Abstract
Background:
This dose-finding study evaluated lenvatinib, an oral multitargeted receptor tyrosine kinase inhibitor, in combination with carboplatin/paclitaxel in chemotherapy-naïve non-small-cell lung cancer (NSCLC) patients.
Patients and Methods:
Patients received lenvatinib twice daily (BID) with carboplatin (area under the curve 6 mg ml−1 min−1, day 1)/paclitaxel (200 mg m−2, day 1) every 3 weeks. The initial dose of lenvatinib was 6 mg BID. The primary end point was maximum tolerated dose (MTD) of lenvatinib. At the MTD, the cohort was expanded by 16 patients. Safety, pharmacokinetics, pharmacodynamics, and antitumor effects were evaluated.
Results:
Twenty-eight patients were treated. At 6 mg BID, dose-limiting toxicities (DLTs) included febrile neutropenia/gingival infection (n=2). No DLTs occurred with 4 mg BID, the recommended MTD for the expansion. Common grade 3/4 toxicities included neutropenia, leukopenia, hypertension, and thrombocytopenia. The combination had no significant impact on individual drug pharmacokinetics. Response rate and median progression-free survival were 68% and 9.0 months, respectively, with 4 mg BID. In the plasma biomarker analysis, stromal cell-derived factor 1α, stem cell factor, and granulocyte colony-stimulating factor correlated with antitumor activity.
Conclusion:
The MTD for lenvatinib with carboplatin/paclitaxel is 4 mg BID in advanced NSCLC patients. This regimen demonstrated manageable tolerability and encouraging antitumor activity.
Keywords: angiogenesis inhibitor, carboplatin, lenvatinib, lung cancer, paclitaxel, tyrosine kinase inhibitor
Non-small-cell lung cancer (NSCLC) accounts for ∼85% of lung cancer cases and is a leading cause of cancer death (Molina et al, 2008; NSCLC Meta-Analyses Collaborative Group, 2008; Jemal et al, 2011). Platinum-based regimens remain the standard first-line chemotherapy options for patients with advanced or metastatic NSCLC. However, the prognosis remains poor and 1-year survival rates have not exceeded 60% (Ohe et al, 2007). Hence, there is an urgent need for novel therapeutic strategies to prolong survival for these patients.
The vascular endothelial growth factor (VEGF) family of proteins, particularly VEGF-A and VEGF receptor 2 (VEGFR2), are key mediators of angiogenesis, which is a fundamental process for tumour cell survival, local invasion, and metastasis in multiple tumour types (Ferrara, 2004; Shibuya, 2010). Several studies have reported that high tumour expression of VEGF protein is predictive of poor survival in NSCLC patients (Baillie et al, 2001; Inoshima et al, 2002; Nakashima et al, 2004). Bevacizumab, an anti-VEGF-A antibody, has been shown to enhance objective response rate (ORR) and prolong survival when used in combination with paclitaxel and carboplatin in patients with non-squamous-cell NSCLC (Sandler et al, 2006; Niho et al, 2012). A number of tyrosine kinase inhibitors (TKIs) that target VEGFR signalling have been developed in combination with a standard platinum doublet regime for the treatment of NSCLC. Although some clinical studies of VEGFR inhibitors did not show clinical benefit (Scagliotti et al., 2010), some TKIs may still offer potential benefits depending on their profile of kinase inhibition.
In addition to VEGF-mediated signalling, preclinical evidence suggests that aberrant signalling mediated by both fibroblast growth factor receptors (FGFR) and platelet-derived growth factor receptors (PDGFR) also contribute to tumour growth and angiogenesis in multiple solid tumour types (Turner and Grose, 2010; Kono et al, 2012). These pathways have also been linked with the development of tumour resistance to agents that target VEGFR (Kono et al, 2012).
Lenvatinib (E7080) is an orally administered multi-kinase inhibitor of VEGFR-1–3, FGFR1–4, PDGFR-β, and c-kit (Matsui et al, 2008a, 2008b). Lenvatinib's potency against PDGFR, FGFR, and c-kit may enhance its antitumor activity in various tumour types (Matsui et al, 2008a).
In phase 1 studies, lenvatinib was commonly associated with hypertension, proteinuria, fatigue, and gastrointestinal (GI) symptoms, which were manageable with routine medical care. Encouraging antitumor activity in patients with melanoma, differentiated thyroid cancer, renal cancer, and endometrial cancer was observed (Nemunaitis et al, 2008; Yamada et al, 2011; Boss et al, 2012).
The primary objective of this dose-finding study was to determine the maximum tolerated dose (MTD) and assess the safety, pharmacokinetics, pharmacodynamics, and antitumor effects of lenvatinib administered by continuous twice daily (BID) dosing with carboplatin and paclitaxel in patients with stage IIIB/IV NSCLC.
Patients and Methods
Study design
This multicenter, open-label, phase 1 dose-finding study (ClinicalTrials.gov identifier NCT00832819; E7080-J081-110) was designed to establish the MTD for lenvatinib in combination with carboplatin/paclitaxel. This study consisted of a dose-finding and an expansion cohort. In the dose-finding cohort, six patients were enrolled per dose level of lenvatinib in order to determine the MTD. Sixteen patients were enrolled at the MTD level in the expansion cohort. Safety, pharmacokinetics, pharmacodynamics, and antitumor effects were evaluated.
Lenvatinib was orally administered by continuous BID dosing in combination with paclitaxel (200 mg m−2) intravenously administered over 3 h followed by a 1-h infusion of carboplatin (area under the curve (AUC) 6.0 min mg ml−1) on day 1 every 3 weeks from cycle 1. Paclitaxel/carboplatin was administered for up to six cycles until progressive disease (PD) was observed. Lenvatinib monotherapy was continued as maintenance therapy when paclitaxel/carboplatin was discontinued for reasons other than PD.
In the dose-finding cohort, the initial dose was 6 mg BID; lenvatinib was administered during a 7 day run-in period (cycle 0) before being administered in combination with carboplatin/paclitaxel. If tolerability at 6 mg BID was confirmed, dose escalation to 8 mg BID and subsequently to 10 mg BID was allowed. If tolerability at 6 mg BID was not confirmed, the subsequent dose was de-escalated to 4 mg BID. Tolerability was confirmed by the frequency of occurrence of dose-limiting toxicities (DLTs) observed by the end of cycle 1 in six patients.
The following toxicities were regarded as DLTs: grade 3 thrombocytopenia requiring blood transfusion; grade 4 thrombocytopenia; grade 4 neutropenia persisting longer than 7 days; and any grade 3 or higher non-haematological toxicity, with the exception of transient abnormal clinical laboratory values not requiring treatment; drug hypersensitivity without dose-dependency; and controllable hypertension, diarrhoea, vomiting, and nausea.
Patient and eligibility criteria
Patients aged 20–74 years with a histologically or cytologically confirmed diagnosis of NSCLC (stage IIIB/IV), at least 1 measurable tumour lesion by Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0 (Therasse et al, 2000), and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 were eligible for the study. Patients were required to have adequate organ function and were excluded from the study if they had received previous therapy for NSCLC, including prior chemotherapy, and surgery and radiotherapy for the primary site. Patients who had hemoptysis of more than 1/2 teaspoon, clinically significant haemorrhagic or thrombotic events within 4 weeks of enrollment or hypertension ⩾150/90 mm Hg at screening (one antihypertensive medication was permitted) were excluded. Other key exclusion criteria included the presence of clinically significant pulmonary or cardiovascular disease, symptomatic brain metastasis, or brain metastasis requiring treatment. Concomitant use of antiplatelet and anticoagulant drugs such as aspirin, warfarin, and ticlopidine was prohibited throughout the study. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and was approved by the Institutional Review Board of each medical institution; all patients provided written, informed consent.
Study assessments
Safety assessments
Adverse events (AEs) were recorded and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) (CTCAE, 2006), and were coded based on Medical Dictionary for Regulatory Activities (version 14.0).
Antitumor activity
Tumour assessment was performed by investigators according to RECIST version 1.0 (Therasse et al, 2000) every two cycles. Tumour shrinkage was defined as the per cent change in sum of the longest diameter of target lesions from baseline to nadir.
Pharmacokinetic/pharmacodynamic studies
Blood samples were collected on day 1 of cycle 1 predose, and 1, 2, 3 (immediately before the end of paclitaxel infusion), 4 (immediately before the end of carboplatin infusion), 6, 8, and 10 h post-lenvatinib oral dosing, on day 2 of cycle 1 predose and 3 h after lenvatinib dosing (only for carboplatin and paclitaxel) for the dose-finding cohort, only. Blood samples were also collected on days 8 and 22 of cycle 1 before lenvatinib dosing. Plasma concentrations of lenvatinib, paclitaxel, and carboplatin were measured by liquid chromatography with tandem mass spectrometry (LC/MS/MS) for lenvatinib (0.08 ng ml−1 as lower limit of quantification (LLOQ)), LC/MS/MS for paclitaxel (1 ng ml−1 as LLOQ), and inductively coupled plasma mass spectrometry for total platinum (10 ng ml−1 as LLOQ), respectively. Exploratory pharmacodynamic analyses were conducted on blood samples collected at predose on day 1 of cycle 0 (dose-finding cohort only) and on days 1, 8, and 22 of cycle 1. Plasma concentrations of biomarkers, including FGF-2, granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), interleukin-8 (IL-8), PDGF-B, stem cell factor (SCF), stromal cell-derived factor 1α (SDF1α), and VEGF were measured by enzyme-linked immunosorbent assay using a Bio-Plex assay kit.
Statistical analysis
All patients were included in the safety, efficacy, pharmacokinetic, and pharmacodynamic analyses. All statistical tests were performed at the 5% significance level (two-sided) without adjustment of multiplicity. Pharmacokinetic parameters of lenvatinib, carboplatin, and paclitaxel were calculated by non-compartmental analysis.
For efficacy analyses, waterfall plots showing per cent changes in sum of the longest diameters of target lesions from baseline to the nadir are presented. Progression-free survival (PFS) was defined as the time from the start of lenvatinib treatment to PD or death, whichever occurred first. PFS was summarised by the Kaplan–Meier method.
For the pharmacodynamic analyses of plasma biomarkers, the per cent change from baseline in each item was analysed by Wilcoxon signed-rank test. Correlations between baseline value and maximum per cent change of plasma marker and efficacy parameters of interest, including maximum decrease in sum of the longest diameters of target lesions from baseline and PFS, were analysed using Spearman's rank correlation coefficient. In this analysis, time to the last adequate tumour assessment was used as PFS when patients were discontinued for reasons other than PD or death.
All analyses were performed using SAS for Windows (release 8.2 or later), SAS Drug Development 3.0, WinNonlin Professional (version 5.01 or later), and Microsoft Excel.
Results
Patients
The study was conducted from February 2009 to July 2011 at three sites in Japan. The study enrolled 28 patients. Twelve patients entered the dose-finding cohort for determination of the MTD (4 mg BID (n=6) and 6 mg BID (n=6)) and 16 patients entered in the 4 mg BID group in an expansion cohort. Baseline demographics and disease characteristics are shown in Table 1. Two patients discontinued the study before completion of cycle 1. With the exception of one patient who discontinued the study during the run-in period, all patients (n=27) received lenvatinib in combination with carboplatin/paclitaxel. Lenvatinib monotherapy was continued as maintenance therapy after completion of carboplatin/paclitaxel in 19 patients.
Table 1. Baseline demographics and disease characteristics.
|
Dose-finding cohort |
Expansion cohort |
|
||
|---|---|---|---|---|
| 4 mg BID (n=6) | 6 mg BID (n=6) | 4 mg BID (n=16) | Total (n=28) | |
| Mean age, years (range) |
52.8 (38, 60) |
54.8 (41, 68) |
58.4 (38, 73) |
56.4 (38, 73) |
|
Gender, n (%) | ||||
| Male | 4 (67) | 4 (67) | 13 (81) | 21 (75) |
| Female |
2 (33) |
2 (33) |
3 (19) |
7 (25) |
|
ECOG PS, n (%) | ||||
| 0 | 5 (83) | 5 (83) | 9 (56) | 19 (68) |
| 1 |
1 (17) |
1 (17) |
7 (44) |
9 (32) |
|
Stage, n (%) | ||||
| IIIB | 2 (33) | 0 | 6 (38) | 8 (29) |
| IV |
4 (67) |
6 (100) |
10 (63) |
20 (71) |
|
Tissue type, n (%) | ||||
| Adenocarcinoma | 5 (83) | 6 (100) | 12 (75) | 23 (82) |
| Squamous cell carcinoma | 0 | 0 | 2 (13) | 2 (7) |
| Others | 1 (17) | 0 | 2 (13) | 3 (11) |
Abbreviations: BID=twice daily; ECOG PS=Eastern Cooperative Oncology Group performance status.
Safety
Evaluation of DLTs and MTD
Two patients in the 6 mg BID group of the dose-finding cohort experienced DLTs (grade 3 febrile neutropenia and grade 3 gingival infections in each). Another patient discontinued from the study due to deterioration of performance status. None of the patients in the 4 mg BID group experienced DLTs. Considering the DLTs and the overall toxicities observed, the MTD was defined as 4 mg BID and this dose was estimated as the recommended dose for the expansion cohort.
Adverse events
Adverse events were evaluated in 28 patients enrolled in the dose-finding and expansion cohorts. In 22 patients at the 4 mg level, common toxicities included thrombocytopenia (100%), neutropenia, leukopenia, peripheral sensory neuropathy, arthralgia, and alopecia (95%). Of interest, proteinuria was reported in 77% and hypertension in 73% of patients. In addition, nausea was reported by 82% of patients, along with other GI-associated AEs such as diarrhoea, constipation, or decreased appetite, each having a 77% incidence rate. The most frequent grade 3 or 4 toxicity was neutropenia (95%), followed by leukopenia (50%), hypertension (36%), thrombocytopenia (27%), and febrile neutropenia (23%) (Table 2). A grade 3 thrombosis was observed only in the maintenance phase of lenvatinib monotherapy. Nine patients (4 mg BID, n=7; 6 mg BID, n=2) experienced a treatment-related serious AE. The most frequently reported SAEs were febrile neutropenia (7%) and pneumonia (7%). No deaths were reported. There were no changes of clinical importance in mean QT interval and QTcF seen in either combination or maintenance therapy.
Table 2. Grades 3 and 4 treatment-related adverse events.
|
Dose of lenvatinib |
||||
|---|---|---|---|---|
| |
4 mg BID (n=22) |
6 mg BID (n=6) |
||
| Adverse event | Grade 3, n (%) | Grade 4, n (%) | Grade 3, n (%) | Grade 4, n (%) |
| Neutropenia |
0 |
21 (95) |
0 |
5 (83) |
| Leukopenia |
10 (46) |
1 (5) |
2 (33) |
1 (17) |
| Thrombocytopenia |
4 (18) |
2 (9) |
0 |
0 |
| Anaemia |
3 (14) |
0 |
0 |
0 |
| Lymphopenia |
1 (5) |
0 |
0 |
0 |
| Febrile neutropenia |
5 (23) |
0 |
1 (17) |
0 |
| Pneumonia |
1 (5) |
0 |
2 (33) |
0 |
| Gingival infection |
1 (5) |
0 |
1 (17) |
0 |
| Hypertension |
8 (36) |
0 |
4 (67) |
0 |
| Proteinuria |
2 (9) |
0 |
0 |
0 |
| Peripheral sensory neuropathy |
1 (5) |
0 |
0 |
0 |
| Nausea |
1 (5) |
0 |
0 |
0 |
| Diarrhoea |
1 (5) |
0 |
0 |
0 |
| Decreased appetite |
1 (5) |
0 |
0 |
0 |
| Weight decreased |
1 (5) |
0 |
0 |
0 |
| Vomiting |
1 (5) |
0 |
0 |
0 |
| Hypocalcemia |
1 (5) |
0 |
0 |
0 |
| Hyponatremia |
1 (5) |
0 |
0 |
0 |
| Anal abscess |
1 (5) |
0 |
0 |
0 |
| Syncope |
0 |
0 |
1 (17) |
0 |
| Pyelonephritis |
1 (5) |
0 |
0 |
0 |
| Neurogenic bladder |
1 (5) |
0 |
0 |
0 |
| Thrombosis | 1 (5) | 0 | 0 | 0 |
Abbreviation: BID=twice daily.
Pharmacokinetics
Pharmacokinetic parameters for each analyte are presented in Table 3. The mean maximum concentration (Cmax) for lenvatinib ranged from 97.5 ng ml−1 in the 4 mg BID group to 138 ng ml−1 in the 6 mg BID group. Median time of maximum concentration (tmax) was 4.18–4.27 h and the mean AUC(0−t) ranged from 692 to 848 ng h ml−1. Both Cmax and AUC(0−t) increased with the dose of lenvatinib after multiple-dose administration in combination with carboplatin/paclitaxel. Blood samples collected on days 8 and 22 of cycle 1 before lenvatinib dosing showed that mean lenvatinib concentrations at predose after repeated administration were almost equivalent regardless of carboplatin/paclitaxel (data not shown).
Table 3. Pharmacokinetic parameters for plasma concentrations of lenvatinib, carboplatin, and paclitaxel.
| |
|
Dose of lenvatinib |
|
|---|---|---|---|
| Analyte | Pharmacokinetic parameter | 4 mg BID (n=6) | 6 mg BID (n=5) |
| Lenvatiniba | Cmax (ng ml−1) | 97.5±38.4 | 138±35.3 |
| tmax (h) | 4.18 (2.00–6.42) | 4.27 (1.92–10.1) | |
| |
AUC(0−t) (ng h ml−1) |
692±245 |
848±216 |
| Carboplatinb | Cend (μg ml−1) | 27.1±4.14 | 26.4±2.23 |
| (AUC 6) |
AUC(0−t) (μg h ml−1) |
79.9±12.7 |
70.8±5.19 |
| Paclitaxelc | Cend (μg ml−1) | 7.27±2.10 | 6.56±1.57 |
| (200 mg m−2) | AUC(0−inf) (μg h ml−1) | 25.0±8.18 | 22.1±5.26 |
| t1/2 (h) | 6.65±0.39 | 6.77±0.42 | |
| CL (l h−1) | 14.4±6.42 | 16.2±2.91 | |
| Vss (l) | 69.1±27.7 | 80.9±16.6 | |
Abbreviations: AUC=area under the curve; BID=twice daily; Cmax=maximum concentration; CL=clearance; Cend=end of concentration; tmax=time to maximum concentration; t1/2=half-life; Vss=volume at steady state.
Data are mean±s.d., except for tmax, where median (minimum–maximum) is shown.
Last sampling time point was 10 h after administration of lenvatinib.
Based on total platinum concentration, last sampling time point was 24 h after starting intravenous infusion of carboplatin.
Last sampling time point was 27 h after starting infusion of paclitaxel.
For paclitaxel, end of concentration (Cend), AUC(0−inf), half-life (t1/2), clearance, and volume at steady state were generally consistent across the doses of lenvatinib. The Cend and AUC(0−t) of carboplatin were also similar across lenvatinib doses.
Antitumor activity
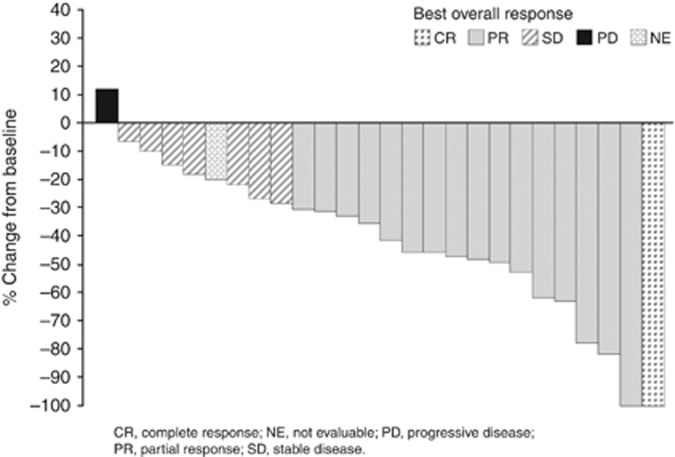
Post-baseline assessments of target lesions were not available for 2 of 28 enrolled patients. Most patients experienced tumour shrinkage after treatment (Figure 1). The best overall responses are summarised in Table 4. In 22 patients at the 4 mg level, 14 patients (64%) had a partial response and 1 patient (5%) had a complete response. The ORR was 68% and 61%, and the median duration of response was 7.9 (95% confidence interval (CI): 4.9, 9.5) and 7.0 (95% CI: 4.9, 9.5) months in the 4 mg BID groups and total, respectively. Median PFS was 9.0 (95% CI: 6.5, 9.5) months each in the 4 mg BID groups and total.
Figure 1.
Waterfall plot of per cent change in sum of the longest diameters of target lesion from baseline to the nadir (n=26). CR=complete response; NE=not evaluable; PD=progressive disease; SD=stable disease.
Table 4. Best overall response.
| |
Dose of lenvatinib |
|
|
|---|---|---|---|
| Parameter | 4 mg BID (n=22) | 6 mg BID (n=6) | Total (n=28) |
|
Best response, n (%) | |||
| CR | 1 (5) | 0 (0) | 1 (4) |
| PR | 14 (64) | 2 (33) | 16 (57) |
| SD | 5 (23) | 2 (33) | 7 (25) |
| PD | 1 (5) | 0 (0) | 1 (4) |
| NE |
1 (5) |
2 (33) |
3 (11) |
| ORR, n (%) | 15 (68) | 2 (33) | 17 (61) |
| (95% CI) | (45–86) | (4–78) | (41–79) |
Abbreviations: BID=twice daily; CI=confidence interval; CR=complete response; NE=not evaluable; ORR=objective response rate (CR+PR); PD=progressive disease; PR=partial response; SD=stable disease.
Pharmacodynamics
Plasma angiogenic proteins and cytokines were quantified at baseline and periodically during the first cycle of combination therapy. During the first cycle of combination therapy, there were significant increases from baseline in VEGF on days 8 and 22 (P<0.001 each), IL-8, and SDF1α on day 8 (P<0.001 each), and HGF on day 22 (P=0.024), whereas PDGF-B was significantly decreased on day 22 (P=0.001). Other plasma markers were not significantly changed during the first cycle.
Correlations between plasma markers and antitumor activity in terms of tumour shrinkage and PFS were explored. Lower baseline levels of SDF1α were significantly correlated with greater tumour shrinkage and longer PFS (Table 5). Furthermore, patients showing a greater increase of SCF and SDF1α had greater tumour shrinkage and longer PFS, respectively. In contrast, patients showing less increase of G-CSF had longer PFS.
Table 5. Baseline and treatment-related change of plasma angiogenic proteins and cytokines and their correlation with efficacy.
|
Observed values (pg ml−1) |
Correlation analysis |
||||||
|---|---|---|---|---|---|---|---|
| |
Median (minimum–maximum) |
Baseline |
Maximum per cent change |
||||
| Angiogenic marker | Baseline (n=28) | Cycle 1 day 8 (n=26) | Cycle 1 day 22 (n=26) | Tumor shrinkage (n=26) | PFS (n=28) | Tumor shrinkage (n=26) | PFS (n=26) |
| FGF-2 |
19.4 (9–55) |
17.2 (7–59) |
16.0 (7–57) |
0.13 |
0.16 |
0.22 |
−0.15 |
| G-CSF |
6.8 (2–80) |
7.6 (3–27) |
7.5 (2–17) |
0.35 |
0.05 |
−0.28 |
−0.42a |
| HGF |
234.9 (119––480) |
244.0 (163–353) |
259.8b (120–1161) |
−0.31 |
−0.29 |
0.14 |
−0.02 |
| IL-8 |
6.3 (3–57) |
9.4b (5–16) |
4.4 (3–15) |
0.25 |
−0.15 |
−0.31 |
−0.10 |
| PDGF-B |
228.0 (27–2700) |
237.9 (14–2849) |
85.4b (13–707) |
0.32 |
0.04 |
−0.20 |
−0.02 |
| SCF |
101.8 (40–205) |
101.0 (54–173) |
102.9 (43–184) |
−0.30 |
0.21 |
0.46a |
0.11 |
| SDF1α |
62.9 (30–195) |
129.9b (62–196) |
76.9 (30–140) |
−0.48a |
−0.48a |
0.39 |
0.41a |
| VEGF | 16.1 (4–62) | 31.3b (17–196) | 28.7b (11–171) | −0.01 | −0.35 | 0.07 | −0.01 |
Abbreviations: FGF=fibroblast growth factor; G-CSF=granulocyte colony-stimulating factor; HGF=hepatocyte growth factor; IL=interleukin; PDGF-B=platelet-derived growth factor B; SCF=stem cell factor; SDF1α=stromal cell-derived factor 1α; VEGF=vascular endothelial growth factor.
Concentration at baseline and per cent change from it were shown as median (minimum–maximum). Correlation analysis indicated Spearman's rank correlation coefficient.
P<0.05, Spearman's rank correlation between plasma marker and efficacy parameter.
P<0.05, Wilcoxon signed-rank test based on per cent change from baseline.
Discussion
The primary objective of this study was to determine the MTD of lenvatinib confirmed by the frequency of DLTs associated with its twice-daily oral administration in combination with carboplatin/paclitaxel in patients with advanced NSCLC. The starting dose of lenvatinib was 6 mg BID. Two out of six patients experienced DLTs, namely febrile neutropenia and gingival infection. In the subsequent enrollment, when the lenvatinib dose was reduced to 4 mg BID, no DLTs were observed. The appropriate dose-reduction of carboplatin or paclitaxel in combination with a higher dose of lenvatinib was not determined in this study. Of 22 patients who received lenvatinib 4 mg BID, the most common grade 3 or 4 haematological toxicities were neutropenia, leukopenia, thrombocytopenia, and febrile neutropenia. The AE profile was similar to that previously reported for Japanese patients with NSCLC who received combination carboplatin/paclitaxel (Ohe et al, 2007). However, the incidence of grade 4 neutropenia and grade 3 or 4 thrombocytopenia in this study was slightly higher than that in the aforementioned study. The combined regimen of carboplatin/paclitaxel with cediranib, another VEGFR inhibitor, demonstrated a higher incidence of grade 3 or 4 of neutropenia, thrombocytopenia, and febrile neutropenia in patients with advanced NSCLC vs carboplatin/paclitaxel alone (Goss et al, 2010). A previous phase 1 study of lenvatinib in Japanese patients with solid tumours reported thrombocytopenia among other DLTs (Yamada et al, 2011). These results suggest that neutropenia and thrombocytopenia resulting from treatment with carboplatin/paclitaxel may be slightly increased in a combined regimen with lenvatinib. Neutropenia was well managed with the use of G-CSF in 17 patients (77%) and thrombocytopenia was generally controlled by dose interruption without transfusion.
The most common non-haematological toxicity in this study was hypertension, which has been reported with lenvatinib monotherapy (Yamada et al, 2011; Boss et al, 2012). Hypertension was generally managed with antihypertensive drugs, and grade 4 hypertension was not observed. This study did not report safety concerns regarding unexpected toxicities related to the treatment combination. However, the results suggest that patients with NSCLC who were administered lenvatinib in combination with carboplatin/paclitaxel should be monitored for potential increases in haematological toxicities.
Results from this study suggested encouraging antitumor activity with an ORR of 68% and a median PFS of 9.0 months at the MTD of 4 mg BID. Therefore, lenvatinib administered at 4 mg BID was the recommended dose for further study in combination with carboplatin/paclitaxel for NSCLC. However, the lenvatinib dose in this combination was lower than the MTD for lenvatinib monotherapy, which was10 mg BID (Nemunaitis et al, 2008).
Pharmacokinetic analyses in the current study indicated that lenvatinib exposure with coadministration of carboplatin and paclitaxel was similar to that observed with lenvatinib monotherapy in patients with advanced solid tumours (Yamada et al, 2011; Boss et al, 2012). The estimated pharmacokinetic parameters for carboplatin and paclitaxel were generally consistent across the 4 and 6 mg BID doses of lenvatinib and similar to historical data (Kimura et al, 1988; Tamura et al, 1995). Overall, these results suggest that concomitant administration of lenvatinib, carboplatin, and paclitaxel does not significantly impact the pharmacokinetics of any of these three drugs in this treatment schedule.
A relatively high ORR and a long PFS were observed in this study. The pathogenesis and progression of NSCLC may involve multiple convergent and compensatory growth factor signalling pathways that emerge upon inhibition of the VEGF pathway, which may account for inherent or acquired resistance to VEGF-targeted therapy (Ellis and Hicklin, 2008). A multivariate analysis of tumour samples from 335 patients who had undergone resection for stage I–IIIA NSCLC found that high expression of PDGF-A correlated with lymph node metastasis, whereas high co-expression of PDGF-B and VEGFR3 was a strong and independent predictor of poor survival in patients with NSCLC (Donnem et al, 2010). Aberrant FGFR-mediated signalling, polymorphisms, and increased expression of FGFR1 and FGFR2 have also been implicated in the pathogenesis and progression of NSCLC (Semrad and Mack, 2012). Other preclinical evidence suggests that PDGFR- and FGFR-mediated signalling may act synergistically to promote tumour angiogenesis and metastasis (Cao et al, 2003; Nissen et al, 2007). Thus, by targeting VEGRs, FGFRs, and PDFGRs, lenvatinib has the potential to inhibit multiple signalling pathways involved in tumour angiogenesis.
Biomarker analysis has become increasingly important in identifying potential responders to treatment in several cancer types. However, to date, there is a need for validated biomarkers for anti-angiogenesis therapy that have prognostic or predictive relevance. Plasma angiogenic proteins and cytokines may be useful predictors of response and resistance to antiangiogenic treatment. Several bone marrow-derived cell populations promote or inhibit tumour progression via various processes. These cells include circulating endothelial progenitor cells (CEP), Gr1+ and CD11b+ myeloid-derived suppressor cells, and VEGFR-1+ hemangiocytes. SDF1α, SCF, and G-CSF may have a role in recruiting these cells to angiogenic sites, thereby contributing to resistance to chemotherapy and antiangiogenic therapy (Bergers and Hanahan, 2008; Shaked et al, 2008). In a phase 1 study of lenvatinib in patients with advanced solid tumours, a lower predose level of SDF1α was correlated with longer treatment duration (Yamada et al, 2011). Similarly, the current study showed that a lower predose level of SDF1α was significantly correlated with greater tumour shrinkage and longer PFS. Therefore, a higher predose level of SDF1α may predict resistance to lenvatinib treatment in patients with NSCLC. In addition, treatment-related increase of G-CSF was correlated with shorter PFS. Granulocyte colony-stimulating factor increase might promote resistance to the lenvatinib combination possibly due to paclitaxel-induced G-CSF increased CEP accumulation (Shaked et al, 2008). In contrast, patients with treatment-related increase of SDF1α and SCF had longer PFS and greater tumour shrinkage, respectively. Although the function of these factors in tumour tissues remains to be elucidated, monitoring these factors in plasma during treatment might be a potential approach to identify biomarkers predictive of clinical activity of this combination regimen.
Conclusions
The MTD for lenvatinib administered orally in combination with carboplatin/paclitaxel is 4 mg BID in patients with advanced or metastatic NSCLC. Concomitant administration of lenvatinib, carboplatin, and paclitaxel does not appear to have a significant impact on the pharmacokinetic profile of any of the three drugs in this treatment schedule. The combination regimen of lenvatinib with carboplatin/paclitaxel had a manageable tolerability profile and encouraging antitumor activity in patients with advanced or metastatic NSCLC. The results of the exploratory biomarker analyses support and warrant further study.
Acknowledgments
We thank Yuki Nishioka, Kenichi Saito and Mikiko Kawakatsu (Eisai Co., Ltd) for the PK, PD, and medical writing. In addition, we would like to thank Phase Five Communications Inc. for medical editorial assistance with this manuscript. We also gratefully acknowledge the commitment of participating patients, their families, and the study investigators for their invaluable contribution to this research. This work was supported by Eisai Co., Ltd.
WY and NK are employees of Eisai Co., Ltd. The remaining authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Baillie R, Carlile J, Pendleton N, Schor AM. Prognostic value of vascularity and vascular endothelial growth factor expression in non-small cell lung cancer. J Clin Pathol. 2001;54:116–120. doi: 10.1136/jcp.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss DS, Glen H, Beijnen JH, Keesen M, Morrison R, Tait B, Copalu W, Mazur A, Wanders J, O'Brien JP, Schellens JH, Evans TR. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer. 2012;106:1598–1604. doi: 10.1038/bjc.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0, DCTD, NCI, NIH, DHHS http://ctep.cancer.gov ( 2006 ) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf . Accessed (2012
- Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- Donnem T, Al-Saad S, Al-Shibli K, Busund LT, Bremnes RM. Co-expression of PDGF-B and VEGFR-3 strongly correlates with lymph node metastasis and poor survival in non-small-cell lung cancer. Ann Oncol. 2010;21:223–231. doi: 10.1093/annonc/mdp296. [DOI] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I, Hara N. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clin Cancer Res. 2002;8:3480–3486. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kato T, Takamizawa H, Tari K, Suzuoki Y, Sekiba K, Fukuoka L, Akimoto M, Abe O, Santo M, Niitani H, Furuse K, Ohta K, Kimura I, Konno K, Honma T, Tominaga K, Niijima T, Inagaki J. A phase I study of carboplatin. Oncologia. 1988;21:88–94. [Google Scholar]
- Kono SA, Heasley LE, Doebele RC, Camidge DR. Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets. 2012;12:107–123. doi: 10.2174/156800912799095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008a;122:664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumour MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008b;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Huang CL, Liu D, Kameyama K, Masuya D, Ueno M, Haba R, Yokomise H. Expression of vascular endothelial growth factor-A and vascular endothelial growth factor-C as prognostic factors for non-small cell lung cancer. Med Sci Monit. 2004;10:BR157–BR165. [PubMed] [Google Scholar]
- Nemunaitis JJ, Senzer NN, Kurzrock R, Ng CS, Das A, Atienza RS, Zang EA, Jansen M, Ashworth S, Hong DS.2008Phase I dose-escalation study of E7080, a multikinase inhibitor, in patients with advanced solid tumors [abstract] J Clin Oncol 26(Suppl634s):abstract 14583. [Google Scholar]
- NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K, Matsui K, Negoro S, Yokoyama A, Kudoh S, Kiura K, Mori K, Okamoto H, Sakai H, Takeda K, Yokota S, Saijo N, Fukuoka M, JO19907 Study Group Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76:362–367. doi: 10.1016/j.lungcan.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Bråkenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumour neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrão Miziara JE, Balint B, De Marinis F, Keller A, Arén O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- Semrad TJ, Mack PC. Fibroblast growth factor signaling in non-small-cell lung cancer. Clin Lung Cancer. 2012;13:90–95. doi: 10.1016/j.cllc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, Daenen LG, Man S, Xu P, Emmenegger U, Tang T, Zhu Z, Witte L, Strieter RM, Bertolini F, Voest EE, Benezra R, Kerbel RS. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Tyrosine kinase receptor Flt/VEGFR family: its characterization related to angiogenesis and cancer. Genes Cancer. 2010;1:1119–1123. doi: 10.1177/1947601910392987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Sasaki Y, Nishiwaki Y, Saijo N. Phase I study of paclitaxel by three-hour infusion: hypotension just after infusion is one of the major dose-limiting toxicities. Jpn J Cancer Res. 1995;86:1203–1209. doi: 10.1111/j.1349-7006.1995.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yamamoto N, Yamada Y, Nokihara H, Fujiwara Y, Hirata T, Koizumi F, Nishio K, Koyama N, Tamura T. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin Cancer Res. 2011;17:2528–2537. doi: 10.1158/1078-0432.CCR-10-2638. [DOI] [PubMed] [Google Scholar]