Abstract
Cyclooxygenase-2 (COX-2) is associated with aggressive breast cancers. The COX-2 product prostaglandin E2 (PGE2) acts through four G-protein-coupled receptors designated EP1–4. Malignant and immortalized normal mammary epithelial cell lines express all four EP. The EP4 antagonist AH23848 reduced the ability of tumor cells to colonize the lungs or to spontaneously metastasize from the mammary gland. EP4 gene silencing by shRNA also reduced the ability of mammary tumor cells to metastasize. Metastasis inhibition was lost in mice lacking either functional Natural Killer (NK) cells or interferon-γ. EP4 antagonism inhibited MHC class I expression resulting in enhanced ability of NK cells to lyse mammary tumor target cells. These studies support the hypothesis that EP4 receptor antagonists reduce metastatic potential by facilitating NK-mediated tumor cell killing and that therapeutic targeting of EP4 may be an alternative approach to the use of COX inhibitors to limit metastatic disease.
Keywords: Metastasis, COX-2, Prostaglandin EP receptors, Natural Killer cells
Introduction
The cyclooxygenase-2 (COX-2) enzyme is frequently over expressed in solid cancers and is associated with a poor prognosis in breast, as well as other malignancies [1, 2]. Epidemiological studies demonstrate that chronic use of COX inhibitors is associated with lower risk of developing breast and other malignancies [3, 4]. In tumors, the major COX-2 product is PGE2. Although PGE2 synthesis clearly contributes to malignant behavior, the mechanisms are complex and include direct effects on tumor cell apoptosis and proliferation [5] as well as indirect effects on tumor angiogenesis [6, 7] and immune effector functions [8–12].
While preclinical studies indicate that COX-2 is a promising therapeutic target in breast and other malignancies, recent concerns regarding the safety of selective COX inhibitors [13] prompted us to seek alternative means to target this pathway. Activities of PGE2 are mediated by a family of G-protein-coupled receptors that are linked to diverse intracellular signaling pathways [14]. We recently showed that EP2, EP3 and EP4 are highly expressed on murine mammary tumors and that pharmacologic antagonism of EP4, but not EP3, inhibits experimental metastasis [15]. Since no pharmacologic antagonist is absolutely specific, we determined the effect of inhibiting EP4 gene expression on mammary tumor behavior in the current study. These data confirm that EP4, expressed on malignant epithelial cells, contributes to metastatic potential. Endogenous Natural Killer cell activity exerts partial control of metastasis and this protective effect is enhanced by COX-2 inhibitors [16]. We have now investigated the role of NK cells in the mechanism by which receptor antagonism inhibits tumor metastasis. We now show that EP4 antagonist-mediated inhibition of metastasis depends on NK cells and that decreased tumor cell MHC class I antigen expression in favor of increased NK recognition may contribute to control of metastasis.
Materials and methods
Cell lines, tumors, mice
Murine mammary tumor cell lines 410.4 and 66.1 and immortalized normal mammary epithelial line EpH4, derived from the pregnant mammary gland of a Balb/c mouse were maintained in DMEM supplemented with 10% FCS (Gemini BioProducts, Inc., Calabasas, CA), 2 mM glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, 1.5 g/l sodium bicarbonate and 0.1 mM nonessential amino acids. For experimental metastasis assays, 1 × 105 tumor cells were injected into the lateral tail vein of syngeneic immune competent Balb/cByJ or Balb/cIfngtm1Ts (IFNγ−/−) (Jackson Laboratories, Bar Harbor, ME) or NK-depleted Balb/cByJ female mice and 21 days later, mice were euthanized and surface pulmonary metastases were quantified under a dissecting microscope. To deplete NK cells, mice were injected with rabbit asialoGM1 ganglioside antibody (20 µl, Wako Bioproducts, Richmond, VA) or normal rabbit serum 1 day prior to and 3 days after tumor cell injection. For spontaneous metastasis assays, 3 × 105 tumor cells were injected subcutaneously proximal to the mammary gland and tumor growth monitored by caliper twice weekly. When tumors achieved an average diameter of 18 mm, or earlier if animals appeared moribund, mice were sacrificed and soft tissues examined for surface tumor colonies.
EP inhibitor treatments
The EP4 antagonist AH23848 was purchased from Sigma Chemical Co. (St. Louis, MO). Line 410.4 or 66.1 cells were cultured in the presence of AH23848 (5 µM) or DMSO for 48 h, washed, and 1 × 105 viable tumor cells were injected into the tail vein of untreated mice. No further drug treatments were carried out. Alternatively, mice were treated with AH23848 or vehicle by i.p. route (10 mg/kg) beginning on day −1 and continuing for 15 days.
EP4 gene silencing
Line 66.1 and 410.4 tumor cells were transfected with a plasmid expressing shRNA targeting the murine EP4 gene or control vector (OpenBiosystems, Huntsville, AL). EP4 expression levels were determined by RT-PCR and western blotting of cell lysates. Stable clones expressing reduced levels of EP4 were established and evaluated for tumorigenic and metastatic properties.
RT-PCR
RNA was extracted from cultured cells using TRIAZOL reagent (Invitrogen), reverse transcribed and amplified using EP-specific primers. Initial denaturation at 94°C for 2 min, followed by 36 cycles of 94°C for 30 s, 60°C for 35 s, 72°C for 45 s and a 10 min extension at 72°C.
cAMP assay
Cells were pretreated with indomethacin (1.0 µM) for 24 h and transferred to complete cell culture medium containing IBMX (100 µM, Sigma Chemical Co., St. Louis, MO). The global agonist PGE2 or the selective EP4 agonist, PGE1-0H, or the EP4 antagonists ONO-208 or AH23848 were added to cells and incubated for 15 min, after which media was aspirated and cell lysates prepared and intracellular cAMP levels determined as per manufacturer’s instructions using the cAMP biotrak EIA system (Amersham).
Flow cytometry
For MHC class I characterization, unfixed cells were stained for MHC class I H-2Ld (clone 30-5-7) or H-2Kd (clone SF1-1.1.1) isotype-control antibody, followed by FITC-conjugated goat-anti-mouse IgG (BD Biosciences Pharmingen, San Diego, CA). For EP receptor studies, tumor cells were fixed in 70% cold ethyl alcohol, blocked with 1% FBS, washed and reacted with polyclonal antibody to EP1, EP2, EP3 or EP4 (Cayman Chemicals), followed by FITC-conjugated goat-anti-rabbit IgG (KPL Inc., Gaithersburg, MD) and fixation in 1% paraformaldehyde. Cells were analyzed by FACScan flow cytometer in the Flow Cytometry Facility of the University of Maryland Greenebaum Cancer Center.
NK cytotoxicity assays
Assays were carried out as described in Ref. [16]. Tumor target cells were labeled with [3H] proline, washed and plated in 96-well flat-bottom microtiter plates. Twenty-four hours later, EP receptor antagonist at the indicated concentrations and single cell suspensions of spleen effector cells from normal Balb/cByJ female mice (stimulated with 100 µg of poly-IC i.p. 24 h prior to sacrifice) were added at a 100:1 effector to target ratio. Lytic activity is linear using ratios of 10:1, 50:1 or 100:1. After a further 18 h incubation, nonadherent cells were aspirated, and radioactivity in the remaining adherent cells was determined. Cytotoxicity was determined as:
% Cytotoxicity = (1 − cpm remaining in experimental well / cpm remaining in medium control well) × 100.
Statistical analysis
Descriptive statistics are described as mean and standard error and compared by Student’s t test. Wilcoxon exact two sample test was used to compare the number of metastases in different treatment groups.
Results
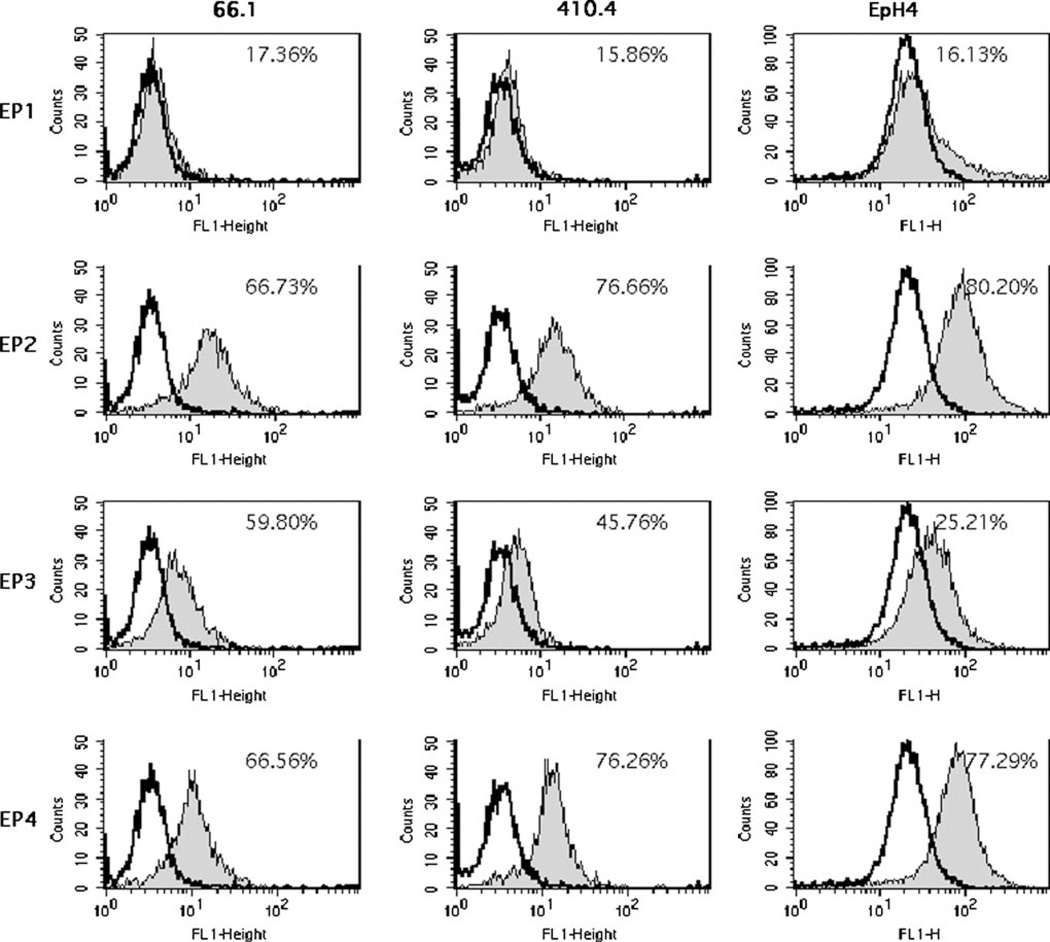
COX-2 expression contributes to tumorigenic and metastatic properties in a murine model of metastatic breast cancer [9, 16–18]. Due to recent concerns regarding the safety of COX-2 inhibitors, we have initiated studies to test the hypothesis that PGE2 directly affects tumor cell behavior in an autocrine manner and that these direct effects are mediated by one or more EP receptor expressed on the tumor cell. Further, that inhibition of EP receptor signaling could, like inhibition of PGE2 synthesis, limit metastasis. Cellular effects of PGE2 are mediated through a family of G-protein-coupled receptors designated EP1, EP2, EP3 and EP4 [14]. We characterized EP receptor expression and function in two murine mammary tumor cell lines (66.1, 410.4) as well as the immortalized murine mammary epithelial cell line EpH4. Both murine breast tumor and mammary epithelial cells express EP1, EP2, EP3 and EP4 (Fig. 1). There is considerably less EP1 detected in comparison to EP2, EP3 and EP4.
Fig. 1.
Flow cytometric analysis of EP staining on two murine mammary tumor cell lines (410.4, 66.1) and immortalized mammary epithelial cells (EpH4). Shaded peak is specific EP staining, open peak is staining with isotype-control antibody
COX inhibitors are highly effective at reducing murine mammary tumor metastasis [9, 16, 18]. Murine mammary tumor cells spontaneously secrete high levels of PGE2. We have hypothesized that production of PGE2 by tumor cells contributes to metastatic ability in an autocrine fashion in which tumor-PGE2 signals through EP receptors on the tumor cells to enhance tumor dissemination. We further hypothesized that blockade of PGE-mediated signaling, downstream of PGE2 synthesis, might have therapeutic effects similar to those observed when PGE2 synthesis is prevented with COX inhibitors. To test this hypothesis, we employed both a pharmacologic antagonist of EP4 as well as a gene-silencing approach to determine the role of EP4 in tumor metastasis.
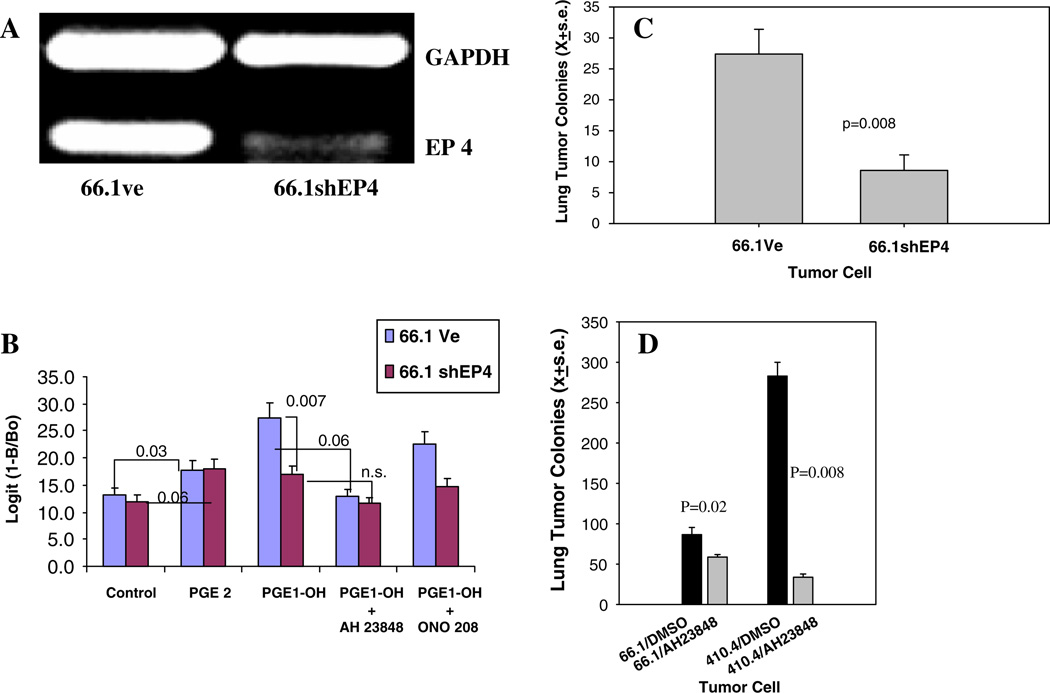
Figure 2a shows the reduced EP4 expression in 66.1 cells transfected with a plasmid expressing shRNA directed to murine EP4. Ligand binding to EP2 and EP4 is coupled to PKA/adenyl cyclase and mediates elevations in intracellular cAMP. The reduction in EP4 expression in 66.1 cells compromised their ability to elevate cAMP in response to the EP4 selective agonist PGE1-OH in comparison to 66.1vector cells (Fig. 2b). The EP4 antagonists AH23848 or ONO208 inhibited the cAMP response to PGE1-OH in 66.1vector cells but had no impact on the cAMP response in 66.1shEP4 cells. When 66.1vector or 66.1shEP4 cells were introduced into Balb/cByJ mice, lung colonizing ability of cells expressing less EP4 was significantly compromised (Fig. 2c, P = 0.008). We derived four additional independent clones expressing reduced levels of EP4 and lung colonization was reduced by 43%, 53%, 53% and 84% when these cells were injected into mice. Likewise, systemic treatment with the EP4 antagonist AH23848 (10 mg/kg) inhibited lung colonization of parental 410.4 or 66.1 cells by 88% and 32%, respectively (Fig. 2d, P = 0.008, P = 0.02, respectively). When tumor cells were transplanted to the mammary gland of mice, EP4 gene silencing did not inhibit local tumor growth (data not shown), however spontaneous metastases were reduced by 77% (P<0.02). Thus, by two independent approaches, we showed that blockade of EP4 reduced the ability of mammary tumor cells to spontaneously metastasize or form tumor cell colonies in the lung.
Fig. 2.
(a) RNA isolated from line 66.1 tumor cells transfected with control plasmid (66.1Ve) or containing shRNA targeting murine EP4 (66.1shEP4) and analyzed for EP4 and GAPDH expression by RT-PCR. (b) Cells treated with PGE2 or the EP4 agonist PGE1-0H and the EP4 antagonists AH23848 or ONO-208 and intracellular cAMP levels determined. (c) 66.1Ve or 66.1shEP4 cells injected into Balb/cByJ mice and lung colonies quantified at day +21. Mean ± SE of 10 mice/group. (d) Balb/cByJ mice treated with AH23848 (i.p., 10 mg/kg/day) or DMSO beginning 1 day before the injection of parental 66.1 or 410.4 tumor cells into Balb/ cByJ mice. Drug treatment continued for 14 additional days
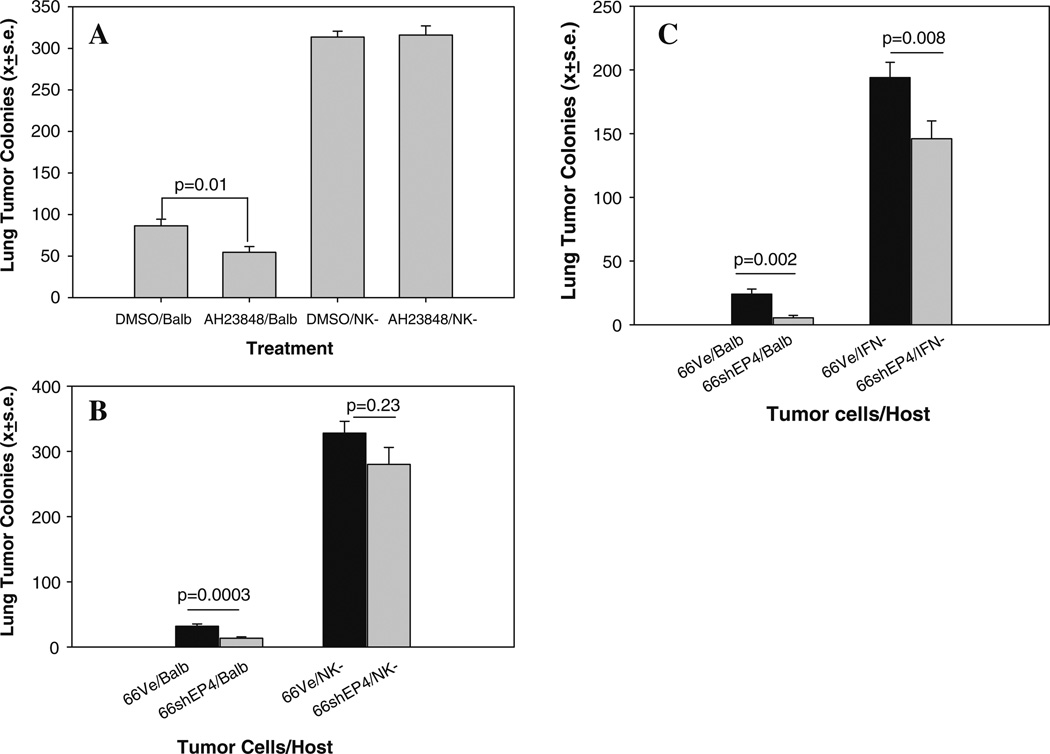
We considered the possibility that EP4 antagonism affected tumor-host cell interactions. Endogenous NK activity exerts some control of metastatic behavior in this model and the ability of indomethacin and celecoxib to further limit metastasis is lost in NK-depleted mice [16]. Therefore, COX inhibitors limit tumor dissemination by an NK-dependent mechanism. We tested the hypothesis that EP4-targeted therapy also depends on NK functions. We compared the ability of AH23848 to control metastatic disease in immune competent Balb/cByJ mice and mice depleted of NK activity. Figure 3a confirms that EP4 pharmacologic antagonism inhibits lung colonization. Injection of vehicle-treated tumor cells into immune competent mice resulted in an average of 86 tumor colonies; AH23848 reduced the mean number of metastases by 37% (P = 0.01). Depletion of NK cells leads to loss of endogenous control of tumor dissemination leading to a fourfold increase in lung metastases and in these mice, AH23848 no longer inhibited metastasis. The reduction of lung metastasis achieved by EP4 silencing (Fig. 3b) was also severely compromised in NK-depleted mice. In this experiment, EP4 silencing reduced lung colonization by 58% in immunologically intact mice (P = 0.0003); in NK-depleted mice, lung colonies were reduced by 16% in mice injected with 66.1shEP4 versus 66.1vector cells, however the difference was not statistically significant. We also compared the metastatic ability of 66.1vector and 66.1shEP4 cells in IFN-γ-deficient mice (Fig. 3c). EP4 gene silencing was much less effective at reducing lung tumors in the absence of IFN-γ expression. Thus, like indomethacin and celecoxib, EP4 antagonists control metastatic disease by immune-dependent mechanisms.
Fig. 3.
(a) Line 66.1 tumor cells were treated with AH23848 (5 µM) or DMSO for 24 h, washed and 1×105 viable cells injected intravenously into Balb/cByJ female mice or Balb/ cByJ mice depleted of NK cells with asialoGM1 antibody. Twenty-one days later, mice were sacrificed and surface lung tumor colonies enumerated. Mean ± SE of lung metastases in 10 mice/group. (b) 1 × 105 66.1vector or 66.1shEP4 cells injected into Balb/cByJ or NK-depleted Balb/cByJ mice. (c) 1 × 105 66.1vector or 66.1shEP4 cells injected into Balb/cByJ or Balb/cIFNγ −/− mice
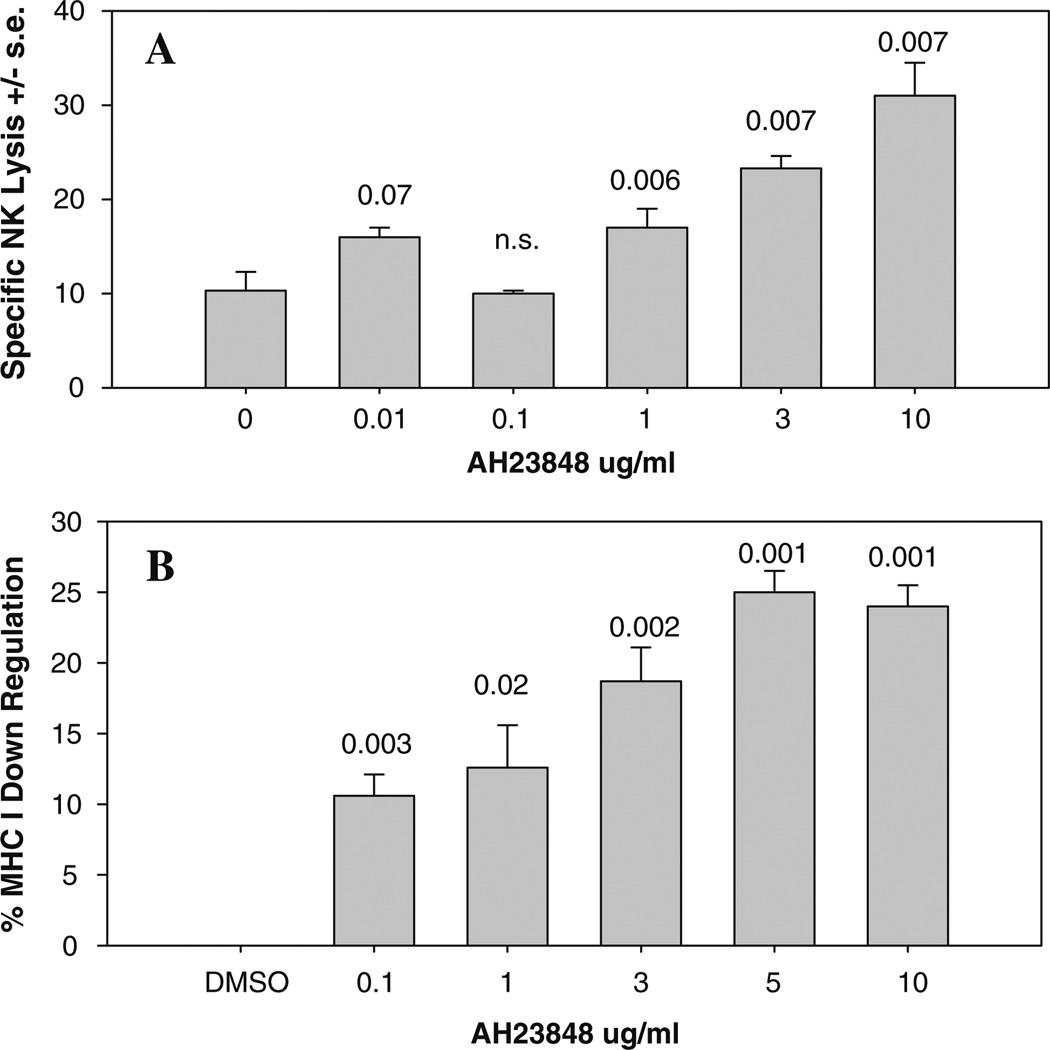
To examine potential mechanisms by which EP4 antagonists and NK cells interact, we determined the effect of EP4 antagonism on NK-mediated lysis of 410.4 tumor cells. Radiolabeled tumor cells and spleen effector cells from normal mice were co-cultured in the presence of AH23848 at concentrations ranging from 0.01 to 10.0 µg/ml and the degree of tumor cell lysis was determined 24 h later. Figure 4a shows that AH23848 enhanced NK-mediated lysis in a concentration-dependent fashion. These results have been replicated in two additional experiments and are consistent with the ability of AH23848 to inhibit tumor metastasis in an NK-dependent manner.
Fig. 4.
(a) Radiolabeled 410.4 tumor cells and spleen cells from normal mice were co-cultured in the presence of DMSO or AH23848 at the indicated concentrations and degree of tumor cell lysis determined 18 h later. (b) Tumor cells were cultured in the presence of AH23848 or DMSO at the indicated concentrations for 48 h after which MHC class I Ld expression was assessed by flow cytometry. Expressed as decrease in antigen levels compared to DMSO-treated cells
The degree of NK-mediated lysis is controlled by many factors including the balance of inhibitory and stimulatory signals delivered by ligands expressed on the potential target cell. Inhibitory signals are mediated by binding of MHC molecules, expressed on the target cell, to cognate inhibitory receptors on the NK effector cell. We asked if an EP4 antagonist would modulate the expression levels of MHC class I. Tumor cells were treated with AH23848 (0.1–10 µg/ml) or DMSO for 24 h after which MHC class I was assayed by flow cytometry. Figure 4b shows one of three representative experiments in which EP4 receptor antagonism downregulated expression of MHC class I in a dose-dependent manner, consistent with the observed increase in NK lytic sensitivity.
Discussion
In tumors, the principal COX-2 product is prostaglandin E2. Cellular effects of PGE2 are mediated through a family of G-protein-coupled receptors designated EP1, EP2, EP3 and EP4 which are coupled to different intracellular signaling pathways [18]. EP expression on diverse cell types has been documented but the precise role of each EP in malignant behavior is not established. EP2 and EP3 may have opposing actions on growth, with EP2 signaling linked to growth stimulation and EP3 linked to either growth inhibition or cellular senescence [19]. Proliferation of some tumor cells is directly supported by EP4 activation [20, 21]. A role for EP1, EP2 and EP4 receptors has been identified in primary colon carcinogenesis. Mice deficient in EP4, but not EP2, are protected in the Min model of colon carcinogenesis [22]. In separate studies, EP1 or EP2 have been implicated in polyp formation in the Apcdelta716 model [23–25]. Similar complexity is apparent in models of UVB or chemically-induced skin carcinogenesis where a role for EP1, EP2 or EP4 has been described [26–29].
Little clinical information regarding EP receptors is available, however, a recent study reports that coexpression of Cox-2 and EP4 is associated with poor prognosis in upper urinary tract tumors [30]. In a small series, EP4 was detected in all seven colon cancer specimens examined and in 8/22 adenomas whereas normal colon was weakly positive or negative for EP4 [31]. In striking contrast, another laboratory reported very little EP4 in colon carcinomas in comparison to normal colon mucosa [32]. In that study, EP1 and EP2 were highly expressed in malignant tissues and EP2 levels were correlated with poor survival. These data indicate that one or more EP receptor is likely to play an important role in the behavior of colon cancer although the precise role for individual EP remains to be clarified.
The role that EP receptors play in behavior of breast cancers is beginning to emerge. Some years ago, we described heterogeneous PGE2 binding activity on mammary tumor cells but, at the time, it was not known that there are a family of receptors that bind PGE2 [33, 34]. More recently, we showed that EP receptors mediate chemotaxis in response to PGE2 and that EP2 and EP4 are linked to adenylate cyclase activation [15]. Other labs have shown that PGE2 positively regulates aromatase, which catalyzes estrogen biosynthesis, through EP1 and EP2 expressed on stromal cells [35] or through EP2 and EP4 [36]. In contrast, EP3 receptor activation inhibits aromatase expression [35]. Murine mammary tumors arising in COX-2 transgenic mice have increased expression of EP1, EP2 and EP4, but decreased expression of EP3 in comparison to normal mammary gland [37]. EP1 antagonism prevents chemically-induced breast carcinogenesis in a mouse model [38], however, in advanced human breast cancer, EP1 is associated with lack of lymph node involvement indicating a better prognosis [39]. Several groups have shown that EP receptors mediate migration of tumor cells in vitro [15, 40, 41]. We recently reported that selective EP4 pharmacologic antagonists inhibit tumor metastasis in vivo and that the therapeutic potential is comparable to that observed with a dual COX-1/COX-2 inhibitor [15]. Thus, EP receptors clearly contribute to both early and late breast cancer progression.
The Carbone Laboratory has confirmed a role for EP4 in cancer metastasis by demonstrating that pharmacologic antagonism or gene silencing of EP4 inhibits metastasis in murine models of lung and colon cancer [42]. One possible mechanism by which EP contribute to metastatic potential was provided by recent data showing that PGE2, acting through EP2 and EP4, induces expression of the chemokine receptor CCR7 on MCF-7 breast cancer cells [43]. CCR7 potentially mediates trafficking of tumor cells to sites of CCR7 ligand (CCL21) expression. The tumor cells used in the current study have a constitutively active COX-2 that produces high levels of PGE2 and metastatic potential is positively correlated with higher PGE2 [9, 17]. These studies suggest that EP receptors, expressed on tumor cells, mediate metastasis directly by responding to autocrine or paracrine production of PGE2. Antagonism of EP4 on 3LL cells leads to reduced tumor cell adhesion, motility, invasion, colony formation and AKT phosphorylation [41].
In addition to EP on tumor cells, many host cells including lymphocytes, dendritic cells, myeloid-derived suppressor cells and NK cells express one or more EP, thus, there are many potential targets of PGE2 action in the tumor-bearing host [12, 44–47]. Experimental metastasis of 3LL cells is reduced in EP4−/− mice although the critical EP4-positive host cell was not identified [42]. Growth of either MC26 or Lewis Lung carcinoma cells was inhibited in EP2 mutant mice and this was attributed to protection of dendritic cells from PGE2-mediated suppression [47]. Likewise, growth of mammary tumors is delayed in EP2 knockout mice and is correlated with decreased numbers of myeloid-derived suppressor cells [12].
We considered the possibility that EP receptors might mediate interactions between tumor cells and other potential immune effector cells. Wehave shown that the ability of Cox inhibitors to control tumor metastasis in this model is highly dependent on functioning NK cells [9, 16]. We also showed that COX inhibitors increase NK lytic activity. The current study shows that, like Cox inhibitors, EP4 antagonists are dependent on NK cells to inhibit metastasis. Furthermore, EP4 antagonists enhance NK lytic activity and at the same time decrease inhibitory MHC class I antigen expression. These studies suggest a novel mechanism by which antagonism of the EP4 receptor on tumor cells enhances effective NK cytolytic activity leading to better control of metastasis. Suppressive effects of PGE2 on NK function have been reported [10], and studies are in progress to determine which EP receptors participate in regulation of NK function.
Angiogenesis is also regulated directly or indirectly by EP receptor functions although different EP have been implicated in each model. Tumor-induced angiogenesis in the Apcdelta716 model of colon carcinogenesis is mediated by EP2 [48], whereas EP3 regulates angiogenesis in a murine sarcoma model [49]. EP4 mediates migration of endothelial cells through an ERK pathway [50]. Using a selective EP4 antagonist, it was shown that PGE2 protects tumor cells from apoptosis induced by camptothecin [51]. EP4 protects cells from chemotherapy-induced apoptosis through EP4 linked to a PI3K/AKT pathway. Resistance to spontaneous apoptosis was also attributed to EP4; in this case through a cAMP-dependent mechanism [52].
A substantial body of data now exists indicating that COX-2 expression in malignancies contributes to aggressive behavior, particularly the occurrence of disseminated disease. In an attempt to avoid potential toxicities of conventional COX-2 inhibitors and to increase specificity and efficacy, we are investigating the possibility of downstream targeting of EP receptors that are expressed on many malignant cells. The results of several laboratories indicate that targeting of EP4 may prevent metastatic disease and the current study supports a mechanism involving activation of NK cells. These findings support the further investigation of specific antagonists of selective EP receptors to prevent disease progression.
Acknowledgments
This work was supported by the United States Department of Defense and the Department of Health and Human Services. We thank the Biostatistics and Flow Cytometry Core Facilities of the University of Maryland Greenebaum Cancer Center.
Contributor Information
Namita Kundu, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Xinrong Ma, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Dawn Holt, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Olga Goloubeva, Marlene and Stewart Greenebaum Cancer Center, University of Maryland School of Medicine, 655 W. Baltimore St., Baltimore, MD 21201, USA.
Suzanne Ostrand-Rosenberg, Department of Biological Sciences, University of Maryland Baltimore County, Baltimore, MD 21250, USA.
Amy M. Fulton, Email: afulton@umaryland.edu, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD 21201, USA; Marlene and Stewart Greenebaum Cancer Center, University of Maryland School of Medicine, 655 W. Baltimore St., Baltimore, MD 21201, USA.
References
- 1.Bennett A, Charlier EM, McDonald AM, Simpson JS, Stanford IF. Prostaglandin and breast cancer. Lancet. 1977;2:624–626. doi: 10.1016/s0140-6736(77)92496-5. [DOI] [PubMed] [Google Scholar]
- 2.Ristimaki A, Sivula A, Lundin M, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 3.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW., Jr. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 4.Harris RE, Beebe-Donk J, Alshafie GA. Reduction of the risk of human breast cancer by selective cyclooxygenase-2 [COX-2] inhibitors. BMC Cancer. 2006;6:27–31. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prosta-glandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 6.Tsujii M, Kawano S, Tsujii S, Sawaoka H, Hori H, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 7.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner M, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 8.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 9.Fulton AM, Heppner GH. Relationships of prostaglandin E and Natural Killer sensitivity to metastatic potential in murine mammary adenocarcinomas. Cancer Res. 1985;45:4779–4784. [PubMed] [Google Scholar]
- 10.Lala PK, Parhar RS, Singh P. Indomethacin therapy abrogates the prostaglandin-mediated suppression of Natural Killer activity in tumor-bearing mice and prevents tumor metastasis. Cell Immunol. 1986;99:108–118. doi: 10.1016/0008-8749(86)90220-0. [DOI] [PubMed] [Google Scholar]
- 11.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase-2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Wang M, Cheng Y, Fitzgerald GA. Cardiovascular hazard and nonsteroidal anti-inflammatory drugs. Curr Opin Pharmacol. 2005;2:204–210. doi: 10.1016/j.coph.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures properties and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 16.Kundu N, Walser TC, Ma X, Fulton AM. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother. 2005;54:981–987. doi: 10.1007/s00262-005-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundu N, Yang Q, Dorsey R, Fulton AM. Increased cyclooxygenase-2 [COX-2] expression and activity in a murine model of metastatic breast cancer. Int J Cancer. 2001;93:681–686. doi: 10.1002/ijc.1397. [DOI] [PubMed] [Google Scholar]
- 18.Kundu N, Fulton AM. Selective cyclooxygenase [COX]-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 19.Watanabe T, Satoh H, Togoh M, Taniguchi S, Hashimoto Y, Kurokawa K. Positive and negative regulation of cell proliferation through prostaglandin receptors in NIH-3T3 cells. J Cell Physiol. 1996;69:401–409. doi: 10.1002/(SICI)1097-4652(199611)169:2<401::AID-JCP20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Cherukuri DP, Chen XBO, Goulet A-C, Young RN, Han Y, Heimark R, et al. The EP4 receptor antagonist, L-161982, blocks prostaglandin E2-induced signal transduction and cell proliferation in HCA-7 colon cancer cells. Exp Cell Res. 2007;313:2969–2979. doi: 10.1016/j.yexcr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambe A, Iguchi G, Moon Y, Kamitani H, Watanabe T, Eling TE. Regulation of EP4 expression via the Sp-1 transcription factor: inhibition of expression by anti-cancer agents. Biochim Biophys Acta. 2008;1783:1211–1219. doi: 10.1016/j.bbamcr.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutoh MK, Watanabe T, Kitamura Y, et al. Involvement of prostaglandin E receptor subtype EP4 in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- 23.Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc[Delta 716] knockout mice. Nat Med. 2001;9:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Kawamori T, Nakatsugi S, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- 25.Kitamura T, Itoh M, Noda T, et al. Combined effects of prostaglandin E receptor subtype EP1 and subtype EP4 antagonists on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. Cancer Sci. 2003;94:618–621. doi: 10.1111/j.1349-7006.2003.tb01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP1 receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126:205–211. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- 27.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 28.Konger RL, Scott GA, Landt Y, Ladenson JH, Pentland AP. Loss of EP2 prostaglandin E2 receptor in immortalized human keratinocytes results in increased invasiveness and decreased paxillin expression. Am J Pathol. 2002;26:2065–2078. doi: 10.1016/S0002-9440(10)64485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chun K-S, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyata Y, Kanda S, Nomata K, Eguchi J, Kanetake H. Expression of cyclooxygenase-2 and EP4 receptor in transitional cell carcinoma of the upper urinary tract. J Urol. 2005;173:56–60. doi: 10.1097/01.ju.0000148272.77539.2d. [DOI] [PubMed] [Google Scholar]
- 31.Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M, Wang W, et al. EP1-4 subtype, COX and PPARc receptor expression in colorectal cancer in prediction of disease-specific mortality. Int J Cancer. 2007;121:232. doi: 10.1002/ijc.22582. [DOI] [PubMed] [Google Scholar]
- 33.Fulton AM, Laterra JJ, Hanchin CM. Prostaglandin E2 receptor heterogeneity and dysfunction in mammary tumor cells. J Cell Physiol. 1989;139:93–99. doi: 10.1002/jcp.1041390114. [DOI] [PubMed] [Google Scholar]
- 34.Fulton AM, Zhang S-Z, Chong YC. Role of the prostaglandin E2 receptor in mammary tumor metastasis. Cancer Res. 1991;51:2047–2050. [PubMed] [Google Scholar]
- 35.Richards JA, Brueggemeier RW. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab. 2003;88:2810–2816. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- 36.Subbaramaiah K, Hudis C, Chang S-H, Hla T, Dannenberg AJ. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. J Biol Chem. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- 37.Chang S-H, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase-2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamori T, Uchiya N, Nakatsugi S, et al. Chemopreventive effects of ONO-8711, a selective prostaglandin E receptor EP1 antagonist, on breast cancer development. Carcinogenesis. 2001;22:2001–2004. doi: 10.1093/carcin/22.12.2001. [DOI] [PubMed] [Google Scholar]
- 39.Thorat MA, Morimiya A, Mehrotra S, Konger R, Badve S. Prostanoid receptor EP1 expression in breast cancer. Mod Pathol. 2008;21:15–21. doi: 10.1038/modpathol.3800970. [DOI] [PubMed] [Google Scholar]
- 40.Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C. Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res. 2003;289:265–274. doi: 10.1016/s0014-4827(03)00269-6. [DOI] [PubMed] [Google Scholar]
- 41.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Huang Y, Porta R, Yanagisawa K, Gonzalex A, Segi E, et al. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006;66:9665–9672. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]
- 43.Pan M-R, Hou M-F, Chang H-C, Hung W-C. Cyclooxygenase-2 upregulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem. 2008;283:11155–11163. doi: 10.1074/jbc.M710038200. [DOI] [PubMed] [Google Scholar]
- 44.Chang S-H, Ai Y, Breyer RM, Lane TF, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase-2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496–4499. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- 45.Narumiya S. Prostanoids in immunity: roles revealed by mice deficient in their receptors. Life Sci. 2003;74:391–395. doi: 10.1016/j.lfs.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Nataraj CD, Thomas W, Tilley SL, Nguyen M, Mannon R, Koller BH, et al. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang LN, Yamagata R, Yadav S, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seno HM, Oshima T, Ishikawa H, et al. Cyclooxygenase 2 and prostaglandin E2 receptor EP2-dependent angiogenesis in Apcdelta716 mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 49.Amano HI, Hayashi H, Endo H, et al. Host prostaglandin E2-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197:221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282:16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 51.George RJ, Sturmoski MA, Anant S, Houchen CW. EP4 mediates PGE2 dependent cell survival through the PI3 Kinase/ AKT pathway. Prostaglandins Other Lipid Mediat. 2007;83:112–120. doi: 10.1016/j.prostaglandins.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawcroft G, Ko CWS, Hull MA. Prostaglandin E2-EP4 receptor signaling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–3019. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]