Abstract
The Mouse Metabolic Phenotyping Centers (MMPCs) were founded in 2001 by the National Institutes of Health (NIH) to advance biomedical research by providing the scientific community with standardized, high-quality phenotyping services for mouse models of diabetes, obesity, and their complications. The intent is to allow researchers to take optimum advantage of the many new mouse models produced in labs and in high-throughput public efforts. The six MMPCs are located at universities around the country and perform complex metabolic tests in intact mice and hormone and analyte assays in tissues on a fee-for-service basis. Testing is subsidized by the NIH in order to reduce the barriers for mouse researchers. Although data derived from these tests belong to the researcher submitting mice or tissues, these data are archived after publication in a public database run by the MMPC Coordinating and Bioinformatics Unit. It is hoped that data from experiments performed in many mouse models of metabolic diseases, using standard protocols, will be useful in understanding the nature of these complex disorders. The current areas of expertise include energy balance and body composition, insulin action and secretion, whole-body and tissue carbohydrate and lipid metabolism, cardiovascular and renal function, and metabolic pathway kinetics. In addition to providing services, the MMPC staff provides expertise and advice to researchers, and works to develop and refine test protocols to best meet the community’s needs in light of current scientific developments. Test technology is disseminated by publications and through annual courses.
Introduction and rationale
Since development of transgenic technology three decades ago, the number of mouse models has exploded. Gene-targeting protocols involving homologous recombination in mouse embryonic stem (ES) cells were developed shortly after the advent of transgenic technology and have resulted in innumerable mutant lines with specific phenotypes and well-defined structural changes in DNA. The discovery of the principles for introducing specific gene modifications in mice by the use of ES cells had such an impact that it resulted in the awarding of pioneers in the field, Drs. M. Capecchi, Sir M. Evans and O. Smithies, with Nobel Prize in Physiology and Medicine Prize in 2007.
The human genome was sequenced over a decade ago. With this achievement the daunting challenge of ascribing function to the genes that comprise it has become paramount. This is a critical undertaking in terms of basic science and health care. With regard to the latter, advancements permit potential target sites responsible for disease susceptibility, new therapeutic targets, and the development of personalized medical approaches. The ability to manipulate the mouse genome has emerged as an important vehicle for establishing the means by which gene expression is regulated and the role of specific gene products. Moreover, transgenesis in the mouse has provided insight into the interaction between genes and the interaction of genes with the environment. One index of the prominent role of mice in medical research is that in many academic animal facilities mice comprise 99 % of the mammalian animal census.
The development of transgenic and gene-targeting methods has resulted in marked gains in our knowledge in virtually every area of biomedical research, including diabetes, obesity, and related disorders. Candidate genes for metabolic diseases have been identified and transgenic mice have been generated; moreover, other murine models with metabolic disorders have been created by large-scale mutagenesis. Tremendous inroads into our understanding of metabolic disease and their complications have been made since the start of the “transgenic era” and yet we are still only at the tip of the iceberg.
There are transgenic and mutant models of numerous other species. However, genetic mouse models clearly predominate in biomedical research. There are several reasons why mice are the chosen species. The techniques for manipulating the mouse germline are well established, the mouse genome is well defined, and an extensive database exists already on mouse models of disease. Mice are among the most cost- and space-effective mammals to house and breed. Approximately threefold more space is required to house rats compared to mice of equivalent census. The relatively small size of the mouse is good for economical reasons but creates difficulties for the study of metabolism. The size of a mouse depends on age, strain, diet, gender, and the presence of specific mutations. The C57Bl6 strain is the most frequently used for studies of metabolism. At 3 months of age a wild-type male C57Bl6 mouse will weigh ~25 g and have a blood volume of ~2 ml. Therefore, studies in these animals can be difficult, and the skills, techniques, and equipment necessary to study metabolic diseases in the mouse are highly specialized. It has been necessary to scale analytical tests for the small blood volumes and tissue mass of the mouse. A challenge in the research community has been to develop and apply complex phenotyping tests to the burgeoning number of genetic mouse models with application to diabetes, metabolism, obesity, and its complications.
The Mouse Metabolic Phenotyping Centers
In recognition of the need for studying the burgeoning number of mouse models of metabolic disease and the difficulty in measuring subtle quantitative phenotypes, The National Institute for Diabetes, Digestive and Kidney Diseases (NID-DK) embarked on an ambitious experiment in centralized mouse phenotyping with the inception of the Mouse Metabolic Phenotyping Centers (MMPC; www.mmpc.org) in 2001. The mission of the MMPC Consortium is to “advance medical and biological research by providing the scientific community with standardized, high quality metabolic and physiologic phenotyping services for mouse models of diabetes, diabetic complications, obesity and related disorders.” The National Heart, Lung, and Blood Institute (NHLBI) became a partner in this project in 2006, providing additional funding and guidance to expand efforts in the area of cardiovascular physiology and the complications of diabetes.
The MMPC Consortium has six phenotyping sites (Box 1). A Central Bioinformatics Unit (CBU) coordinates the activities of the consortium and manages a database of MMPC phenotyping test results that is populated by each MMPC. The MMPC CBU maintains the consortium’s web site (www.mmpc.org), which includes a comprehensive, search-able list of tests and services, database access, and funding opportunities available to the scientific community.
Box 1. Mouse Metabolic Phenotyping Centers at a glance (www.mmpc.org).
| Test catalog | www.mmpc.org/shared/catalog.aspx |
| Application for services | https://www.mmpc.org/secure/order.aspx |
| Database | www.mmpc.org/shared/search.aspx |
| Courses | www.mmpc.org/shared/courses.aspx |
| MICROMouse funding program | www.mmpc.org/shared/fundingPrograms.aspx |
| Case Western Reserve | www.case.edu/med/mmpc/index.html |
| University of California Davis | www.mmpc.ucdavis.edu |
| University of Cincinnati | www.mousephenotype.uc.edu/UC |
| University of Massachusetts | www.umassmed.edu/umpc/index.aspx |
| Vanderbilt University | www.mc.vanderbilt.edu/mmpc |
| Yale University | www.mouse.yale.edu |
| National Institute of Diabetes and Digestive and Kidney Diseases | www.niddk.nih.gov |
| National Heart Lung and Blood Institute | www.nhlbi.nih.gov |
| Mutant Mouse Regional Resource Centers (MMRRC) | www.mmrrc.org |
| Knock Out Mouse Project | www.komp.org |
| NIDDK Consortium Interconnectivity Network (dkCOIN) | www.dkcoin.org |
| Diabetic Complications Consortium (DCC) | www.diacomp.org |
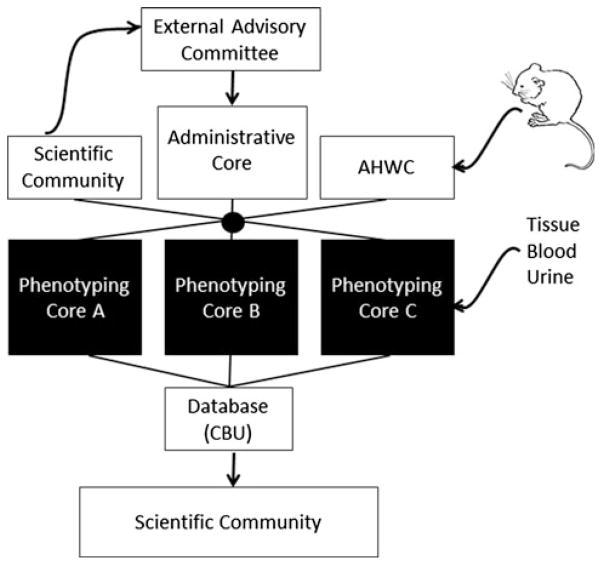
All MMPCs consist of the general structure depicted in Fig. 1. The participating centers are selected to cover the scope of needs of the broad scientific community, although there is overlap among centers in certain areas (e.g., energy balance, tests of insulin sensitivity) to cover projected demands. Each site consists of an administrative core that establishes center priorities, handles billing, and manages data and the operations of experimental phenotyping cores; an animal husbandry core that receives, pathogen tests, and houses mice; and one or more phenotyping cores that provide testing services to mouse researchers in the US and abroad.
Fig. 1.
The functional unit of the MMPC. All administrative activities are coordinated by the Central Bioinformatics Unit (CBU) at the Georgia Health Science Center. AHWC, Animal Health and Welfare Core
The phenotyping cores that part of each MMPC are the foundation upon which the consortium is built. The benefits of the MMPC stem from the creation of these national centralized cores. The core concept broadens the scope, expedites the completion, improves the quality, and reduces the overall costs of the research being performed.
The core concept enables the acquisition, development, operation, and maintenance of equipment that is beyond the grasp of individual investigators. This thereby broadens the research scope available to investigators. The existence of this equipment within a core structure assures the most efficient use of expensive items. Second, the core concept allows technically complex experiments to be available to investigators who would otherwise not be able to invest the time or resources to develop them.
The completion of research projects is expedited through the greater proficiency of a staff highly experienced in specific procedures.
Accuracy and precision are high due to the advantages of a specialized staff that is dedicated to specific tests.
Expediting research, sharing use of expensive equipment, and utilizing a skilled technical staff will necessarily achieve the last goal of decreasing research costs.
One of the primary goals of the MMPC Consortium is to be responsive to the needs of the national scientific community of mouse researchers. Programmatic changes are assessed at the consortium level and at each individual center. The consortium is guided by a steering committee comprising NIH program officers, external scientific advisors, and representatives of each center. This steering committee identifies areas for development and expansion. Standing committees established by the steering committee standardize administrative operations and policies on animal husbandry and transfer. Each MMPC has an organizational structure that decides on areas of emphasis based on the demands on specific services and the technical strengths and expertise at the specific academic institution (Fig. 1).
Center activities
Phenotyping tests
The range of phenotyping tests among the six MMPCs is extensive. Over 200 tests are offered in the MMPC catalog (http://www.mmpc.org/shared/catalog.aspx). These can be clustered into tests of cardiovascular function and the complications of diabetes, insulin sensitivity and metabolism, pancreatic islet function, weight management and energy balance, mouse pathology, and a range of analytical services (hormones, analytes, metabolomics, cell signaling pathways) (Table 1). The long-term success of the MMPC program rests with the responsiveness of the centers to the needs of the scientific community. Scientists are encouraged to inquire if a desired phenotyping test is not listed, as it may be possible to implement it at one of the six centers.
Table 1.
Broad areas of current emphasis
| Cardiovascular function and the complications of diabetes |
| Insulin sensitivity and glucose metabolism |
| Pancreatic islet morphology and function |
| Energy balance |
| Bariatric surgery |
| Incretin secretion and gastrointestinal function |
| Mouse pathology |
| Analytical services |
| Hormones |
| Analytes |
| Metabolomics |
| Cell signaling |
Centers operate on a fee-for-service basis. The costs are underwritten by the grants from the NIH, making the service less expensive than the actual cost. Besides the tests that require interinstitutional mouse transfer, the centers also offer a range of analytical tests that do not require mouse transfer. These analyses are specific for the unique properties of the mouse. On occasion, some MMPC analytical tests are also available commercially. In those instances the MMPC, insofar as we are aware, are able to provide those services at a lower cost.
The capacity to perform multiple measurements in a single mouse allows for more precise analysis of physiological and pathophysiological responses. Considering the expense and time involved in shipping or breeding, it is cost effective and efficient to obtain as much data from a single mouse as possible. Several of the tests utilized by the MMPCs are noninvasive or minimally invasive, thus allowing application of multiple assessments to a single mouse. Some of those tests include tail cuff blood pressure monitoring, echocardiography, urine analysis, exercise tolerance, body composition, food absorption, food intake, energy expenditure, and activity monitoring. Data obtained from a single mouse are further maximized in terminal procedures by the excising of tissue that can be immediately processed or suitably archived for further analysis. Genetically modified mice can develop unexpected phenotypes that can be detected through a broad range of testing without compromising the primary research test (e.g., glucose clamp).
The investigators that use an MMPC range from those who have considerable expertise in metabolic diseases to those who have no prior experience in metabolic disease but have a transgenic mouse that, perhaps surprisingly, presents with a phenotype indicative of a metabolic disease. An authority on cancer biology, for example, may delete a gene from a mouse that results in a germline that exhibits hyperglycemia and increased adiposity. The cancer biologist, who would normally think little about the determinants of hyperglycemia and obesity, can initiate an informal investigation on the means by which the gene deletion causes the metabolic phenotype by beginning a dialog with one of several MMPC contacts listed at www.mmpc.org. An important and unique part of the MMPC experience is the interaction with MMPC faculty and staff who have vast experience and novel expertise with a range of phenotyping tests. MMPC directors and core directors can advise on preliminary screening tests that can be performed by the primary investigator and on screening strategies for MMPC tests based on preliminary results and the physiology of the expressed/deleted gene.
Database
A material transfer form (www.mmpc.org/MTA.pdf) is in place to protect the interests of investigators. All data and intellectual property that are generated by the MMPC belong to the investigator who has submitted the request for services. However, NIH requests that investigators allow data generated by an MMPC to be placed in the public MMPC database after one of the following two conditions has been met: (1) the data have been published and are therefore in the public domain, or (2) 2 years have passed since completion of services by the MMPC. Investigators may request that specific data be withheld from the public database for an additional period of time. The database is searchable for protocols as well as for specific experiments, by mouse strain, scientific research area, or output from a single center.
Standardization of phenotyping procedures
It has become clear over time that while there is consensus on the need to improve and standardize phenotyping approaches for the study of metabolic disease in mouse models, actually doing so is a daunting task. A primary reason is the evolving nature of some of the complex tests, where considerable experience making a measurement in mice with a variety of genetic alterations can result in the intellectual basis for further development and validation of the test. An example is an increasing awareness that different approaches to analysis and normalization of indirect calorimetry data for energy balance can result in diametrically opposed interpretations of the data when the control and experimental groups have very different average mass (Kaiyala et al. 2010). Another primary obstacle to the standardization of complex tests is the need to customize experimental parameters for specific mouse models in order to uncover subtle phenotypes. For instance, one might choose to clamp glucose at a higher level than typical when studying a mouse that experiences fasting hyperglycemia, or choose a higher insulin infusion rate for extremely insulin-resistant mice. Finally, it may not be possible to account for or control all the environmental variables, e.g., water and air, microbiome, noise and light levels, length of quarantine, that can contribute to a metabolic phenotype, and these may change from one animal facility to the next, or even from rack to rack within a given animal room. To overcome any experimental issues arising from test or environmental parameters, for every experimental model, all MMPC tests are also performed on appropriate control groups and results are typically reported both as absolute values and as the difference from those of the control. For these reasons, each MMPC standardizes its procedures to suit its strengths and its overall operational strategy while doing as much as possible to understand and document the differences among centers. The consortium has published standard operating procedures for measuring food intake (Ellacott et al. 2010) and for performing glucose clamps (Ayala et al. 2010). Individual MMPCs have published papers describing methods (Ayala et al. 2006; Jandacek et al. 2004; Kaiyala and Schwartz 2011; Kaiyala et al. 2010; Rottman et al. 2007; Shearer et al. 2008; Tong et al. 2010; Yin et al. 2011), standards (Ayala et al. 2006; McGuinness et al. 2009; Rottman et al. 2003), and strain comparisons (Berglund et al. 2008; Burgess et al. 2005; Qi et al. 2005). The Vanderbilt MMPC maintains a comprehensive online laboratory manual for in vivo testing (http://www.mc.vanderbilt.edu/mmpc) and has a video of the surgery and glucose clamp that can be viewed online (Ayala et al. 2011). The MMPC, through education and outreach, serves as a resource to open dialog on standardization of procedures to study the mouse. For many procedures the approaches are relatively easy to learn and can be implemented by any laboratory. Others are more complex and require more advanced skills, expertise, and/or equipment.
Development
The engine for technical development is the needs of the scientific community. Feedback from investigators, the vision of the executive committee, and the insight of MMPC faculty provide input to areas where phenotype tests need to be further developed or introduced. Recent examples of MMPC development efforts are sophisticated new imaging techniques, bariatric surgical procedures, sampling of gastrointestinal lymph, and the implementation of new ways to measure energy expenditure.
There are mechanisms for development built into the MMPC Consortium. These target important new areas as put forward through consortium working groups that are formed to identify technical needs and resolve those deficits. Funding is available to MMPC staff and to the broad scientific community through a grant funding program, MICROMouse: MMPC Initiative for Collaborative Research on the Mouse (http://www.mmpc.org/shared/fundingPrograms.aspx). The MMPC has an annual budget for the MICROMouse grant program for competitive funding of one-year grants. These grants focus on (1) projects that develop or miniaturize metabolic phenotyping tests for use in mice (available to all interested researchers), and (2) research-driven projects that arise from, or take advantage of, MMPC activities that combine the interests of two or more investigators and that fall outside of those research activities currently funded by an MMPC or an outside investigator’s established research program. With regard to the latter, the MMPCs provide tests to characterize many of the mouse models of metabolic diseases that are generated across the country and therefore are in a unique position to identify opportunities for new or expanded studies as they arise.
Education programs
The MMPC commitment to dissemination of technology is described at www.mmpc.org/shared/courses.aspx. The MMPC commitment to education began 8 years ago with the inaugural edition of the course Glucose Clamping the Conscious Mouse. This course, which is offered annually by the Vanderbilt MMPC, combines didactic sessions with over 20 h of guided “hands-on” time. This course is designed to address two major needs of the scientific community. The first is to aid those laboratories needing to perform glucose clamps on a regular basis in the use of this challenging technique. The second is to make the mouse clamp technology more transparent so that scientists wishing to make sense of the growing literature better understand the factors involved in performing clamps in the conscious mouse.
The course Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis is sponsored by the NIDDK and the Donald W. Reynolds Institute on Aging at the University of Arkansas for Medical Sciences in collaboration with the MMPC. It is a week-long course in the theory and practice of isotopic tracers (stable and radioactive) for the study of metabolism in humans and animals by means of mass spectrometry and NMR. A special effort is made to emphasize application to mice. The course also emphasizes isotopomer analysis for metabolic flux rates and metabolic regulation.
Two other courses are conducted at the Vanderbilt MMPC. One is an intensive 2-week course with the objective of providing registrants with the tools needed to measure the effect of an experimental intervention on macronutrient metabolism, energy balance, cardiovascular homeostasis, and animal behavior. The course combines lectures, hands-on laboratories, and demonstrations and data problem sessions. More information on this course can be obtained at www.mc.vanderbilt.edu/diabetes/msshortcourse. The success of the MMPC education program led to the formation of a course conducted by the faculty of the NIH-funded Vanderbilt Mouse Kidney Physiology and Disease Center using the resources of the Vanderbilt MMPC. This course is entitled Experimental Techniques in Mouse Kidney Injury Workshop. This is a 5-day training course for scientists interested in the kidney, including those maladies that are complications of diabetes. This is a hands-on workshop designed to teach surgical and phenotyping techniques for commonly used mouse renal injury models. This course is scheduled annually. More information can be obtained at https://www.mc.vanderbilt.edu/mkpdc.
Availability of mutant mouse lines
Currently, thousands of new mutant mouse models are being generated by high-throughput mutagenesis programs underway in the US (Knockout Mouse Project, KOMP), Europe (European Conditional Mouse Mutagenesis Programme, EUCOMM), Canada (North American Conditional Mouse Mutagenesis Program, NorCOMM), and Asia. Some of the nearly 20,000 new mutant mouse models being generated will exhibit phenotypes consistent with diabetes, obesity, diabetic complications, and diabetes-related disorders.
To take advantage of these mutant mouse resources, the MMPC provides users with a pipeline enabling them to request metabolic phenotyping tests on mutant mouse lines generated at a large-scale production site. This new feature allows investigators access to mutant mouse lines that can be obtained from public repositories anywhere in the world. To facilitate access to available mutant mice, the UC Davis MMPC is located at the site of the largest of four NIH-supported Mutant Mouse Regional Resource Centers (MMRRC) having an archive of over 30,000 mutant mouse alleles. The MMRRC accepts donated mice, verifies their authenticity, archives them, and then makes them available without restriction of use to academic investigators for scientific research (some restrictions apply to requests from commercial, for-profit entities). Further, UC Davis also serves as the archive for the KOMP program, which houses ~8,500 conditional and deletion knockout lines as ES cells, germplasm, and live mice, all available to users of the MMPC. Finally, if a particular mutant mouse line does not exist for an investigator’s gene of interest, then a user can arrange to have it created and sent to an MMPC for metabolic phenotyping. For example, the UC Davis Mouse Biology Program offers all the services necessary to generate a mutant mouse de novo (e.g., transgenesis, homologous recombination in ES cells) and then can turn the mouse over to the MMPC at UC Davis or send it to another MMPC for phenotyping and analysis. All of the above saves time and expense for an investigator because a metabolic testing plan can be initiated without the need for shipping mice between a researcher’s laboratory and the MMPC.
Scientific contributions
The MMPC focuses on developing, standardizing, and disseminating phenotyping tests and not strictly on describing or defining metabolic disease in mouse models. However, its members are also leading scientists in the fields of diabetes, obesity, liver, kidney, and heart disease. Core and center Directors typically spend a great deal of time consulting with clients prior to testing their animal models in order to identify the best set of experiments to do and thereby ensure that interpretable data are acquired.
The consortium has over 1,200 members, defined as researchers who staff or advise the centers or who have submitted mice or tissue samples for study. It has produced more than 635 papers (that acknowledge the MMPC contribution) since its inception in 2001, with over 280 between 2009 and 2012. Many of these papers have been in high-impact journals such as Nature, Science, and Proceedings of the National Academy of Sciences. Although the MMPC specializes in relatively low-throughput studies that require specialized equipment or expertise, the volume of studies in the six centers is considerable. There have been 496 orders placed since the beginning of 2009, comprising about 2.5 million assays performed on plasma and tissue extracts and 215 experiments done on 3,700 mice of 54 different strains. Almost all of these experiments would be difficult for investigators to do in their own laboratories. For instance, in a single year (2008) the MMPC performed 1,085 euglycemic clamps in mice, 1,093 measurements of energy balance by calorimetry or doubly-labeled water, 7,661 body composition studies, 886 cardiac echocardiography studies, 161 lymph fistulas, and 400 measures of fat absorption. Data from these experiments can be found in the MMPC database after publication or no later than 2 years after the tests are completed.
Current scientific interests and consortium projects
Glucose clamping
Services that remain among the most heavily used are those tests that involve glucose clamping the conscious mouse. In the presence of hyperinsulinemia, glucose can be clamped at euglycemia to provide an index of insulin sensitivity, or at hypoglycemia to assess the neuroendocrine response to a fall in blood glucose. Clamping glucose (in the absence of insulin) at hyperglycemia can provide a test of insulin secretion in vivo. These procedures are described in a recent review of the MMPC procedures (Ayala et al. 2010). Considering the utility of these procedures, it is of continued interest to devise new ways to improve these approaches. These include combining clamp technology with isotopes for measuring nutrient flux, metabolomics, and microspheres and fluorescence for measuring blood flow.
Energy balance
Energy balance at the level of the whole body is determined by energy expenditure and energy intake. Kaiyala and colleagues (Kaiyala and Schwartz 2011; Kaiyala et al. 2010) began a rigorous effort to develop a multilinear model for calculating energy expenditure in mice by incorporating the contributions of both lean and fat compartments. The MMPC Consortium is testing the feasibility of developing a web-based calculator for performing multilinear regression to calculate more meaningful rates of energy expenditure.
Although energy balance at the most fundamental level is determined by energy expenditure and energy intake, each of these components is determined by covariates such as physical activity, gender, feeding behavior, strain, and diet. An objective of the MMPC Consortium is to further define the effects of these covariates to better define and isolate differences in energy balance due to transgenesis.
Bariatric surgery
Given the effectiveness of bariatric surgery to treat severe obesity and metabolic syndrome, the development of model systems of bariatric surgery are of great value. Understanding the mechanism(s) of action of the various bariatric surgeries may help improve existing procedures, identify genetic and other factors that impact the variability of outcomes within a procedure type, identify genetic and other factors that determine the optimal procedure for each individual, lead to new pharmacological approaches to the treatment of obesity and metabolic syndrome, and advance understanding of the basic mechanisms underlying control of energy and glucose homeostasis. The development of bariatric surgery in the laboratory mouse is particularly important since the mouse is the only organism in which transgenesis can be readily used to probe the role of individual genes and cell types in the efficacy of the procedures. The Vanderbilt and UC Davis MMPCs have developed and published or are in the process of developing mouse models of bariatric surgery (Yin et al. 2011). This multicenter proposal utilizes unique strengths in the MMPC Consortium with the specific aims of (1) developing, (2) refining, (3) standardizing, and (4) characterizing mouse models of bariatric surgery. Extensive characterization of unique procedures will be performed using the strengths of multiple MMPCs in energy balance, gastrointestinal histological alterations, micronutrient status, microbiota, gastrointestinal absorption, lymph composition, incretin secretion, and the pancreatic islet cell morphology and function. The efforts of the MMPC Bariatric Surgery Working Group will provide the MMPC Consortium with well-defined models of bariatric surgery and a truly novel service that will further the research efforts of the greater scientific community.
Enhanced imaging resources
The imaging resources and expertise at the institutions where the MMPCs are located offer unique opportunities for molecular imaging of metabolism at the molecular, cellular, tissue, organ, and whole-body levels. Advances in biophotonics, such as laser-trap Raman and single-molecule fluorescence spectroscopy, make possible the nondestructive analyses of individual living cells for characterizing the dynamics of intracellular molecular interactions of lipids and proteins. Biophotonics methodologies and magnetic resonance (MR) microscopy offer complementary approaches for tracking cells in real time. MR spectroscopy can be used to obtain real-time measurements of whole-body and tissue-specific fat distribution, TCA cycle flux, ATP production, and glycogen synthesis rates. SPECT imaging provides a means to evaluate cardiac function and angiogenesis in relation to diabetes, while PET has been useful for imaging physiological functions, including blood flow, glucose metabolism, neurotransmitter synthesis, receptor availability, second messengers, transporters, diffusion, inflammation, and drug delivery. A significant advantage of many of these imaging techniques is that longitudinal studies are possible, and mice can be used later for other phenotyping tests. In addition, imaging studies provide complementary data to other metabolomics approaches such as mass spectroscopy, leading to a comprehensive understanding of mechanisms contributing to metabolic dysregulation in disease. A major impediment to the researcher who may want to employ an imaging technique is access to the instrumentation and associated infrastructure, and thus validation of the technology for metabolic measurements. The incorporation of the imaging cores within the UC Davis, Vanderbilt, and Yale MMPCs seeks to lower this hurdle for investigators.
Has the MMPC experiment been successful?
The consortium is competitively funded for 5 years at a time. It is evaluated by the NIH and its advisory committee 2 years prior to each competition, and each center must undergo successful peer evaluation for continued funding. It has very clearly achieved success in developing and validating metabolic tests for mouse models and has allowed many more laboratories access to these tests than would have been possible without it. The large number of publications that report MMPC-derived data provides a minimum estimate of the impact of the program. The full impact of the MMPC Consortium is difficult to capture, as achievements from outside the consortium that are made possible by MMPC test standardization, minimal standards for data reporting, and educational outreach are difficult to quantify.
The centralized phenotyping approach is not without its challenges and limitations, however. Planning experiments takes considerable effort and time for both the investigator and MMPC personnel. Even with cost-sharing from the NIH awards, many of the phenotyping tests offered are expensive (albeit less expensive than commercial costs or costs of tests in individual laboratories) and applicants must weigh cost against the value of the data. Many of the tests are time consuming, and when demand is high (as it is for tests such as the glucose clamps), the wait for data can be as long as several months. Shipping precious animals can present logistical problems and occasionally fragile animals do not do well in transit. The need for quarantine can make timing difficult, especially when animal age is an important factor. Recognizing these hurdles, the MMPCs have worked to streamline the process of animal shipment and to minimize quarantine residence time. Another challenge occurs once data are received, when it becomes the responsibility of the investigator to interpret it with the assistance of the MMPC core director. A goal of the MMPC is that data be presented in a form that can be easily understood and used appropriately, and therefore data presentation and interpretation take considerable time and energy on the part of the consortium members. In summary, the MMPC has worked diligently to improve and perform tests, to disseminate the technology to other laboratories, and to educate researchers about the strengths and limitations of the technology.
The MMPC Consortium: a summary
The MMPC Consortium has been important over the last 10 years in the discovery and characterization of metabolic and associated phenotypes in mice. Mice characterized by an MMPC may originate in individual investigator laboratories or in large-scale, global mutant mouse production programs (e.g., International Knockout Mouse Consortium). The latter are incredibly valuable sources of new, genetically modified alleles available as vectors, ES cells, germplasm, and/or live mice at related, publicly accessible repositories (e.g., KOMP Repository, European Mouse Mutant Program). Complementing these entities are a number of other archives and repositories (e.g., MMRRC, The Jackson Laboratory) that distribute mutant mouse lines created and deposited by individual researchers and laboratories. Once created and/or obtained, the MMPC can provide sensitive “tertiary” analysis to test specific hypotheses on mutant mouse lines. Other phenotyping programs (e.g., International Mouse Phenotyping Consortium) conduct broad, high-throughput primary screening for abnormalities across multiple body systems in order to identify interesting phenotypes worthy of secondary and tertiary metabolic analysis. The MMPCs can assist researchers by conducting metabolism-related tests and analyses on mutant mouse lines preselected for their likelihood of being of interest in the fields of diabetes, obesity, and metabolic diseases based on primary or secondary screens. The standardized, sensitive tests in place at the MMPC can be combined with provocative stimuli (e.g., dietary modification, exercise, pharmacological interventions) to detect phenotypes that may otherwise be silent.
The MMPCs develop, standardize, and implement phenotyping procedures for mouse models of diabetes. The impact of the MMPCs is further broadened by courses and papers that serve to disseminate protocols and methods for metabolic research to investigators who may wish to establish procedures for more sophisticated testing in their own laboratory. The strategic plan of the MMPCs is summarized in Fig. 2. The goal of disseminating protocols and methods increases the impact of the consortium well beyond that which would be achieved by performing the services alone.
Fig. 2.
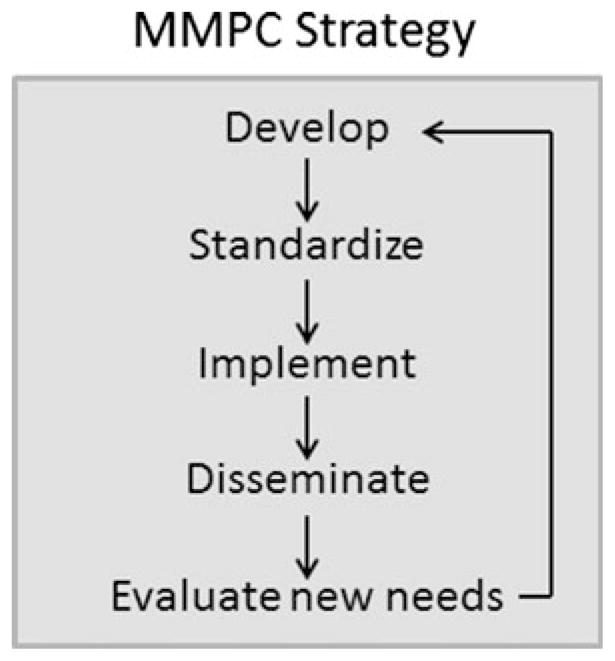
The guiding strategy of the MMPCs
Acknowledgments
The MMPCs are supported by U24 Grants DK059630, DK059632, DK059635, DK59637, DK076126, DK076169, DK076174, DK092993, and DK093000.
Footnotes
This study was conducted on behalf of the Mouse Metabolic Phenotyping Centers Consortium.
Contributor Information
Maren R. Laughlin, Email: maren.laughlin@nih.gov, Division of Diabetes, Endocrinology and Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, 6707 Democracy Blvd, Rm. 787, MSC 5460, Bethesda, MD 20892-5460, USA
K. C. Kent Lloyd, Mouse Biology Program, School of Veterinary Medicine, Center for Comparative Medicine, University of California, Davis, Davis, CA, USA
Gary W. Cline, Department of Diagnostic Radiology, Yale University School of Medicine, New Haven, CT, USA
David H. Wasserman, Department of Molecular Physiology and Biophysics and Mouse Metabolic Phenotyping Center, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Malabanan C, James FD, Ansari T, Fueger PT, et al. Hyperinsulinemic–euglycemic clamps in conscious, unrestrained mice. J Vis Exp. 2011;57:e3188. doi: 10.3791/3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, et al. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab. 2005;289(1):E53–E61. doi: 10.1152/ajpendo.00601.2004. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010;12:10–17. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127(1):139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60(1):17–23. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59(7):1657–1666. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297(4):E849–E855. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54(9):2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME, et al. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr. 2003;16(11):1150–1157. doi: 10.1067/S0894-7317(03)00471-1. [DOI] [PubMed] [Google Scholar]
- Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography. 2007;24(1):83–89. doi: 10.1111/j.1540-8175.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- Shearer J, Coenen KR, Pencek RR, Swift LL, Wasserman DH, Rottman JN. Long chain fatty acid uptake in vivo: comparison of [125I]-BMIPP and [3H]-bromopalmitate. Lipids. 2008;43(8):703–711. doi: 10.1007/s11745-008-3183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Tschöp MH, Aulinger BA, Davis HW, Yang Q, Liu J, Gaylinn BD, Thorner MO, D’Alessio D, Tso P. The intestinal lymph fistula model–a novel approach to study ghrelin secretion. Am J Gastrointest Liver Physiol. 2010;298(3):G474–G480. doi: 10.1152/ajpgi.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DP, Gao Q, Ma LL, Yan W, Williams PE, McGuinness OP, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254(1):73–82. doi: 10.1097/SLA.0b013e3182197035. [DOI] [PMC free article] [PubMed] [Google Scholar]