Abstract
It has long been established that hyperglycemia with or without a prior diagnosis of diabetes increases both mortality and disease-specific morbidity in hospitalized patients1–4 and that goal-directed insulin therapy can improve outcomes.5–9 During the past decade, since the widespread institutional adoption of intensified insulin protocols after the publication of a landmark trial,5,10 the pendulum in the inpatient diabetes literature has swung away from achieving intensive glucose control and toward more moderate and individualized glycemic targets.11,12 This change in clinical practice is the result of several factors, including challenges faced by hospitals to coordinate glycemic control across all levels of care,13,14 publication of negative prospective trials,15,16 revised recommendations from professional organizations,17,18 and increasing evidence on the deleterious effect of hypoglycemia.19–22 This article reviews the pathophysiology of hyperglycemia during illness, the mechanisms for increased complications and mortality due to hyperglycemia and hypoglycemia, beneficial mechanistic effects of insulin therapy and provides updated recommendations for the inpatient management of diabetes in the critical care setting and in the general medicine and surgical settings.23,24
Keywords: Inpatient diabetes, Hyperglycemia, Hypoglycemia, Critical illness, Hospital, Nutrition
PREVALENCE OF HYPERGLYCEMIA AND DIABETES IN HOSPITALIZED PATIENTS
The prevalence of diabetes around the world is alarmingly high and is growing. In 2007, it was estimated that approximately 23.6 million people in the United States had diabetes, approximately 7.8% of the population, of whom 90% to 95% of these had type 2 diabetes mellitus.25 Patients with diabetes have a 3-fold greater chance of hospitalization compared with those without diabetes,26,27 and it is estimated that more than 20% of all adults discharged have diabetes, with 30% of them requiring 2 or more hospitalizations in any given year.4,26,27
The exact prevalence of hospital hyperglycemia is not known but it varies based on study populations and definitions used in previous reports. Observational studies have reported a prevalence of hyperglycemia ranging from 32% to 38% in community hospitals4,28 to approximately 70% of diabetic patients with acute coronary syndrome29 and approximately 80% of cardiac surgery patients in the perioperative period.5,30 Patients with newly identified, or “stress,” hyperglycemia may be at the highest risk of hyperglycemia-related morbidity and mortality. The American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) consensus on inpatient hyperglycemia defined stress hyperglycemia or hospital-related hyperglycemia as any blood glucose (BG) concentration greater than 7.8 mmol/L (140 mg/dL). Although stress hyperglycemia typically resolves as the acute illness or surgical stress abates, it is important to identify and track patients because 60% of patients admitted with new hyperglycemia had confirmed diabetes at 1 year.13 Cross-sectional studies of patients without a known history of diabetes with hyperglycemia revealed that between 30% and 60% of patients have impaired carbohydrate intolerance or diabetes during follow-up.13 Until recently, clinical guidelines recommended that all patients with stress hyperglycemia should be tested with an oral glucose tolerance test shortly after discharge to assess carbohydrate tolerance.10 More recently, the use of the hemoglobin A1c (HbA1c) test has been recommended versus the oral glucose tolerance test as the preferred diagnostic testing in hospitalized patients with hyperglycemia.31 Measurement of HbA1c levels during periods of hospitalization provides an opportunity to differentiate patients with stress hyperglycemia from those with diabetes who were previously undiagnosed and to identify patients with known diabetes who would benefit from intensification of their glycemic management regimen.32,33 The ADA recommendations indicate that patients with HbA1c level of 6.5% or higher can be identified as having diabetes.
PATHOPHYSIOLOGY OF HYPERGLYCEMIA DURING ILLNESS
Hyperglycemia is a frequent manifestation of critical and surgical illness, resulting from the acute metabolic and hormonal changes associated with the response to injury and stress.34,35 Acute illness, surgery, and trauma raise levels of stress mediators, namely stress hormones, cytokines, and central nervous system, that interfere with carbohydrate metabolism, leading to excessive hepatic glucose output and reduced glucose uptake in peripheral tissues. In addition, acute illness increases proinflammatory cytokines, which increase insulin resistance by interfering with insulin signaling.
Regulation of Blood Glucose in Healthy Individuals
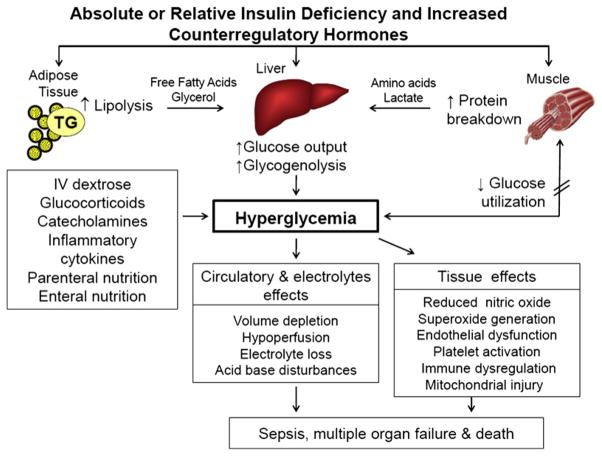
Maintenance of a constant BG level is essential for normal physiology in the body, particularly for the central nervous system. The brain can neither synthesize nor store the amount of glucose required for normal cellular function.36 In the postabsorptive state, systemic glucose balance is maintained, and hypoglycemia and hyperglycemia are prevented, by dynamic, minute-to-minute regulation of endogenous glucose production from the liver and kidneys and of glucose use by peripheral tissues (Fig. 1).37,38 Glucose production is accomplished by gluconeogenesis or glycogenolysis. By way of gluconeogenesis, noncarbohydrate precursors, such as lactate, alanine, and glycerol, are converted to glucose. Excess glucose is polymerized to glycogen, which is mainly stored in the liver and muscle. Glycogenolysis breaks down glycogen to the individual glucose units for mobilization during times of metabolic need. These steps are dependent on the interaction of different mechanisms, including glucoregulatory hormones (insulin and counterregulatory hormones) and gluconeogenic substrate supply (lactate, glycerol, and amino acids). Insulin is the main glucoregulatory hormone and inhibits hepatic glucose production and stimulates peripheral glucose uptake.36,39 In the liver, insulin directs glucose-6-phosphate to glycogen by increasing the activity of glycogen synthase and decreasing the activity of glycogen phosphorylase, which stimulates the breakdown of glycogen to glucose. In addition, insulin inhibits gluconeogenesis by inhibiting gene transcription and expression of phosphoenolpyruvate carboxykinase, the rate-limiting step in hepatic gluconeogenesis,40,41 and by increasing the transcription of pyruvate kinase, the main glycolytic enzymes that produces pyruvate molecules, the final product of aerobic glycolysis.42
Fig. 1.
Pathogenesis of hyperglycemia. Hyperglycemia during acute illness results from increased hepatic glucose production and impaired glucose use in peripheral tissues. Excess counterregulatory hormones (glucagon, cortisol, catecholamines, and growth hormone) increase lipolysis and protein breakdown (proteolysis) and impaired glucose use by peripheral tissues. Hyperglycemia causes osmotic diuresis that leads to hypovolemia decreased glomerular filtration rate and worsening hyperglycemia. At the cellular level, increased BG levels results in mitochondrial injury by generating reaction oxygen species and endothelial dysfunction by inhibiting nitric oxide production. Hyperglycemia increases levels of inflammatory cytokines, such as TNF-α; IL-6, leading to immune system dysfunction; and plasminogen activator inhibitor-1 and fibrinogen, causing platelet aggregation and hyper-coagulable state. These changes can eventually lead to increased risk of infection, impaired wound healing, multiple organ failure, prolonged hospital stay, and death.
Pathogenesis of Hyperglycemia During Stress and Illness
Given the obligatory role of glucose to maintain normal physical function, it is not surprising that the normal response to stress or illness includes the release of counterregulatory hormones, which counteract insulin to increase the availability of glucose.43 Counterregulatory hormones leads to several alterations in carbohydrate metabolism, including insulin resistance, increased hepatic glucose production, impaired peripheral glucose use, and relative insulin deficiency. In addition, high epinephrine levels stimulate glucagon secretion and inhibit insulin release by pancreatic β cells (Table 1).44 High cortisol levels increase hepatic glucose production, stimulate protein catabolism, and increase circulating amino acids concentration, providing precursors for gluconeogenesis.45,46
Table 1.
Important counterregulatory hormones and mediators of inflammation known to be associated with acute hyperglycemia
| Glucoregulatory Hormone | Metabolic Effect |
|---|---|
| Cortisol | ↑ Skeletal muscle IR, ↑ lipolysis → ↑ gluconeogenesis |
| Epinephrine | ↑ Skeletal muscle IR, ↑ gluconeogenesis and glycogenolysis, ↑ Lipolysis, ↓ insulin secretion from β cell |
| Norepinephrine | ↑ Gluconeogenesis (at high levels), ↑ lipolysis |
| Glucagon | ↑ Gluconeogenesis and glycogenolysis |
| Growth hormone | ↑ Skeletal muscle IR, ↑ gluconeogenesis, ↑ lipolysis |
| Inflammation Mediators | Metabolic Effect |
| TNF-α | ↑ Skeletal and hepatic IR |
| IL-1 | ↑ Skeletal and hepatic IR |
| IL-6 | ↑ Skeletal and hepatic IR |
| IL-18 | ↑ Skeletal and hepatic IR |
| FFAs | ↑ Skeletal and hepatic IR, ↑ gluconeogenesis |
Abbreviation: IR, insulin resistance.
Acute stress increases proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1,34,47–49 which increase insulin resistance by interfering with insulin signaling. TNF-α activates c-Jun N-terminal kinase, a signaling protein molecule that phosphorylates insulin receptor substrate-1 and prevents insulin-mediated activation of phosphatidylinositol 3-kinase involved in tissue glucose uptake. Downstream effect process decreases insulin stimulation of glucose uptake and causes hyperglycemia.50,51
Mechanisms of Detrimental Effects of Hyperglycemia
Although there are still no proved mechanisms to explain the detrimental effects of hyperglycemia in critically ill patients, several mechanisms may explain the higher risk of complications and mortality in hyperglycemic patients in the hospital (see Fig. 1). Severe hyperglycemia causes osmotic diuresis that leads to hypovolemia, decreased glomerular filtration rate, and prerenal azotemia. Hyperglycemia has also been shown to increase rate of hospital infections and poor wound healing.52,53 Hyperglycemia is associated with impaired leukocyte function, including decreased phagocytosis, impaired bacterial killing, and chemotaxis.54 Hyperglycemia has also been shown to impair collagen synthesis and to impair wound healing among patients with poorly controlled diabetes.52 In addition, acute hyperglycemia results in nuclear factor κB (NF-κB) activation and production of inflammatory cytokines, such as TNF-α, IL-6, and plasminogen activator inhibitior-1, which cause increased vascular permeability and leukocyte and platelet activation.55
Acute hyperglycemia activates oxidative pathway through increased generation of reactive oxygen species (ROS). ROS and the cellular redox state are increasingly thought to be responsible for affecting different biologic signaling pathways. ROS are formed from the reduction of molecular oxygen or by oxidation of water to yield products, such as superoxide anion, hydrogen peroxide, and hydroxyl radical; the mitochondria and NADP oxidase are the major sources of ROS production.56 In moderate amounts, ROS are involved in several physiologic processes that produce desired cellular responses. Large quantities of ROS, however, can lead to cellular damage of lipids, membranes, proteins, and DNA.57 Oxidative stress activates a series of stress pathways involving a family of serine/threonine kinases, which in turn have a negative effect on insulin signaling. Oxidative stress has also been implicated as a contributor to β-cell and mitochondrial dysfunction, which can lead to the development and worsening of hyperglycemia.57
In patients with acute ischemic cardiac events, acute hyperglycemia has been shown to attenuate ischemic preconditioning of the heart, a protective mechanism for ischemic injury, possibly by inhibiting activation of ATP-sensitive potassium channels that activates glycolysis.58 Increasing evidence indicates that hyperglycemia may induce cardiac myocyte death through apoptosis or by exaggerating ischemia-reperfusion cellular injury.53,59 High glucose concentrations also have deleterious effect on endothelial function by suppressing formation of nitric oxide and impairing endothelium-dependent flow-mediated dilation.60 In addition, hyperglycemia induces abnormalities in hemostasis, including increased platelet activation, adhesion, and aggregation61; reduced plasma fibrinolytic activity; and increased plasminogen activator inhibitor-1 activity.62 Adding to the effects of hyperglycemia in acute coronary syndrome, high free fatty acid (FFA) levels seen in diabetes and stress can also aggravate ischemia/reperfusion damage by limiting the ability of cardiac muscle to uptake glucose for anaerobic metabolism.63,64 Although FFAs are the normal substrate of choice for healthy myocardium, high FFA levels are toxic to an ischemic myocardium,63,64 leading to cardiac arrhythmias, sympathetic overactivity, increased blood pressure, oxidative stress, and endothelial dysfunction.65–67 Increased FFAs also produce dose-dependent insulin resistance in peripheral tissues and increase hepatic glucose output in both diabetic and nondiabetic individuals.35,68
In patients with ischemic stroke, hyperglycemia has been shown to aggravate neuronal damage after brain ischemia.53,69–71 During the progression of stroke, the area of ischemic penumbra (the region of the brain tissue surrounding the core of infarcted tissue where neurons still viable) is more sensitive to ischemic injury.70,72 Studies using animal models of stroke have shown that hyperglycemia decreases reperfusion to the ischemic tissue and increases infarct volumes compared with normoglycemia.73 Hemispheric cerebral blood flow has been shown reduced by as much as 37% in hyperglycemia compared with normoglycemia.74 Increased tissue acidosis due to accumulation of lactate in the ischemic brain mediates ischemic injury by enhancing lipid peroxidation and free radical formation, accumulation of intracellular calcium, and impairing mitochondrial function.70
HYPERGLYCEMIA IN ACUTE ILLNESS: RATIONALE FOR PROACTIVE TREATMENT
Extensive observational and prospective randomized trials in patients with critical illness indicate a strong association between hyperglycemia and poor clinical outcome, such as mortality, morbidity, length of stay, infections, and overall complications.4,75 This association is well documented on admission and also for the mean glucose level during the hospital stay.2,3,76 Cross-sectional studies have shown that the risk of complications and mortality relates to the severity of hyperglycemia, with a higher risk observed in patients without a history of diabetes (new-onset and stress-induced hyperglycemia) compared with those with a known diagnosis of diabetes.
Glycemic Control Trials in Critical Care Setting
A large retrospective cohort study of more than 250,000 veterans admitted to various ICUs in the United States found that the development of hyperglycemia is an independent risk for mortality in individuals with cardiac diagnoses, sepsis, and respiratory failure.76 In cardiac surgery patients, perioperative hyperglycemia has been associated with increased length of stay, delayed extubation, increased risk of perioperative complications, and mortality.77 Similarly, several observational studies and meta-analyses in patients with myocardial infarction29,78 and with stroke and subarachnoid hemorrhage70,73,79 have consistently reported that hyperglycemia on admission or during the hospital stay is associated with poor clinical outcome and a higher risk of mortality, independently from other predictors of a poor prognosis, such as age and diabetic status. In a nonrandomized, prospective study, Furnary and colleagues2 followed 3554 patients with diabetes who underwent coronary artery bypass graft and were treated with either subcutaneous insulin or continuous insulin infusion (CII) for hyperglycemia. Compared with patients treated with subcutaneous insulin who had average BG level of 11.9 mmol/L (214 mg/dL), patients treated with CII with average BG level of 9.8 mmol/L (177 mg/dL) had significantly less deep sternal wound infections and a reduction in risk-adjusted mortality by 50%.7 A follow-up analysis in a subset of this study population revealed that patients with BG levels greater than 11.1 mmol/L (>200 mg/dL) had higher mortality (5.0% vs 1.8%, P<.001) than those with BG levels less than 11.1 mmol/L.2 Similarly, the Leuven surgical ICU study reported that intensive therapy to maintain target glucose levels 4.4 mmol/L to 6.1 mmol/L (80–110 mg/dL) compared with conventional therapy to maintain target levels between 10 mmol/L and 11.1 mmol/L (180–200 mg/dL) significantly reduced the frequency of bacteremia, antibiotic requirements, length of ventilator dependency, number of ICU days, and an an overall mortality (34% reduction).5 In a different study, these investigators following the same protocol in medical ICU patients also reported less ICU and hospital mortality after 3 days of treatment with CII.15
Recent randomized controlled trials, however, have shown that intensive glycemic control (target glucose <110 mg/dL) has been difficult to achieve without increasing the risk for severe hypoglycemia,16,80–82 causing some to be discontinued early (Table 2). In addition, several multicenter trials have failed to show significant improvement in clinical outcome or have resulted in increased mortality risk.16,80–83 The largest and recent Normoglycemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial, with more than 6000 subjects from different ICUs,16 randomized patients to receive either conventional glycemic control (<10 mmol/L [<180 mg/dL]) or intensive glycemic control (4.5–6 mmol/L [81–108 mg/dL]) and reported that intensive glycemic control was associated with increased mortality at 90 days (24.9% vs 27.5%, P = .02) and higher incidence of hypoglycemia (6.8% vs 0.5%, P<.001).16
Table 2.
Summary of key randomized clinical trials designed to test effect of glycemic control in critical illness
| Author | Population | Design/Endpoint | No. of Patients | Achieved Glycemic Endpoints (Mean ± SD [mg/dL]) | Hospital Mortality OR (95% CI)a | Comments | |
|---|---|---|---|---|---|---|---|
| Control | Glycemic Control | ||||||
| Van den Berghe et al5,15 | Surgical, mechanical ventilation | Randomized 80–110 vs 180–200 mg/dL Research RN-titrated insulin per protocol |
1548 | Daily 153 ± 33 | Daily 103 ± 19 (P<.001) | 0.64 (0.45–0.91) | High use of parenteral dextrose (in TPN); stopped early for benefit |
| Van den Berghe et al5,15 | Medical, expected ICU stay >72 h | Randomized Bedside RN-titrated per paper protocol |
1200 | Daily 153 ± 31 | Daily 111 ± 29 (P<.001) | 0.89 (0.71–1.13) | High use of parenteral dextrose (in TPN) |
| Presier GLUCONTROL163 | Medical, surgical | Randomized 80–110 vs 140–180 mg/dL Bedside RN-titrated per protocol |
1078 | 144 (IQR 128–162) median— all values | 117 (IQR 108–130) median—all values | 1.27 (0.94–1.7) | Stopped early for hypoglycemia; many protocol violations |
| NICE-SUGAR16 | Medical, surgical | Randomized 80–110 vs 140–180 mg/dL Adjusted via computerized algorithm |
6104 | 144 ± 23 | 115 ± 18 (P<.001) | 28-Day mortality: 1.09 (0.96–1.23) 90-Day mortality: 1.14 (1.02–1.28) |
GC group did not achieve target |
| Brunkhorst et al80 (VISEP) | Sepsis | Randomized 80–110 vs 180–200 mg/dL Bedside RN-titrated per Van den Berghe protocol |
537 | Median—daily 138 (IQR 111–184) | Median—daily 130 (IQR 108–167) (P = .05) | 28-Day mortality: 0.94 (0.63–1.38) | GC group did not achieve target; study stopped early for hypoglycemia risk; underpowered |
| De La Rosa et al81 | Medical, surgical | Randomized 80–110 vs 180–200 mg/dL Bedside RN-titrated per protocol |
504 | Median— all values 149 (IQR 124–180) |
Median— all values 120 (IQR 110–134) (P<.001) |
28-Day mortality: 1.09 (0.75–1.54) | GC group did not achieve target; underpowered |
| Arabi et al165 | Medical, surgical | Randomized 80–110 vs 180–200 mg/dL Bedside RN-titrated per paper protocol |
523 | 171 ± 34 | 115 ± 18 (P<.0001) | 0.78 (0.53–1.13) | Underpowered |
| Farah et al168 | Medical with >3-day LOS | Randomized 110–140 vs 140–200 mg/dL | 89 | 174 ± 20 | 142 ± 14 | 28-Day mortality: 54% vs 46% (P>.05) | Underpowered; vascular morbidity lower with GC |
| Gray and Perdrizet169 | Surgical, excluded patients with diabetes | Randomized 80–120 mg/dL vs 180–220 mg/dL | 61 | 179 ± 61 | 125 ± 36 mg/dL; daily mean value lower on each day | 21% vs 11% (P = .5) | Underpowered; lower nosocomial infection incidence with GC |
| Mackenzie et al170 | Medical, surgical, trauma | Randomized 72–108 vs 180–198 mg/dL | 240 | 144 ± 40 Time- weighted mean | 113 ± 22 Time- weighted mean | 40% vs 32% (P = .28) | Stopped early due to financial limits |
Abbreviations: GC, glycemic control; IQR, interquartile; IV, intravenous; LOS, length of stay; OR, odds ratio; RN, registered nurse; SQ, subcutaneous; TPN, total parenteral nutrition.
Hospital mortality unless otherwise specified. OR <1 indicates benefit due to glycemic control. Outcome prevalence written as control group vs glycemic control.
Hyperglycemia and Noncritical Illness
The importance of hyperglycemia also applies to non–critically ill patients admitted to general medicine and surgery services. In such patients, hyperglycemia is associated with poor hospital outcomes, including prolonged hospital stay, infections, disability after hospital discharge, and death.4,84 In a retrospective study of 1886 patients admitted to a community hospital, mortality was significantly higher in patients with newly diagnosed hyperglycemia and those with known diabetes compared with those with normoglycemia (10% vs 1.7% vs 0.8%, respectively; P<.01).4 Admission hyperglycemia has also been linked to worse outcomes in patients with community-acquired pneumonia.85 In a prospective cohort multicenter study of 2471 patients, those with admission glucose levels greater 11 mmol/L (198 mg/dL) had a greater risk of mortality and complications than those with glucose less than 11 mmol/L. The risk of in-hospital complications increased 3% for each 1 mmol/L increase in admission glucose. In a retrospective study of 348 patients with chronic obstructive pulmonary disease and respiratory tract infection, the relative risk of death was 2.10 in those with a BG levels of 7 mmol/L to 8.9 mmol/L (126–160 mg/dL) and 3.42 for those with BG levels greater than 9.0 mmol/L (162 mg/dL) compared with patients with BG levels 6.0 mmol/L (110 mg/dL).86 Furthermore, each 1 mmol/L increase in BG level was associated with a 15% increase in the risk of an adverse clinical outcome, which was defined as death or length of stay of greater than 9 days.
Several observational studies in general surgery patients admitted to noncritical care areas have also shown that hyperglycemia is associated with increased risks of perioperative complications, length of stay, and mortality.87–90 Patients with glucose levels of 5.6 mmol/L to 11.1 mmol/L (110–200 mg/dL) and those with glucose levels greater than 11.1 mmol/L (>200 mg/dL) had, respectively, a 1.7-fold and 2.1-fold increased mortality compared with those with glucose levels less than 5.6 mmol/L (<110 mg/dL). General surgery patients with glucose levels greater than 12.2 mmol/L (>220 mg/dL) on the first postoperative day had a 2.7-times increased rate of infection.89 The risk of postoperative infection rate in patients undergoing noncardiac general surgery was estimated to increase by 30% for every 2.2 mmol/L (40 mg/dL) rise in the presence of hyperglycemia.91 A recent study in 3184 general surgery patients in non-ICU setting reported a strong association between presurgery and postsurgery hyperglycemia and postoperative cases of pneumonia, systemic blood infection, urinary tract infection, acute renal failure, and acute myocardial infarction.87 In that study, multivariate analysis adjusted for age, gender, race, and surgery severity found that the risk of death increased in proportion to perioperative glucose levels; however, this association was significant only for patients without a history of diabetes compared with patients with a known history of diabetes.87 Patients without a history of diabetes (stress hyperglycemia) experiencing worse outcome and higher mortality at a same glucose level than those with known history of diabetes suggests a lack of adaptation to acute hyperglycemia and its associated inflammatory and oxidative state.
Beneficial Mechanistic Effects of Insulin Therapy
The adverse outcomes associated with hyperglycemia may be attributed to the inflammatory and pro-oxidant effects observed with increased glucose levels. Many of the adverse outcomes can be prevented by administration of insulin. The positive effects of insulin administration are attributed to its anti-inflammatory, vasodilatory, and antioxidant effects as well as its ability to inhibit lipolysis and platelet aggregation. Several studies have reported that elevated levels of cytokines and inflammatory markers associated with severe hyperglycemia return to normal shortly after the treatment with insulin and resolution of hyperglycemia.48 Insulin acts to suppress counterregulatory hormones and proinflammatory transcription factors and may even suppress the formation of ROS.34,92 Insulin suppresses the proinflammatory transcription factors, NF-κB and early growth response-1.93–95 Recent studies have also shown that insulin administration is associated with a decrease in the concentration of compounds whose gene transcription is modulated by these factors, including plasminogen activator inhibitor-1, intercellular adhesion molecule-1, monocyte chemotactic protein-1, and monocyte chemotactic protein-9.93,95 Additionally, insulin induces vasodilation and inhibits lipolysis and platelet aggregation. The vasodilation that accompanies insulin administration may be attributed to its ability to stimulate nitric oxide release and induce the expression of endothelial nitric oxide synthase.
GLYCEMIC TARGETS IN ICU AND NON-ICU SETTINGS
The AACE/ADA Task Force on Inpatient Glycemic Control recommended targeting a BG level between 7.8 mmol/L and 10.0 mmol/L (140 and 180 mg/dL) for the majority of ICU patients and lower glucose targets between 6.1 mmol/L and 7.8 mmol/L (110 and 140 mg/dL) in selected ICU patients (ie, centers with extensive experience and appropriate nursing support, cardiac surgical patients, and patients with stable glycemic control without hypoglycemia). Glucose targets greater than 10 mmol/L (>180 mg/dL) or less than 6.1 mmol/L (<110 mg/dL) are not recommended in ICU patients due to lack of proved benefit and potential risk in both large and small prospective randomized trials (see Table 2).
In non-ICU settings, the AACE/ADA Practice Guideline18,96 recommends a premeal glucose level less than 140 mg/dL (7.8 mmol/L) and a random BG level less than 10.0 mmol/L (180 mg/dL) for the majority of non–critically ill patients treated with insulin.96 To avoid hypoglycemia (<3.9 mmol/L), the total basal and prandial insulin dose should be reduced if glucose levels fall between less than 3.9 mmol/L and 5.6 mmol/L (70–100 mg/dL). In contrast, higher glucose ranges may be acceptable in terminally ill patients or in patients with severe comorbidities as well as in those in patient-care settings where frequent glucose monitoring or close nursing supervision is not feasible.18,96,97 In such patients, however, it is prudent to maintain a reasonable degree of glycemic control (BG <11.1 mmol/L [200 mg/dL]) as a way of avoiding symptomatic hyperglycemia.
MANAGING HYPERGLYCEMIA IN THE HOSPITAL ENVIRONMENT
Despite solid evidence in support of glycemic control in hospitalized patients, BG control continues to be deficient and is frequently overlooked in critically ill patients and in general medicine and surgery services.4,98 Many factors could explain the physician’s inactivity in addressing in-hospital hyperglycemia. First, hyperglycemia is rarely the focus of care during the hospital stay, because the overwhelming majority of hospitalizations in patients with hyperglycemia occur for comorbid conditions.4,99 Second, fear of hypoglycemia constitutes a major barrier to efforts to improve glycemic control in hospitalized subjects, especially in patients with poor caloric intake.100 Third, in the presence of altered nutrition and associated medical illness, physicians frequently hold a patient’s previous outpatient diabetes regimen and initiate sliding scale coverage with regular insulin. Finally, the specific morbidities due to secondary causes of hyperglycemia, such as steroid-exacerbated hyperglycemia, remain largely unknown.101 Hospital care of patients with diabetes and hyperglycemia is complex, involving multiple providers with varying degrees of expertise who are dispersed across many different areas of the hospital. A multidisciplinary systems approach with identifiable hospital champions can help guide meaningful progress away from clinical inertia and toward safe glycemic control, insulin management, and hypoglycemia prevention.18,102
Glucose Monitoring in Hospital
Bedside capillary point of care (POC) testing is the preferred method for guiding ongoing glycemic management of individual patients.18 POC testing is usually performed 4 times a day: before meals and at bedtime for patients who are eating.53,96 For patients who are restricted to nothing by mouth or are receiving continuous enteral nutrition, POC testing is recommended every 4 to 6 hours. More frequent glucose monitoring is indicated in patients treated with continuous intravenous insulin infusion103,104 or after a medication change that could alter glycemic control (eg, corticosteroid use or abrupt discontinuation of enteral or parenteral nutrition)101,105,106 or in patients with frequent episodes of hypoglycemia.11,53
Health care workers should keep in mind that the accuracy of most hand-held glucose meters is far from optimal.107 There is an accepted variance between meter readings and central laboratory results (up to 20% allowed by Food and Drug Administration regulations),96,108 which can potentially lead to inappropriate therapy. Many patient factors are known to affect the accuracy of the POC testing including pH changes, oxygenation status, and low hematocrit, among others.107,109
Medical Nutrition Therapy in Hospitalized Patients with Diabetes
Medical nutrition therapy (MNT) plays an important role in management of hyperglycemia in hospitalized patients with diabetes mellitus and often requires specifically designed insulin programs. The goals of inpatient MNT for patients with diabetes are to help optimize glycemic control, provide adequate calories to meet metabolic demands, address individuals needs based on personal food preferences, and provide a discharge plan for follow-up care.53,97,110 MNT in the hospital can be challenging in the presence of acute medical illness, poor appetite, inability to eat, increased nutrient and calorie needs due to catabolic stress, and variation in diabetes medications.
The metabolic needs of most hospitalized subjects can be supported by providing 25 to 35 cal/kg/d111,112 whereas critically ill patients require less caloric intake at 15 to 25 cal/kg/d.113 This translates into a diet on average containing 1800 to 2000 cal/d53 or a diet containing approximately 200 g/d of carbohydrates divided between meals.112 Care must be taken not to overfeed patients because this can exacerbate hyperglycemia. There is no single meal planning system that is ideal for hospitalized patients. It is suggested, however, that hospitals consider implementing a consistent carbohydrate diabetes meal-planning system.112 This systems uses meal plans without a specific calorie level but with consistency in the carbohydrate content of meals. The carbohydrate components of breakfast, lunch, dinner, and snacks may vary, but the day-to-day carbohydrate content of specific meals and snacks is kept constant.53,112 It is recommended that the term, ADA diet, no longer be used, because the ADA no longer endorses a single nutrition prescription or percentages of macronutrients.112
Patients requiring clear or full liquid diets should receive approximately 200 g/day of carbohydrates in equally divided amounts at meal and snack times. Liquids should not all be sugar-free because patients require sufficient carbohydrate and calories, and sugar-free liquids do not meet these nutritional needs. After surgery, food intake should be initiated as quickly as possible with progression from clear liquids to full liquids to solid foods as rapidly as tolerated.112,114 Increasing evidence, however, indicates that early enteral feeding in the perioperative period is safe and well tolerated and results in reduction of wound morbidity and healing, fewer septic complications, diminished weight loss, and improved protein kinetics.114
Enteral Nutrition
Although the majority of non–critically ill hospitalized patients receive nutrition support as 3 discrete meals with or without scheduled snacks each day, some patients may require enteral nutrition support. Standard enteral formulas reflect the reference values for macronutrients and micronutrients for a healthy population and contain 1 to 2 calories per milliliter. Standard diabetes-specific formulas provide low amounts of lipids (30% of total calories) combined with a high carbohydrate content (55%–60% of total calories); however, newer diabetic formulas have replaced part of carbohydrates with monounsaturated fatty acids (up to 35% of total calories), 10 g/L to 15 g/L dietary fiber, and up to 30% fructose.115,116 Several outpatient and inpatient studies in subjects with type 2 diabetes mellitus have reported better glycemic control (lower mean, fasting, and/or postprandial glucose levels), a trend toward decreased HbA1c levels, and lower insulin requirements with a low-carbohydrate, high–monounsaturated fatty acids (LCHM) formulas compared with a standard high-carbohydrate formulas.117,118 In a meta-analysis of studies comparing enteral LCHM formulas with standard formulations, the postprandial glucose rise was reduced by 18 mg/dL to 29 mg/dL with the newer formulations.119
Parenteral Nutrition
The beneficial effect of parenteral nutrition in improving the nutritional status of critically ill patients is well established.120 Recent randomized trials and meta-analyses, however, have suggested that parenteral nutrition may be associated with increased risk of infectious complications and mortality in critically ill patients.120,121 In addition, its use has been linked to aggravation of hyperglycemia independent of a prior history of diabetes.121,122 BG level measures above 150 mg/dL before and within 24 hours of initiation of parenteral nutrition are predictors of both increased inpatient complications and hospital mortality. Although randomized controlled studies to guide effective and safe administration of insulin during parenteral nutrition are lacking, patients with or without history of diabetes with persistent hyperglycemia (>140 mg/dL) should be treated with insulin therapy. To correct hyperglycemia, regular insulin can be added to parenteral nutrition solutions or can be given as continuous insulin infusion.
PHARMACOLOGIC TREATMENT OF INPATIENT HYPERGLYCEMIA
Insulin, given either intravenously or subcutaneously, is the preferred regimen for effectively treating hyperglycemia in the hospital. The use of oral antidiabetic agents should be avoided in the hospital setting because no data are available on their safety and efficacy in the inpatient setting.53,98 Major limitations to the use of oral agents for hospital use are their slow onset of action that does not allow rapid glycemic control and dose adjustments to meet the changing needs of acutely ill patients and risk of hypoglycemia with insulin secretagogues. Sulfonylureas may increase the risk of hypoglycemia in hospitalized patients with poor appetite or ordered dietary restrictions. In addition, they may worsen cardiac and cerebral ischemia123–126 by inhibiting ATP-sensitive potassium channels, resulting in cell membrane depolarization and increased intracellular calcium concentration.127 Many patients have one or more contraindications to the use of metformin on admission,128 including acute congestive heart failure, renal or liver dysfunction, and chronic pulmonary disease. The use of thiazolidinediones is limited because they can increase intravascular volume and may precipitate or worsen congestive heart failure and peripheral edema.129,130 Despite their benign side-effect profile and single oral daily dose of dipeptidyl peptidase-IV inhibitors,131 these agents have a minor reduction in glucose concentration, and no previous studies have assessed their efficacy and safety in hospitalized patients.
Insulin Therapy in Critical Care
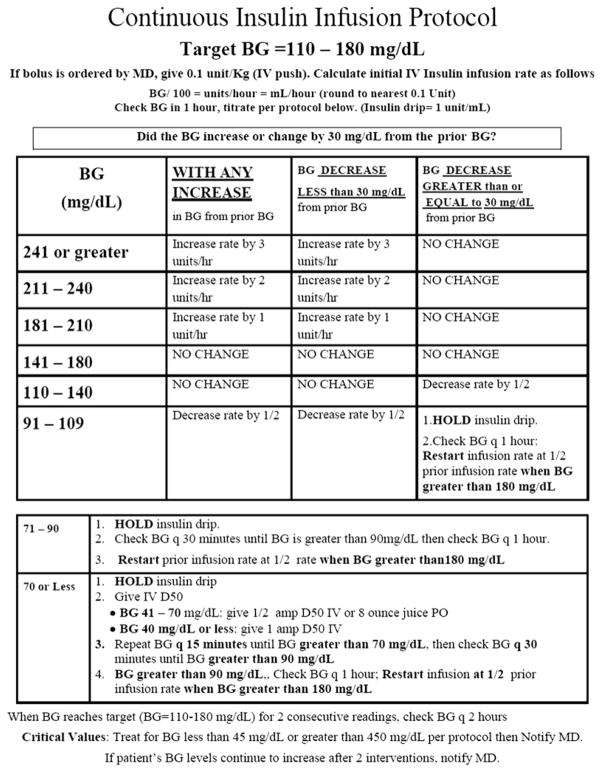
In the critical care setting, a variety of CII protocols have been shown effective in achieving glycemic control and in improving hospital outcome with a low-rate of hypoglycemic events.2,132 In most patients, CII lowers BG levels to target range in approximately 4 to 8 hours and allows for rapid titration of dose for both anticipated (eg, initiation or discontinuation of vasopressors) or unanticipated (eg, acute deteriorations in clinical status) changes in clinical status. Essential elements that increase protocol success of CII are (1) rate adjustment considers the current and previous glucose value and the current rate of insulin infusion, (2) rate adjustment considers the rate of change (or lack of change) from the previous reading, and (3) frequent glucose monitoring (hourly until stable glycemia is established, and then every 2–3 h) (Fig. 2).132–134 BG level greater than140 mg/dL should trigger initiation of insulin therapy, titrated to maintain glucose values absolutely between 140 mg/dL and 180 mg/dL while avoiding hypoglycemia. Using a higher trigger value to start insulin treatment could allow excursion to glucose values above 180 mg/dL, which is undesirable with respect to the immunosuppressive effects.
Fig. 2.
Example of insulin infusion protocol. Essential elements that increase protocol success of CII are (1) rate adjustment considers the current and previous glucose value and the current rate of insulin infusion, (2) rate adjustment considers the rate of change (or lack of change) from the previous reading, and (3) frequent glucose monitoring.
Recently, computer-based algorithms aiming to direct the nursing staff in adjusting insulin infusion rates have become commercially available.135,136 Controlled trials have reported more rapid and tighter glycemic control with computer-guided algorithms than with standard paper form protocols in ICU patients.137 Uuse of computer-based algorithms has been associated with lower glycemic variability and a higher percentage of BG level readings within target range than treating patients with the standard regimen. The clinical importance of the degree of variability and rapidity of fluctuations in glucose levels in critically ill patients is a topic of recent interest because it has been identified as an independent contributor to the risk of mortality in critically ill patients.138 Despite differences in glycemic control between insulin algorithms, no clinical outcome differences have been reported in the frequency of severe hypoglycemic events, length of ICU and hospital stay, or mortality. Thus, most insulin algorithms seem appropriate alternatives for the management of hyperglycemia in critically ill patients, and the choice depends on physician preferences and cost considerations.132,139,140
The use of subcutaneous insulin has not been formally studied in ICU patients and should be avoided in critical ill patients, in particular during hypotension or shock. Many factors can affect insulin absorption during critical illness and in the perioperative period.141 The net of these factors is increased potential for overlapping dose effects, administration timing errors, and unexpected hypoglycemia.
Transition from Intravenous Insulin Infusion to Subcutaneous Insulin
All patients with type 1 diabetes mellitus and most patients with type 2 diabetes mellitus receiving CII in critical care setting require transitioning to a subcutaneous insulin regimen.9,18,142,143 Several models have been proposed for transition from insulin infusion to subcutaneous insulin therapy.144–147 In general, the initial dose and distribution of subcutaneous insulin at the time of transition can be determined by extrapolating the intravenous insulin requirement over the preceding 6 to 8 hours to a 24-hour period.53,146 The total daily dose of insulin can also be calculated based on body weight, which could emulate general subcutaneous weight-based insulin guidelines. It is important that consideration be given to a patient’s nutritional status and medications, with continuation of glucose monitoring to guide ongoing adjustments in the insulin dose, because changes in insulin sensitivity can occur during acute illness.
Recent literature on transition methodology discusses 2 general principles behind safe and effective transition from intravenous to subcutaneous insulin: (1) the 24-hour insulin requirement is extrapolated from an appropriately selected hourly insulin rate and (2) the subcutaneous insulin program is designed to fit a patient’s nutrition program.146,148 Strategies to find the basal dose include taking 80% of the total amount of insulin used in the preceding 24 hours and splitting it into basal and prandial insulin to maintain glucose in the optimal range.146 To prevent recurrence of hyperglycemia during the transition to subcutaneous insulin, it is important to allow an overlap of 1 to 2 hours between discontinuation of intravenous insulin and the administration of subcutaneous insulin. For patients who are not receiving significant amount of calories, the basal dose can be given in 1 single, long-acting, daily insulin (eg, glargine or detemir) dose or 2 intermediate-acting insulin (neutral protamine Hagedorn [NPH]) doses every 12 hours. Short-acting insulins (regular) or rapid-acting insulins (aspart, glulisine, and lispro) can be added as needed depending on nutritional intake and glucose levels.
Most patients with stress hyperglycemia and with normal HbA1c levels who have been on CII in the ICU at rates less than or equal to 1 to 2 U/h at the time of transition do not require a scheduled subcutaneous insulin regimen. Many of these patients can be treated with correction insulin to determine if they require scheduled subcutaneous insulin.
Insulin Therapy in the Non–Critical Care Setting
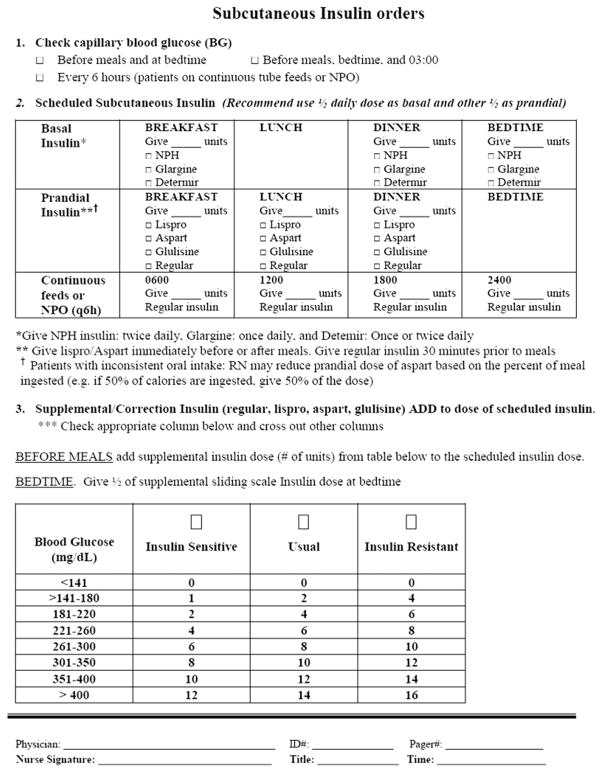
Subcutaneous insulin is the preferred therapeutic agent for BG control in the non-ICU setting. No single insulin regimen meets the needs for all subjects with hyperglycemia. Scheduled subcutaneous insulin therapy with basal or intermediate acting insulin given once or twice a day in combination with short-acting or rapid-acting insulin administered before meals is preferred as an effective and safe strategy for glycemic management in non–critically ill patients.18,149 Subcutaneous insulin programs should address the 3 components of insulin requirement: basal (what is required in the fasting state), nutritional (what is required for glucose elevations or to dispose of glucose in hyperglycemia) (Fig. 3).149
Fig. 3.
Use of insulin ordering forms to prescribe basal/bolus insulin programs. Insulin order forms are useful to illustrate and encourage the use of the 3 components of a patient-tailored insulin program (eg, basal, nutritional, and supplemental/correction).
The practice of discontinuing oral diabetes medications and/or insulin therapy and starting sliding scale insulin (SSI) results in undesirable levels of hypoglycemia and hyperglycemia.150,151 The SSI regimen, although straightforward and easy to use, is faced with several challenges that include inadequate coverage of glycemic excursions and insulin stacking.152 The authors recently reported the results of a prospective, randomized multicenter trial comparing the efficacy and safety of a basal/bolus insulin regimen with basal bolus regimen and SSI in patients with type 2 diabetes mellitus admitted to general medicine wards.153 The authors found that among 130 insulin-naïve patients with an admission BG level between 140 mg/dL and 400 mg/dL, the use of basal-bolus insulin had greater improvement in BG control than sliding scale alone. A BG target of less than 140 mg/dL was achieved in 66% of patients in the glargine plus glulisine group and 38% in the sliding scale group. One-fifth of patients treated with an SSI without a basal component had persistently elevated BG level greater than 240 mg/dL during the hospital stay. The incidence of hypoglycemia, defined as a BG level less than 3.3 mmol/L (<60 mg/dL), was low in this study. In general surgery patients, the recent RAndomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes Undergoing General Surgery (RABBIT 2 Surgery) trial154 compared the efficacy and safety of basal bolus regimen to SSI in 211 patients with type 2 diabetes mellitus. Study outcomes included differences in daily glucose levels and a composite of postoperative complications, including wound infection, pneumonia, respiratory failure, acute renal failure, and bacteremia. Patients were randomized to receive basal bolus regimen with glargine and glulisine (starting dose of 0.5 U/kg/d) or SSI (4 times/d). The basal bolus regimen resulted in significant improvement in glucose control and a reduction in the frequency of the composite complications. The results of these trials indicate that basal bolus regimen is preferred to SSI and results in improved glycemic control and lower rate of hospital complications in general medical and surgical patients with type 2 diabetes mellitus.
An open-label, controlled, multicenter trial randomly assigned 130 medical patients with type 2 diabetes mellitus to receive detemir once daily and aspart before meals with NPH and regular insulin twice daily.155 Both treatment regimens resulted in significant improvements in inpatient glycemic control with a glucose target of less than 140 mg/dL before meals achieved in 45% in the detemir/aspart group and in 48% of NPH/regular insulin group. Hypoglycemia (<60 mg/dL) was observed in approximately one-fourth of patients treated with detemir/aspart and NPH/regular insulin during the hospital stay. There were no differences in length of hospital stay or mortality between groups. Thus, it seems that similar improvement in glycemic control can be achieved with either basal bolus therapy with detemir/aspart or with NPH/regular insulin in general medical patients with type 2 diabetes mellitus.
Initial insulin doses of basal bolus protocols vary widely from 0.3 U/kg/d to 1.5 U/kg/d,53,153,156,157 although only the initial starting dose range between 0.4 U/kg/d and 0.5 U/kg/d, divided into a balanced basal bolus regimen, has been studied prospectively.153,154 A case-control analysis of 1990 patients with diabetes using hypoglycemia as a tool to identify insulin dose ranges based on risk reported that a total daily dose of 0.6 U/kg seems to be the threshold below which the odds of hypoglycemia are low and that doses more than 0.79 U/kg/d are associated with a 3-fold higher odds of hypoglycemia than doses lower than 0.2 U/kg/d. Thus, it may be reasonable to consider lower initial daily doses (≤0.3 U/kg) in patients with hypoglycemia risk factors (eg, elderly patients and those with renal insufficiency). Approximately 50% of the calculated dose can be administered as basal insulin and 50% as prandial or nutritional insulin in divided doses administered with meals. Daily or twice-daily adjustment of the initial insulin doses is required to achieve and maintain glucose targets and to avoid hypoglycemia in nearly all cases of hyperglycemia during hospitalization.
RECOGNITION AND MANAGEMENT OF HYPOGLYCEMIA IN THE HOSPITAL SETTING
Hypoglycemia is defined as any glucose level less than 3.9 mmol/L (70 mg/dL).106,158 Severe hypoglycemia has been defined by many investigators as less than 2.2 mmol/L (40 mg/dL).158 The incidence of severe hypoglycemia among the different trials ranged between 5% and 28%, depending on the intensity of glycemic control in the ICU,133 whereas rates from trials using subcutaneous insulin in non–critically ill patients range from less than 1% to 33%.153,154 The key predictors of hypoglycemic events in hospitalized patients include older age, greater illness severity, diabetes, and the use of oral glucose-lowering medications and insulin.159,160 In-hospital processes of care that contribute to risk for hypoglycemia include unexpected changes in nutritional intake that are not accompanied by associated changes in the glycemic management regimen (eg, cessation of nutrition for procedures and adjustment in the amount of nutritional support), interruption of the established routine for glucose monitoring, deviations from the established glucose control protocols, and failure to adjust therapy when glucose is trending down or steroid therapy is tapered.156,161
Increasing evidence from observational studies and clinical trials indicates that the development of severe hypoglycemia is independently associated with increased risk of mortality.15,20,22,83,162–166 The odds ratio (95% CI) for mortality associated with 1 or more episodes was 2.28 (1.41–3.70, P = .0008) among a cohort of 5365 patients admitted to a mixed medical-surgical ICU.159 In a larger cohort of more than 60,000 patients, hypoglycemia was associated with longer ICU stay and greater hospital mortality, especially for patients with more than 1 episode of hypoglycemia. In patients with acute myocardial infarction, those with hypoglycemia had higher mortality compared with patients without hypoglycemic event (12.7% vs 9.6%, P = .03), and the relationship between hypoglycemia and mortality was similar in patients with and without known history of diabetes.22 Despite these observations, the direct causal effect of iatrogenic hypoglycemia on adverse outcome is still debatable. In a recent study assessing the impact of iatrogenic versus spontaneous hypoglycemia in critical illness, Kosiborod and colleagues22 reported that spontaneous hypoglycemia is associated with higher in-hospital mortality and that insulin-induced hypoglycemia was not associated with increased risk of death compared with subjects without hypoglycemia. Similarly, a recent study of 31,970 patients also reported that hypoglycemia is associated with increased in-hospital mortality (hazard ratio 1.67; 95% CI, 1.33–2.09); however, the greater risk was limited to patients with spontaneous hypoglycemia and not to patients with drug-associated hypoglycemia.167 After adjustment for patient comorbidities, the association between spontaneous hypoglycemia and mortality was eliminated. These studies raised the possibility that hypoglycemia is a marker of disease burden rather than a direct cause of death.22,162,167
SUMMARY
Based on currently available literature, insulin should not be viewed passively as an optional therapy in the hospital. Current evidence supports proactive, scheduled insulin regimens for any patient with consistent hyperglycemia, not only patients with known diabetes and/or who were taking insulin before hospitalization. The proactive approach considers that hyperglycemia is at once both deleterious and related to the endocrinology of the stressed state and requires patient-tailored and situation-tailored insulin therapy. Exposed patients with newly identified hyperglycemia may be at the highest risk of hyperglycemia-related morbidity and mortality. In consideration of the hospital environment, namely unpredictable changes in care and patient condition, imprecision of glucose monitoring, and the variable effects of interventions and nutrition therapy on glycemia, moderate glycemic goals that seek to control glucose while avoiding hypoglycemia are prudent for the majority of hospitalized patients.
Acknowledgments
Dr Umpierrez is supported by research grants from the National Institutes of Health (UL1 RR025008) (Atlanta Clinical and Translational Science Institute) and American Diabetes Association (7-03-CR-35).
References
- 1.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 2.Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–60. doi: 10.1016/s0003-4975(99)00014-4. [discussion: 360–2] [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–8. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 5.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Weekers F, Giulietti AP, Michalaki M, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144(12):5329–38. doi: 10.1210/en.2003-0697. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–21. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic L, Peterson CM. Insulin and glucose requirements during the first stage of labor in insulin-dependent diabetic women. Am J Med. 1983;75(4):607–12. doi: 10.1016/0002-9343(83)90441-2. [DOI] [PubMed] [Google Scholar]
- 9.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 10.Garber AJ, Moghissi ES, Bransome ED, Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 11.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903–11. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med. 2010;363(26):2540–6. doi: 10.1056/NEJMcp1001115. [DOI] [PubMed] [Google Scholar]
- 13.Greci LS, Kailasam M, Malkani S, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26(4):1064–8. doi: 10.2337/diacare.26.4.1064. [DOI] [PubMed] [Google Scholar]
- 14.Moghissi ES, Hirsch IB. Hospital management of diabetes. Endocrinol Metab Clin North Am. 2005;34(1):99–116. doi: 10.1016/j.ecl.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 16.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 17.Qaseem A, Humphrey LL, Chou R, et al. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260–7. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turchin A, Matheny ME, Shubina M, et al. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153–7. doi: 10.2337/dc08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vriesendorp TM, DeVries JH, van Santen S, et al. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med. 2006;34(11):2714–8. doi: 10.1097/01.CCM.0000241155.36689.91. [DOI] [PubMed] [Google Scholar]
- 21.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217–24. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301(15):1556–64. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 23.Braithwaite SS, Magee M, Sharretts JM, et al. The case for supporting inpatient glycemic control programs now: the evidence and beyond. J Hosp Med. 2008;3(Suppl 5):6–16. doi: 10.1002/jhm.350. [DOI] [PubMed] [Google Scholar]
- 24.Magee MF, Clement S. Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation. Endocr Pract. 2004;10(Suppl 2):81–8. doi: 10.4158/EP.10.S2.81. [DOI] [PubMed] [Google Scholar]
- 25.Association AD. [Accessed June 11, 2010];Diabetes Statistics. Diabetes Basics. Available at: http://www.diabetes.org/diabetes-basics/diabetes-statistics/
- 26.Jiang HJ, Stryer D, Friedman B, et al. Multiple hospitalizations for patients with diabetes. Diabetes Care. 2003;26(5):1421–6. doi: 10.2337/diacare.26.5.1421. [DOI] [PubMed] [Google Scholar]
- 27.Donnan PT, Leese GP, Morris AD. Hospitalizations for people with type 1 and type 2 diabetes compared with the nondiabetic population of Tayside, Scotland: a retrospective cohort study of resource use. Diabetes Care. 2000;23(12):1774–9. doi: 10.2337/diacare.23.12.1774. [DOI] [PubMed] [Google Scholar]
- 28.Cook CB, Kongable GL, Potter DJ, et al. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. J Hosp Med. 2009;4(9):E7–14. doi: 10.1002/jhm.533. [DOI] [PubMed] [Google Scholar]
- 29.Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119(14):1899–907. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 30.Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30(4):823–8. doi: 10.2337/dc06-2184. [DOI] [PubMed] [Google Scholar]
- 31.Sonksen PH. Home monitoring of blood glucose by diabetic patients. Acta Endocrinol Suppl (Copenh) 1980;238:145–55. [PubMed] [Google Scholar]
- 32.Mazurek JA, Hailpern SM, Goring T, et al. Prevalence of hemoglobin A1c greater than 6.5% and 7. 0% among hospitalized patients without known diagnosis of diabetes at an urban inner city hospital. J Clin Endocrinol Metab. 2010;95(3):1344–8. doi: 10.1210/jc.2009-1151. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin D, Villanueva G, McNutt R, et al. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008–11. doi: 10.2337/diacare.28.5.1008. [DOI] [PubMed] [Google Scholar]
- 34.Umpierrez GE, Kitabchi AE. ICU care for patients with diabetes. Curr Opin Endocrinol. 2004;11:75–81. [Google Scholar]
- 35.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 36.Corssmit EP, Romijn JA, Sauerwein HP. Review article: regulation of glucose production with special attention to nonclassical regulatory mechanisms: a review. Metabolism. 2001;50(7):742–55. doi: 10.1053/meta.2001.24195. [DOI] [PubMed] [Google Scholar]
- 37.Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117(4):868–70. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boden G. Gluconeogenesis and glycogenolysis in health and diabetes. J Investig Med. 2004;52(6):375–8. doi: 10.1136/jim-52-06-31. [DOI] [PubMed] [Google Scholar]
- 39.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981;240(6):E630–9. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- 40.Edgerton DS, Ramnanan CJ, Grueter CA, et al. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes. 2009;58(12):2766–75. doi: 10.2337/db09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabbay RA, Sutherland C, Gnudi L, et al. Insulin regulation of phosphoenolpyruvate carboxykinase gene expression does not require activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271(4):1890–7. doi: 10.1074/jbc.271.4.1890. [DOI] [PubMed] [Google Scholar]
- 42.Parks WC, Drake RL. Insulin mediates the stimulation of pyruvate kinase by a dual mechanism. Biochem J. 1982;208(2):333–7. doi: 10.1042/bj2080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Losser MR, Damoisel C, Payen D. Bench-to-bedside review: glucose and stress conditions in the intensive care unit. Crit Care. 2010;14(4):231. doi: 10.1186/cc9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherpereel PA, Tavernier B. Perioperative care of diabetic patients. Eur J Anaesthesiol. 2001;18(5):277–94. doi: 10.1046/j.0265-0215.2001.00876.x. [DOI] [PubMed] [Google Scholar]
- 45.Chan TM. The permissive effects of glucocorticoid on hepatic gluconeogenesis. Glucagon stimulation of glucose-suppressed gluconeogenesis and inhibition of 6-phosphofructo-1-kinase in hepatocytes from fasted rats. J Biol Chem. 1984;259(12):7426–32. [PubMed] [Google Scholar]
- 46.McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 48.Stentz FB, Umpierrez GE, Cuervo R, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–86. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 49.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130(1):43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 50.Fan J, Li YH, Wojnar MM, et al. Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock. 1996;6(3):164–70. [PubMed] [Google Scholar]
- 51.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276(5 Pt 1):E849–55. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 52.Edwards FH, Grover FL, Shroyer AL, et al. The Society of Thoracic Surgeons National Cardiac Surgery Database: current risk assessment. Ann Thorac Surg. 1997;63(3):903–8. doi: 10.1016/s0003-4975(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 53.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–97. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 54.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 55.Garg R, Chaudhuri A, Munschauer F, et al. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37(1):267–73. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 56.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kersten JR, Montgomery MW, Ghassemi T, et al. Diabetes and hyperglycemia impair activation of mitochondrial K(ATP) channels. Am J Physiol Heart Circ Physiol. 2001;280(4):H1744–50. doi: 10.1152/ajpheart.2001.280.4.H1744. [DOI] [PubMed] [Google Scholar]
- 59.Ceriello A, Quagliaro L, D’Amico M, et al. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51(4):1076–82. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 60.Title LM, Cummings PM, Giddens K, et al. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36(7):2185–91. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- 61.Gresele P, Guglielmini G, De Angelis M, et al. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol. 2003;41(6):1013–20. doi: 10.1016/s0735-1097(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 62.Pandolfi A, Giaccari A, Cilli C, et al. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001;38(2):71–6. doi: 10.1007/s005920170016. [DOI] [PubMed] [Google Scholar]
- 63.Oswald GA, Smith CC, Betteridge DJ, et al. Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 1986;293(6552):917–22. doi: 10.1136/bmj.293.6552.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22(11):1827–31. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 65.Trence DL, Kelly JL, Hirsch IB. The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change. J Clin Endocrinol Metab. 2003;88(6):2430–7. doi: 10.1210/jc.2003-030347. [DOI] [PubMed] [Google Scholar]
- 66.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100(5):1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343(8890):155–8. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 68.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15(1):45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Pulsinelli WA, Levy DE, Sigsbee B, et al. Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med. 1983;74(4):540–4. doi: 10.1016/0002-9343(83)91007-0. [DOI] [PubMed] [Google Scholar]
- 70.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 71.Kawai N, Keep RF, Betz AL. Hyperglycemia and the vascular effects of cerebral ischemia. Stroke. 1997;28(1):149–54. doi: 10.1161/01.str.28.1.149. [DOI] [PubMed] [Google Scholar]
- 72.Prado R, Ginsberg MD, Dietrich WD, et al. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J Cereb Blood Flow Metab. 1988;8(2):186–92. doi: 10.1038/jcbfm.1988.48. [DOI] [PubMed] [Google Scholar]
- 73.Kruyt ND, Biessels GJ, Devries JH, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145–55. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 74.Quast MJ, Wei J, Huang NC, et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17(5):553–9. doi: 10.1097/00004647-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(Suppl 2):21–33. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 76.Falciglia M, Freyberg RW, Almenoff PL, et al. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37(12):3001–9. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 78.Goyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006;27(11):1289–97. doi: 10.1093/eurheartj/ehi884. [DOI] [PubMed] [Google Scholar]
- 79.Mazighi M, Labreuche J, Amarenco P. Glucose level and brain infarction: a prospective case-control study and prospective study. Int J Stroke. 2009;4(5):346–51. doi: 10.1111/j.1747-4949.2009.00329.x. [DOI] [PubMed] [Google Scholar]
- 80.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 81.De La Rosa Gdel C, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12(5):R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Preiser JC, Brunkhorst F. Tight glucose control and hypoglycemia. Crit Care Med. 2008;36(4):1391. doi: 10.1097/CCM.0b013e31816a16d0. [author reply: 1391–2] [DOI] [PubMed] [Google Scholar]
- 83.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–7. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA. 2002;288(17):2167–9. doi: 10.1001/jama.288.17.2167. [DOI] [PubMed] [Google Scholar]
- 85.McAlister FA, Majumdar SR, Blitz S, et al. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–5. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 86.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–9. doi: 10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783–8. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turnbull PJ, Sinclair AJ. Evaluation of nutritional status and its relationship with functional status in older citizens with diabetes mellitus using the mini nutritional assessment (MNA) tool—a preliminary investigation. J Nutr Health Aging. 2002;6(3):185–9. [PubMed] [Google Scholar]
- 89.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 90.Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156(1):137–42. doi: 10.1530/eje.1.02321. [DOI] [PubMed] [Google Scholar]
- 91.Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248(4):585–91. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- 92.Hirsch IB, McGill JB. Role of insulin in management of surgical patients with diabetes mellitus. Diabetes Care. 1990;13(9):980–91. doi: 10.2337/diacare.13.9.980. [DOI] [PubMed] [Google Scholar]
- 93.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86(7):3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 94.Aljada A, Ghanim H, Mohanty P, et al. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab. 2002;87(3):1419–22. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 95.Dandona P, Aljada A, Bandyopadhyay A. The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia. Diabetes Care. 2003;26(2):516–9. doi: 10.2337/diacare.26.2.516. [DOI] [PubMed] [Google Scholar]
- 96.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–69. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 97.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levetan CS, Magee MF. Hospital management of diabetes. Endocrinol Metab Clin North Am. 2000;29(4):745–70. doi: 10.1016/s0889-8529(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 99.Levetan CS, Passaro M, Jablonski K, et al. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21(2):246–9. doi: 10.2337/diacare.21.2.246. [DOI] [PubMed] [Google Scholar]
- 100.Ben-Ami H, Nagachandran P, Mendelson A, et al. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med. 1999;159(3):281–4. doi: 10.1001/archinte.159.3.281. [DOI] [PubMed] [Google Scholar]
- 101.Donihi AC, Raval D, Saul M, et al. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12(4):358–62. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- 102.Korytkowski M, Dinardo M, Donihi AC, et al. Evolution of a diabetes inpatient safety committee. Endocr Pract. 2006;12(Suppl 3):91–9. doi: 10.4158/EP.12.S3.91. [DOI] [PubMed] [Google Scholar]
- 103.Juneja R, Foster SA, Whiteman D, et al. The nuts and bolts of subcutaneous insulin therapy in non-critical care hospital settings. Postgrad Med. 2010;122(1):153–62. doi: 10.3810/pgm.2010.01.2109. [DOI] [PubMed] [Google Scholar]
- 104.Smiley D, Rhee M, Peng L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5(4):212–7. doi: 10.1002/jhm.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seley JJ, D’Hondt N, Longo R, et al. Position statement: inpatient glycemic control. Diabetes Educ. 2009;35(Suppl 3):65–9. [Google Scholar]
- 106.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dungan K, Chapman J, Braithwaite SS, et al. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–9. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 108.Scott MG, Bruns DE, Boyd JC, et al. Tight glucose control in the intensive care unit: are glucose meters up to the task? Clin Chem. 2009;55(1):18–20. doi: 10.1373/clinchem.2008.117291. [DOI] [PubMed] [Google Scholar]
- 109.Kanji S, Buffie J, Hutton B, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–85. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 110.Schafer RG, Bohannon B, Franz MJ, et al. Diabetes nutrition recommendations for health care institutions. Diabetes Care. 2004;27(Suppl 1):S55–7. doi: 10.2337/diacare.27.2007.s55. [DOI] [PubMed] [Google Scholar]
- 111.McMahon MM, Rizza RA. Nutrition support in hospitalized patients with diabetes mellitus. Mayo Clin Proc. 1996;71(6):587–94. doi: 10.4065/71.6.587. [DOI] [PubMed] [Google Scholar]
- 112.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 113.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N) JPEN J Parenter Enteral Nutr. 2009;33(3):277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 114.Warren J, Bhalla V, Cresci G. Postoperative diet advancement: surgical dogma vs evidence-based medicine. Nutr Clin Pract. 2011;26(2):115–25. doi: 10.1177/0884533611400231. [DOI] [PubMed] [Google Scholar]
- 115.Kreymann KG, Berger MM, Deutz NE, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25(2):210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 116.Via MA, Mechanick JI. Inpatient enteral and parental nutrition for patients with diabetes. Curr Diab Rep. 2010;11(2):99–105. doi: 10.1007/s11892-010-0168-5. [DOI] [PubMed] [Google Scholar]
- 117.Craig LD, Nicholson S, SilVerstone FA, et al. Use of a reduced-carbohydrate, modified-fat enteral formula for improving metabolic control and clinical outcomes in long-term care residents with type 2 diabetes: results of a pilot trial. Nutrition. 1998;14(6):529–34. doi: 10.1016/s0899-9007(98)00062-8. [DOI] [PubMed] [Google Scholar]
- 118.Leon-Sanz M, Garcia-Luna PP, Sanz-Paris A, et al. Glycemic and lipid control in hospitalized type 2 diabetic patients: evaluation of 2 enteral nutrition formulas (low carbohydrate-high monounsaturated fat vs high carbohydrate) JPEN J Parenter Enteral Nutr. 2005;29(1):21–9. doi: 10.1177/014860710502900121. [DOI] [PubMed] [Google Scholar]
- 119.Elia M, Ceriello A, Laube H, et al. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2005;28(9):2267–79. doi: 10.2337/diacare.28.9.2267. [DOI] [PubMed] [Google Scholar]
- 120.Heyland DK, Montalvo M, MacDonald S, et al. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44(2):102–11. [PubMed] [Google Scholar]
- 121.Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361(11):1088–97. doi: 10.1056/NEJMct0806956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1(8263):68–71. doi: 10.1016/s0140-6736(82)90212-4. [DOI] [PubMed] [Google Scholar]
- 123.Brady PA, Terzic A. The sulfonylurea controversy: more questions from the heart. J Am Coll Cardiol. 1998;31(5):950–6. doi: 10.1016/s0735-1097(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 124.Meinert CL, Knatterud GL, Prout TE, et al. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 125.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 126.Deutsch E, Berger M, Kussmaul WG, et al. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation. 1990;82(6):2044–51. doi: 10.1161/01.cir.82.6.2044. [DOI] [PubMed] [Google Scholar]
- 127.Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269(3 Pt 1):C525–45. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 128.Horlen C, Malone R, Bryant B, et al. Frequency of inappropriate metformin prescriptions. JAMA. 2002;287(19):2504–5. doi: 10.1001/jama.287.19.2504-a. [DOI] [PubMed] [Google Scholar]
- 129.Delea TE, Edelsberg JS, Hagiwara M, et al. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2003;26(11):2983–9. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 130.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27(1):256–63. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 131.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 132.Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27(2):461–7. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 133.Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Curr Opin Clin Nutr Metab Care. 2010;13(2):198–204. doi: 10.1097/MCO.0b013e32833571db. [DOI] [PubMed] [Google Scholar]
- 134.Donihi A, Rea R, Haas L, et al. Safety and effectiveness of a standardized 80–150mg/dl iv insulin infusion protocol in the Medical Intensive care unit: >11,000 hours of experience. Diabetes. 2006;55(Suppl 1):459-P. [Google Scholar]
- 135.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418–23. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 136.Juneja R, Roudebush C, Kumar N, et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9(3):232–40. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 137.Newton CA, Smiley D, Bode BW, et al. A comparison study of continuous insulin infusion protocols in the medical intensive care unit: computer-guided vs. standard column-based algorithms. J Hosp Med. 2010;5(8):432–7. doi: 10.1002/jhm.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dossett LA, Cao H, Mowery NT, et al. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74(8):679–85. doi: 10.1177/000313480807400802. [discussion: 685] [DOI] [PubMed] [Google Scholar]
- 139.DeSantis AJ, Schmeltz LR, Schmidt K, et al. Inpatient management of hyperglycemia: the Northwestern experience. Endocr Pract. 2006;12(5):491–505. doi: 10.4158/EP.12.5.491. [DOI] [PubMed] [Google Scholar]
- 140.Rea RS, Donihi AC, Bobeck M, et al. Implementing an intravenous insulin infusion protocol in the intensive care unit. Am J Health Syst Pharm. 2007;64(4):385–95. doi: 10.2146/ajhp060014. [DOI] [PubMed] [Google Scholar]
- 141.Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110(2):478–97. doi: 10.1213/ANE.0b013e3181c6be63. [DOI] [PubMed] [Google Scholar]
- 142.Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mao CS, Riegelhuth ME, Van Gundy D, et al. An overnight insulin infusion algorithm provides morning normoglycemia and can be used to predict insulin requirements in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82(8):2466–70. doi: 10.1210/jcem.82.8.4121. [DOI] [PubMed] [Google Scholar]
- 144.O’Malley CW, Emanuele M, Halasyamani L, et al. Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia. J Hosp Med. 2008;3(Suppl 5):55–65. doi: 10.1002/jhm.355. [DOI] [PubMed] [Google Scholar]
- 145.Donaldson S, Villanuueva G, Rondinelli L, et al. Rush University guidelines and protocols for the management of hyperglycemia in hospitalized patients: elimination of the sliding scale and improvement of glycemic control throughout the hospital. Diabetes Educ. 2006;32(6):954–62. doi: 10.1177/0145721706294918. [DOI] [PubMed] [Google Scholar]
- 146.Schmeltz LR, DeSantis AJ, Schmidt K, et al. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia. Endocr Pract. 2006;12(6):641–50. doi: 10.4158/EP.12.6.641. [DOI] [PubMed] [Google Scholar]
- 147.Grainger A, Eiden K, Kemper J, et al. A pilot study to evaluate the effectiveness of glargine and multiple injections of lispro in patients with type 2 diabetes receiving tube feedings in a cardiovascular intensive care unit. Nutr Clin Pract. 2007;22(5):545–52. doi: 10.1177/0115426507022005545. [DOI] [PubMed] [Google Scholar]
- 148.Avanzini F, Marelli G, Donzelli W, et al. Transition from intravenous to subcutaneous insulin: effectiveness and safety of a standardized protocol and predictors of outcome in patients with acute coronary syndrome. Diabetes Care. 2011;34(7):1445–50. doi: 10.2337/dc10-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.King AB, Armstrong DU. Basal bolus dosing: a clinical experience. Curr Diabetes Rev. 2005;1(2):215–20. doi: 10.2174/1573399054022794. [DOI] [PubMed] [Google Scholar]
- 150.Umpierrez G, Maynard G. Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? J Hosp Med. 2006;1(3):141–4. doi: 10.1002/jhm.101. [DOI] [PubMed] [Google Scholar]
- 151.Hirsch IB. Sliding scale insulin–time to stop sliding. JAMA. 2009;301(2):213–4. doi: 10.1001/jama.2008.943. [DOI] [PubMed] [Google Scholar]
- 152.Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563–7. doi: 10.1016/j.amjmed.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 153.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30(9):2181–6. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 154.Umpierrez E, Smiley D, Jacobs S, et al. Randomized study of basal bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34(2):256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hor T, Smiley D, Munoz C, et al. Comparison of inpatient insulin regimens: DE-temir plus Aspart vs. NPH plus regular in Medical Patients with Type 2 Diabetes (DEAN Trial) Diabetes. 2008;57(Suppl 1):458A. doi: 10.1210/jc.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maynard G, Lee J, Phillips G, et al. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15. doi: 10.1002/jhm.391. [DOI] [PubMed] [Google Scholar]
- 157.Pietras SM, Hanrahan P, Arnold LM, et al. State-of-the-art inpatient diabetes care: the evolution of an academic hospital. Endocr Pract. 2010;16(3):512–21. doi: 10.4158/EP09319.CO. [DOI] [PubMed] [Google Scholar]
- 158.Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–28. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 159.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–7. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 160.Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163(15):1825–9. doi: 10.1001/archinte.163.15.1825. [DOI] [PubMed] [Google Scholar]
- 161.Smith WD, Winterstein AG, Johns T, et al. Causes of hyperglycemia and hypoglycemia in adult inpatients. Am J Health Syst Pharm. 2005;62(7):714–9. doi: 10.1093/ajhp/62.7.714. [DOI] [PubMed] [Google Scholar]
- 162.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 163.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–48. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 164.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 165.Arabi YM, Tamim HM, Rishu AH. Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit Care Med. 2009;37(9):2536–44. doi: 10.1097/CCM.0b013e3181a381ad. [DOI] [PubMed] [Google Scholar]
- 166.Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care. 2011;15(4):R173. doi: 10.1186/cc10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med. 2011;124(11):1028–35. doi: 10.1016/j.amjmed.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farah R, Samokhvalov A, Zviebel F, et al. Insulin therapy of hyperglycemia in intensive care. Isr Med Assoc J. 2007;9(3):140–2. [PubMed] [Google Scholar]
- 169.Grey NJ, Perdrizet GA. Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract. 2004;10(Suppl 2):46–52. doi: 10.4158/EP.10.S2.46. [DOI] [PubMed] [Google Scholar]
- 170.Mackenzie IM, Ercole A, Blunt M, et al. Glycaemic control and outcome in general intensive care: the East Anglian GLYCOGENIC study. Br J Intensive Care. 2008;18:121–6. [Google Scholar]