age, socioeconomic status, tumour stage and diabetes. Physical activity, body mass index and smoking were all mutually adjusted for each other.
The association between recent recreational physical activity and colorectal cancer-specific mortality was significantly modified by sex (Pinteraction=0.03) (Table 3). Performing any amount of recreational physical activity significantly reduced the risk of dying from colorectal cancer in females; the HR for increasing levels of physical activity were 0.34 (95% CI=0.15, 0.75), 0.37 (95% CI=0.17, 0.81) and 0.41 (95% CI=0.18, 0.90). No significant association was found in males.
In terms of intensity and timing, both recent and lifetime moderate-intensity physical activity were associated with a significantly reduced risk of overall and colorectal-cancer mortality in females (Table 4). Neither recent nor lifetime vigorous-intensity physical activity was significantly associated with mortality in females, and neither recent nor lifetime moderate-intensity or vigorous-intensity physical activity was significantly associated with mortality in males. Resistance training and sedentary work were not significantly associated with colorectal cancer-specific mortality or overall mortality in males or females (Table 4).
Table 4. Adjusted HR for the association between moderate-intensity and vigorous-intensity recent and lifetime physical activity, resistance training and sedentary work, and overall and colorectal cancer-specific mortality.
|
All-cause mortality |
Colorectal cancer-specific mortality |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
Males |
Females |
Males |
Females |
||||
| n (%) | HRa | 95% CI | HRa | 95% CI | HRa | 95% CI | HRa | 95% CI | |
|
Total recent moderate MET-hours per week | |||||||||
| 0 | 182 (20.7) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 0.1–5.9 | 129 (14.7) | 0.77 | 0.43, 1.35 | 0.63 | 0.29, 1.35 | 0.95 | 0.49, 1.84 | 0.42 | 0.17, 1.02 |
| 6–17.9 | 219 (24.9) | 0.68 | 0.42, 1.09 | 0.41 | 0.20, 0.85 | 1.00 | 0.59, 1.69 | 0.44 | 0.21, 0.94 |
| ⩾18 |
239 (39.7) |
0.78 |
0.52, 1.18 |
0.41 |
0.21, 0.79 |
1.04 |
0.65, 1.68 |
0.40 |
0.20, 0.83 |
|
Total recent vigorous MET-hours per week | |||||||||
| 0 | 626 (71.2) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 0.1–5.9 | 97 (11.0) | 0.45 | 0.19, 1.04 | 0.54 | 0.24, 1.25 | 0.60 | 0.25, 1.42 | 0.45 | 0.17, 1.22 |
| 6–17.9 | 80 (9.1) | 0.46 | 0.21, 1.01 | 0.80 | 0.34, 1.89 | 0.56 | 0.25, 1.25 | 0.98 | 0.41, 2.35 |
| ⩾18 |
76 (8.7) |
0.93 |
0.51, 1.70 |
1.55 |
0.57, 4.18 |
0.83 |
0.42, 1.63 |
2.06 |
0.73, 5.81 |
|
Lifetime total physical activity | |||||||||
| Low in all-age periods | 216 (24.6) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High in one-age period | 177 (20.1) | 0.90 | 0.55, 1.48 | 0.52 | 0.26, 1.05 | 1.03 | 0.60, 1.79 | 0.73 | 0.35, 1.55 |
| High in two-age periods | 153 (17.4) | 1.17 | 0.72, 1.89 | 0.83 | 0.40, 1.70 | 1.14 | 0.66, 1.99 | 1.13 | 0.50, 2.54 |
| High in all-age periods |
333 (37.9) |
1.02 |
0.67, 1.56 |
0.48 |
0.26, 0.91 |
1.18 |
0.74, 1.89 |
0.65 |
0.33, 1.30 |
|
Lifetime moderate physical activity | |||||||||
| Low in all-age periods | 225 (25.6) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High in one-age period | 187 (21.3) | 0.98 | 0.62, 1.55 | 0.75 | 0.37, 1.50 | 1.26 | 0.76, 2.10 | 1.07 | 0.50, 2.29 |
| High in two-age periods | 185 (21.0) | 1.06 | 0.66, 1.69 | 0.73 | 0.36, 1.46 | 1.12 | 0.64, 1.94 | 0.90 | 0.41, 1.97 |
| High in all-age periods |
282 (32.1) |
0.89 |
0.56, 1.41 |
0.31 |
0.15, 0.64 |
1.14 |
0.68, 1.90 |
0.41 |
0.18, 0.91 |
|
Lifetime vigorous physical activity | |||||||||
| Low in all-age periods | 412 (46.9) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High in one-age period | 239 (27.2) | 0.94 | 0.64, 1.38 | 0.75 | 0.38, 1.47 | 1.03 | 0.68, 1.57 | 0.98 | 0.48, 1.99 |
| High in two-age periods | 124 (14.1) | 0.87 | 0.51, 1.49 | 0.81 | 0.36, 1.82 | 0.88 | 0.48, 1.59 | 1.09 | 0.47, 2.52 |
| High in all-age periods |
104 (11.8) |
0.76 |
0.41, 1.39 |
1.41 |
0.56, 3.56 |
0.70 |
0.35, 1.39 |
1.56 |
0.59, 4.08 |
|
Lifetime resistance training | |||||||||
| None | 772 (87.8) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Possible | 56 (6.4) | 1.08 | 0.54, 2.15 | 0.31 | 0.09, 1.09 | 1.20 | 0.57, 2.53 | 0.31 | 0.08, 1.13 |
| Definite |
51 (5.8) |
0.64 |
0.26, 1.60 |
0.46 |
0.13, 1.66 |
0.81 |
0.32, 2.05 |
0.50 |
0.14, 1.84 |
|
Years in sedentary work | |||||||||
| 0 | 685 (77.9) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 0.1–9.9 | 80 (9.1) | 0.72 | 0.34, 1.50 | 1.28 | 0.59, 2.77 | 0.90 | 0.43, 1.90 | 1.51 | 0.68, 3.34 |
| ⩾10 | 114 (13.0) | 0.82 | 0.51, 1.32 | 1.84 | 0.66, 5.12 | 0.81 | 0.48, 1.35 | 1.60 | 0.52, 4.96 |
Abbreviations: CI=confidence intervals; HR=hazards ratio; MET-hours=metabolic-equivalent hours.
Adjusted for age, socioeconomic status, tumour stage and diabetes. Moderate-intensity and vigorous-intensity physical activity are mutually adjusted.
Body mass index
Compared with normal weight participants, overweight participants had a higher risk of colorectal cancer-specific mortality (HR=1.51, 95% CI=1.04, 2.18) and obese participants had a non-significant increased risk (HR=1.33, 95% CI=0.89, 1.99) (Table 2). The significant association between BMI and both overall mortality and colorectal cancer-specific mortality appeared to be limited to females and rectal cancer patients, although neither sex nor cancer site significantly modified the effect of BMI on mortality (Table 3).
Smoking
As with physical activity and BMI, the association between current smoking and mortality appeared to be more pronounced in females than in males (Table 3). Females who were current smokers had ∼2.5 times the risk of both overall mortality (HR=2.64, 95% CI=1.18, 5.93) and colorectal cancer-specific mortality (HR=2.70, 95% CI=1.16, 6.29). Being a former smoker was not associated with colorectal cancer-specific mortality or overall mortality in neither males nor females; however, it was significantly associated with mortality among people with stage IV colorectal cancer.
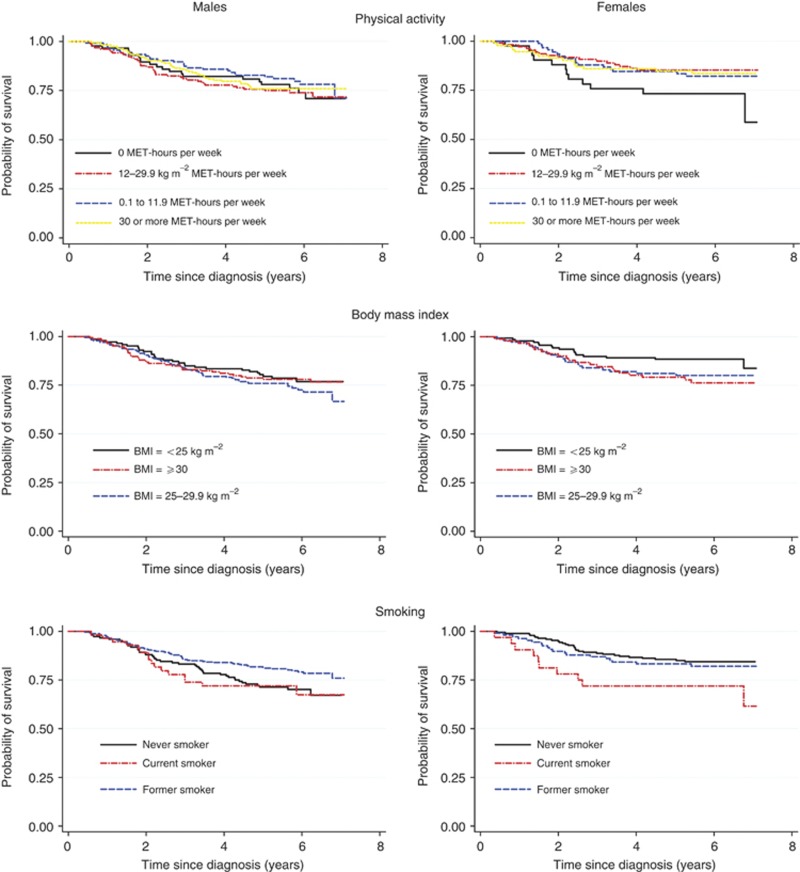
Kaplan–Meier curves of colorectal cancer-specific survival across physical activity, BMI and smoking categories, for males and females separately, are presented in Figure 1.
Figure 1.
Kaplan–Meier curves of colorectal cancer-specific survival across physical activity, BMI and smoking categories, by sex.
Discussion
In this study, we found that physical activity, BMI and smoking were significantly associated with all-cause mortality and/or colorectal cancer-specific mortality in females with colorectal cancer. For females, recent recreational physical activity was associated with a 50–60% reduced risk of both overall mortality and disease-specific mortality; being overweight or obese almost doubled the risk of overall and colorectal cancer-specific mortality, and being a current smoker increased the risk of overall and colorectal cancer-specific mortality by 2.5 times. The results were less pronounced in males, with no significant associations found between physical activity, BMI or smoking and mortality.
Our finding of an inverse association between physical activity and mortality in female colorectal cancer patients adds to the growing body of literature, indicating that physical activity may have an important role in the prognosis of colorectal cancer. Seven previous studies have examined this association (Haydon et al, 2006; Meyerhardt et al, 2006a, 2006b, 2009; Baade et al, 2011; Kuiper et al, 2012; Campbell et al, 2013). Five of these studies have investigated the effect of pre-diagnosis physical activity, with three studies finding a significant inverse association (Haydon et al, 2006; Kuiper et al, 2012; Campbell et al, 2013) and two studies finding no association (Meyerhardt et al, 2006a, 2006b). Post-diagnosis physical activity has been more consistently associated with survival among colorectal cancer patients than pre-diagnosis physical activity, with all of the six studies on this topic finding an inverse association, and risk reductions ranging from 40 to 70% (Meyerhardt et al, 2006a, 2006b, 2009; Baade et al, 2011; Kuiper et al, 2012; Campbell et al, 2013). A randomised controlled trial (the Colon Health and Life-Long Exercise Change trial) to confirm the results from this and other observational studies is currently underway (Courneya et al, 2008).
In our study, we found that physical activity was not inversely associated with mortality in patients with stage IV colorectal cancer. The only other study to investigate this reported similar findings (Haydon et al, 2006), suggesting that physical activity does not increase survival in patients diagnosed with metastatic colorectal cancer. However, most previous studies on physical activity and survival in cancer patients have excluded people with metastatic disease (Ballard-Barbash et al, 2012), and larger studies are needed to confirm this finding.
The results of this study suggest that being overweight or obese is associated with poorer survival in colorectal cancer patients. This finding is consistent with much of the previous literature in this area (Vrieling and Kampman, 2010). It has been proposed that obesity may influence colorectal cancer survival by increasing insulin resistance and increasing the levels of insulin and insulin-like growth factors (Vrieling and Kampman, 2010). Conversely, physical activity may lead to increased survival in colorectal cancer patients by decreasing insulin resistance and lowering the concentrations of insulin and insulin-like growth factors (Vrieling and Kampman, 2010). Other possible mechanisms through which physical activity may influence survival in colorectal cancer patients include reduced weight and modulation of oxidative DNA damage (Davies et al, 2011).
Our finding of increased mortality in colorectal cancer patients who are current smokers is consistent with some (Munro et al, 2006; Phipps et al, 2011, 2013), but not all (Yu et al, 1997; Park et al, 2006; McCleary et al, 2010; Nordenvall et al, 2013), previous research on this topic. Two studies have found that colon/colorectal cancer patients who were current smokers had poorer survival than those who were non-smokers (Munro et al, 2006; Phipps et al, 2013), whereas another study found that colon cancer-specific mortality, but not rectal cancer-specific mortality, was higher in current smokers than in never or former smokers (Phipps et al, 2011). The remaining four studies found no association between current smoking and survival in colorectal cancer patients (Yu et al, 1997; Park et al, 2006; McCleary et al, 2010; Nordenvall et al, 2013).
Moderate-intensity but not vigorous-intensity physical activity was significantly associated with mortality in this study. Only one of the previous six studies on physical activity and survival in colorectal cancer patients has taken intensity into account. That study found that intensity did not influence the effect of physical activity on survival in females (Kuiper et al, 2012), suggesting that moderate-intensity physical activity is sufficient to improve survival. Lifetime physical activity as well as recent physical activity was associated with mortality in females in this study, suggesting that women who have been consistently physically active before a diagnosis of colorectal cancer have improved survival.
Sedentary work and resistance training, both of which may be independent risk factors for colorectal cancer (Boyle, 2012; Boyle et al, 2012a), were not significantly associated with colorectal cancer-specific or overall survival in this study. It has been suggested that sedentary behaviour may potentially have an important role in morbidity and mortality in cancer patients (Lynch et al, 2013), but only one previous study has investigated the association between sedentary behaviour and colorectal-cancer survival, with the results indicating a positive association between pre-and post-diagnosis leisure time sitting and both overall and colorectal cancer-specific mortality (Campbell et al, 2013). No previous studies have examined the association between resistance training and survival in colorectal cancer patients.
In this study, we found that the effects of physical activity, BMI and smoking on mortality in colorectal cancer patients were more pronounced in females than in males.
Previous research indicates that the effect of obesity on mortality after a colorectal cancer diagnosis may differ according to sex, although the results are inconsistent (Vrieling and Kampman, 2010). A study by Meyerhardt et al (2003) also found that obesity may have a greater effect on mortality among female colorectal cancer patients than male colorectal cancer patients, whereas a study by Sinicrope et al (2010) found that class 1 obesity (BMI=30–34.9 kg m−2) was associated with increased mortality in female but not male colon cancer patients, but the opposite for class 2–3 obesity (BMI⩾35 kg m−2). However, several other studies report no sex differences (Dignam et al, 2006; Baade et al, 2011; Campbell et al, 2012). Several plausible explanations for this potential gender disparity have been raised, including the different effects that obesity may have on leptin levels, insulin resistance, adult-onset diabetes, C-reactive protein levels and circulating oestrogen levels in females and males (Meyerhardt et al, 2003). Five previous studies have conducted sex-specific analyses in relation to smoking and mortality in colorectal cancer patients. Three of these studies found no association in males (Park et al, 2006; McCleary et al, 2010; Nordenvall et al, 2013) and/or females (McCleary et al, 2010), whereas the remaining two studies found a significantly increased risk of colorectal cancer-specific mortality in female smokers but not male smokers, although there was no significant effect modification (Phipps et al, 2011, 2013). No prior studies have found that the effect of physical activity on mortality in colorectal cancer patients differ by sex.
This study had several limitations that should be taken into account when interpreting the results. It is possible that the colorectal cancer patients who participated in this study were healthier (i.e., more likely to be physically active and less likely to be overweight or be a smoker) than those who did not take part. It is also possible that people with more advanced colorectal cancer were under-represented in this cohort, as they may have died before being invited to take part in the WABOHS. Participants were recruited from a population-based cancer registry soon after diagnosis, thus limiting potential patient loss and increasing the generalisibility of our results; however, 105 colorectal cancer patients died before they were invited to take part in the WABOHS. We also did not have any information about the treatment that the participants received for their colorectal cancer, so were not able to investigate its role as a possible confounder or effect modifier. However, as treatment is highly correlated with disease extent (stage), which we adjusted for in our analyses, it is probable that its inclusion in the analyses would have had little influence on our results. We were not able to investigate the effect of post-diagnosis physical activity, BMI or smoking as these data were not collected. Information about physical activity and sedentary behaviour was only collected in the recreational and occupational domains, respectively. Although the questions used in this study to measure physical activity, BMI and smoking were based on other reliable questionnaires, it is possible that some exposure misclassification may have occurred. However, any such exposure misclassification is likely to have been non-differential, and would therefore have attenuated the risk estimates seen in this study. With the exception of diabetes, we did not have information on co-morbidity, which may potentially be a confounder and/or mediator between lifestyle factors and mortality. Finally, we had limited statistical power to detect significant differences in stratified analyses, particularly those involving cancer stage.
In summary, the results of this study, along with previous research, suggest that lifestyle factor such as physical activity, obesity and smoking may have an important role in the prognosis of colorectal cancer patients, particularly females. Larger studies and, where appropriate, randomised controlled trials are needed to confirm and improve our understanding of these associations.
Acknowledgments
We thank Barry Iacopetta, Kieran McCaul, David Crawford, Tim Threlfall, Cassandra Clayforth, Jenny Landrigan, Mehdi Tabatabaei, Jen Girschik, Clare Tran and Beatriz Cuesta-Briand for their contributions to the Western Australian Bowel Health Study. This work was supported by the Australian National Health and Medical Research Council (Project Grant Number 353568 and Fellowship Number 37614900 to LF).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, Jacobs DR, Leon AS. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32 (9:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics 2008Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), AustraliaCatalogue Number 2033.0.55.001Australian Bureau of Statistics: Canberra, Australia [Google Scholar]
- Australian Institute of Health and Welfare, Australasian Association of Cancer Registries 2010Cancer in Australia: An Overview, 2010Cancer Series Number 60. Catalogue Number CAN 56AIHW: Canberra [Google Scholar]
- Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20 (7:1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104 (11:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle T. Physical activity and colon cancer: timing, intensity and sedentary behavior. Am J Lifestyle Med. 2012;6 (3:204–215. [Google Scholar]
- Boyle T, Bull F, Fritschi L, Heyworth J. Resistance training and the risk of colon and rectal cancers. Cancer Causes Control. 2012a;23 (7:1091–1097. doi: 10.1007/s10552-012-9978-x. [DOI] [PubMed] [Google Scholar]
- Boyle T, Heyworth J, Bull F, McKerracher S, Platell C, Fritschi L. Timing and intensity of recreational physical activity and the risk of subsite-specific colorectal cancer. Cancer Causes Control. 2011a;22 (12:1647–1658. doi: 10.1007/s10552-011-9841-5. [DOI] [PubMed] [Google Scholar]
- Boyle T, Heyworth J, Fritschi L, Bull F. Long term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol. 2011b;176 (10:1183–1191. doi: 10.1093/aje/kwq513. [DOI] [PubMed] [Google Scholar]
- Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012b;104 (20:1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- Boyle T, Leong S. Comparing ratings of occupational physical activity. Epidemiology. 2012;23 (6:934–936. doi: 10.1097/EDE.0b013e31826d08e4. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30 (1:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31 (7:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155 (3:282–291. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- Clapin HF, Fritschi L, Iacopetta B, Heyworth JS. Dietary and supplemental folate and the risk of left- and right-sided colorectal cancer. Nutr Cancer. 2012;64 (7:937–945. doi: 10.1080/01635581.2012.715718. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Booth CM, Gill S, O'Brien P, Vardy J, Friedenreich CM, Au HJ, Brundage MD, Tu D, Dhillon H, Meyer RM. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15 (6:279–285. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105 (S1:S52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98 (22:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- Faivre-Finn C, Bouvier-Benhamiche A-M, Phelip JM, Manfredi S, Dancourt V, Faivre J. Colon cancer in France: evidence for improvement in management and survival. Gut. 2002;51 (1:60–64. doi: 10.1136/gut.51.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM.2010GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide:IARC CancerBase No. 10International Agency for Research on Cancer: Lyon, France [Google Scholar]
- Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30 (2:266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55 (1:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J, Phipps A, Neuhouser M, Chlebowski R, Thomson C, Irwin M, Lane D, Wactawski-Wende J, Hou L, Jackson R, Kampman E, Newcomb P. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23 (12:1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch BM, Dunstan DW, Vallance JK, Owen N. Don't take cancer sitting down. Cancer. 2013;119 (11:1928–1935. doi: 10.1002/cncr.28028. [DOI] [PubMed] [Google Scholar]
- McCleary NJ, Niedzwiecki D, Hollis D, Saltz LB, Schaefer P, Whittom R, Hantel A, Benson A, Goldberg R, Meyerhardt JA. Impact of smoking on patients with stage III colon cancer. Cancer. 2010;116 (4:957–966. doi: 10.1002/cncr.24866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98 (3:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006a;24 (22:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169 (22:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006b;24 (22:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- Munro AJ, Bentley AHM, Ackland C, Boyle PJ. Smoking compromises cause-specific survival in patients with operable colorectal cancer. Clin Oncol. 2006;18 (6:436–440. doi: 10.1016/j.clon.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nordenvall C, Nilsson PJ, Ye W, Andersson TML, Nyrén O. Tobacco use and cancer survival: a cohort study of 40,230 Swedish male construction workers with incident cancer. Int J Cancer. 2013;132 (1:155–161. doi: 10.1002/ijc.27587. [DOI] [PubMed] [Google Scholar]
- Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24 (31:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival. Cancer. 2011;117 (21:4948–4957. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps AI, Shi Q, Newcomb PA, Nelson GD, Sargent DJ, Alberts SR, Limburg PJ. Associations between cigarette smoking status and colon cancer prognosis among participants in North Central Cancer Treatment Group Phase III Trial N0147. J Clin Oncol. 2013;31 (16:2016–2023. doi: 10.1200/JCO.2012.46.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69 (1:239–241. [Google Scholar]
- Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16 (6:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Labor 1991Dictionary of occupational titles4th ednUS Government Printing Office: Washington, DC [Google Scholar]
- Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2010;92 (3:471–490. doi: 10.3945/ajcn.2010.29005. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research . AICR: Washington, DC; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP. Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev. 1997;21 (6:497–509. [PubMed] [Google Scholar]
- Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61 (5:561–569. doi: 10.1136/jcp.2007.054858. [DOI] [PubMed] [Google Scholar]