Abstract
Objective
Parenteral nutrition has been associated with metabolic and infectious complications in intensive care unit patients. The underlying mechanism for the high risk of complications is not known but may relate to the proinflammatory effects of soybean oil–based lipid emulsions, the only Food and Drug Administration–approved lipid formulation for clinical use.
Design
Prospective, double-blind, randomized, controlled trial.
Setting
Medical–surgical intensive care units from a major urban teaching hospital and a tertiary referral university hospital.
Patients
Adult medical–surgical intensive care unit patients.
Intervention
Parenteral nutrition containing soybean oil–based (Intralipid) or olive oil–based (ClinOleic) lipid emulsions.
Measurements
Differences in hospital clinical outcomes (nosocomial infections and noninfectious complications), hospital length of stay, glycemic control, inflammatory and oxidative stress markers, and granulocyte and monocyte functions between study groups.
Results
A total of 100 patients were randomized to either soybean oil–based parenteral nutrition or olive oil–based parenteral nutrition for up to 28 days. A total of 49 patients received soybean oil–based parenteral nutrition (age 51 ± 15 yrs, body mass index 27 ± 6 kg/m2, and Acute Physiology and Chronic Health Evaluation II score 15.5 ± 7 [±SD]), and a total of 51 patients received olive oil–based lipid emulsion in parenteral nutrition (age 46 ± 19 yrs, body mass index 27 ± 8 kg/m2, and Acute Physiology and Chronic Health Evaluation II score 15.1 ± 6 [±SD]) for a mean duration of 12.9 ± 8 days. The mean hospital blood glucose concentration during parenteral nutrition was 129 ± 14 mg/dL, without differences between groups. Patients treated with soybean oil–based and olive oil–based parenteral nutrition had a similar length of stay (47 ± 47 days and 41 ± 36 days, p = .49), mortality (16.3% and 9.8%, p = .38), nosocomial infections (43% vs. 57%, p = .16), and acute renal failure (26% vs. 18%, p = .34). In addition, there were no differences in inflammatory and oxidative stress markers or in granulocyte and monocyte functions between groups.
Conclusion
The administration of parenteral nutrition containing soybean oil–based and olive oil–based lipid emulsion resulted in similar rates of infectious and noninfectious complications and no differences in glycemic control, inflammatory and oxidative stress markers, and immune function in critically ill adults.
Keywords: hyperglycemia, inpatient diabetes, lipid emulsion, nutrition support, olive oil, parenteral nutrition, soybean oil
Protein-calorie malnutrition is common in critically ill patients (1) and is associated with increased risk of hospital complications and mortality (2-5), longer hospital stay, and hospital costs (6). Improving nutrition status may restore immunologic competence and reduce the frequency and severity of infectious complications in critically ill patients (7-10). The beneficial effects of parenteral nutrition (PN) administration in improving nutritional status and reducing complications in significantly malnourished intensive care unit (ICU) patients are well established (11-14). Recent randomized trials and metaanalyses, however, have suggested that treatment with PN compared to enteral nutrition may increase the risk for infectious complications and hospital mortality (15-17).
The increased rate of PN-associated complications is likely multifactorial but may be related to the development of hyperglycemia (18-20) or to effects of specific PN components, including administration of conventional soybean oil–based lipid emulsions, which contain a high content of linoleic acid and ω-6 polyunsaturated fatty acids (9, 21). Soybean oil–based lipid emulsions are the only FDA-approved lipid formulations available for PN use in the United States. Experimental reports suggest that such ω-6 polyunsaturated fatty acid–rich lipid emulsions may promote the generation of arachidonic acid–derived eicosanoids, which, in turn, could potentially exaggerate the inflammatory response during stress and trauma (22), contribute to immunosuppression by impairing neutrophil and macrophage function (21, 23), and adversely affect endothelial function (9, 24). In addition, soybean oil–based lipid emulsion inhibits lymphocytes, macrophages, and neutrophil functions, besides impairing reticuloendothelial function and reducing plasma lipid clearance (9, 21).
The concern regarding the potential complications associated with the use of high ω-6 polyunsaturated fatty acid formulations has led to the development of alternative lipid emulsions for PN (9, 25). Limited data suggest that lowering linoleic acid content by partly replacing soybean oil with olive oil may improve the safety of PN (26, 27). Several in vitro animal and human studies have shown that the impairment of immune function, oxidative stress, glucose metabolism, endothelial function and inflammation that occurs with soybean oil–based emulsions may be attenuated with olive oil–based lipid emulsions (21-24, 27, 28). However, it is unclear whether the use of olive oil–based lipid emulsions in PN results in improved clinical outcomes compared to PN containing conventional soybean oil–based formulations. Accordingly, we designed this investigator-initiated, randomized, double-blind, controlled clinical trial to compare clinical outcomes and metabolic responses to PN containing either olive oil–based or soybean oil–based lipid emulsion in adult medical–surgical ICU patients.
RESEARCH DESIGN AND METHODS
This prospective, double-blind controlled clinical trial randomized 100 medical and surgical ICU patients to receive standard PN formulations containing soybean oil–based (Intralipid, Deerfield, IL) or olive oil–based (ClinOleic, Baxter Healthcare, Deerfield, IL) lipid emulsions. The olive oil–based lipid emulsion is comprised of ~80% olive oil and ~20% soybean oil plus glycerol, purified egg phospholipids, sodium oleate, sodium hydroxide, and water for injection. The soybean oil–based lipid emulsion is comprised of 100% soybean oil, plus egg yolk phospholipids, glycerin, and water for injection. We enrolled male and female patients between the ages of 18 and 80 yrs who were expected to require PN for longer than 5 days by conventional criteria (11). We excluded patients who had received PN within 48 hrs prior to study entry, those with septic shock (defined as unstable blood pressure despite pressor support and mean arterial pressure <60 mm Hg on more than two occasions within 24 hrs prior to study entry), patients with a history of organ transplantation, active malignancy (defined as requiring chemotherapy, radiation, and/or surgical intervention within 90 days prior to entry), cirrhosis or with a serum total bilirubin level ≥10.0 mg/dL, acquired immunodeficiency syndrome, chronic renal failure (defined as requirement for hemodialysis or peritoneal dialysis therapy, or with a serum creatinine >3.5 mg/dL), or a mental condition rendering the subject unable to understand the scope and possible consequences of the study or when the legally authorized representative were not available. Also excluded were patients who were pregnant or breast-feeding, those with terminal illness and a life expectancy of <7 days, and patients with a baseline serum triglyceride concentration of >400 mg/dL.
The study was conducted at Grady Memorial Hospital, a major urban teaching hospital affiliated with Emory University and at Emory University Hospital, a tertiary referral academic institution in Atlanta, GA. The study protocol and consent forms were approved by the Institutional Review Board at Emory University. Informed consent was obtained prior to randomization from study subjects and/or their legally authorized representative. A research pharmacist at each institution coordinated treatment assignment following a computer-generated randomization table.
The study PN was administered using uniform guidelines for PN support in ICU settings established at both study sites. Briefly, the nutritional goals aimed to provide total daily calorie (kcal) intake at 1.3 times basal energy expenditure (per the Harris–Benedict equation using actual body weight or adjusted body weight in obese subjects per standard methods) (11). The total amino acid/protein intake goal was 1.5 g/kg day. Parenteral vitamin K, multivitamins, electrolytes, and trace elements were added as required per standard methods (11).
Study subjects received a maximum of 28 days of the study PN formulations. If a subject continued to require PN after day 28, the type of PN and need for lipid emulsion was decided by the primary care physician and the local nutrition support team. PN was started and continued only if deemed indicated by both the primary physicians and investigators. If a subject’s PN was discontinued but was later restarted during the initial 28 days after entry, study PN was restarted based on the initial randomization and continued, as clinically indicated, until day 28. All patients were managed by their primary care ICU team, who determined when to initiate and discontinue PN and transition to enteral feeds, in consultation with the investigator, per usual practices in the two participating hospitals. In general, transition of PN to enteral feeds (oral diet or conventional tube feedings) occurred as soon as tolerated; when enteral intake was ≥50% of energy requirements for ≥48 hrs, PN was discontinued.
The primary outcome of this study was the rate of new nosocomial infections, defined as culture-proven infection including wound, drain, bloodstream, respiratory tract, and urinary tract infections (25). The presence of nosocomial infections was diagnosed based on standardized Centers for Disease Control criteria (29). We followed these Centers for Disease Control guidelines for laboratory-confirmed bloodstream infection and did not distinguish catheter-related infections per se. New nosocomial infections were diagnosed after 48 hrs of PN initiation in order to minimize the chance that the infection were present (but undiagnosed) prior to study PN initiation. The following daily information was evaluated by the study team for nosocomial infection surveillance: temperature (fever) curve, white blood cell counts (to evaluate leukocytosis/leucopenia), review of daily progress notes in the medical record, daily clinical microbiology laboratory culture data, orders for antimicrobial agents (agent, daily dose, and start/stop times will be recorded), review of all relevant dictated radiographic reports (e.g., chest radiographs, abdominal computer tomography), communication, as needed, with primary physicians and site infectious disease consultants, and use of the Centers for Disease Control guidelines for diagnosis of specific nosocomial infections (25).
Secondary outcomes included differences between treatment groups in ICU and hospital length of stay, glycemic control, specific neutrophil and monocyte functions, inflammatory and oxidative stress markers, respiratory failure and need for mechanical ventilation, cardiac complications (defined as myocardial infarction, cardiac arrhythmia, or cardiac arrest documented by electrocardiogram and/or cardiac enzyme evidence), acute renal failure (new development of serum creatinine concentration >2.2 mg/dL or an increment >0.5 mg/dL from baseline), and ICU and hospital mortality (during PN infusion or after PN treatment was completed).
Blood glucose (BG) monitoring and glycemic management followed similar protocols approved by both participating institutions. Capillary BG was measured with a glucose meter at bedside during PN infusion. Regular insulin was added to the PN solutions at a starting dose of 0.1 units per gram of dextrose in nondiabetic patients and at 0.15 units per gram of dextrose in patients with a history of diabetes when the BG concentration was >120 mg/dL. Patients with repeated BG values >140 mg/dL received continuous intravenous insulin infusion following a standard algorithm, adjusted to maintain a target BG level between 80 and 140 mg/dL in the ICU and <180 mg/dL in the less-controlled setting of the general medical–surgical wards. Nosocomial infections were diagnosed following standardized Centers for Disease Control criteria (25). New nosocomial infections were diagnosed after 48 hrs of PN initiation in order to minimize the chance that the infection was actually present (but undiagnosed) prior to study PN initiation. The investigators reviewed each subject’s records regarding potential new infection diagnosis daily on each weekday from Monday to Friday. Data from the weekends were collected and entered in the database the following Monday. Data on respiratory failure and need for mechanical ventilation were collected daily. The day the subject was weaned from the ventilator was recorded as a ventilator day. The presence or absence of acute respiratory distress syndrome was monitored and recorded daily using criteria set by The Acute Respiratory Distress Syndrome Network (30).
Laboratory Assays
Plasma glucose was measured on the CX7 Chemistry Analyzer (Beckman Diagnostics, Fullerton, CA) using reagents and calibrators from Beckman Diagnostics. Levels of interleukin-6, tumor necrosis factor-α, C-reactive protein, insulin, and C-peptide were measured in plasma using a solid phase, two-site sequential chemiluminescent immunometric assays on the DPC Immulite analyzer (Diagnostic Products, Los Angeles, CA). Plasma glutathione, cysteine and related redox potential of these pools were measured as indicators of oxidative stress, as previously outlined (31). Briefly, samples were collected in a preservation solution and stored at −80° under conditions known to result in negligible oxidation. Samples showing visual evidence of hemolysis were discarded. Samples were treated to form dansyl derivatives, analyzed by high performance liquid chromatography with fluorescence detection and quantified relative to γ-glutamyl-glutamate as an internal standard. Redox potential values were calculated using the Nernst equation with Eo −264 mV for the glutathione/glutathione disulfide couple and −250 mV for the cysteine/cystine couple at pH 7.4 (31). Coefficients of variation for concentration measurements were 5%–6% for all parameters except cysteine and glutathione disulfide, which were 9%–10%.
Immune Function
The phagocytic and oxidative burst activity of monocytes and granulocytes in heparinized whole blood was assessed according to manufacturer’s instructions using specific reagent kits for this purpose at baseline and again at day 7. Briefly, granulocyte and monocyte phagocytic activity was quantitated by incubation of whole blood with fluorescein isothiocynate-labeled, opsonized Escherichia coli bacteria at 37°C (Phagotest, Orpegen Pharma, Heidelberg, Germany) with detection of fluorescence of internalized particles as a percentage of positive cells by flow cytometry (FACSort Becton Dickinson Biosciences, Franklin Lakes, NJ), analyzed using FlowJo software (Tree Star, Ashland, OR). Oxidative burst activity of monocytes and granulocytes was quantitated in whole blood using a kit containing unlabeled opsonized bacteria (E. coli), phorbol-12-myristate-13 acetate and the chemotactic peptide N formyl-Met-Leu-Phe as stimulants, and dihydrorhodamine-123 as a fluorogenic substrate to determine the percentage of phagocytic cells that produce reactive oxidants (Phagoburst, Orpegen Pharma, Heidelberg, Germany). A sample without stimulants served as a negative background control for each experiment. The percentage of positive cells was determined by flow cytometry and analyzed using FlowJo software.
Statistical Analysis
The primary outcome of this study was the rate of nosocomial infections diagnosed after entry including wound, drain, catheter, respiratory tract, and urinary tract infections while receiving PN. Chi-square test (or Fisher’s exact test) was used to compare rates of infections between the soybean oil–based PN and olive oil–based PN study groups. For continuous secondary outcomes, we adopted nonparametric Wilcoxon tests to assess the difference between treatment groups. Differences in categorical secondary outcomes between treatment groups were evaluated using chi-square tests (or Fisher’s exact tests when needed). The Cochran–Mantel–Haenszel test was used to examine the difference in categorical outcomes between the two treatment groups while controlling for study center and Acute Physiology and Chronic Health Evaluation group. The absolute change in monocyte and granulocyte endpoints from baseline to day 7 was calculated.
A power analysis was conducted prior to the study based on preliminary data from Grady Memorial Hospital that indicated an overall infection rate of 0.36 during PN therapy (32). Using an approximate two-sample proportion test, two-sided and α = 0.05, we estimated >80% power with 50 patients per group in order to detect a difference >0.24 in the occurrence rates of nosocomial infection between the two PN lipid emulsion study groups.
RESULTS
A total of 100 patients received soybean oil–based (n = 49) and olive oil–based (n = 51) lipid emulsion. The groups were well-matched for gender, age, racial distribution, body mass index or Acute Physiology and Chronic Health Evaluation II, and Sequential Organ Failure Assessment scores (Table 1). In addition, there were no differences in the mean glucose, albumin, and serum creatinine on admission to the hospital or at the time of recruitment between treatment groups in the ICU (not shown). The majority of patients were recruited from the surgical ICUs (89%) following trauma (39.6%), major gastrointestinal (48.4%), or oncological surgery (12 %).
Table 1.
Clinical characteristics of study patients treated with soybean oil– and olive oil–based parenteral nutrition infusion
| All | Soybean Oil PN | Olive Oil PN | p | |
|---|---|---|---|---|
| No. of patients | 100 | 49 | 51 | |
| Age, yrs | 48.8 ± 17 | 51.3 ± 15 | 46.4 ± 19 | .19 |
| Race: Black/Hispanic/White/Other | 40/2/57/1 | 18/2/29/0 | 22/0/28/1 | .41 |
| Gender, male/female | 55/45 | 27/22 | 28/23 | .98 |
| Body mass index, kg/m2 | 27.4 ± 7 | 27.3 ± 6 | 27.4 ± 8 | .61 |
| Weight, kg | 81.2 ± 21 | 80.6 ± 20 | 81.8 ± 22 | .75 |
| Intensive care unit type: surgical/medical/other | 89/9/2 | 46/3/0 | 43/6/2 | .29 |
| Start of PN after admission, days | 12.6 ± 14 | 13.1 ± 13 | 12.2 ± 15 | .3–8 |
| Duration of PN infusion, days | 12.9 ± 8 | 13.1 ± 8 | 12.8 ± 8 | .87 |
| Acute Physiology and Chronic Health Evaluation II score | 15.1 ± 6 | 15.5 ± 7 | 15.1 ± 6 | .89 |
| Sequential Organ Failure Assessment score | 5.7 ± 4 | 5.2 ± 3 | 6.27 ± 4 | .23 |
| Hospital length of stay, days | 43.7 ± 42 | 46.7 ± 48 | 40.8 ± 36 | .49 |
| Intensive care unit length of stay, days | 16.1 ± 16 | 15.2 ± 14 | 17.0 ± 18 | .77 |
| BG pre-PNa | 137.5 ± 33 | 140.7 ± 31 | 134.6 ± 35 | .073 |
| BG during first day of PN, mg/dL | 144.6 ± 27 | 144.9 ± 28 | 144.3 ± 28 | .83 |
| BG during total PN | 125.4 ± 9 | 126.1 ± 8 | 124.8 ± 9 | .49 |
| BG during hospital stay, mg/dL | 128.9 ± 14 | 130.2 ± 15 | 127.8 ± 13 | .54 |
| Serum creatinine pre-PN, mg/dL | 1.33 ± 2 | 1.0 ± 0.5 | 1.65 ± 3 | .51 |
| Serum creatinine during PN, mg/dL | 1.14 ± 0.8 | 1.01 ± 0.6 | 1.26 ± 1.0 | .48 |
| Serum albumin pre-PN, g/dL | 2.07 ± 0.6 | 1.95 ± 0.5 | 2.20 ± 0.7 | .13 |
| Serum albumin during PN, g/dL | 2.58 ± 2.5 | 2.72 ± 3.0 | 2.45 ± 1.9 | .83 |
PN, parenteral nutrition; BG, blood glucose.
To convert the values for glucose from mg/dL to mmol/L, multiply by 0.05551.
Values are mean ± SD.
For the entire cohort, PN was started 12.6 ± 13.9 days after hospital admission and was administered for a mean duration of 12.9 ± 8.3 days (Table 1). The hospital and ICU length of stay were 43.6 ± 42 days and 16.1 ± 16 days, respectively, without differences between treatment groups. Macronutrient intake (kcal, amino acids, dextrose, and lipid emulsion) as a function of body weight from study PN was similar between the two study groups. For example, mean daily kcal/kg per day in the soybean oil–based PN group was 22 ± 5 vs. 22 ± 6 kcal/kg per day in the olive oil–based PN group (p = not significant); PN amino acid intake was also similar between the study groups (1.21 ± 0.26 vs. 1.16 ± 0.28 g/kg per day, NS). For the entire cohort, the mean total PN kcal intake was 22 ± 6 kcal/kg per day, amino acids 1.19 ± 0.27 g/kg per day, dextrose 3.3 ± 1.0 g/kg per day, and total fat 0.59 ± 0.2 g/kg per day.
The results of the primary and other secondary outcomes of the trial are shown in Table 2. The overall hospital mortality during the entire hospital stay, including the period following discontinuation of PN, was 13% without a difference between patients treated with soybean oil–based and olive oil–based PN (16.3% and 9.8%, respectively, p = .38). The hospital mortality during PN therapy was 9%, without differences between patients treated with soybean oil–based (10.2%) and olive oil–based PN (7.8%), p = .74. Patients treated with PN–olive oil had similar rates of nosocomial infections (43% vs. 57%, p = .16), pneumonia (10% vs. 14%, p = .76), wound infections (8.1% vs. 21.5%, p = .09), bloodstream infection (22% vs. 21%, p = .91), urinary tract infection (14% vs. 14%, p > .99), acute renal failure (26% vs. 18%, p = .34), length of ICU stay (15 ± 14 vs. 17 ± 18 days, p = .77), and mortality (10% vs. 8%, p = .74) compared to soybean oil–based PN.
Table 2.
Mortality and hospital complications in patients treated with soybean oil– and olive oil–based parenteral nutrition
| All | Soybean Oil Parenteral Nutrition | Olive Oil Parenteral Nutrition | p | |
|---|---|---|---|---|
| Deaths during hospital stay, n (%) | 13 (13) | 8 (16.3) | 5 (9.8) | .38 |
| Acute renal failure, n (%) | 22 (22) | 13 (26.5) | 9 (17.6) | .34 |
| Infectious complications | ||||
| Any infection, n (%) | 50 (50) | 21 (42.8) | 29 (56.8) | .16 |
| Pneumonia, n (%) | 12 (12) | 5 (10.2) | 7 (13.7) | .76 |
| Urinary tract infection, n (%) | 12 (12) | 7 (14.3) | 7 (13.7) | >.99 |
| Bacteremia | 22 (22) | 11 (22.4) | 11 (21.5) | .92 |
| Wound infection, n (%) | 15 (15) | 4 (8.1) | 11 (21.5) | .09 |
| Cardiac complications | ||||
| Acute myocardial infarction, n (%) | 2 (2) | 0 (0) | 2 (3.9) | .50 |
| Congestive heart failure, n (%) | 3 (3) | 1 (2.04) | 2 (3.9) | >.99 |
| Cardiac arrhythmia, n (%) | 11 (11) | 5 (10.2) | 6 (11.7) | >.99 |
Values are mean ± SD.
A total of 23 patients had a history of diabetes and 77 had no history of diabetes prior to admission. The overall mean BG concentration during PN was 129 ± 14 mg/dL. There were no differences in mean BG concentration between patients with history of diabetes compared to those with no history of diabetes or between patients treated with soybean oil–based or olive oil–based PN (Fig. 1). The mean hospital BG in diabetic patients treated with soybean oil–based PN was 128.4 ± 6 mg/dL and olive oil–PN was 125.0 ± 9 mg/dL. The mean BG in nondiabetic patients treated with soybean oil–based PN was 125.3 ± 9 mg/dL and olive oil–PN was 124.7 ± 9 mg/dL. There were no significant differences in the frequency of hypoglycemic events between treatment groups. A total of 74 (74%) of patients experienced hypoglycemia episodes during the study period (defined as BG <70 mg/dL); however, only 4 patients (4%) had a documented BG <40 mg/dL. There were no differences in the frequency of hypoglycemic events between treatment groups. From a total of 8661 BG readings in the olive oil–based PN group, a total of 163 readings (1.88%) were <70 mg/dL and four readings (0.05%) were <40 mg/dL. In the soybean oil–based PN cohort, a total of 125 of 8188 BG readings (1.52%) were <70 mg/dL and two BG readings (0.02%) were <40 mg/dL. None of the episodes of hypoglycemia in either group were associated with loss of consciousness or seizure.
Figure 1.
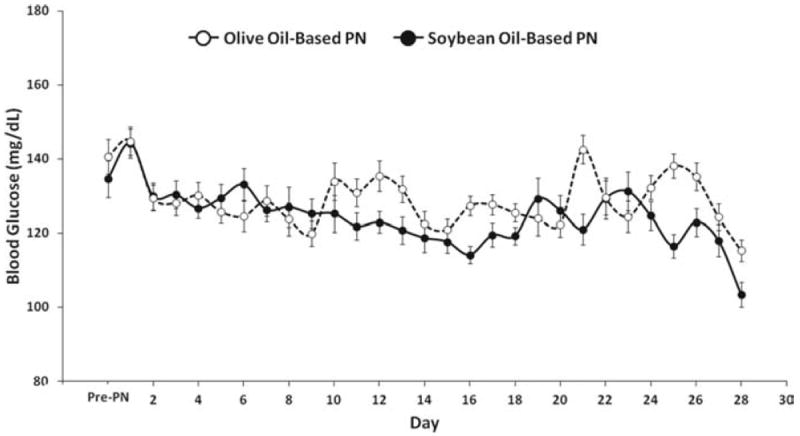
Changes in mean daily blood glucose concentration during parenteral nutrition (PN) containing soybean oil– and olive oil–based lipid emulsion in critically ill patients. Values are mean ± SEM.
No significant differences were noted between groups in plasma levels of inflammatory markers (C-reactive protein, interleukin-6, and tumor necrosis factor-α) (Table 3). Indices of oxidative stress (plasma cystine, cysteine, glutathione, glutathione disulfide concentrations, and the redox potential of the glutathione/glutathione disulfide cysteine/cystine pools, respectively) were similar between study groups at baseline and on day 3 and day 7 of study PN infusion.
Table 3.
Plasma concentration of circulating levels of inflammatory markers and oxidative stress markers at baseline, day 3, and day 7 of soybean oil– and olive oil–based parenteral nutrition infusion
| Variable | Soybean Oil Parenteral Nutrition | Olive Oil Parenteral Nutrition | p |
|---|---|---|---|
| C-reactive protein, mg/L | |||
| Day 1 | 169.67 ± 125.05 | 152.45 ± 123.09 | .17 |
| Day 3 | 109.48 ± 58.42 | 115.33 ± 66.80 | .66 |
| Day 7 | 122.71 ± 80.68 | 146.63 ± 115 | .53 |
| Tumor necrosis factor-α, pg/mL | |||
| Day 1 | 0.12 ± 0.08 | 0.09 ± 0.03 | .20 |
| Day 3 | 0.12 ± 0.05 | 0.09 ± 0.03 | .09 |
| Day 7 | 0.10 ± 0.04 | 0.10 ± 0.07 | .48 |
| Cystine, uM | |||
| Day 1 | 82.22 ± 43.74 | 75.80 ± 31.09 | .87 |
| Day 3 | 88.65 ± 39.09 | 93.54 ± 36.04 | .53 |
| Day 7 | 90.06 ± 42.28 | 87.7 ± 42.04 | .80 |
| Cysteine, uM | |||
| Day 1 | 11.95 ± 9.44 | 19.60 ± 20.46 | .024 |
| Day 3 | 11.86 ± 10.01 | 11.53 ± 7.72 | .95 |
| Day 7 | 13.56 ± 8.14 | 12.95 ± 11.24 | .23 |
| Glutathione, uM | |||
| Day 1 | 1.40 ± 1.32 | 1.38 ± 1.01 | .82 |
| Day 3 | 0.92 ± 0.68 | 2.56 ± 6.39 | .41 |
| Day 7 | 0.95 ± 0.63 | 0.90 ± 0.54 | .80 |
| Glutathione disulfide, uM | |||
| Day 1 | 0.19 ± 0.33 | 0.31 ± 0.56 | .65 |
| Day 3 | 0.14 ± 0.40 | 0.37 ± 0.88 | .01 |
| Day 7 | ±0.34 ± 0.85 | 0.71 ± 2.17 | .82 |
| Glutathione redox potential, mV | |||
| Day 1 | −116.99 ± 19.54 | −116.45 ± 19.10 | .67 |
| Day 3 | −115.96 ± 20.90 | −111.44 ± 34.88 | .32 |
| Day 7 | −111.48 ± 23.07 | −109.77 ± 24.72 | .75 |
| Cysteine redox potential, mV | |||
| Day 1 | −74.17 ± 14.33 | −83.73 ± 18.78 | .017 |
| Day 3 | −71.7 ± 15.40 | −71.02 ± 16.67 | .90 |
| Day 7 | −77.35 ± 12.46 | −73.33 ± 20.42 | .38 |
p values before multiple comparison adjustment.
Values are mean ± SD.
No significant difference occurred in the change from baseline to study day 7 in granulocyte and monocyte phagocytosis and reactive oxygen species generation, respectively, between groups (Table 4).
Table 4.
Change in immune function after 7-day administration of parenteral nutrition containing soybean oil– and olive oil–based lipid emulsion in intensive care unit patients
| Soybean Oil Parenteral Nutrition | Olive Oil Parenteral Nutrition | p | |
|---|---|---|---|
| Granulocyte phagocytosis, % | 3.86 ± 18.8 | 6.36 ± 38.4 | .55 |
| Monocyte phagocytosis, % | −3.40 ± 24.9 | −9.25 ± 29.3 | .35 |
| Granulocyte reactive oxygen species generation, % | −16.36 ± 33.6 | −7.92 ± 35.8 | .64 |
| Monocyte reactive oxygen species generation, % | −3.91 ± 31.3 | −26.35 ± 45.8 | .21 |
Values are mean ± SD.
DISCUSSION
This prospective, double-blind randomized trial aimed to compare differences in clinical outcomes in medical–surgical ICU patients receiving PN containing standard soybean oil–based lipid emulsion vs. PN containing olive oil–based lipid emulsion. We observed similar rates of infectious and noninfectious complications, and no significant differences in hospital or ICU length of stay, glycemic control, and inflammatory and oxidative stress markers in medical and surgical ICU patients.
Malnutrition is common with a prevalence of 20%–40% in ICU patients (1, 11) and has been shown to be an independent risk factor for increased complications, mortality, length of hospital stay, and costs (6). In patients unable to be fed adequately by the enteral route, PN improves nutritional status and provides essential substrates and micronutrients for cellular and organ functions in hospitalized patients (7-10). Despite improving nutritional status, the use of PN is associated with an increased risk of some hospital complications and even mortality in some critically ill patients (33-35). The increased risk of complications may be related, among other factors, to hyperglycemia due to PN dextrose infusion and, theoretically, by the administration of soybean oil–based lipid emulsions have been suggested to lead to proinflammatory responses, altered leukocyte function, and endothelial dysfunction (21, 36, 37). Although data are still limited, administration of soybean oil–based lipid emulsions as a component of PN has been shown to exert immunosuppressive effects (23) and increase nitric oxide production, proinflammatory responses, and sympathetic activity in some studies (9, 24). Further, these formulations have been shown to induce dose-dependent insulin resistance in diabetic and nondiabetic individuals when given in short-term studies at high doses (38, 39). In a recent prospective, randomized, cross-over study we compared the vascular, metabolic, immune, and inflammatory effects of a 24-hr infusion of soybean oil–based PN, olive oil–based PN, lipid-free PN, and normal saline in healthy subjects (40). Soybean oil–based PN increased blood pressure and reduced brachial artery flow-mediated dilatation from baseline by 23% at 4 hrs and by 25% at 24 hrs, both p < .01; in contrast, olive oil–PN and lipid-free PN did not change either blood pressure or endothelial function compared to normal saline infusion in these individuals (40).
Recent in vitro animal and human studies have shown that immune system dysfunction, oxidative stress, insulin resistance, endothelial dysfunction and inflammation that occur with soybean oil–based lipid emulsions could potentially be avoided with the use of olive oil–based PN solutions (23, 24, 27). However, in the current study of adult ICU patients receiving otherwise identical PN, we observed no differences between the two lipid emulsion groups with regard to clinical outcomes and no differences in key systemic inflammatory, oxidative stress, or immune markers.
Several factors may explain the lack of beneficial effects and outcome differences between previous studies and our current report. First, in our trial, PN support was started after an average of 11 days of hospital admission when many patients had already experienced a significant number of serious medical and surgical complications. Current American and European nutrition support guidelines differ but suggest that critically ill patients unable to consume adequate nutrients orally should be candidates for PN after 2–7 days (41).
Limitations of our study include the relatively small number of patients, the preponderance of surgical ICU subjects, and the fact that our study was not powered to demonstrate differences in mortality between treatment groups. Nonetheless, our trial is unique in that it is, to our knowledge, the first rigorous, double-blind trial to compare infection rates and other clinical outcomes and inflammatory, oxidative, and immune parameters after feeding with olive oil–based vs. soybean oil–based PN in critically ill patients with comparable tight BG control. The observed mortality in both of our treatment groups was lower than previous observational studies in patients receiving PN (19, 32) and in large randomized controlled studies focused on BG control in critically ill patients (42-44). The low mortality rate observed in this study as well as that reported by Casaer et al (45) could be explained by the intensified glycemic control regimen with a mean daily glucose concentration <130 mg/dL. This underscores the importance of intensive glycemic control in reducing hospital complications and mortality in patients receiving nutritional support.
In summary, our results indicate that the administration of PN containing soybean oil–based and olive oil–based lipid emulsions results in similar overall rates of infectious and noninfectious complications, mortality, and ICU length of stay and no significant differences in metabolic, inflammatory, or immune markers in critically ill adult patients.
Acknowledgments
This investigator-initiated study was supported by a research grant from Baxter Pharmaceuticals (Dr. Umpierrez) and National Institutes of Health UL1 RR025008 (Atlanta Clinical and Translational Science Institute), American Diabetes Association 7-03-CR-35 (Dr. Umpierrez). Dr. Smiley receives research support from the National Institute of Health (K08 DK0830361). Dr. Ziegler receives research support from the National Institute of Health (K24 RR023356).
Drs. Umpierrez and Smiley received funding from the National Institutes of Health.
Footnotes
This study was presented as an oral presentation at the Annual Endocrine Society Meeting in June 2011.
Dr. Umpierrez contributed to study design, interpreting data, and in writing the manuscript. Mr. Spiegelman, Mr. Zhao, Dr. Pinzon, Mr. Griffith, Dr. Garcia, Dr. Luo, and Mr. Thomas contributed to acquiring data, checking accuracy of data, and interpreting data. Drs. Smiley and Newton contributed to study design, checking accuracy of data, interpreting data, and revising the manuscript. Dr. Peng conducted the statistical analysis and contributed to interpreting the data and revising the manuscript. Mr. Morris contributed to database management and interpreting data. Dr. Ziegler contributed to study design, interpreting data, and in revising the manuscript.
The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Giner M, Laviano A, Meguid MM, et al. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996;12:23–29. doi: 10.1016/0899-9007(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 2.Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: Results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 3.Warnold I, Lundholm K. Clinical significance of preoperative nutritional status in 215 noncancer patients. Ann Surg. 1984;199:299–305. doi: 10.1097/00000658-198403000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dempsey DT, Mullen JL, Buzby GP. The link between nutritional status and clinical outcome: Can nutritional intervention modify it? Am J Clin Nutr. 1988;47(2 Suppl):352–356. doi: 10.1093/ajcn/47.2.352. [DOI] [PubMed] [Google Scholar]
- 5.Dark DS, Pingleton SK. Nutrition and nutritional support in critically ill patients. J Int Care Med. 1993;8:16–33. [Google Scholar]
- 6.Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–239. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 7.Martindale RG, Cresci G. Preventing infectious complications with nutrition intervention. JPEN J Parenter Enteral Nutr. 2005;29(1 Suppl):S53–S56. doi: 10.1177/01486071050290S1S53. [DOI] [PubMed] [Google Scholar]
- 8.Strickland A, Brogan A, Krauss J, et al. Is the use of specialized nutritional formulations a cost-effective strategy? A national database evaluation. JPEN J Parenter Enteral Nutr. 2005;29(1 Suppl):S81–S91. doi: 10.1177/01486071050290S1S81. [DOI] [PubMed] [Google Scholar]
- 9.Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr. 2006;30:351–367. doi: 10.1177/0148607106030004351. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004;328:1407. doi: 10.1136/bmj.38118.593900.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361:1088–1097. doi: 10.1056/NEJMct0806956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1:68–71. doi: 10.1016/s0140-6736(82)90212-4. [DOI] [PubMed] [Google Scholar]
- 13.The Veterans Affairs Total Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 14.Detsky AS, Baker JP, O’Rourke K, et al. Perioperative parenteral nutrition: A meta-analysis. Ann Intern Med. 1987;107:195–203. doi: 10.7326/0003-4819-107-2-195. [DOI] [PubMed] [Google Scholar]
- 15.Zaloga GP. Parenteral nutrition in adult inpatients with functioning gastrointestinal tracts: Assessment of outcomes. Lancet. 2006;367:1101–1111. doi: 10.1016/S0140-6736(06)68307-4. [DOI] [PubMed] [Google Scholar]
- 16.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med. 2005;33:213–220. doi: 10.1097/01.ccm.0000150960.36228.c0. discussion 260. [DOI] [PubMed] [Google Scholar]
- 18.der Voort PH, Feenstra RA, Bakker AJ, et al. Intravenous glucose intake independently related to intensive care unit and hospital mortality: An argument for glucose toxicity in critically ill patients. Clin Endocrinol (Oxf) 2006;64:141–145. doi: 10.1111/j.1365-2265.2006.02437.x. [DOI] [PubMed] [Google Scholar]
- 19.Cheung NW, Napier B, Zaccaria C, et al. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care. 2005;28:2367–2371. doi: 10.2337/diacare.28.10.2367. [DOI] [PubMed] [Google Scholar]
- 20.Schloerb PR. Glucose in parenteral nutrition: A survey of U.S. medical centers. JPEN J Parenter Enteral Nutr. 2004;28:447–452. doi: 10.1177/0148607104028006447. [DOI] [PubMed] [Google Scholar]
- 21.Yaqoob P. Lipids and the immune response: From molecular mechanisms to clinical applications. Curr Opin Clin Nutr Metab Care. 2003;6:133–150. doi: 10.1097/00075197-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Yaqoob P. Monounsaturated fatty acids in parenteral nutrition; evaluation of risks and benefits. Br J Nutr. 2005;94:867–868. doi: 10.1079/bjn20051576. [DOI] [PubMed] [Google Scholar]
- 23.Buenestado A, Cortijo J, Sanz MJ, et al. Olive oil-based lipid emulsion’s neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. JPEN J Parenter Enteral Nutr. 2006;30:286–296. doi: 10.1177/0148607106030004286. [DOI] [PubMed] [Google Scholar]
- 24.Sala-Vila A, Barbosa VM, Calder PC. Olive oil in parenteral nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:165–174. doi: 10.1097/MCO.0b013e32802bf787. [DOI] [PubMed] [Google Scholar]
- 25.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-de-Lorenzo A, Denia R, Atlan P, et al. Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: A randomised double-blind study of an olive oil-based lipid emulsion v. medium/long-chain triacylglycerols. Br J Nutr. 2005;94:221–230. doi: 10.1079/bjn20051467. [DOI] [PubMed] [Google Scholar]
- 27.Yaqoob P. Monounsaturated fats and immune function. Proc Nutr Soc. 1998;57:511–520. doi: 10.1079/pns19980075. [DOI] [PubMed] [Google Scholar]
- 28.McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: Effectiveness in prevention of hyperglycemia and infectious complications–a randomized clinical trial. Crit Care Med. 2000;28:3606–3611. doi: 10.1097/00003246-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 30.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 31.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: An important marker of poor outcome and mortality in hospitalized patients. Diabetes Care. 2010;33:739–741. doi: 10.2337/dc09-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson AD, Jain PK, MacFie J. Parenteral nutrition in the critically ill. Intensive Care Med. 2003;29:2103. doi: 10.1007/s00134-003-1996-4. [DOI] [PubMed] [Google Scholar]
- 34.Heyland DK, Montalvo M, MacDonald S, et al. Total parenteral nutrition in the surgical patient: A meta-analysis. Can J Surg. 2001;44:102–111. [PubMed] [Google Scholar]
- 35.Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: Review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr. 1997;66:683–706. doi: 10.1093/ajcn/66.3.683. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC. Hot topics in parenteral nutrition. Rationale for using new lipid emulsions in parenteral nutrition and a review of the trials performed in adults. Proc Nutr Soc. 2009;68:252–260. doi: 10.1017/S0029665109001268. [DOI] [PubMed] [Google Scholar]
- 37.Umpierrez GE, Smiley D, Robalino G, et al. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:609–614. doi: 10.1210/jc.2008-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gosmanov AR, Smiley DD, Robalino G, et al. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am J Physiol Endocrinol Metab. 2010;299:E953–E958. doi: 10.1152/ajpendo.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siqueira J, Smiley D, Newton C, et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: Comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab. 2011;96:3207–3216. doi: 10.1210/jc.2011-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClave SA, Martindale RG, Vanek VW, et al. A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine: Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 42.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 43.De La Rosa Gdel C, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: A randomised clinical trial. Crit Care. 2008;12:R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finfer S, Chittock DR, Su SY, et al. NICE-SUGAR Study Investigators: Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 45.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]


