Abstract
Hyperglycemia and elevated free fatty acids (FFA) are implicated in the development of endothelial dysfunction. Infusion of soy-bean oil-based lipid emulsion (Intralipid®) increases FFA levels and results in elevation of blood pressure (BP) and endothelial dysfunction in obese healthy subjects. The effects of combined hyperglycemia and high FFA on BP, endothelial function and carbohydrate metabolism are not known. Twelve obese healthy subjects received four random, 8-h IV infusions of saline, Intralipid 40 mL/h, Dextrose 10% 40 mL/h, or combined Intralipid and dextrose. Plasma levels of FFA increased by 1.03±0.34 mmol/L (p=0.009) after Intralipid, but FFAs remained unchanged during saline, dextrose, and combined Intralipid and dextrose infusion. Plasma glucose and insulin concentrations significantly increased after dextrose and combined Intralipid and dextrose (all, p<0.05) and were not different from baseline during saline and lipid infusion. Intralipid increased systolic BP by 12±9 mmHg (p<0.001) and diastolic BP by 5±6 mmHg (p=0.022), and decreased flow-mediated dilatation (FMD) from baseline by 3.2%±1.4% (p<0.001). Saline and dextrose infusion had neutral effects on BP and FMD. The co-administration of lipid and dextrose decreased FMD by 2.4%±2.1% (p=0.002) from baseline, but did not significantly increase systolic or diastolic BP. Short-term Intralipid infusion significantly increased FFA and BP; in contrast, FFA and BP were unchanged during combined infusion of Intralipid and dextrose. Combined Intralipid and dextrose infusion resulted in endothelial dysfunction similar to Intralipid alone.
1. Introduction
Hyperglycemia and dyslipidemia are implicated in pathogenesis of atherosclerosis, hypertension, and vascular dysfunction. Several studies have reported that infusion of Intralipid results in an elevation of free fatty acids (FFA) levels and rapid and sustained increase in blood pressure and endothelial dysfunction [1-5]. The mechanisms for Intralipid-induced endothelial dysfunction and hypertension are not completely understood but are likely mediated by FFA level rise which, in turn, decreases nitric oxide production and increases autonomic nervous system activity [6-8]. Intralipid is a soybean-based lipid infusion that contains significant amount of ω-6 polyunsaturated fatty acids (PUFA) that are easily oxidized and generate reactive oxygen species [9]. Infusion of PUFA can induce pro-oxidative and pro-inflammatory states[5]and cause insulin resistance by decreasing peripheral glucose uptake [10], down-regulating intracellular insulin signaling [11,12] and decreasing insulin-mediated recruitment of microvasculature in peripheral tissues [13,14]. Additionally, previous studies have shown that PUFA can precipitate endothelial dysfunction and increase blood pressure in healthy subjects [3,4,15-17].
Increasing evidence also indicates that acute hyperglycemia is associated with endothelial dysfunction and blood pressure changes in non-diabetic lean and obese humans [18,19]. High blood glucose levels above 10–15 mmol/L lead to a pro-oxidative state and endothelial dysfunction [18-22]. Hyperglycemia inhibits endothelial nitric oxide synthase (eNOS) activity which may explain glucose-mediated endothelial dysfunction [23].
The hypothesis of “glucolipotoxicity” has been put forward to explain β-cell dysfunction secondary to combined effects of hyperglycemia and elevated FFA [24-26]. Most of the evidence supporting the phenomenon of glucolipotoxicity comes from in vitro and in vivo animal models [24-26], and thus, we lack data ascertaining how the combination of hyperglycemia and elevated FFA would affect carbohydrate metabolism in human subjects. Also, we do not know whether the combination of physiologically relevant elevation of blood glucose and FFA would have synergistic effects in worsening endothelial dysfunction and vascular function in healthy subjects. Accordingly, we investigated the effects of acute exposure to elevated plasma concentration of PUFA and glucose separately and in combination on blood pressure and endothelial function in obese, healthy subjects.
2. Research design and methods
2.1. Participants
We studied 12 obese, normotensive, healthy subjects. Obesity was defined as a body mass index (BMI) ≥30 kg/m2. All participants had a blood pressure <140/90 mm Hg and had no prior history of pre-diabetes, diabetes mellitus, hypertension or use of antihypertensive or lipid medications prior to the study. Diabetes mellitus was excluded with a 2-h glucose of <200 mg/dL during a 75 g oral glucose tolerance test and a fasting glucose of <126 mg/dL. Subjects with fasting triglyceride levels >250 mg/dL, hepatic disease (transaminases >3 times the upper limit of normal), renal insufficiency (creatinine >1.5 mg/dL), pregnancy, breast-feeding status, recent drug abuse (<3 months), significant psychiatric illness or tobacco use were excluded. The Institutional Review Board at Emory University approved the research protocol, and all subjects gave written and signed consent prior to participation in the study.
2.2. Study protocol
Participants were admitted to the Clinical Research Unit at Grady Memorial Hospital in random order, on four occasions to receive an 8-h IV infusion of Intralipid 20% at 40 mL/h, dextrose 10% solution at 40 mL/h, combination Intralipid 20%+dextrose 10% at 40 mL/h, or normal saline at 40 mL/h. The Intralipid 20% solution is a long-chain triglyceride emulsion composed of 50% polyunsaturated fatty acids (PUFA), 26% monounsaturated fatty acids, and 19% saturated fatty acids. Intravenous catheters were placed in each forearm, one for infusion and one for blood sampling. During the 8-h infusion, patients remained in the supine position and were kept NPO except for water ad-lib.
Blood pressure was measured in triplicate with a manual cuff prior to and every 4 h during the infusions. Blood samples for glucose, insulin, C-peptide and FFA levels were drawn at baseline and at 4 and 8 h during the infusions.
2.3. Endothelial function
Endothelial function was assessed at baseline and at 4 h during infusion using established methodology [27]. Briefly, ultrasound images of the brachial artery were obtained at baseline under standardized conditions and 60 s after induction of reactive hyperemia by cuff occlusion of the forearm for 5-min. Image landmarks as well as surface markers were utilized to ensure anatomical consistency between serial imaging studies. All images were digitized online, and arterial diameters were measured with customized software (Medical Imaging Applications, Coralville, Iowa) by individuals blinded to the clinical and laboratory status of the subjects. Flow mediated dilatation (FMD) was expressed as the percentage increase in diameter from baseline. Based on our previous studies, the mean difference in FMD between two consecutive assessments at least one week apart is 1.26%±0.76% (r=0.75); the mean difference in the FMD between 2 readings of the same subjects is 0.82±0.48% (r=0.97).
2.4. Laboratory methods
Plasma glucose and triglycerides were measured on the CX7 Chemistry Analyzer (Beckman Diagnostics, Fullerton, CA) using reagents and calibrators from Beckman Diagnostics. FFA levels were determined by a colorimetric method (Genzyme Diagnostics, Elkton, PA). Levels of insulin and C-peptide were measured in plasma using a solid phase, two-site sequential chemiluminescent immunometric assays on the DPC Immulite analyzer (Diagnostic Products, Los Angeles, CA). The coefficient of variance for the assays was <5%.
2.5. Statistical analysis
The primary endpoints of the study included changes in FMD and BP from baseline during Intralipid infusion without and with dextrose, dextrose infusion alone and normal saline. Comparison between baseline data and values during infusions was carried out using paired t tests. Repeated measures ANOVA was further used to evaluate differences in outcome changes over time among different groups. Statistical significance was defined as a p value <0.05. All data are expressed as mean±standard deviation (SD) or mean ±standard error of mean (SEM) where indicated.
3. Results
The clinical characteristics of study subjects are listed in Table 1. All subjects were African American, and none of them had metabolic syndrome as defined by the American Heart Association/National Heart Lung Blood Institute (AHA/NHLBI) diagnostic criteria [28].
Table 1.
Baseline clinical and metabolic features of the study subjects.
| Age, years | 41±7 |
| Gender, M/F | 7/5 |
| Weight, kg | 90±15 |
| Body mass index, kg/m2 | 32±2 |
| Systolic BP, mm Hg | 113±21 |
| Diastolic BP, mm Hg | 65±12 |
| Fasting glucose, mg/dL | 86±9 |
| 2-h OGTT glucose, mg/dL | 110±26 |
| Plasma insulin, μU/mL | 8±5 |
| C-peptide, ng/mL | 2.21±1 |
| Free fatty acids, mmol/L | 0.72±0.3 |
| Triglycerides, mg/dL | 102±58 |
| HDL-Cholesterol, mg/dL | 45±17 |
Values are mean±SD.
BP: blood pressure; OGTT: oral glucose tolerance test.
3.1. Blood pressure and endothelial function
The infusion of normal saline did not result in any significant changes in systolic and diastolic blood pressure from baseline (Fig. 1). The administration of Intralipid resulted in a significant rise in systolic blood pressure from baseline by 12.6±7.2 mm Hg(p<0.001) at 4 h and 12.1±8.6 mm Hg at 8 h, (p=0.001) (Fig. 1A). Changes in systolic blood pressure during Intralipid infusion were also higher than blood pressure changes observed during saline administration (p=0.053 between group by repeated measures ANOVA). The administration of dextrose solution alone did not result in significant changes in systolic or diastolic blood pressure from baseline (p=0.39 for 4-h change, p=0.42 for 8-h change). In contrast, the co-administration of Intralipid and dextrose only non-significantly increased systolic BP from baseline by 12.9±22.0 mm Hg at 8-h of infusion (p=0.08) (Fig. 1A).
Fig. 1.
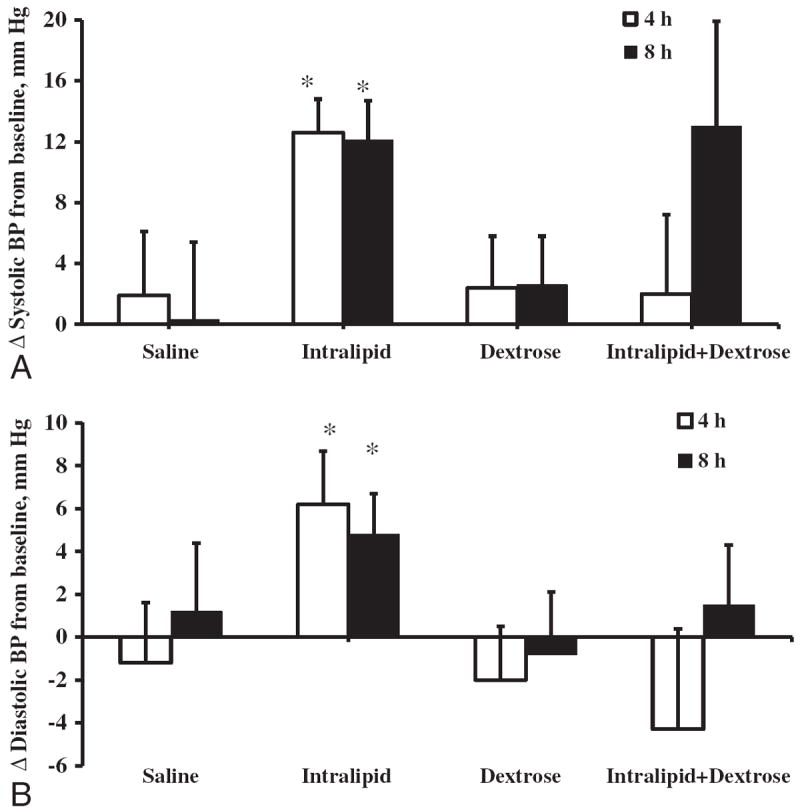
Changes in systolic (A) and diastolic (B) blood pressure during 8-h intravenous infusion of Saline, Intralipid, Dextrose or Intralipid+Dextrose in obese subjects. Values are mean±SEM. †p<0.05; *p<0.01 compared to baseline.
The administration of Intralipid raised diastolic blood pressure from baseline by 6.2±8.1 mm Hg (p=0.024) at 4 h and 4.8±6.2 mm Hg (p=0.022) at 8 h from baseline (Fig. 1B) and these changes were larger than the observed blood pressure changes during saline infusion (p=0.073 by repeated measures ANOVA). Dextrose infusion did not alter diastolic blood pressure (Fig. 1B) just as the co-administration of Intralipid and dextrose had no significant effects on diastolic blood pressure at 4 h and 8 h from baseline (p=NS). Diastolic blood pressure changes over time among saline, dextrose and combined Intralipid and dextrose groups were similar (p=0.75 by repeated measures ANOVA).
Endothelial function, measured as FMD, was similar among study groups before cuff occlusion at 0 and 4 h of study. The mean baseline FMD before any intervention ranged from 8.9% to 10.3%. Flow-mediated dilatation did not change after saline infusion and non-significantly decreased by 0.9%±1.8% (p=0.103) after dextrose infusion alone. Intralipid infusion markedly diminished FMD by 3.2%±1.4% from baseline at 4 h (p<0.001) and this decrease was significantly different from the effects of saline infusion (p=0.032 by repeated measures ANOVA) (Fig. 2). FMD decreased at 4 h by 2.4%±2.1% (p=0.002) after the addition of dextrose to Intralipid. FMD changes over time were different among saline, dextrose, and combined Intralipid and dextrose groups (p=0.037 by repeated measures ANOVA).
Fig. 2.
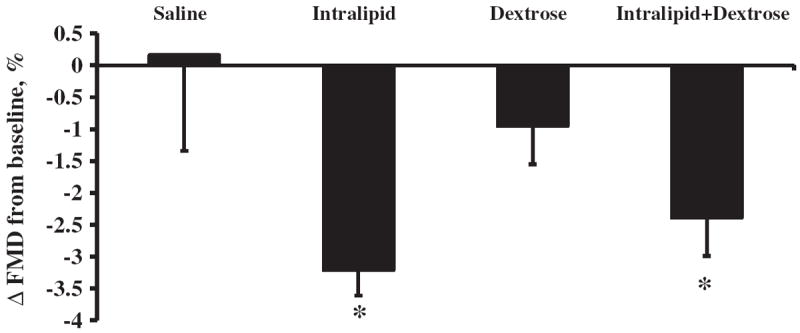
Changes in flow-mediated dilatation (FMD) at 4 h after intravenous infusion of Saline, Intralipid, Dextrose or Intralipid+Dextrose in obese subjects. Baseline FMD was on average 9.4%. Values are mean±SEM. †p<0.05; *p<0.01 compared to baseline.
3.2. Plasma FFA and triglycerides concentrations
Both saline and dextrose infusion had no effect on levels of FFA (Fig. 2A). The administration of Intralipid almost doubled FFA concentration (p<0.01). Mean FFA concentration was increased by 0.84 mmol/L (p=0.004) and 1.03 mmol/L (p=0.009) after 4 h and 8 h of Intralipid infusion, respectively (Fig. 2A). In contrast to Intralipid alone, we observed no increase in FFA levels from baseline during combined infusion of Intralipid and dextrose at 4 h (p=0.46) and 8 h (p=0.30) (Fig. 2A). Comparison of FFA level changes among saline, dextrose, and Intralipid and dextrose groups demonstrated similar trends over time (p=0.91, by repeated measures ANOVA).
Intralipid infusion raised triglycerides from baseline by 119 mg/dL (p<0.001) after 4 h and 121 mg/dL(p=0.001) after 8 h of the infusion (Fig. 2B). The co-administration of Intralipid and dextrose increased triglycerides levels by 40 mg/dL at 4 h and by 47 mg/dL at 8 h (both, p<0.01) (Fig. 2B). The increase in triglyceride level after Intralipid alone was significantly higher than the co-administration of Intralipid and dextrose infusion (p=0.016).
3.3. Plasma glucose, insulin, and C-peptide concentrations
Changes in plasma glucose, insulin, and C-peptide levels during saline, dextrose, and Intralipid alone and with dextrose infusions are shown in Table 2. Concentration of plasma glucose did not significantly change during saline and Intralipid infusion. Dextrose in combination with Intralipid resulted in sustained increase in plasma glucose levels from baseline with a peak increase of 25±9 mg/dL (p=0.034) at 4 h and 30±26 mg/dL (p=0.008) after 4 h of Intralipid plus dextrose infusion. Compared to baseline, there were no significant changes in insulin and C-peptide levels during saline or Intralipid infusion (Table 2). In contrast, dextrose alone and Intralipid plus dextrose infusions resulted in a 3 to 4-fold elevation in insulin levels (p<0.05) and a 2 to 3-fold increase in C-peptide from the baseline (p<0.05). Changes in insulin and C-peptide levels from baseline were similar between dextrose alone and Intralipid plus dextrose infusion at both 4 h and 8 h (p=NS).
Table 2.
The effects of Intralipid and dextrose administration alone or in combination on glucose, insulin, C-peptide.
| Glucose (mg/dL) | Insulin (μU/mL) | C-peptide (ng/mL) | C-peptide/Glucose | ||
|---|---|---|---|---|---|
| Saline | 0 h | 86.0±9.6 | 8.4±4.9 | 2.21±1.04 | 2.54±1.03 |
| 4 h | 87.0±20.7 | 11.3±6.4 | 2.06±0.96 | 2.34±1.00 | |
| 8 h | 88.0±15.0 | 10.1±6.6 | 2.71±1.36 | 3.05±1.33 | |
| Intralipid | 0 h | 78.4±8.0 | 9.1±5.5 | 2.14±1.12 | 2.72±1.63 |
| 4 h | 75.6±9.4 | 11.3±7.7† | 2.39±1.16 | 3.03±1.01 | |
| 8 h | 73.7±10.6 | 9.3±7.5 | 2.40±1.23 | 2.95±1.45 | |
| Dextrose | 0 h | 77.4±12.4 | 8.4±9.6 | 1.96±1.58 | 2.47±1.63 |
| 4 h | 100.4±19.5* | 25.1±10.2* | 4.73±1.61† | 4.61±1.01† | |
| 8 h | 93.0±13.9 | 33.4±21.0* | 5.79±2.08† | 6.28±2.14† | |
| Intralipid+Dextrose | 0 h | 71.6±12.3 | 10.4±9.6 | 2.23±1.53 | 3.11±2.21 |
| 4 h | 101.2±16.6† | 28.0±12.5† | 4.97±1.74† | 4.93±1.74† | |
| 8 h | 97.8±11.1† | 40.5±22.8† | 6.43±2.11† | 6.58±2.38† | |
Values are Mean±SD;
=p<0.05 vs 0 h,
=p<0.01 vs 0 h without multiple comparison adjustments.
4. Discussion
This study aimed to compare the acute metabolic and vascular effects of high FFA during Intralipid infusion alone vs the co-administration of lipid and dextrose in obese healthy individuals. We observed that the infusion of Intralipid alone increased FFA from baseline and resulted in a rapid and sustained elevation in blood pressure and significant reduction in endothelial function. In contrast, the co-administration of Intralipid and dextrose resulted in impaired endothelial function but did not change FFA or blood pressure levels from baseline. The infusion of dextrose alone or in combination with Intralipid resulted in significantly higher glucose and insulin concentrations compared to Intralipid alone. Our results suggest that changes in FFA and insulin concentrations may explain the observed differences in blood pressure and endothelial function during lipid and dextrose infusions in obese subjects.
Several studies have shown that the infusion of Intralipid is associated with impaired endothelial function and in significant elevation in blood pressure from baseline [1,3-5,8]. Similarly, several studies have also demonstrated that dextrose infusion [18,20-22] results in increased blood pressure and impaired endothelial function; however; the deleterious vascular effects seen with dextrose infusion are related to the rise in blood glucose concentration [29-31]. In agreement with our study, previous studies in which plasma blood glucose increased less than 10 mmol/L during dextrose infusion resulted in no significant changes in endothelial function or blood pressure [29,30,32]. In contrast, in studies where blood glucose levels rose more than 15 mmol/L, dextrose infusion resulted in a significant impairment in endothelial function [18,20-22,33]. No prior studies have determined how the combined infusion of Intralipid and dextrose affects vascular function. Our results indicate that changes in circulating FFA and insulin levels may mediate the blood pressure changes and endothelial dysfunction following dextrose and lipid load in obese subjects.
Hyperglycemia acutely increases insulin production in non-diabetic humans. In our study, the combined lipid plus dextrose infusion resulted in a similar insulin concentration increment as observed during dextrose infusion (Table 2). These findings have been registered along with consistent elevation of plasma glucose by 20–30 mg/dL during dextrose and Intralipid plus dextrose infusion. It is unlikely that the hyperinsulinemia observed in our experiments was due to delayed hormone degradation as previously demonstrated in African Americans [34] because changes in the C-peptide and C-peptide/glucose ratios followed similar trends (Table 2). Previous studies reported that in long-term Intralipid infusion (24–72 h), high FFA levels inhibit insulin production in non-diabetic individuals [35-37]. In contrast, several authors have reported unchanged rates of basal and glucose-induced insulin secretion during acute Intralipid infusion despite elevation of FFA in healthy individuals [38,39]. Our short-term studies also suggest no deleterious effects of high FFA on β-cell function. This adaptation may occur due to effective peripheral utilization of triglycerides and FFAs mediated by dextrose-induced hyperinsulinemia as shown in our experiments (Fig. 3) and previous studies [40] in which mild hyperinsulinemia abolished Intralipid-induced elevation of FFA in healthy individuals. Future studies utilizing clamping of insulin production should further characterize the role of hyperinsulinemia in vascular responses produced by combined Intralipid and dextrose infusion.
Fig. 3.
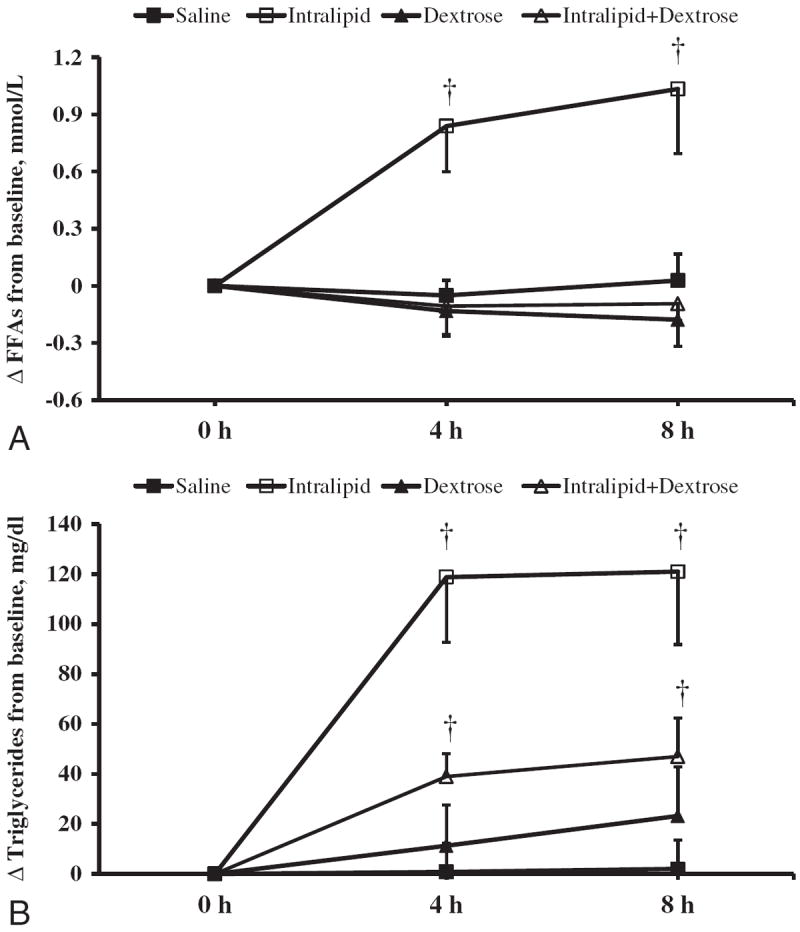
Changes in plasma free fatty acids (A) and triglycerides (B) concentrations during 8-h intravenous infusion of Saline, Intralipid, Dextrose or Intralipid +Dextrose in obese subjects. Values are mean±SEM. †p<0.05; *p<0.01 compared to baseline.
Obesity, insulin resistance, and hyperinsulinemia lead to the development of hypertension and cardiovascular disease [41,42]. In the states of preserved insulin sensitivity as in our subjects, insulin is a potent vasodilator acting via stimulation of nitric oxide (NO) release by endothelial cells in resistant blood vessels and microvasculature [14,17,29,43-47]. Insulin activation of phosphatidylinositol 3-kinase (PI 3-kinase)/Akt pathway results in eNOS phosphorylation, NO synthesis and vasorelaxation [48-50]. The vasodilatory effects of insulin are endothelium-dependent because circulating insulin potentiates acetylcholine-induced but not sodium nitroprusside-induced vasorelaxation [43,51]. On the other hand, increased FFA achieved by either lipid infusion, high fat diet, or direct in vitro incubation has been demonstrated to diminish endothelium-dependent vasodilation by downregulation of PI 3-kinase, eNOS phosphorylation, and NO production [2,52-55]. In our studies, physiological hyperinsulinemia ranging between 30–40 μU/mL over the length of dextrose and Intralipid infusion was sufficient to reduce Intralipid-induced FFA elevation, which, we believe, can be responsible for the attenuation of deleterious vascular effects of the lipid infusion. Furthermore, several studies performed either in vitro [56] or in non-diabetic individuals [57] suggest that mild transient hyperglycemia can even potentiate insulin-induced vasodilation. In addition to the direct effect of insulin on eNOS activation, Cadrillo et al postulated that systemic hyperinsulinemia may activate additional mechanisms leading to vasodilation [58] and our studies suggest that insulin-mediated suppression of FFA levels maybe one of those mechanisms.
This study has several strengths. We used a control group that received normal saline infusion. Also, the rate of the infusion of Intralipid with or without dextrose is similar to the rates that are prescribed for parenteral nutrition in the clinical setting. We recruited obese, healthy individuals without other comorbid conditions such that we eliminated many confounding factors that could affect the interpretation of our results. This study, however, has several limitations. We studied African American subjects and our findings cannot be applied to other ethnic groups. To further generalize the results of this study, a larger sample size and inclusion of subjects from different ethnicities would be required. Recently, Chow et al elegantly demonstrated that Black non-diabetic subjects have higher levels of glucose load-induced insulin release and much more effective mechanisms in FFA utilization compared with White subjects [59]. Subjects in our studies were obese, insulin-sensitive and without family history of diabetes; therefore, the vascular outcomes of combined Intralipid and dextrose infusion found in our study cannot be applied to patients with diabetes mellitus or insulin resistance. There is evidence that obese, insulin-resistant (in terms of glucose regulation) individuals may still have intact endothelial function [60]. However, the physiologic consequences of having underlying elevations in both insulin and free fatty acids as it occurs in insulin resistant state are not elucidated by this study.
In summary, our studies demonstrate that combined Intralipid and dextrose infusion impairs endothelial function. The co-administration of dextrose abolishes Intralipid-induced blood pressure increases in obese subjects. Our findings indicate that elevations of FFA levels observed during Intralipid infusion may mediate some of the observed vascular effects of the lipid load in obese subjects. The results of this study may have important clinical implications for the patients receiving nutrition support. We have demonstrated that the combined infusion of Intralipid and dextrose can be relatively safe for the patients. Endothelial dysfunction, however, induced by Intralipid independent of dextrose loading necessitates the use of lipid infusions characterized by less inflammatory potential.
Acknowledgments
We appreciate the support of the nursing and technical staff of the Grady Memorial Hospital General Clinical Research Center.
Funding
This study was supported by research grants from the American Diabetes Association (7-03-CR-35) and National Institutes of Health: MO1-RR00039.
Abbreviations
- BP
blood pressure
- FFA
free fatty acids
- FMD
flow-mediated dilatation
- PUFA
ω-6 polyunsaturated fatty acids
- eNOS
endothelial nitric oxide synthase
Footnotes
Author contributions
Aidar R. Gosmanov - conduct of the study, data collection and analysis, data interpretation, and manuscript writing; Dawn D. Smiley - conduct of the study, data collection and analysis, data interpretation; Limin Peng - data collection and analysis, data interpretation; Joselita Siquiera - conduct of the study, data collection; Gonzalo Robalino - conduct of the study, data collection; Christopher Newton - conduct of the study; Guillermo E. Umpierrez - design and conduct of the study, data collection and analysis, data interpretation, and manuscript writing.
Conflict of interest
Authors have nothing to disclose.
References
- 1.Gosmanov AR, Smiley DD, Robalino G, et al. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am J Physiol Endocrinol Metab. 2010;299:E953–8. doi: 10.1152/ajpendo.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg HO, Paradisi G, Hook G, et al. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–8. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stojiljkovic MP, Zhang D, Lopes HF, et al. Hemodynamic effects of lipids in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1674–9. doi: 10.1152/ajpregu.2001.280.6.R1674. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Robalino G, et al. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African–Americans with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:609–14. doi: 10.1210/jc.2008-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grekin RJ, Dumont CJ, Vollmer AP, et al. Mechanisms in the pressor effects of hepatic portal venous fatty acid infusion. Am J Physiol. 1997;273:R324–30. doi: 10.1152/ajpregu.1997.273.1.R324. [DOI] [PubMed] [Google Scholar]
- 7.Egan BM, Hennes MM, Stepniakowski KT, et al. Obesity hypertension is related more to insulin’s fatty acid than glucose action. Hypertension. 1996;27:723–8. doi: 10.1161/01.hyp.27.3.723. [DOI] [PubMed] [Google Scholar]
- 8.Florian JP, Pawelczyk JA. Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin Sci (Lond) 2010;118:61–9. doi: 10.1042/CS20090063. [DOI] [PubMed] [Google Scholar]
- 9.Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN JPEM JPEM J Parenter Enteral Nutr. 2006;30:351–67. doi: 10.1177/0148607106030004351. [DOI] [PubMed] [Google Scholar]
- 10.Brehm A, Krssak M, Schmid AI, et al. Acute elevation of plasma lipids does not affect ATP synthesis in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299:E33–8. doi: 10.1152/ajpendo.00756.2009. [DOI] [PubMed] [Google Scholar]
- 11.Itani SI, Ruderman NB, Schmieder F, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 12.Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol Sci. 2004;19:92–6. doi: 10.1152/nips.01459.2003. [DOI] [PubMed] [Google Scholar]
- 13.Boden G, Chen X, Ruiz J, et al. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–46. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Liu J, Jahn LA, et al. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–9. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong PJ, Dean TS, Hayward CS, et al. Effect of fat and carbohydrate consumption on endothelial function. Lancet. 1999;2134:354. doi: 10.1016/s0140-6736(99)03374-7. [DOI] [PubMed] [Google Scholar]
- 16.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 17.de Jongh RT, Serne EH, Ijzerman RG, et al. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–82. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 18.Mullan BA, Ennis CN, Fee HJ, et al. Protective effects of ascorbic acid on arterial hemodynamics during acute hyperglycemia. Am J Physiol Heart Circ Physiol. 2004;287:H1262–8. doi: 10.1152/ajpheart.00153.2003. [DOI] [PubMed] [Google Scholar]
- 19.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 20.Ceriello A, Esposito K, Piconi L, et al. Glucose “peak” and glucose “spike”: impact on endothelial function and oxidative stress. Diabetes Res Clin Pract. 2008;82:262–7. doi: 10.1016/j.diabres.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano D, Marfella R, Coppola L, et al. Vascular effects of acute hyperglycemia in humans are reversed by l-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–90. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 23.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacca A, Xiao C, Oprescu AI, et al. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–62. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- 25.Poitout V. Glucolipotoxicity of the pancreatic beta-cell: myth or reality? Biochem Soc Trans. 2008;36:901–4. doi: 10.1042/BST0360901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poitout V, Amyot J, Semache M, et al. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–98. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 29.Natali A, Baldi S, Vittone F, et al. Effects of glucose tolerance on the changes provoked by glucose ingestion in microvascular function. Diabetologia. 2008;51:862–71. doi: 10.1007/s00125-008-0971-6. [DOI] [PubMed] [Google Scholar]
- 30.Siafarikas A, Watts K, Beye P, et al. Lack of effect of oral glucose loading on conduit vessel endothelial function in healthy subjects. Clin Sci (Lond) 2004;107:191–6. doi: 10.1042/CS20040004. [DOI] [PubMed] [Google Scholar]
- 31.Reed AS, Charkoudian N, Vella A, et al. Forearm vascular control during acute hyperglycemia in healthy humans. Am J Physiol Endocrinol Metab. 2004;286:E472–80. doi: 10.1152/ajpendo.00348.2003. [DOI] [PubMed] [Google Scholar]
- 32.Houben AJ, Schaper NC, de Haan CH, et al. Local 24-h hyperglycemia does not affect endothelium-dependent or -independent vasoreactivity in humans. Am J Physiol. 1996;270:H2014–20. doi: 10.1152/ajpheart.1996.270.6.H2014. [DOI] [PubMed] [Google Scholar]
- 33.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643–8. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11:755–62. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 35.Carpentier A, Mittelman SD, Bergman RN, et al. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes. 2000;49:399–408. doi: 10.2337/diabetes.49.3.399. [DOI] [PubMed] [Google Scholar]
- 36.Hagman DK, Latour MG, Chakrabarti SK, et al. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes. 2008;57:424–31. doi: 10.2337/db07-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung N, Sakaue T, Carpentier A, et al. Prolonged increase of plasma non-esterified fatty acids fully abolishes the stimulatory effect of 24 hours of moderate hyperglycaemia on insulin sensitivity and pancreatic beta-cell function in obese men. Diabetologia. 2004;47:204–13. doi: 10.1007/s00125-003-1301-7. [DOI] [PubMed] [Google Scholar]
- 38.Amery CM, Round RA, Smith JM, et al. Elevation of plasma fatty acids by ten-hour intralipid infusion has no effect on basal or glucose-stimulated insulin secretion in normal man. Metabolism. 2000;49:450–4. doi: 10.1016/s0026-0495(00)80007-4. [DOI] [PubMed] [Google Scholar]
- 39.Storgaard H, Jensen CB, Vaag AA, et al. Insulin secretion after short- and long-term low-grade free fatty acid infusion in men with increased risk of developing type 2 diabetes. Metabolism. 2003;52:885–94. doi: 10.1016/s0026-0495(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 40.Aberg V, Thorne A, Alvestrand A, et al. Combined hypertriglyceridemic and insulin-glucose clamps for the characterization of substrate oxidation and plasma elimination of a long-chain triglyceride emulsion in healthy men. Metabolism. 2012;61:221–8. doi: 10.1016/j.metabol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–22. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowers JR, Frohlich ED. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med Clin North Am. 2004;88:63–82. doi: 10.1016/s0025-7125(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 43.Taddei S, Virdis A, Mattei P, et al. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation. 1995;92:2911–8. doi: 10.1161/01.cir.92.10.2911. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–86. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–45. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 46.Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 47.Cardillo C, Nambi SS, Kilcoyne CM, et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–5. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- 48.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 49.Muniyappa R, Montagnani M, Koh KK, et al. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 50.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–8. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611–8. doi: 10.2337/diabetes.50.11.2611. [DOI] [PubMed] [Google Scholar]
- 52.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–94. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 53.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–7. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 54.Wang XL, Zhang L, Youker K, et al. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–10. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 55.Symons JD, McMillin SL, Riehle C, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–94. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taubert D, Rosenkranz A, Berkels R, et al. Acute effects of glucose and insulin on vascular endothelium. Diabetologia. 2004;47:2059–71. doi: 10.1007/s00125-004-1586-1. [DOI] [PubMed] [Google Scholar]
- 57.Major-Pedersen A, Ihlemann N, Hermann TS, et al. Effects of oral glucose load on endothelial function and on insulin and glucose fluctuations in healthy individuals. Exp Diabetes Res. 2008;2008:672021. doi: 10.1155/2008/672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardillo C, Kilcoyne CM, Nambi SS, et al. Vasodilator response to systemic but not to local hyperinsulinemia in the human forearm. Hypertension. 1998;32:740–5. doi: 10.1161/01.hyp.32.4.740. [DOI] [PubMed] [Google Scholar]
- 59.Chow CC, Periwal V, Csako G, et al. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African–American women. J Clin Endocrinol Metab. 2011;96:2456–63. doi: 10.1210/jc.2011-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckman JA, Goldfine AB, Dunaif A, et al. Endothelial function varies according to insulin resistance disease type. Diabetes Care. 2007;30:1226–32. doi: 10.2337/dc06-2142. [DOI] [PubMed] [Google Scholar]


