Abstract
Background:
NKG2D recognises several ligands, including polymorphic major histocompatibility complex class I chain-related chain-related proteins A and B (MICA/B) and unique long 16-binding proteins (ULBPs). These ligands are present on cancer cells and are recognised by NKG2D in a cell-structure-sensing manner, triggering natural killer (NK) cell cytotoxicity. However, the mechanisms that control the expression of NKG2D ligands in malignant cells are poorly understood. 1-α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) was recently shown to enhance the susceptibility of melanoma cells to the cytotoxicity of NK cells. However, the function of 1,25(OH)2D3 in other cancers and its potential mechanisms of action remain unknown.
Methods:
The expression levels of miR-302c and miR-520c in Kasumi-1, K562, MCF7 and MDA-MB-231 cells were evaluated using quantitative real-time PCR. The targets of miR-302c and miR-520c were confirmed by luciferase reporter assay. The killing effects of NK92 cells against Kasumi-1, K562, MCF7 and MDA-MB-231 cells were examined using the CytoTox 96 Non-Radioactive Cytotoxicity Assay. The levels of cytokines IFN-γ and granzyme B, which indicate the activation of NK cells, were also measured by enzyme-linked immunosorbent assay.
Results:
Treatment with 1,25(OH)2D3 enhanced the susceptibility of both the haematological tumour cell line Kasumi-1 and solid tumour cell line MDA-MB-231 to NK92 cells. miR-302c and miR-520c expression was induced, and their levels inversely correlated with the levels of NKG2D ligands MICA/B and ULBP2 upon 1,25(OH)2D3 treatment. A luciferase reporter assay revealed that miR-302c and miR-520c directly targeted the 3′-UTRs of MICA/B and ULBP2 and negatively regulated the expression of MIA/B and ULBP2. Moreover, upregulation of miR-302c or miR-520c by transfection of their mimics remarkably reduced the viability of Kasumi-1 cells upon NK cell co-incubation. By contrast, the suppression of the activity of miR-302c or miR-520c by their respective antisense oligonucleotides improved the resistance of Kasumi-1 cells to NK cells.
Conclusion:
1,25(OH)2D3 facilitates the immuno-attack of NK cells against malignant cells partly through downregulation of miR-302c and miR-520c and hence upregulation of the NKG2D ligands MICA/B and ULBP2.
Keywords: NKG2D ligands; miR-302c; miR-520c; 1,25(OH)2D3
As a first line of defence of the innate immune system, natural killer (NK) cells exert direct and indirect antitumour effects via their cytotoxic and immune-regulatory capacities. Their activity is controlled by signals derived from a variety of activating and inhibitory receptors that recognise ligands specifically expressed by malignant cells or stressed cells (Pegram et al, 2011; Fernandez-Messina et al, 2012; Mondelli, 2012; Saito et al, 2012). NKG2D is one of the most important receptors mediating the cytotoxicity of NK cells against malignant cells (Ljunggren, 2008; Obeidy and Sharland, 2009; Tsuboi et al, 2011). Ligands that bind NKG2D receptors include major histocompatibility complex (MHC) class I chain-related proteins A and B (MICA/B) and unique long 16-binding proteins 1 through 6 (ULBP1-6) (Nausch and Cerwenka, 2008; Brandt et al, 2009; Eisenring et al, 2010). The expression of these ligands may be induced upon infection and other inducers of cellular stress and is unusual in normal cells (Champsaur and Lanier, 2010). By binding to the NKG2D receptors on NK and T cells, NKG2D ligands may initiate an immune response against cells expressing these ligands. In mouse models, transfection with NKG2D ligands results in NKG2D-mediated tumour rejection (Cerwenka et al, 2001; Diefenbach et al, 2001). Downregulation or complete knockout of NKG2D in mice results in an impaired immune response against tumour cells, higher expression levels of NKG2D ligands, and an increased incidence of certain tumours (Guerra et al, 2008; Wiemann et al, 2005). One of the most important strategies by which tumour cells adapt to elude the immune response is the downregulation or loss of expression of certain ligands (Mistry and O'Callaghan, 2007). Despite the importance of NKG2D in antitumour immunity, the mechanisms that control the expression of its ligands in malignant cells are poorly understood.
1-α,25-Dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, primarily regulates calcium homoeostasis in intestine, bone, kidneys, and parathyroid gland. It binds to the vitamin D receptor and regulates gene and miRNA expression to execute its antitumour effect. 1-α,25-Dihydroxyvitamin D3 shows antiproliferative and pro-differentiation effects in many malignant cells and reduces the development and growth of tumours in preclinical models (Trump et al, 2010). In addition to these antiproliferative effects, 1,25(OH)2D3 modulates the expression of mediators of apoptosis such as Bax, Bcl-2, and Bcl-XL and directly induces apoptosis via caspase activation (Kizildag et al, 2012; Vuolo et al, 2012; Zhang et al, 2013). More recently, 1,25(OH)2D3 was reported to enhance the susceptibility of melanoma cells to the cytotoxicity of NK cells (Lee et al, 2011).
In this study, we extended the function of 1,25(OH)2D3 to the enhancement of NK susceptibility of other malignant cells, including the haematological tumour cell line Kasumi-1 and the solid tumour cell line MDA-MB-231, and explored the possible underlying mechanisms of the observed effects.
Materials and methods
Cell culture and treatment with 1,25(OH)2D3
Kasumi-1and K-562 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. MDA-MB-231, MCF-7, and HEK-293T cells were cultured in DMEM supplemented with 10% fetal bovine serum. NK92 cells were maintained in RPMI 1640 supplemented with 10% human A+serum and 20 ng ml−1 IL-2. All cell lines were maintained at 37 °C in a humidified atmosphere of 5% CO2 and used in the log phase of growth for all experiments. For 1,25(OH)2D3 treatment, cells were treated with 0.01, 0.05, 0.1, or 0.5 nM 1,25(OH)2D3 for 24 h. For the control group, 0.1% ethanol was added to culture medium.
Enrichment of primary polyclonal NK cells
CD3−CD56+ NK cells were enriched from human peripheral blood mononuclear cells (PBMC) of healthy donors, using the MACS technology (Miltenyi Biotec, Cologne, Germany). PBMCs were first depleted of CD3+ cells, and then positive selection of CD56+ cells was carried out according to the manufacturer's protocol. Enrichment of CD3-CD56+ NK cells was confirmed by flow cytometry, using the anti-human mAb anti-CD3-PE-Cy-7 and anti-CD56-APC (BD Bioscience, San Diego, CA, USA). Natural killer cells were cultured for 48 h in the presence of 250–500 IU ml−1 IL-2 (Chiron Cooperation, Emeryville, CA, USA) before assaying.
Killing activity analysis of NK cells
CytoTox 96 Non-Radioactive Cytotoxicity Assay was performed as described previously (Tsuboi et al, 2011). Briefly, target cells were washed with PBS, resuspended with fresh NK92 culture medium, and seeded in a 96-well plate at a density of 5000 cells per well. NK92 cells or primary human NK cells were added at effector-to-target (E:T) ratios of 32 : 1, 16 : 1, and 8 : 1 and incubated for 4 h at 37 °C in a humidified atmosphere of 5% CO2. The supernatant was harvested and subjected to analysis by the CytoTox 96 Non-Radioactive Cytotoxicity Assay. The killing effect of NK cells against target cells was assessed with the following equation: Cytotoxicity=(Experimental−Effector spontaneous−Target spontaneous)/(Target maximum−Target spontaneous) × 100%.
Construction of the vectors expressing MICA, MICB, and ULBP2
The coding sequences of MICA, MICB, and ULBP2 lacking 3′-UTR were amplified and cloned into pCDNA6B plasmid (Invitrogen, San Diego, CA, USA) to generate pCDNA6B-MICA, pCDNA6B-MICB, and pCDNA6B-ULBP2. The primers used for amplifying and cloning MICA, MICB, and ULBP2 were the following: for MICA: upstream 5′-ACGGATCCACCATGGGGCTGGGCCCGGTCTT-3′, downstream 5′-AAGATATCGGCGCCCTCAGTGGAGCCAG-3′ for MICB upstream 5′-ATGGGGCTGGGCCGGGTCCTG-3′, downstream 5′-AACTCGAGGCGGTGCCCTCAGTGGAACCAG-3′ and for ULBP2 upstream 5′-ACGGATCCACCATGGGGCTGGGCCGGGTCCT-3′, downstream 5′-AAGATATCGGTGCCCTCAGTGGAACCAG-3′.
Luciferase reporter assay
The 3′-UTRs of MICA, MICB, and ULBP2, which contain putative miRNA-binding sites, were amplified and cloned into the psi-CHECK2 vector (Promega, Madison, WI, USA). The following primers were used: MICA: upstream 5′-AACTCGAGGGACGAGTGACCACAGGGAT-3′, downstream 5′-TAGCGGCCGCCAGCCTCCAACAACAATA-3′ MICB: upstream 5′-AACTCGAGTGTTTCTGCTGCTATGCC-3′, downstream 5′-TAGCGGCCGCAGGCACGGTGGCTCATTC-3′ and ULBP2: upstream 5′-AACTCGAGCTCCTGTGAGCACGGTCTT-3′, downstream 5′-TAGCGGCCGCTTGTTTAGTCAGCCAGAA-3′. Mutagenesis was performed using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA), and all the adenines of miRNA-binding sequences were substituted with thymines. The empty vector (psi-CHECK2) was used as a negative control. Wild-type (wt) or mutant (mut) 3′-UTR vectors were cotransfected with miR-302c and miR-520c mimics or negative controls into 293T cells using Lipofectamine 2000 (Invitrogen). The luciferase activity was measured 48 h later using the Dual-Luciferase Reporter Assay System (Promega). The Renilla luciferase values were normalised to firefly luciferase values, and the ratio of Renilla/firefly values is presented. The experiments were performed independently in triplicate.
RNA extraction, reverse transcription, and quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesised with the MLV transcriptase Kit (Invitrogen). miR-302c and miR-520c expression was assayed using a Bulge-LoopTM miRNA qRT–PCR primer (GenePharma, Shanghai, China) and Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen) on a LightCycler 480 (Roche, Basel, Switzerland) according to the manufacturer's instructions. U6 small nuclear RNA, purchased from RiboBio (Guangzhou, China), was used as an internal control. The inhibitory effects on MICA, MICB, and ULBP2 mRNAs were evaluated using qRT–PCR, with GAPDH as an endogenous control. The sequences of the qRT–PCR primers were the following: for MICA: upstream 5′-ACAATGCCCCAGTCCTCCAGA-3′, downstream 5′-ATTTTAGATATCGCCGTAGTTCCT-3′ for MICB: upstream 5′-TGAGCCCCACAGTCTTCGTTAC-3′, downstream 5′-TGCCCTGCGTTTCTGCCTGTCATA-3′ for ULBP2: upstream 5′-CCCTGGGGAAGAAACTAAATGTC-3′, downstream 5′-ACTGAACTGCCAAGATCCACTGCT-3′. The fold changes were calculated using relative quantification with the 2−ΔΔCt method. All of the reactions were performed in a 20-μl reaction volume in triplicate.
Western blot analysis
The cells were washed three times with PBS and then lysed in RIPA buffer in the presence of proteinase inhibitor cocktail (Shanghai Shenergy Biocolor BioScience and Technology Company, Shanghai, China). The protein concentration was determined using a BCA assay (Bios, Beijing, China). Aliquots (25 mg) were separated by 10% SDS–PAGE and transferred to nitrocellulose membranes. The membranes were probed with primary antibodies against ULBP2 and MICA/B (sc-80419, sc-23870, and sc-80527, respectively, mouse monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h, washed extensively with 0.1% Tween-20 in PBS, and incubated with secondary antibodies conjugated to horseradish peroxidase at 1 : 1000 dilutions. The intensity of the protein fragments was visualised using an X-ray image film processor (Kodak, Rochester, NY, USA).
Flow-cytometry analysis
For analysis of MICA, MICB, and ULBP2 surface expression, Kasumi-1 and MDA-MB-231 cells were incubated with anti-MICA/B (Human MICA/B Allophycocyanin MAb; R&D Com, Minneapolis, MN, USA) and anti-ULBP2 antibody (Human ULBP2 Phycoerythrin MAb; R&D Com). The resulting fluorescence was measured by flow-cytometry analysis using a FACS flow cytometer (Becton Dickinson, San Jose, CA, USA).
Measurement of the secretion of IFN-γ by enzyme-linked immunosorbent assay
Cells treated with different concentrations of 1,25(OH)2D3 for 24 h were washed with PBS and resuspended with fresh NK92 culture medium and seeded into 96-well plates at a density of 5000 cells per well. NK92 cells were added at E:T ratios of 16 : 1 and incubated for 4 h at 37 °C in a humidified atmosphere of 5% CO2. The supernatant was harvested, and IFN-γ was quantified using an enzyme-linked immunosorbent assay (ELISA) (Human IFN-γ ELISA Kit II, Cat 550612; BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. The intensities of the cytokine signals were analysed using an ELISA reader (Bio-Rad; Hercules, CA, USA).
Results
1-α,25-Dihydroxyvitamin D3 enhances the susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells
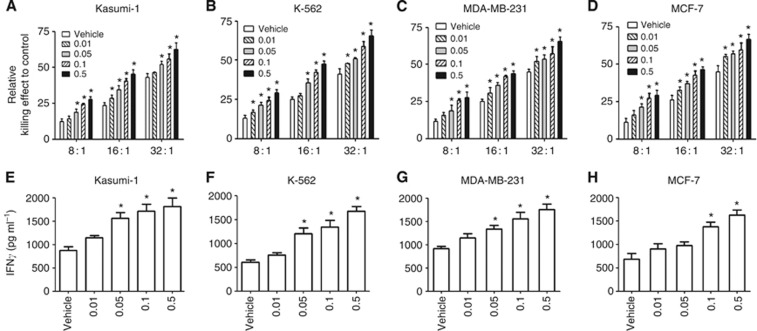
To explore whether 1,25(OH)2D3 increases the malignant cell-killing effect of NK cells, both the haematological tumour cell line Kasumi-1 and K-562 and the solid tumour cell line MDA-MB-231and MCF-7 were treated with 1,25(OH)2D3 and co-cultured with NK92 cells. The addition of 1,25(OH)2D3 increased the killing effect of NK92 cells against Kasumi-1 cells in a dose-dependent manner (Figure 1A–D, P<0.01). Besides, the level of cytokine IFN-γ, which represented the activity of NK cells, was measured. As shown in Figure 1, 1,25(OH)2D3 treatment remarkably increased the secretion of IFN-γ in Kasumi-1, K-562, MDA-MB-231, and MCF-7 cells (Figure 1A–D, P<0.01) in a dose-dependent manner. To further confirm the role of 1,25(OH)2D3 on the NK cell-mediated cytotoxicity on malignant cells, we performed the killing assay using primary NK cells. CD3−CD56+ NK cells were enriched from human PBMC (Supplementary Figure S1A). Kasumi-1 and MDA-MB-231 cells were chosen for the primary NK cell-mediated killing assay. In agreement with the observations made by NK92 cells, 1,25(OH)2D3 obviously increases secretion of INF-γ (Supplementary Figure S1B) and the killing effect of the primary NK cells to kasumi-1 and MDA-MB-231 cells (Supplementary Figure S1C and D). Taken together, these data indicate that 1,25(OH)2D3 increases the susceptibility of Kasumi-1 and MDA-MB-231 cells to the cytotoxicity of NK92 cells.
Figure 1.
1-α,25-Dihydroxyvitamin D3 enhances the cytotoxicity of NK cells to Kasumi-1, K-562, MDA-MB-231, and MCF-7 cells. (A–D) Malignant cells treated with the indicated concentration of 1,25(OH)2D3 for 24 h were co-incubated with NK cells at an indicated E:T ratio for another 4 h. The killing effect of NK92 cells against Kasumi-1 cells was evaluated using the CytoTox 96 Non-Radioactive Cytotoxicity Assay. (E–H) Malignant cells treated with the indicated concentration of 1,25(OH)2D3 for 24 h were co-incubated with NK cells at an indicated E:T ratio of 16 : 1 for another 4 h. The supernatant was collected and subjected to the measurement of IFN-γ. The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
1-α,25-Dihydroxyvitamin D3 downregulates miR-302c and miR-520c
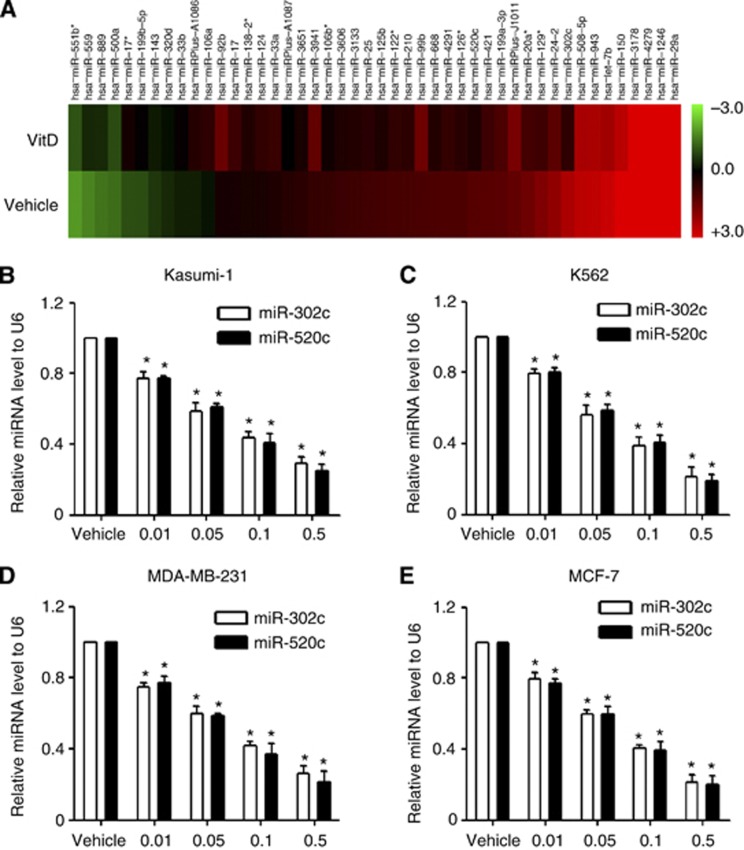
To explore the potential mechanisms by which 1,25(OH)2D3 enhances the susceptibility of Kasumi-1 and MDA-MB-231 cells to NK92 cells, we screened the miRNAs that were modulated by 1,25(OH)2D3 in Kasumi-1 cells using a miRNA microarray (Figure 2A). Two highly downregulated miRNAs, miR-302c and miR-520c, provoked our interest because of their tumour-suppressing roles in cancer cells (Keklikoglou et al, 2012; Cai et al, 2013; Liang et al, 2013). The effect of 1,25(OH)2D3 in downregulating miR-302c and miR-520c was confirmed using qRT–PCR. As shown in Figure 2, 1,25(OH)2D3 treatment resulted in the dose-dependent downregulation of miR-302c and miR-520c in Kasumi-1, K-562, MDA-MB-231 and MCF-7 cells (Figure 2B–D, P<0.01).
Figure 2.
1-α,25-Dihydroxyvitamin D3 treatment downregulates miR-302c and miR-520c expression. (A) miRNA microarray profiling of the 46 miRNAs that are differentially expressed between vehicle and 1,25(OH)2D3-treated Kasumi-1 cells. (B–E) Kasumi-1, K-562, MDA-MB-231, or MCF-7 cells were treated with the indicated concentration of 1,25(OH)2D3 for 24 h. Total RNA was extracted, and the miR-302c and miR-520c levels were evaluated using qRT–PCR. The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
miR-302c and miR-520c regulate the 1,25(OH)2D3-mediated susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells
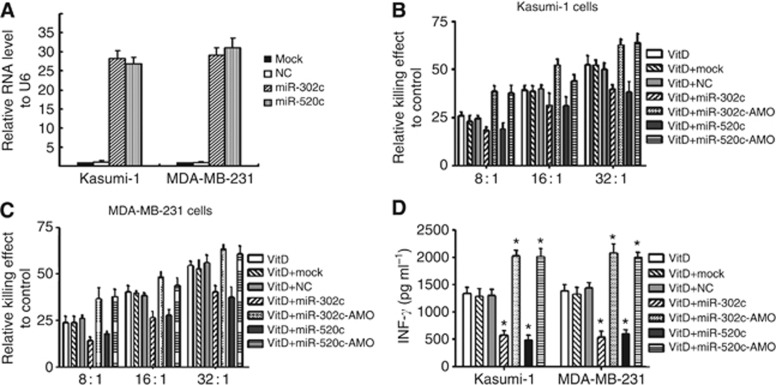
To evaluate whether miR-302c or miR-520c affects the 1,25(OH)2D3-mediated susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells, Kasumi-1 and MDA-MB-231 cells were transiently transfected with miR-302c and miR-520c mimic or anti-miRNA oligonucleotide (AMO) and were treated with 1,25(OH)2D3. The overexpression of miR-302c or miR-520c was evaluated by qRT–PCR (Figure 3A). A FAM-tagged NC-AMO was used as the positive control of the transfection efficiency of the miRNA AMOs (Supplementary Figure S2). Upregulation of miR-302c or miR-520c by their mimics remarkably reduced the viability of Kasumi-1 cells upon co-incubation with NK92 cells, whereas suppression of the activity of miR-302c or miR-520c by their respective AMOs improved the resistance of Kasumi-1 cells to NK92 cells (Figure 3B, P<0.01). Similar observations were made in MDA-MB-231 cells (Figure 3C, P<0.01). We next evaluated the secretion of INF-γ. As shown in Figure 3D, transfection of miR-302c or miR-520c mimics reduced the secretion of INF-γ to <50% (P<0.01) compared with the negative control in both Kasumi-1 and MDA-MB-231 cells. By contrast, miR-302c or miR-520c ASO transfection dramatically increased INF-γ secretion. Taken together, these observations indicate that miR-302c and miR-520c regulate 1,25(OH)2D3-mediated susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells.
Figure 3.
miR-302c and miR-520c regulate the 1,25(OH)2D3-mediated susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells. (A) The expression levels of miR-302c and miR-520c were measured in Kasumi-1 and MDA-MB-231 cells transfected with miRNA mimics using qRT–PCR. (B, C) Malignant cells transfected with miR-302c and miR-520c mimics or AMO for 24 h were treated with 0.1 μM 1,25(OH)2D3 for another 24 h and then co-incubated with NK92 cells at the indicated E:T ratio for 4 h. The killing effects of NK92 cells against Kasumi-1 cells (B) and MDA-MB-231 cells (C) are shown. (D) Malignant cells transfected with miR-302c and miR-520c mimics or AMO for 24 h were treated with 0.1 μM 1,25(OH)2D3 for another 24 h and then co-incubated with NK92 cells at an E:T ratio of 16 : 1 for 4 h. The supernatant was collected and subjected to the measurement of IFN-γ. The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
Both miR-302c and miR-520c target the NKG2D ligands MICA, MICB, and ULBP2
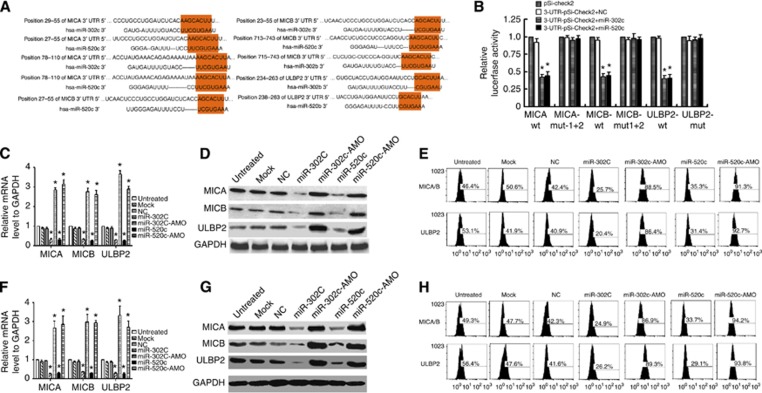
To determine the mechanisms by which miR-302c and miR-520c regulate 1,25(OH)2D3-mediated susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cells, we used the bioinformatic prediction algorithm TargetScan to analyse the direct mRNA targets of miR-302c and miR-520c. Both miR-302c and miR-520c have the same ‘seed' sequence and have the same targets (Figure 4A). Of all the hypothetical targets of miR-302c and miR-520c, MICA, MICB, and ULBP2 were of the most interest because these are NKG2D ligands reported to be associated with NK cell-mediated cytotoxicity (Chavez-Blanco et al, 2011; Fernandez-Messina et al, 2012; Hilpert et al, 2012). To further identify whether MICA, MICB, and ULBP2 responded to miR-302c and miR-520c through direct 3′-UTR interactions, we cloned the wt 3′-UTRs of the putative miR-302c and miR-520c targets (MICA-wt, MICB-wt, and ULBP2-wt) and mutated sequences (MICA-mt1+2, MICB-mt1+2, and ULBP2-mut) individually into a reporter plasmid (pSi-CHECK2) downstream of the Renilla luciferase gene (Figure 4A, P<0.01). These plasmids, together with miR-302c or miR-520c mimic, were transiently transfected into 293T cells. The transfection of pSi-CHECK2 together with miR-302c or miR-520c mimic was used as the negative control. A dual-luciferase reporter assay system was used to detect luciferase expression 48 h after transfection. The results demonstrated that miR-302c or miR-520c mimic attenuated the fluorescence of MICA-wt, MICB-wt, and ULBP2-wt by more than two-fold compared with the negative control (Figure 4B; P<0.01), whereas MICA-mt1+2, MICB-mt1+2, and ULBP2-mut exhibited abrogated responses to miR-302c and miR-520c (Figure 4B, P<0.01). To further confirm the potential role of miR-302c or miR-520c in the regulation of MICA, MICB, and ULBP2, we evaluated their mRNA and protein expression levels in Kasumi-1 and MDA-MB-231 cells following the introduction of miR-302c and miR-520c mimics or AMOs. Transfection of miR-302c or miR-520c mimics led to notable decreases in MICA, MICB, and ULBP2 mRNA levels, whereas transfection of miR-302c or miR-520c AMOs upregulated MICA, MICB, and ULBP2 mRNA level in Kisumi-1 cells (Figure 4C, P<0.01) compared with the negative control. Consistently, the proteins of MICA, MICB, and ULBP2 in Kisumi-1 cells were downregulated by miR-302c and miR-520c mimics and upregulated by AMO transfection detected by both western blot (Figure 4D) and flow-cytometry analysis (Figure 4E). Similar observations were made in MDA-MB-231 cells (Figure 4F–H). Collectively, these data demonstrate that miR-302c and miR-520c are able to suppress the expression of MICA, MICB, and ULBP2 by targeting their 3′-UTRs.
Figure 4.
miR-302c and miR-520c suppress the expression of MICA, MICB, and ULBP2 by targeting their 3′-UTRs. (A) Sequences of the reporter constructs containing the predicted miR-302c- and miR-520c-binding sites in the 3′-UTRs of MICA, MICB, and ULBP2. (B) The overexpression of miR-302c or miR-520c miRNA mimic significantly attenuated the luciferase activities of the wt-MICA, wt-MICB, and wt-ULBP2 3′-UTRs in 293T cells. The cotransfection of WT or mut 3′-UTR and miRNA is indicated. (C–E) miR-302c and miR-520c mimics or AMO were transfected into Kamusi-1 cells for 48 h, and the mRNA levels of MICA, MICB, and ULBP2 were evaluated by qRT–PCR. (C) The protein levels of MICA, MICB, and ULBP2 were detected by western blot (D) and flow-cytometry analysis (E). (F–H) miR-302c and miR-520c mimics or AMO were transfected into MDA-MB-231 cells for 48 h, and the mRNA levels of MICA, MICB, and ULBP2 were evaluated by qRT–PCR (F). The protein levels of MICA, MICB, and ULBP2 were detected by western blot (G) and flow-cytometry analysis (H). The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
MICA, MICB, and ULBP2 expression increases in response to 1,25(OH)2D3 treatment, an effect that is inversely correlated with miR-302c and miR-520c expression
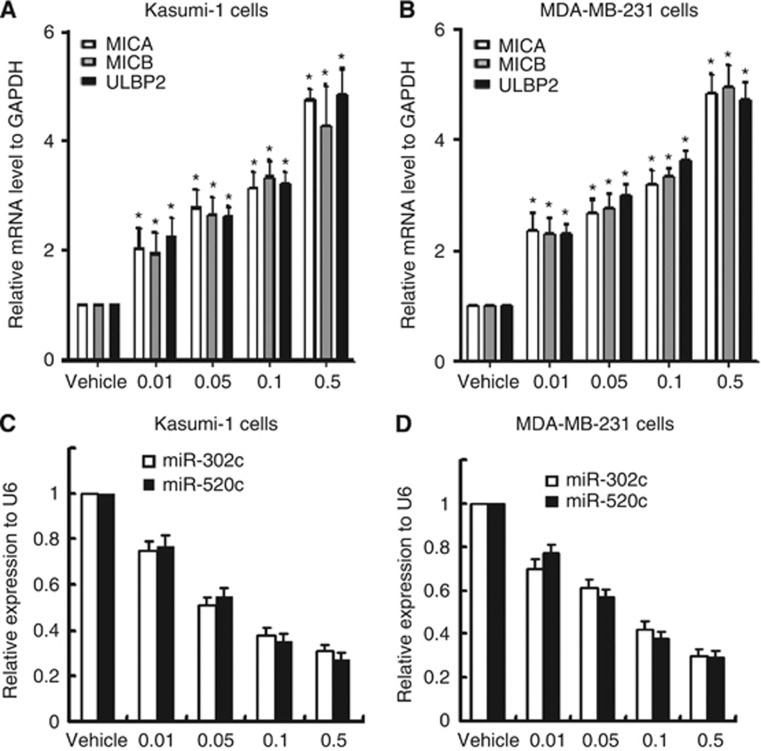
Based on the results that miR-302c and miR-520c suppressed the expression of MICA, MICB, and ULBP2, we assessed the correlations between the three NKG2D ligands and miR-302c and miR-520c in cancer cells upon 1,25(OH)2D3 treatment. MICA, MICB, and ULBP2 mRNAs were upregulated in a 1,25(OH)2D3 dose-dependent manner in Kasumi-1 cells (Figure 5A, P<0.01). Similar observations were made in MDA-MB-231 cells (Figure 5B, P<0.01). By contrast, miR-302c and miR-520c decreased in a dose-dependent manner in Kasumi-1 cells (Figure 5C, P<0.01) and MDA-MB-231 cells (Figure 5D, P<0.01). Taken together, these results indicate that MICA, MICB, and ULBP2 expression levels were inversely correlated with miR-302c and miR-520c expression in Kasumi-1 and MDA-MB-231 cells exposed to 1,25 (OH)2D3.
Figure 5.
MICA, MICB, and ULBP2 expressions are inversely correlated with miR-302c and miR-520c. (A, B) MICA, MICB, and ULBP2 mRNAs were upregulated in a dose-dependent manner in response to 1,25(OH)2D3 treatment in both Kasumi-1 (A) and MDA-MB-231 cells (B). (C, D) miR-302c and miR-520c were downregulated in a dose-dependent manner in response to 1,25(OH)2D3 treatment in both Kasumi-1 (C) and MDA-MB-231 (D) cells. The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
The miR-302c/miR-520c-MICA/MICB/ULBP2 pathways are potentially involved in 1,25(OH)2D3-mediated susceptibility of malignant cells to the cytotoxicity of NK cells
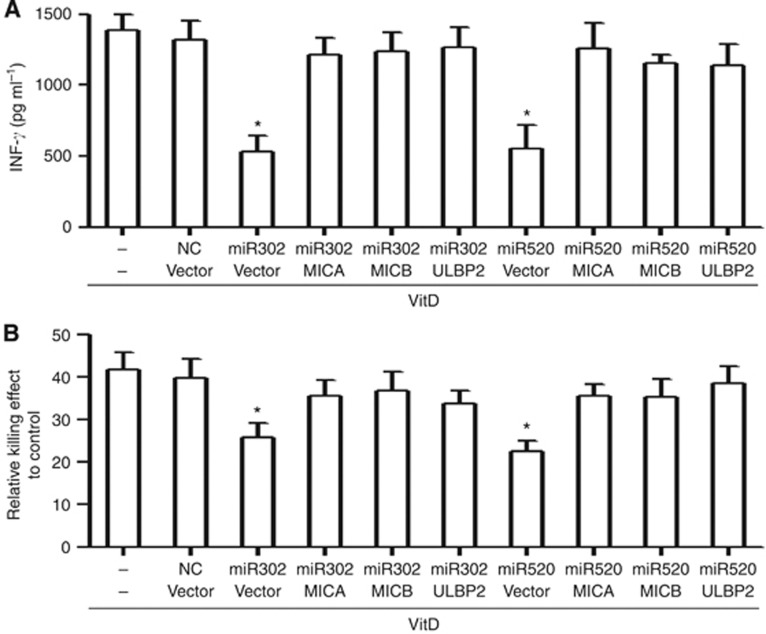
Then we examined whether these NKG2D ligands could reverse the effects of miR-302c and miR-520c in malignant cells upon 1,25(OH)2D3 treatment. Recombinant vectors expressing MICA, MICB, or ULBP2 were transfected into MDA-MB-231 cells, and the overexpression of MICA, MICB, and ULBP2 in MDA-MB-231 cells was examined using flow-cytometry analysis (Supplementary Figure S3). MDA-MB-231 cells transfected with miRNA mimics and plasmids overexpressing NKG2D ligands or negative control for 24 h were treated with 1,25(OH)2D3 for 24 h. Cells were collected and subjected to the killing assay by NK92 cells. MICA, MICB, and ULBP2 overexpression in miRNAs ectopically expressed that MDA-MB-231 cells increase the secretion of IFN-γ to 80% of the vector control (Figure 6A). Besides, the killing effect was also obviously improved in MDA-MB-231cells overexpressing MICA, MICB, or ULBP2 (Figure 6B). Taken together, these results indicate that MICA, MICB, or ULBP2 is functional contribution to the 1,25(OH)2D3-mediated susceptibility of malignant cells to NK cells via the miR-302c/miR-520c-NKG2D ligands pathway.
Figure 6.
MICA, MICB and ULBP2 could reverse the effects of miR-302c- and miR-520c-mediated resistance of malignant cells to NK cells upon 1,25(OH)2D3 treatment. (A) MDA-MB-231 cells transfected with indicated miRNAs and vectors for 24 h were treated with 0.1 μM 1,25(OH)2D3 for another 24 h and then co-incubated with NK92 cells at an E:T ratio of 16 : 1 for 4 h. The killing effects of NK92 cells against MDA-MB-231 cells evaluated using the CytoTox 96 Non-Radioactive Cytotoxicity Assay. (B) The supernatant was collected and subjected to the measurement of IFN-γ. The results are expressed as the means±s.d.; n=3, *P<0.01 compared with the control.
Discussion
Vitamin D is a major cancer-chemopreventive agent. This anticancer potential is because of the role of 1,25(OH)2D3 as a transcription factor that regulates cell growth, differentiation, and apoptosis, which are central mechanisms in the progression of cancer (Deeb et al, 2007). The studies presented here demonstrated that miR-302c and miR-520c were downregulated in response to 1,25(OH)2D3 treatment and contributed to the susceptibility of malignant cells to NK cells. Target analysis identified that both miR-302c and miR-520c targeted the NKG2D ligands MICA, MICB, and ULBP2 and suppressed their mRNA and protein levels. Our data indicate that 1,25(OH)2D3 enhanced the killing effect of NK cells against malignant cells through a miR-302c/miR-520c/NKG2D ligand pathway.
NKG2D ligands are a group of MHC class I-like molecules that act as molecular danger signals to alert the immune system to infected or neoplastic cells. One common strategy that tumour cells employ to elude immune response is the downregulation or loss of expression of certain ligands. In animal models of spontaneous tumour formation, the absence of NKG2D results in enhanced formation of aggressive tumours (Guerra et al, 2008). In other mouse models, transfection with NKG2D ligands results in NKG2D-mediated tumour rejection, whereas higher expression of NKG2D ligands results in an increased incidence of certain tumours (Cerwenka et al, 2001; Diefenbach et al, 2001; Wiemann et al, 2005; Guerra et al, 2008). In the present study, we found evidence that miR-302c and miR-520c modulated the expression of the NKG2D ligands MICA, MICB, and ULBP2 by targeting their 3′-UTRs. The introduction of miR-302c or miR-520c mimics into Kasumi-1 and MDA-MB-231 cells reduced the mRNA and protein expression of those NKG2D ligands, whereas the introduction of miR-302c or miR-520c AMO increased their expression. Upon 1,25(OH)2D3 treatment, MICA, MICB, and ULBP2 expression was elevated and inversely correlated with miR-302c and miR-520c levels. Besides, upon 1,25(OH)2D3 treatment, miR-302c or miR-520c AMO notably increased the susceptibility of Kasumi-1 and MDA-MB-231 cells to NK cell cytotoxicity. More importantly, upon 1,25(OH)2D3 treatment, reintroduction of MICA, MICB, and ULBP2 reverses the miR-302c- and miR-520c-mediated resistance of malignant cells to the cytotoxicity of NK cells. We did not further examine whether MICA, MICB, and ULBP2 modulated 1,25(OH)2D3-mediated cytotoxicity, given that many studies have confirmed the roles of these proteins in this context. (Yadav et al, 2009; Trotta et al, 2012).
Cancer immunotherapy comprises a variety of treatment approaches; great interest has surrounded the activation of the immune system as an oncolytic agent owing to its ability to induce death in a range of human malignancies while sparing normal cells. In this paper, we propose that modulation of the miR-302c/miR-520c-NKG2D ligand pathway may be a valuable strategy to increase the susceptibility of both haematological and solid tumour cells to NK cells.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81172548, 30800489, 81072176 and 81102023) and Guangdong Natural Science Foundation (S2011040004481).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Wang YD, Zheng PS. The microRNA-302-367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA. 2013;19 (1:85–95. doi: 10.1261/rna.035295.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Blanco A, De la Cruz-Hernandez E, Dominguez GI, Rodriguez-Cortez O, Alatorre B, Perez-Cardenas E, Chacon-Salinas R, Trejo-Becerril C, Taja-Chayeb L, Trujillo JE, Contreras-Paredes A, Duenas-Gonzalez A. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int J Oncol. 2011;39:1491–1499. doi: 10.3892/ijo.2011.1144. [DOI] [PubMed] [Google Scholar]
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- Fernandez-Messina L, Reyburn HT, Vales-Gomez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. doi: 10.3389/fimmu.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189:1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Guckel B, Fehm T, Schneeweiss A, Sahin O, Wiemann S, Tschulena U. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- Kizildag S, Ates H, Kizildag S. Treatment of K562 cells with 1,25-dihydroxyvitamin D3 induces distinct alterations in the expression of apoptosis-related genes BCL2, BAX, BCLXL, and p21. Ann Hematol. 2012;89:1–7. doi: 10.1007/s00277-009-0766-y. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park S, Cheon S, Lee JH, Kim S, Hur DY, Kim TS, Yoon SR, Yang Y, Bang SI, Park H, Lee HT, Cho D. 1,25-Dihydroxyvitamin D(3) enhances NK susceptibility of human melanoma cells via Hsp60-mediated FAS expression. Eur J Immunol. 2011;41:2937–2946. doi: 10.1002/eji.201141597. [DOI] [PubMed] [Google Scholar]
- Liang Z, Ahn J, Guo D, Votaw JR, Shim H. MicroRNA-302 replacement therapy sensitizes breast cancer Cells to ionizing radiation. Pharm Res. 2013;30 (4:1008–1016. doi: 10.1007/s11095-012-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ljunggren HG. Cancer immunosurveillance: NKG2D breaks cover. Immunity. 2008;28:492–494. doi: 10.1016/j.immuni.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–447. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli MU. NKG2D and its ligands: key to immunotherapy of liver cancer. J Hepatol. 2012;56:308–310. doi: 10.1016/j.jhep.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- Obeidy P, Sharland AF. NKG2D and its ligands. Int J Biochem Cell Biol. 2009;41:2364–2367. doi: 10.1016/j.biocel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- Saito H, Osaki T, Ikeguchi M. Decreased NKG2D expression on NK cells correlates with impaired NK cell function in patients with gastric cancer. Gastric Cancer. 2012;15:27–33. doi: 10.1007/s10120-011-0059-8. [DOI] [PubMed] [Google Scholar]
- Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J (Sudbury, Mass) 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T, Nakamura T, Saitoh H, Yamaya K, Funyu T, Fukuda M, Ohyama C. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30:3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo L, Di Somma C, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol. 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- Yadav D, Ngolab J, Lim RS, Krishnamurthy S, Bui JD. Cutting edge: down-regulation of MHC class I-related chain A on tumor cells by IFN-gamma-induced microRNA. J Immunol. 2009;182:39–43. doi: 10.4049/jimmunol.182.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Zhang X, Yu Z. Synergistic effect of retinoic acid and vitamin D analog EB1089-induced apoptosis of hepatocellular cancer cells. Cytotechnology. 2013;65 (3:457–465. doi: 10.1007/s10616-012-9500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.