Abstract
Adipose tissue plays a role in obesity-related cancers via increased production of inflammatory factors, steroid hormones, and altered adipokines. The impact of weight loss on adipose-tissue gene expression may provide insights into pathways linking obesity with cancer risk. We conducted an ancillary study within a randomized trial of diet, exercise, or combined diet+exercise vs. control among overweight/obese postmenopausal women. In 45 women, subcutaneous adipose-tissue biopsies were performed at baseline and after 6 months and changes in adipose-tissue gene expression were determined by microarray with an emphasis on pre-specified candidate pathways, as well as by unsupervised clustering of >37,000 transcripts (Illumina). Analyses were conducted first by randomization group, and then by degree of weight change at 6-months in all women combined. At 6 months, diet, exercise and diet+exercise participants lost a mean of 8.8 kg, 2.5 kg, and 7.9 kg (all p<0.05 vs. no change in controls). There was no significant change in candidate-gene expression by intervention group. In analysis by weight-change category, greater weight loss was associated a decrease in 17β-hydroxysteroid dehydrogenase-1 (HSD17B1, p-trend<0.01) and leptin (LEP, p-trend<0.01) expression, and marginally significant increased expression of estrogen receptor-1 (ESR1, p-trend=0.08) and insulin-like growth factor binding protein-3 (IGFBP3, p-trend=0.08). Unsupervised clustering revealed 83 transcripts with statistically significant changes. Multiple gene-expression changes correlated with changes in associated serum biomarkers. Weight-loss was associated with changes in adipose-tissue gene expression after 6 months, particularly in two pathways postulated to link obesity and cancer, i.e., steroid-hormone metabolism and IGF signaling.
Keywords: Adiposity, gene expression, obesity, weight-loss, exercise, diet, leptin, sex hormones, inflammation, adipokines, human, randomized-controlled trial
INTRODUCTION
Overweight or obesity and low levels of physical activity, are associated with an increased risk of several types of cancer, particularly colorectal and postmenopausal breast cancer (1–4). The metabolic and hormonal consequences of excess fat mass and a sedentary lifestyle are associated with disease promotion and progression. Possible mechanistic pathways, many of which are modulated by change in weight or body composition, appear to involve inflammatory factors, steroid hormones, insulin-like growth factors or insulin resistance (5–8).
Biomarkers associated with these mechanisms have been investigated in peripheral blood, but there has been little evaluation directly at the level of adipose tissue in humans. Adipose tissue may play a role in several of these proposed mechanisms (9), as it is an endocrine organ integral to regulation of energy homeostasis, insulin sensitivity, and glucose tolerance (10, 11). In addition to adipocytes, white adipose tissue also contains pre-adipocytes, endothelial cells, fibroblasts, and leukocytes (12). In obesity, the number of adipose-tissue leukocytes increases dramatically, which, together with a switch in their activation status, results in a state of low-grade chronic inflammation (13). The activated leukocytes, including macrophages and T cells, secrete inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα) (14), which likely mediate the inflammation. Inflammation in turn modulates the endocrine functions of the adipocytes, including secretion of adipokines (15).
Systemic inflammation and the changes in circulating adipokine concentrations are now thought to be a major cause of obesity-associated insulin resistance (13), and have also been linked to an increased risk for several types of cancer (16). Systemic inflammation (e.g., elevated serum C-reactive protein (CRP) or serum amyloid A (SAA)) has been associated with multiple cancer types, particularly colorectal cancer (17), while low serum concentrations of the anti-inflammatory and insulin-sensitizing adipokine adiponectin, have been associated with an increased risk for breast cancer (18). Insulin resistance and the associated hyperinsulinemia, as well as type 2 diabetes, are associated with breast, colorectal, endometrial, and pancreatic cancer (16). Finally, increased adipose-tissue estrogen synthesis plays a role in postmenopausal breast, as well as endometrial cancer (3, 19). In this context, adipose tissue expresses aromatase (cytochrome P450 19A1) and is an important site for extra-gonadal conversion of androgens to estrogens (20).
Research on the effect of changes in energy balance on human adipose-tissue biology is limited and primarily focused on the short-term effects of very low calorie diets (21–29). The effect of exercise and/or caloric restriction on gene expression in normal mouse mammary glands has been reported (30); together these experimental studies provide intriguing initial evidence for alterations in mRNA expression of inflammatory cytokines and adipokines, particularly IL-6 and leptin (21, 24, 28, 29). The research presented here adds human data on the effect of weight loss on adipose-tissue biology. The purpose of this study was to examine, within a randomized controlled trial, the 6-month effects of caloric restriction, exercise, or both on subcutaneous adipose-tissue biology in overweight, postmenopausal women. A secondary purpose was to examine the association of degree of weight loss with changes in adipose-tissue biology.
SUBJECTS AND METHODS
This study, conducted from 2008 to 2009, was ancillary to a 12-month randomized, controlled trial that tested the effects of three lifestyle change interventions (caloric restriction weight loss diet, exercise, or both caloric restriction weight loss diet plus exercise) compared to a usual lifestyle control group on biomarkers of breast cancer risk among 439 women (31–34). This ancillary study examined the effect of the interventions from baseline to 6 months on adipose-tissue gene expression, the associations of degree of weight loss with gene-expression change, and correlations between adipose-tissue gene expression and serum concentrations of specific adipokines, pro-inflammatory cytokines, and sex-steroid hormones. All study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board in Seattle, WA, and all participants provided informed consent.
Subject Recruitment
Details of the parent trial have been published previously (31). In brief, participants were female, overweight or obese (BMI ≥25.0 kg/m2 or ≥23.0 kg/m2 if Asian-American), engaging in <100 min/week of moderate or vigorous activity, and postmenopausal (aged 50–75 years). Specific exclusion criteria included: use of postmenopausal hormones within the past 3 months; history of breast cancer or other serious medical conditions; diagnosed diabetes, fasting blood glucose ≥126mg/dL, or use of diabetes medications; alcohol intake >2 drinks/day; current smoking; current or planned participation in another structured weight-loss program; and use of weight-loss medications. Eligible women were randomized into 1 of the 4 study arms, as described previously (31).
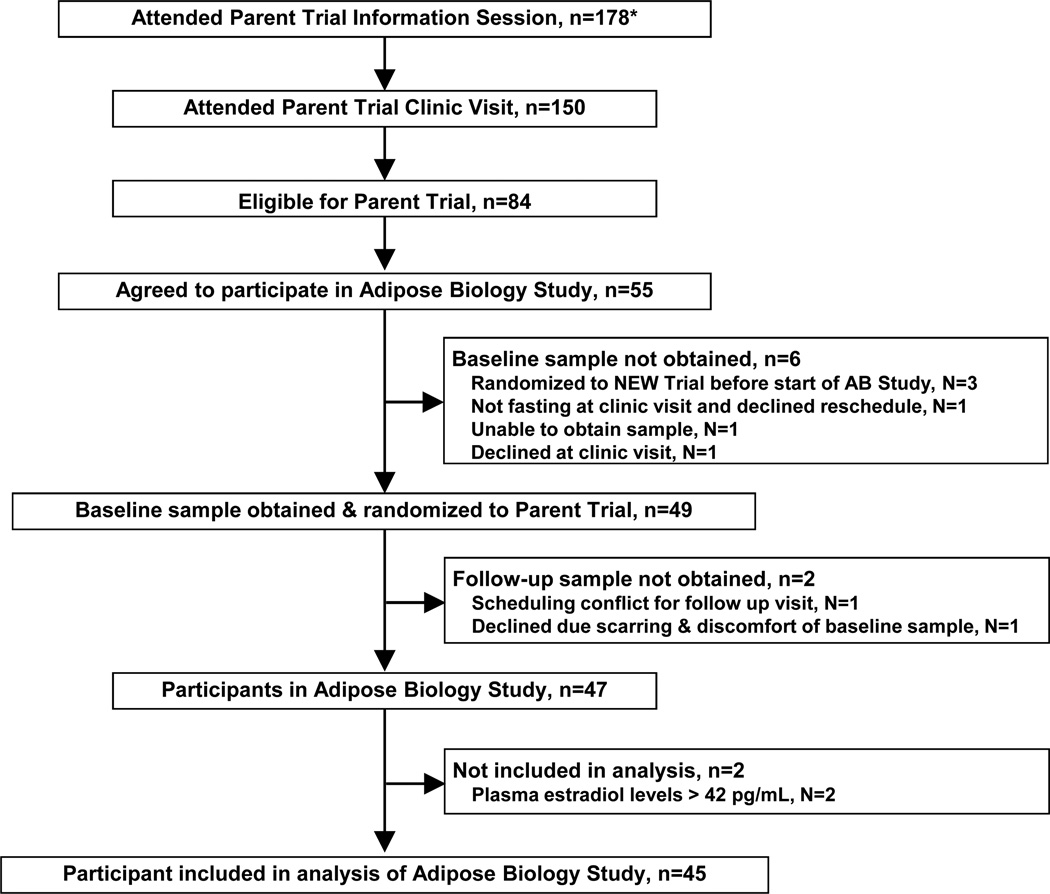
During the yearlong period of recruitment for the ancillary study, 178 women attended an information session and 84 were eligible to participate in the parent trial. Of these, 55 expressed interest in participating in the ancillary study, 49 enrolled and consented to the adipose-tissue biopsy at baseline and 6-months, and 47 provided both baseline and six-month adipose-tissue samples. Consistent with the primary endpoint analysis of the parent trial (34), two participants were removed due to subsequently confirmed high baseline serum estradiol levels (>42 pg/mL); thus, 45 participants were included in the analysis. Recruitment to the ancillary study is presented in Figure 1.
Figure 1.
Flow of participants through the Adipose Biology (AB) Study
Lifestyle Change Interventions
The parent trial interventions have been described in detail previously (31). The calorie-reduced, low-fat diet was modified from the Diabetes Prevention Program (35) and the Look AHEAD trial (36), with a goal of reducing total daily energy intake to 1200–2000 kcal/day based on baseline weight, daily energy intake from fat to <30%, and a 10% reduction in body weight by 6-months with maintenance thereafter. Participants met individually 2–4 times with a study dietitian and attended weekly dietitian-led group meetings in months 1–6.
The exercise intervention goal was ≥45 minutes of moderate-to-vigorous intensity exercise, 5 days per week (≥225 minutes/week). Participants attended ≥3 supervised sessions/week at our study facility and for remaining sessions exercised at home. Activities with ≥4 metabolic equivalents (METs) (37) were counted towards the prescribed exercise target.
Women randomized to the diet+exercise intervention received both the dietary-weight-loss intervention and the aerobic-exercise intervention. The diet sessions were run separately from those for the diet group and participants were instructed not to discuss the diet intervention at the exercise facility. Women randomized to the control group were requested not to change their diet or exercise habits for the duration of the study.
Outcome Measures
Baseline and 6-month study measures were obtained by trained study personnel blinded to randomization status. At baseline, anthropometric measures (height, weight, waist circumference) were obtained and body composition was measured using a dual energy x-ray absorptiometry (DEXA) scanner (GE Lunar, Madison, WI). All participants wore pedometers (Accusplit, Silicon Valley, CA) for 7 consecutive days and maximal aerobic capacity (VO2max) was determined from a graded treadmill test with collection of expired gases. At 6 months, anthropometric measures and DEXA were repeated for the ancillary study, along with the use of pedometers. In addition, intervention women completed diet and/or activity logs, depending on assigned group, to document adherence (31).
Blood samples
At baseline and 6-months, 12-hour fasting blood samples (50 mL) were collected, processed within 1 hour and stored at −70°C. The following analytes were measured: estrone, estradiol, total testosterone, androstenedione and sex hormone binding globulin (Reproductive Endocrine Research Laboratory, University of Southern California; insulin, IL-6, and glucose (Northwest Lipid Research Laboratories, University of Washington); high sensitivity CRP and SAA (Department of Laboratory Medicine, University of Washington). The inter-assay coefficients were: steroid hormones (8–13%), sex hormone-binding globulin (SHBG) (5–7%), insulin (4.5%), glucose (1.4%), CRP (4.7%), SAA (6.2%) and IL-6 (12.4%).
Subcutaneous adipose-tissue biopsy and total RNA preparation
Details of the sampling procedure have been previously published (38). Briefly, following an overnight fast, an abdominal subcutaneous adipose-tissue sample (≥~350 mg) was obtained by needle aspiration under local anesthesia. Tissue was flash-frozen and then stored at −80°C. Total RNA was subsequently extracted using the Qiagen RNeasy lipid tissue mini kit (Qiagen, Valencia, CA), quantified using Ribogreen (Invitrogen, Carlsbad, CA) and integrity was assessed on a Bioanalyzer 2100 (Agilent, Santa Clara, CA).
BeadChip microarray assays and data pre-processing
One-hundred ng of total RNA was transcribed using the Illumina TotalPrep RNA Amplification Kit (Ambion). Expression of over 37,000 mRNA transcripts was assessed using the Human HT-12 v3 Expression Beadchips and the iScan System Reader (Illumina). Each chip was analyzed for assay performance by examination of internal quality control measurements. Data is publicly available in Gene Expression Omnibus (GEO) [http://www.ncbi.nlm.nih.gov/geo/; Accession number GSE43471]. The raw, non-normalized bead-summary values output by BeadStudio (Illumina) were used for subsequent analysis. A final gene-level report was generated after background subtraction and quantile normalization. The data quality was assessed with the R package “arrayQualityMetrics” and no outliers were identified. Using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems), we examined overlaps with known biological functions and canonical pathways.
qRT-PCR
Genes selected for quantitative real-time polymerase chain reaction (qRT-PCR) validation included 5 candidate genes that showed a statistically significant correlation with weight loss (LEP, IGFBP3, HSD17B1, SAA1 and ESR1), plus four candidate genes of high interest (ADIPOQ, CYP19A1, TNF, and IL-6) that were not significant in the microarray analysis of weight change. Individuals were included in the qRT-PCR validation if sufficient RNA was available from adipose tissue at both time points. A total of 20 individuals met this criteria, representing each weight change group: gained weight (N=5), lost ≤ 5% baseline body weight (N=3), lost 5–10% baseline body weight (N=6), and lost > 10% baseline body weight (N=6). Total RNA was reverse transcribed using SuperScript III (Invitrogen) and mRNA expression levels were determined using pre-developed Taqman gene expression assays on a 7900HT real-time PCR system (Applied Biosystems). Reactions were carried out in triplicate using 2x Gene Expression Master Mix (Applied Biosystems). Levels of GUSB were used for normalization, chosen because of its stable expression in adipose tissue in our own results and those of others (39). The ΔΔCt method was used to relatively quantify mRNA levels across all samples tested.
Statistical analyses
We first investigated changes in gene expression by intervention compared with control. We then examined changes in gene expression by degree of weight change, combining all participants together, including controls. We chose the following weight-change categories based on levels of clinically relevant weight loss and approximate quartiles based on our data: gained any weight, lost 0–5%, lost 5–10%, and lost >10% (40). We applied the generalized estimating equation (GEE) approach to account for repeated measurements on the same subjects. We performed trend tests of weight-change category on change of gene expression. A three-tiered approach was used: first, we investigated effects on pre-specified candidate genes; second, we investigated effects on candidate pathways and; third, we explored changes across all genes on the microarray. Baseline gene-expression values and absolute changes in baseline gene expression were correlated to the serum values by Pearson’s correlation. Statistical analyses were performed using SAS (version 9.2, SAS Institute Inc, Cary, NC).
Candidate gene analysis
The R limma Bioconductor package(41) was used to evaluate the significance of differences in change of expression between the control and intervention groups or by degree of weight change, pre- and post-intervention. Eighty-two candidate genes were chosen prior to analysis based on literature reports and involvement with inflammation, immune function, or steroid/hormone metabolism (Supplementary Table 1). These specific genes were evaluated by intervention groups or by a trend test by weight-change category for all women combined. P-values were adjusted to account for 82 simultaneous comparisons, using the Benjamini and Hochberg method (42).
Gene-set analysis
A Gene-Set Enrichment Analysis approach was used to assess expression change differences between control and intervention or weight-change category at the level of biochemical pathways (43, 44). Curated gene sets related to inflammation, immune function, and steroid/hormone metabolism were chosen from the Molecular Signatures Database (43). Fifteen gene sets were analyzed, all of which contained ten or more of those genes (Supplementary Table 2). The gene sets together included 888 genes. As in the candidate gene analysis, the gene sets were evaluated by testing whether the change in expression of each of the intervention group samples was identical to the control. In addition, we assessed the significance of change in expression, post-intervention minus pre-intervention, relative to weight-change category.
Discovery gene analysis
To identify novel genes whose expression in adipose tissue was impacted by weight loss, all genes with measureable mRNA expression in our samples were evaluated for differences by weight change using the same statistical tools as for the candidate gene analysis. P-values were adjusted for all 37,834 genes measured.
RESULTS
Anthropometrics and Main Intervention Effects
The 45 participants were randomly assigned to the diet intervention (n=8), the exercise intervention (n=14), the diet+exercise intervention (n=16), or the control group (n=7) (Figure 1). Unequal group sizes are attributable to this being a subset of consenting participants within a larger trial. Of these participants, 39 underwent DEXA scans at baseline and 6-months [diet (n=7), exercise (n=12), diet+exercise (n=13), control (n=7)] and 35 provided pedometer step counts at baseline and 6-months [diet (n=6), exercise (n=11), diet+exercise (n=12), control (n=6)].
The anthropometric characteristics of the subjects randomized to the four intervention groups were comparable at baseline (Table 1). The mean age of participants was 58.5±4.6 years. Participants had a mean BMI of 31.3± 4.3kg/m2, 47.6±4.2% body fat, and VO2max of 24.4±4.3 ml/kg/min (consistent with poor aerobic fitness). Baseline values for serum levels of selected biomarkers of cancer risk are also presented in Table 1.
Table 1.
Baseline characteristics and fasting serum values of participants in the Adipose Biology Study (n=45).
| All | By Intervention Group | ||||
|---|---|---|---|---|---|
| Mean (SD) n= 45 |
Control Mean (SD) n=7 |
Diet Mean (SD) n=8 |
Exercise Mean (SD) n=14 |
Diet + Exercise Mean (SD) n=16 |
|
| Age (years) | 58.5 (4.6) | 55.5 (2.9) | 54.5 (3.2) | 60.9 (5.3) | 59.7 (3.1) |
| Race/ethnicity (n, %) | |||||
| Non-Hispanic White | 43 (96) | 6 (86) | 8 (100) | 14 (100) | 15 (94) |
| Other* | 2 (4) | 1 (14) | 0 (0) | 0 (0) | 1 (6) |
| Weight (kg) | 84.6 (12.6) | 92.8 (10.3) | 77.1 (10.0) | 85.3 (14.8) | 84.2 (11.2) |
| BMI (kg/m2) | 31.3 (4.3) | 33.2 (3.8) | 28.9 (3.0) | 30.8 (3.5) | 32.1 (5.2) |
| Body fat (%) | 47.6 (4.2) | 49.0 (4.0) | 46.0 (3.4) | 47.1 (4.7) | 48.4 (4.2) |
| Waist (cm) | 96.4 (10.3) | 102.4 (4.4) | 91.3 (11.0) | 97.8 (12.8) | 95.0 (8.4) |
| VO2max (ml/kg/min) | 24.4 (4.3) | 23.3 (4.4) | 25.5 (5.7) | 23.2 (4.0) | 25.6 (3.7) |
| Steps (per week) | 5450 (2253) | 5161(2565) | 4955(2229) | 5828 (1815) | 5518(2581) |
| Fasting Serum Concentrations | |||||
| Insulin (µIU/mL) | 12.1 (6.3) | 15.8 (11.4) | 9.9 (5.4) | 13.1 (4.6) | 10.7 (4.7) |
| Glucose (mg/dL) | 95.8 (8.2) | 92.7 (5.4) | 92.8 (4.1) | 97.7 (10.8) | 97.0 (7.9) |
| HOMA-IR | 2.9 (1.5) | 3.6 (2.4) | 2.3 (1.3) | 3.2 (1.2) | 2.6 (1.3) |
| CRP (mg/L) | 3.5 (3.0) | 2.9 (1.4) | 2.6 (1.9) | 4.5 (4.2) | 3.5 (2.6) |
| SAA (mg/L) | 6.8 (4.4) | 7.4 (3.6) | 5.6 (4.4) | 5.9 (2.8) | 8.0 (5.7) |
| Estradiol(pg/mL) | 12.3 (6.1) | 13.5 (8.4) | 9.7 (5.3) | 13.3 (6.0) | 12.1 (5.7) |
| Estrone(pg/mL) | 39.7 (17.0) | 40.9 (23.8) | 36.2 (20.3) | 43.3 (16.0) | 37.7 (13.5) |
| Total Testosterone(ng/dL) | 32.2 (17.8) | 31.1 (24.1) | 29.8 (15.8) | 32.5 (15.3) | 33.5 (19.4) |
| Androstenedione (ng/dL) | 57.3 (24.0) | 51.9 (24.9) | 57.3 (28.6) | 59.3 (27.4) | 58.0 (19.8) |
| Free estradiol(pg/mL) | 0.33 (0.16) | 0.37 (0.21) | 0.26 (0.15) | 0.34 (0.16) | 0.33 (0.16) |
| Free testosterone(pg/mL) | 6.8 (3.6) | 6.9 (4.7) | 6.3 (3.1) | 6.4 (2.7) | 7.4 (4.4) |
| SHBG(nmol/L) | 38.5 (13.9) | 33.4 (12.0) | 39.2 (17.2) | 44.2 (16.4) | 35.5 (9.5) |
| Leptin (ng/mL) | 28.9 (11.3) | 32.6 (9.4) | 24.3 (11.0) | 27.4 (12.2) | 30.9 (11.4) |
| Adiponectin (µg/mL) | 13.8 (5.9) | 13.5 (5.8) | 12.2 (4.9) | 13.9 (6.3) | 14.7 (6.5) |
Legend:
Hispanic white (n=1) and “other” (n=1).
Results for all participants from the parent trial have been previously reported (31–34). Participants in this ancillary study experienced mean weight changes as follows (P-values in reference to control group): diet, −11.3% (p<0.001); exercise, −3.0% (p=0.03); and diet+exercise, −9.4% (p<0.0001); controls, +1.0%. Compared to no change in controls, the mean percent body fat loss was: diet, −12.6% (p=0.02); exercise, −3.1% (p=0.56); and diet+exercise, −13.2% (p<0.01). Compared to the control group (−333 steps/week), the diet group had no statistically significant change in number of steps per week (+1071 steps/week, p=0.16), while both the exercise (+2570 steps/week, p<0.0001) and diet+exercise (+4182 steps/week, p<0.0001) had an increase in steps per week. Baseline and 6-month serum marker values are presented in Supplementary Table 3.
Changes in Adipose-Tissue Candidate Genes and Candidate Gene Sets: Analyses by Intervention Arm
In the first candidate gene analysis, microarray data from 45 participants were used to compare changes in expression between baseline and 6-months, for 82 candidate genes, between intervention groups and the control group (Supplementary Figure 1). The unadjusted analysis indicated that nine genes had significantly altered expression in the diet intervention compared to control. Four genes had decreased expression: hydroxysteroid (17-beta) dehydrogenase 1 (HSD17B1) (p=0.01, adj. p=0.29); leptin (LEP) (p=0.03/0.29); insulin receptor (INSR) (p=0.02/0.29); and telomeric repeat binding factor 1 (TERF1) (p=0.03/0.29). Five genes had increased expression: telomeric repeat binding factor 2 (TERF2) (p=0.03/0.29); telomeric repeat binding factor 2, interacting protein (TERF2IP) (p=0.02/0.29); cyclooxygenase-2 (also known as prostaglandin-endoperoxide synthase 2, PTGS2) (p=0.004/0.29); interleukin-1 beta (IL-1B) (p=0.03/0.29); and estrogen receptor (ESR1) (p=0.03/0.29). No significant change in gene expression was observed in the exercise-only group. In the diet+exercise intervention, expression of three genes was significantly decreased (HSD17B1: p=0.02/0.45; LEP: p=0.04/0.60; fatty-acid-binding protein 1 FABP1: p=0.02/0.45), and expression of two genes was significantly increased (ESR1: p= 0.02/0.45; TERF2IP: p=0.01/p=0.45).
For the initial gene-set analysis, the microarray data were also used to compare changes in expression between baseline and 6-months visits for 15 predefined gene sets by intervention group (Supplementary Table 2). Differential expression was observed in one gene set in the diet intervention (jak-STAT signaling pathway: p=0.004/0.06), and two gene sets in the diet+exercise intervention (IL-6 pathway: p= 0.0002/p=0.003; and toll pathway: p=0.03/0.20). However, when adjusted for multiple comparisons, only the IL-6 pathway in the diet+exercise intervention remained statistically significant.
Changes in Adipose-Tissue Candidate Genes and Candidate Gene Sets: Analyses by Weight-Change Category
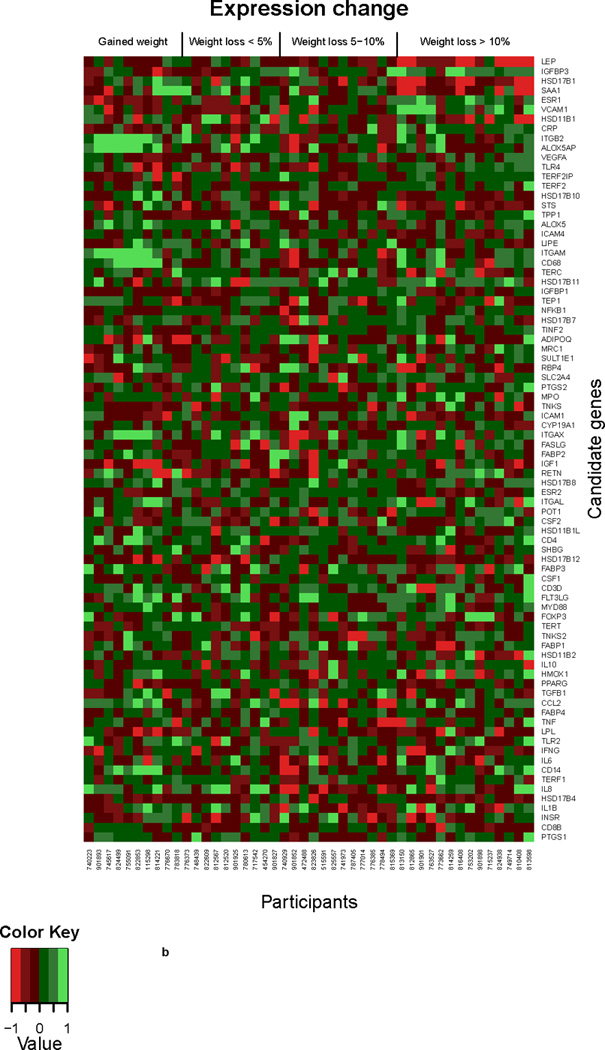
In the second candidate gene analysis, microarray data were used to evaluate the association of percent change by weight-change category between the baseline and 6-months visits on changes in gene expression among all study participants for significantly regulated genes (Figure 2a) and all 82 candidate genes (Figure 2b). In this analysis, the sample was split into approximate quartiles, gained weight (n=10), lost ≤ 5% baseline body weight (n=10), lost 5–10% baseline body weight and lost > 10% baseline body weight (n=14). In sex hormone-related genes, a decrease in expression of HSD17B1 (p=0.0002/0.008), STS (p=0.02/0.20) and HSD17B10 (p=0.045/0.37) was noted with greater weight loss, but only HSD17B1 remained statistically significant after adjustment for multiple comparisons. A trend for an increase in ESR1 (p=0.004/0.08) with greater weight loss was noted.
Figure 2.
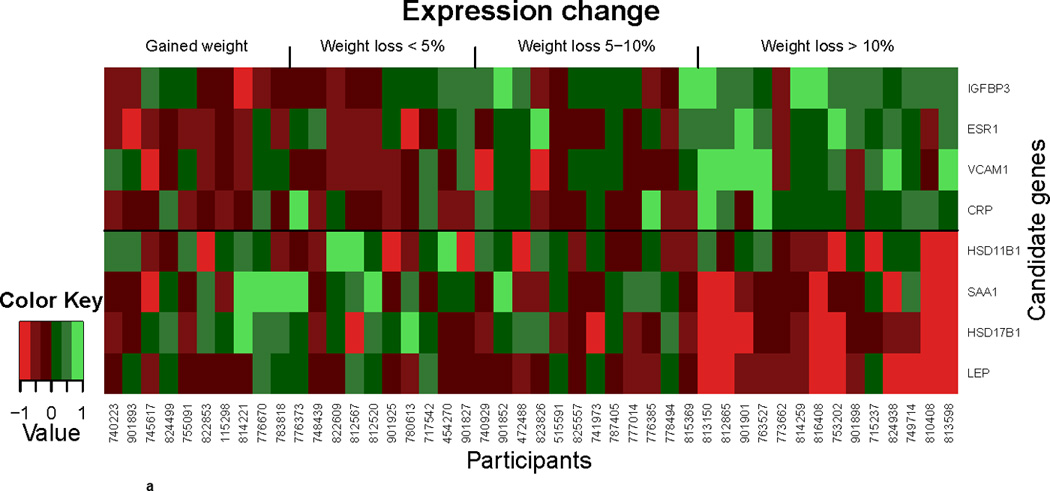
a. Differential gene expression analyzed by weight change. Heat map displaying significant gene expression changes over 6 months in individuals with no weight loss compared to those with <5%, 5–10%, and >10% weight loss. Gradients indicate the level of gene-expression change over time.
b. Differential gene expression analyzed by weight change. Heat map displaying the gene expression change of 82 candidate genes over 6 months in individuals with no weight loss compared to those with <5%, 5–10%, and >10% weight loss. Gradients indicate the level of gene-expression change over time.
In inflammation-related genes, the expression of ICAM4 (p=0.01/0.13) and SAA1 (p=0.01/0.13) decreased, while that of VCAM1 (p=0.04/0.37) and CRP (p=0.01/0.13) increased with weight loss in the unadjusted analysis; however, none remained statistically significant after adjustment for multiple comparisons.
Expression of LEP decreased with greater weight loss (p=0.0003/0.002) and expression of IGFBP3 increased with greater weight loss (p=0.003/0.08).
In the gene-set analysis, percent weight change was associated with two KEGG pathways: C21-steroid hormone metabolism (p=0.005, adj. p=0.07) and jak-STAT signaling (p=0.042, adj. p=0.30), but neither association was significant when adjusted for multiple comparisons (Supplementary Table 2).
To validate our findings, we performed qRT-PCR gene expression analysis on a subset of 20 participants for 9 candidate genes. We observed statistically significant decreases in expression of LEP, SAA1 and HSD17B1 and increased expression of IGFBP3 and ESR with weight loss, which is consistent with our findings in the microarray analysis (Supplementary Figure 2). No statistically significant changes were detected in ADIPOQ, CYP19A1, TNF or IL-6, also consistent with our microarray results.
Changes in all Analyzed Adipose-Tissue Genes: Analyses by Weight Change
Unsupervised clustering of >37,000 transcripts by weight-change category revealed 83 transcripts with statistically significant p-values for trend (p<0.05 after adjustment for multiple comparisons). This included 32 genes with decreased expression and 51 genes whose expression increased with greater weight loss (Supplementary Table 4). Of these, 31 genes had a ≥25% change in expression in the largest weight loss category (Table 2). Of note, LEP was the only one of our pre-specified candidate genes that also was significant in the unsupervised analysis.
Table 2.
Change in adipose tissue gene expression (unsupervised) by weight-change category (all women combined).*
| Weight-Change Group | ||||||
|---|---|---|---|---|---|---|
| Gene | All n=45 % change |
Gained n=10 % change |
<=5% loss n=10 % change |
5–10% loss n=11 % change |
>10% loss n=14 % change |
Adj. p-trend† |
| BCYRN1 | −19% | 78% | 19% | −34% | −59% | 0.009 |
| HSZFP36 | 2% | −17% | −9% | 0% | 30% | 0.009 |
| ABCC6 | −13% | 4% | 1% | −4% | −35% | 0.013 |
| PELI2 | −2% | −25% | −7% | −6% | 26% | 0.013 |
| ZNF33B | 4% | −15% | −6% | 7% | 27% | 0.013 |
| HIST1H4H | −22% | 8% | 9% | −16% | −53% | 0.014 |
| CEBPZ | 4% | −16% | −2% | 2% | 27% | 0.014 |
| C6 | 49% | −15% | 6% | 12% | 253% | 0.014 |
| MRAP | −17% | 9% | −8% | −4% | −44% | 0.017 |
| TMEM189 | −14% | 3% | −2% | −8% | −34% | 0.017 |
| MGC13057 | −11% | 29% | −3% | −13% | −34% | 0.017 |
| MAST4 | −3% | 41% | 6% | −4% | −30% | 0.017 |
| LOC643496 | 0% | −24% | −11% | 2% | 32% | 0.017 |
| HS.333084 | −30% | 42% | 15% | −21% | −74% | 0.024 |
| HIST1H1C | −5% | 31% | 23% | −4% | −38% | 0.024 |
| GPLD1 | −16% | 2% | 2% | −13% | −39% | 0.030 |
| LEP | −19% | 0% | 1% | −13% | −44% | 0.031 |
| BOAT | −4% | 32% | 2% | −3% | −26% | 0.031 |
| HIST2H2AA3 | 0% | 47% | 11% | −6% | −26% | 0.031 |
| LOC402560 | −3% | 19% | 16% | −2% | −26% | 0.031 |
| FLJ10213 | 4% | −15% | −3% | 6% | 25% | 0.035 |
| ATP6V0E2 | −7% | 14% | −4% | −1% | −26% | 0.035 |
| ZNF818 | 2% | −23% | −7% | 8% | 26% | 0.035 |
| ACVR1 | 2% | −14% | −14% | 1% | 30% | 0.036 |
| CSTA | −5% | 34% | 5% | −7% | −30% | 0.038 |
| LOC390349 | −7% | 4% | 28% | −12% | −29% | 0.038 |
| PCDH18 | 1% | −29% | −10% | −1% | 43% | 0.038 |
| C20ORF24 | −11% | 9% | −2% | −11% | −28% | 0.048 |
| ZNF823 | 5% | −10% | −2% | 1% | 25% | 0.048 |
| C9ORF116 | −3% | 26% | 3% | 5% | −27% | 0.049 |
| LOC401115 | −4% | 51% | −1% | −13% | −28% | 0.049 |
Only transcripts with adjusted P trend <0.05 and +/− >=25% change in expression in the >10% weight loss category are shown
adjusted to account for 82 simultaneous comparisons, using the Benjamini and Hochberg method [41]. A full table of all 83 genes is in Supplementary Table 4.
We computed the overlap between the 83 statistically significant genes and canonical pathways and GO (gene ontology) categories used GSEA and the Molecular Signatures Database (MSigDB) (43) (Table 3). The results show overlap with signaling pathways, such as mTOR signaling (p=0.011), and a strong overlap with gene sets related to mRNA metabolism and translation. Using Ingenuity Pathway Analysis, we had similar results. In that analysis, mTOR signaling was the top canonical pathway (p=0.002) (data not shown).
Table 3.
Unsupervised clustering analysis-canonical pathway and GO gene set hits from the Molecular Signatures Database.
| Gene Set Name* | Database | Description | # Genes in Gene Set (K) |
# Genes in Overlap (k) |
k/K | p value |
|---|---|---|---|---|---|---|
| Signaling Pathways | ||||||
| MTOR PATHWAY | Biocarta | mTOR Signaling Pathway | 23 | 2 | 0.087 | 0.011 |
| ABC TRANSPORTERS | KEGG | ABC transporters | 44 | 2 | 0.0455 | 0.037 |
| mRNA Metabolism | ||||||
| DEADENYLATION OF mRNA | Reactome | Genes involved in Deadenylation of mRNA | 22 | 2 | 0.0909 | 0.010 |
| METABOLISM OF mRNA | Reactome | Genes involved in Metabolism of mRNA | 46 | 3 | 0.0652 | 0.004 |
| METABOLISM OF RNA | Reactome | Genes involved in Metabolism of RNA | 96 | 4 | 0.0417 | 0.004 |
| TRANSPORT OF MATURE mRNA DERIVED FROM AN INTRON CONTAINING TRANSCRIPT | Reactome | Genes involved in Transport of Mature mRNA derived from an Intron-containing Transcript | 51 | 2 | 0.0392 | 0.049 |
| Translation | ||||||
| REGULATION OF TRANSLATIONAL INITIATION | Gene Ontology | Genes annotated by the GO term GO:0006446. Any process that modulates the frequency, rate or extent of translational initiation. | 31 | 2 | 0.0645 | 0.019 |
| TRANSLATION FACTOR ACTIVITY NUCLEIC ACID BINDING | Gene Ontology | Genes annotated by the GO term GO:0008135. Functions during translation by binding nucleic acids during polypeptide synthesis at the ribosome. | 39 | 2 | 0.0513 | 0.030 |
| TRANSLATION INITIATION FACTOR ACTIVITY | Gene Ontology | Genes annotated by the GO term GO:0003743. Functions in the initiation of ribosome-mediated translation of mRNA into a polypeptide. | 24 | 2 | 0.0833 | 0.012 |
| TRANSLATION REGULATOR ACTIVITY | Gene Ontology | Genes annotated by the GO term GO:0045182. Any substance involved in the initiation, activation, perpetuation, repression or termination of polypeptide synthesis at the ribosome. | 41 | 2 | 0.0488 | 0.033 |
| TRANSLATIONAL INITIATION | Gene Ontology | Genes annotated by the GO term GO:0006413. The process preceding formation of the peptide bond between the first two amino acids of a protein. This includes the formation of a complex of the ribosome, mRNA, and an initiation complex that contains the first aminoacyl-tRNA. | 39 | 2 | 0.0513 | 0.030 |
| Cell Life Span | ||||||
| APOPTOTIC EXECUTION PHASE | Reactome | Genes involved in Apoptotic execution phase | 48 | 2 | 0.0417 | 0.044 |
| PACKAGING OF TELOMERE ENDS | Reactome | Genes involved in Packaging Of Telomere Ends | 49 | 2 | 0.0408 | 0.045 |
| Others | ||||||
| ATP BINDING | Gene Ontology | Genes annotated by the GO term GO:0005524. Interacting selectively with ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. | 151 | 4 | 0.0265 | 0.021 |
Only gene sets with a p value <0.05 are shown.
Correlation of Gene Expression with Serum Blood Markers
The baseline and end-of-study serum markers are presented in Supplementary Table 3. At baseline, there were statistically significant correlations between serum levels of sex hormones, inflammatory and metabolic markers and adipokines, and gene expression for some sex steroid-related, inflammation-related, adipose-tissue lipid-metabolism genes and other genes of interest (Table 4). For example, serum levels of estradiol were positively associated with the expression of HSD17B1 and HSD11B1, and negatively associated with HSD11B1L and HSD17B8. For adipokine/inflammation-related genes, serum levels of adiponectin were positively associated with the expression of ADIPOQ and RBP4, and negatively with the expression of LEP. Some associations were also observed between CRP levels and expression of SAA1 and LEP, but not CRP. Similarly, there were some associations regarding genes regulating lipid metabolism with serum glucose and insulin. As expected, a strong correlation was observed between serum leptin and the expression of LEP.
Table 4.
Associations (Pearson correlation coefficients) between baseline serum biomarker levels and baseline gene expression.
| Pathway* | Estradiol (pg/mL) |
Testosterone (ng/dL) |
SHBG (nmol/L) |
Free estradiol (pg/mL) |
|
|---|---|---|---|---|---|
| Sex Steroid-Related Genes |
ESR1 | −0.21† | −0.06 | 0.54 | −0.32 |
| 0.17‡ | 0.72 | 0.0001 | 0.03 | ||
| HSD17B11 | 0.34 | 0.04 | −0.09 | 0.34 | |
| 0.02 | 0.79 | 0.54 | 0.02 | ||
| HSD11B1 | 0.33 | 0.08 | −0.17 | 0.37 | |
| 0.03 | 0.59 | 0.27 | 0.013 | ||
| HSD11B1L | −0.38 | −0.18 | −0.09 | −0.34 | |
| 0.01 | 0.24 | 0.56 | 0.02 | ||
| HSD17B8 | −0.31 | −0.08 | 0.13 | −0.29 | |
| 0.04 | 0.58 | 0.39 | 0.049 | ||
|
Leptin (ng/mL) |
Insulin (µU/mL) |
CRP (mg/L) |
Adiponectin (µg/mL) |
||
| Inflammation-Related Genes |
ADIPOQ | 0.13 | 0.17 | −0.28 | 0.44 |
| 0.38 | 0.27 | 0.06 | 0.002 | ||
| IFNG | 0.32 | −0.09 | 0.15 | −0.09 | |
| 0.034 | 0.54 | 0.33 | 0.56 | ||
| IL10 | −0.26 | −0.3 | 0.15 | −0.03 | |
| 0.09 | 0.047 | 0.32 | 0.85 | ||
| SAA1 | 0.2 | −0.15 | 0.44 | 0.071 | |
| 0.18 | 0.34 | 0.0023 | 0.65 | ||
| TLR4 | 0.27 | 0.39 | −0.01 | −0.13 | |
| 0.08 | 0.009 | 0.97 | 0.41 | ||
|
Insulin (µU/mL) |
Glucose (mg/dL) |
Adiponectin (µg/mL) |
|||
| Adipose tissue lipid metabolism-related Genes |
LIPE | −0.44 | −0.3 | 0.42 | NA |
| 0.003 | 0.047 | 0.004 | |||
| ABCA1 | −0.04 | 0.33 | 0.01 | NA | |
| 0.81 | 0.025 | 0.95 | |||
|
Leptin (ng/mL) |
CRP (mg/L) |
Adiponectin (µg/mL) |
|||
| Other Genes | LEP | 0.46 | 0.33 | −0.37 | NA |
| 0.0016 | 0.026 | 0.013 | |||
| RBP4 | −0.1 | −0.01 | 0.43 | NA | |
| 0.51 | 0.97 | 0.0034 | |||
Only genes with at least one statistically significant association (p<0.05) are shown
correlation coefficient
p-value
The correlation between changes in serum levels and changes in gene expression is presented in Table 5. Again, here we observed some expected associations, for example between changes in ESR1, SHBG, and HSD17B7 and corresponding changes in serum estradiol with fairly strong correlation coefficients (r=0.33–0.47). A reduction in serum glucose was associated with a decrease in the expression of ADIPOQ, and increases in IL1B, IL6 and IL8. For genes involved in adipose-tissue lipid metabolism, decreases in serum leptin and insulin were associated with an increase in expression of ABCA1, As expected, changes in LEP were positively associated with serum leptin and glucose, but inversely with adiponectin.
Table 5.
Associations (Pearson correlation coefficients) between changes in serum biomarker levels and changes in gene expression from baseline to 6-months.
| Pathway* | Estrone (pg/mL) |
Estradiol (pg/mL) |
Testosterone (ng/dL) |
SHBG (nmol/L) |
Free estradiol (pg/mL) |
|
|---|---|---|---|---|---|---|
| Sex Steroid-Related Genes |
ESR1 | −0.07† | −0.45 | −0.01 | 0.47 | −0.48 |
| 0.67‡ | 0.0021 | 0.96 | 0.0011 | 0.0009 | ||
| HSD11B1L | −0.07 | −0.13 | −0.36 | −0.21 | −0.1 | |
| 0.64 | 0.4 | 0.016 | 0.17 | 0.5 | ||
| SHBG | 0.31 | −0.06 | 0.2 | −0.23 | −0.04 | |
| 0.036 | 0.68 | 0.2 | 0.12 | 0.78 | ||
| HSD17B7 | −0.02 | −0.33 | 0.08 | 0.16 | −0.34 | |
| 0.91 | 0.028 | 0.59 | 0.3 | 0.023 | ||
|
Glucose (mg/dL) |
||||||
| Inflammation-related Genes |
ADIPOQ | 0.37 | NA | NA | NA | NA |
| 0.012 | ||||||
| IL1B | −0.33 | |||||
| 0.026 | ||||||
| IL6 | −0.38 | |||||
| 0.011 | ||||||
| IL8 | −0.39 | |||||
| 0.008 | ||||||
|
Leptin (ng/mL) |
Insulin (µU/mL) |
|||||
| Adipose tissue lipid metabolism-related Genes |
ABCA1 | −0.35α | −0.35 | NA | NA | |
| 0.022 | 0.018 | |||||
|
Leptin (ng/mL) |
Insulin (µU/mL) |
Glucose (mg/dL) |
Adiponectin (µg/mL) |
|||
| Other Genes | IGF1 | −0.04α | −0.3 | 0.2 | 0.07α | |
| 0.78 | 0.045 | 0.18 | 0.65 | |||
| IGFBP3 | −0.22α | 0.02 | −0.16 | 0.49α | ||
| 0.15 | 0.92 | 0.31 | 0.0011 | |||
| LEP | 0.34α | 0.24 | 0.38 | −0.32α | ||
| 0.028 | 0.11 | 0.01 | 0.039 | |||
| RBP4 | 0.11α | 0.24 | 0.47 | −0.09α | ||
| 0.5 | 0.11 | 0.0012 | 0.58 | |||
N=45, except α, N=42
only genes with at least one statistically significant association (p<0.05) are shown
correlation coefficient
p-value
DISCUSSION
This study is the first to measure gene expression profiles in subcutaneous adipose tissue with an intervention study that examined the impact of dietary weight loss, exercise, and dietary weight loss plus exercise, compared to control. There was a change in gene expression in three genes, hydroxysteroid (17-beta) dehydrogenase 1 (HSD17B1), leptin (LEP), and estrogen receptor 1 (ESR1) in tissue obtained from women in both the diet and diet+exercise interventions. However, these effects by intervention arm did not remain statistically significant after the adjustment for multiple comparisons for 82 candidate genes and 15 candidate pathways. This finding may be due to the small sample size in this pilot study or the rather stringent adjustment. The only other intervention study to date to examine a change in gene expression with a dietary intervention in humans reported a decrease in LEP and ADIPOQ gene expression with a very low calorie diet in 24 obese men (n=18) and women (n=6) (45). LEP expression appears to be consistently altered with weight loss or caloric restriction, as a similar reduction in expression was noted in whole mouse mammary tissue with caloric restriction compared to ad libitum (30).
In gene-set analyses, the IL-6 pathway was significantly modified by the diet & exercise intervention (adj. p=0.003), while jak-STAT signaling was affected by diet alone with marginal significance (adj. p=0.06). We observed more statistically significant signals in the analyses by weight change.
In an analysis combining women from all groups, a decrease in expression of genes involved in estrogen synthesis was noted with greater weight loss, with a statistically significant decrease in expression of HSD17B1 after accounting for multiple comparisons. This is the first report that illustrates the association of weight change with genes involved in synthesis of sex hormones in adipose tissue. HSD17B1 is a critical component of the estrogen metabolism pathway because it catalyzes the conversion of less-active estrone to estradiol.
We hypothesized that a reduction in body weight and fat mass would reduce adipose-tissue inflammation, as supported by animal studies (46, 47), as well as human interventions including reduced or very low calorie diets, or bariatric surgery (28, 29, 48). Surprisingly, we found no association between weight change and the expression of the vast majority of inflammatory cytokines and chemokines, including TNFα, IL-6, and MCP-1. Among the few inflammatory genes that showed a trend towards reduced expression from baseline to 6-months with weight loss, none reached statistical significance after adjustment for multiple comparisons, although some changes of substantial size with greater weight loss were observed, such as SAA1 (p= 0.01, adj p=0.13), where there was a reduction of expression of 38% among those who lost >10%. One potential explanation is that the weight loss achieved in this sub-set of participants was too small to have a sufficient effect on the inflammatory process. This is surprising given that the women in the diet and diet+exercise groups lost >12% of body fat, and reductions in serum levels of inflammatory markers observed in all intervention groups (33). In a recent report from the CALERIE study, Tam et al. (49) also found no impact of a 25% weight reduction with diet or diet + exercise on genes relating to inflammation in subcutaneous adipose tissue among obese individuals. Our data, along with the findings of Tam et al. (49), strongly suggest that adipose-tissue inflammation may be less impacted by changes in energy balance than has previously been hypothesized.
In the gene-set analysis, the unsupervised clustering of >37,000 transcripts by percent weight change revealed 83 transcripts with statistically significant adjusted p-values for trend. Using IPA analysis to examine overlaps with known biological functions and canonical pathways, several hits were noted in genes related to differentiation of adipocytes, the mTOR signaling pathway, and the IGF-1 signaling pathway. We also used GSEA to determine overlap between the genes that had statistically significant changes with weight loss and canonical pathways and GO categories. This analysis supported our IPA analysis as we observed several hits for mRNA metabolism, the mTOR pathway and others. Interestingly, LEP, the strongest signal in our candidate gene analysis, was the 42nd strongest hit in this clustering. This reaffirms the importance of leptin, but also suggests that there are numerous other biologic pathways that are altered by weight change that require further investigation.
The ability to examine the association between serum biomarkers and gene expression is a novel aspect of our study. At baseline, serum levels of estradiol were associated with the expression of genes involved in synthesis of steroid hormones. Furthermore serum levels of adiponectin, leptin and SAA were positively associated with gene expression of ADIPOQ, LEP and SAA1, respectively. Associations between serum insulin, glucose and adiponectin and genes related to adipose-tissue lipid metabolism were also observed. Furthermore, changes in serum levels with the intervention and changes in gene expression were examined. The interpretation of these findings is more difficult but does demonstrate the interplay between serum glucose and gene expression of several inflammatory genes, along with a negative association between serum leptin and insulin and gene expression of ABCA1, which is involved in adipose-tissue lipid metabolism. While these findings suggest that serum levels of biomarkers may serve as a proxy for gene expression at the level of adipose tissue in some cases, more research is needed to understand the complex interplay of pathways at the level of the human adipose tissue.
Our study combined hypothesis-driven approaches at both the candidate gene and candidate pathway level with empirical strategies, using unsupervised clustering of >37,000 transcripts. Overall, the candidate gene/candidate pathway approach had a >10% success rate, even after adjustment of the level of multiple comparisons made (82 candidate genes). The unsupervised clustering (“-omic strategy”) revealed a total of 0.2% hits among all 37,000 transcripts. This approach highlights a number of new signals that require further follow-up. Notably, the very stringent level of multiple comparison adjustment for this approach would have missed significant changes in 8 of our candidate genes for which we had seen a statistically significant effect in the hypothesis-driven analysis. This suggests that the two approaches – hypothesis-driven and empirical – are complementary, because the “–omic strategy” alone would result in a significant number of such false negative results. When based on prior biologic plausibility, significant effects are to be expected when investigated via a targeted approach.
A major strength of this study is the investigation of effects of weight change directly in the target tissue. Here, a number of important processes (including estrogen biosynthesis and inflammatory processes) take place which influence overall metabolic states and cancer risk. We have demonstrated that subcutaneous adipose-tissue collection and gene-expression analysis is feasible and has utility in cancer prevention studies. We observed the strongest associations with gene expression changes when stratifying by weight loss with data from all intervention groups combined. An advantage of the study is the randomized design which included different weight-loss groups (diet, exercise, diet+exercise) and a control group. In addition, the interventions used in this study are consistent with lifestyle interventions which could be adopted by the general population as a cancer prevention strategy (50), in contrast to the very low calorie diet (i.e., 450 kcal/d) examined in the study by Franck et al. (45).
There were several weaknesses in our study. First, the sample size for analyses by intervention arm was too small to reveal any statistically significant results after adjustment for multiple comparisons. Despite the pilot-study nature of the investigation and small sample size, including a small number of participants in the diet and control groups, we were able to identify a number of pre-hypothesized and new candidates. We only assayed one adipose-tissue biopsy sample from one anatomical site; subcutaneous abdominal fat. Gene expression could vary within the same fat depot and when compared to other fat depots, such as visceral, liver, or intra-muscular fat. Visceral fat may be more biological active for some analytes than adipose at other sites (51, 52). Furthermore, a target tissue of interest for breast cancer prevention is breast tissue and our study could not address the microenvironment in the breast itself. Therefore, the reported changes in gene expression in subcutaneous fat by intervention group or weight loss category may not be representative of changes in other biologically relevant tissues, such as breast or visceral fat. Our sample was limited to postmenopausal women aged 50–75 years, most of whom were non-Hispanic whites, and therefore our findings may not apply to men, women of different ages, or persons of other race/ethnic groups. Finally, we tested only one weight loss dietary plan and only one exercise program, and cannot extrapolate to other methods of weight loss.
In conclusion, our study demonstrated that diet- or exercise-induced weight loss results in measurable and sizable changes in adipose-tissue gene expression, particularly in sex-hormone steroid synthesis, leptin, and insulin signaling. Notable was the unexpected lack of effect on inflammatory pathways. The combination of a hypothesis-driven and empirical approach was particularly fruitful in that it supported hypothesized changes while also suggesting novel signals that require confirmatory study.
Supplementary Material
Acknowledgments
Funding Source: This study was supported by funding from NIH R21 CA131676-01(PI: Ulrich) and NCI U54 CA116847 (PI: Foster-Schubert).
References
- 1.Vainio H, Bianchini F, editors. Weight Control and Physical Activity. Lyon: IARC Press; 2002. [DOI] [PubMed] [Google Scholar]
- 2.Friedenreich CM. Review of anthropometric factors and breast cancer risk. European J Cancer Prev. 2001;10:15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 3.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology (Williston Park) 2005;19:871–881. discussion 81–2, 85–6. [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Friedenreich CM. Physical activity and cancer prevention: From observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 6.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 9.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 13.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 15.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 16.Grote VA, Becker S, Kaaks R. Diabetes mellitus type 2 - an independent risk factor for cancer? Exp Clin Endocr Diab. 2010;118:4–8. doi: 10.1055/s-0029-1243193. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol. 2011;28:1288–1295. doi: 10.1007/s12032-010-9617-x. [DOI] [PubMed] [Google Scholar]
- 19.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 20.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and action of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 21.Arvidsson E, Viguerie N, Andersson I, Verdich C, Langin D, Arner P. Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes. 2004;53:1966–1971. doi: 10.2337/diabetes.53.8.1966. [DOI] [PubMed] [Google Scholar]
- 22.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 23.Levine JA, Eberhardt NL, Jensen MD. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. J Clin Endocrinol Metab. 1999;84:2751–2754. doi: 10.1210/jcem.84.8.5910. [DOI] [PubMed] [Google Scholar]
- 24.Bastard JP, Hainque B, Dusserre E, Bruckert E, Robin D, Vallier P, et al. Peroxisome proliferator activated receptor-gamma, leptin and tumor necrosis factor-alpha mRNA expression during very low calorie diet in subcutaneous adipose tissue in obese women. Diabetes Metab Res Rev. 1999;15:92–98. doi: 10.1002/(sici)1520-7560(199903/04)15:2<92::aid-dmrr21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu YM, Lacorte JM, Viguerie N, Poitou C, Pelloux V, Guy-Grand B, et al. Adiponectin gene expression in subcutaneous adipose tissue of obese women in response to short-term very low calorie diet and refeeding. J Clin Endocrinol Metab. 2003;88:5881–5886. doi: 10.1210/jc.2003-030886. [DOI] [PubMed] [Google Scholar]
- 26.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 27.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–1381. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 29.Viguerie N, Vidal H, Arner P, Holst C, Verdich C, Avizou S, et al. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia. 2005;48:123–131. doi: 10.1007/s00125-004-1618-x. [DOI] [PubMed] [Google Scholar]
- 30.Padovani M, Lavigne JA, Chandramouli GV, Perkins SN, Barrett JC, Hursting SD, et al. Distinct effects of calorie restriction and exercise on mammary gland gene expression in C57BL/6 mice. Cancer Prev Res (Phila) 2009;2:1076–1087. doi: 10.1158/1940-6207.CAPR-09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity. 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, et al. Effects of a Caloric Restriction Weight Loss Diet and Exercise on Inflammatory Biomarkers in Overweight/Obese Postmenopausal Women: A Randomized Controlled Trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, et al. Reduced-Calorie Dietary Weight Loss, Exercise, and Sex Hormones in Postmenopausal Women: Randomized Controlled Trial. J Clin Oncol. 2012;30:2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 38.Campbell KL, Makar KW, Kratz M, Foster-Schubert KE, McTiernan A, Ulrich CM. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev Res (Phila Pa) 2009;2:37–42. doi: 10.1158/1940-6207.CAPR-08-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fink T, Lund P, Pilgaard L, Rasmussen JG, Duroux M, Zachar V. Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol. 2008;9:98. doi: 10.1186/1471-2199-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioin-formatics and Computational Biology Solutions using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 43.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 45.Franck N, Gummesson A, Jernas M, Glad C, Svensson PA, Guillot G, et al. Identification of adipocyte genes regulated by caloric intake. The Journal of clinical endocrinology and metabolism. 2011;96:E413–E418. doi: 10.1210/jc.2009-2534. [DOI] [PubMed] [Google Scholar]
- 46.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia. 2009;52:921–931. doi: 10.1007/s00125-009-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 49.Tam CS, Covington JD, Ravussin E, Redman LM. Little evidence of systemic and adipose tissue inflammation in overweight individuals(dagger) Front Genet. 2012;3:58. doi: 10.3389/fgene.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 51.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Bosse Y, Marceau P, Biron S, Lebel S, Richard D, et al. Gene expression variability in subcutaneous and omental adipose tissue of obese men. Gene Expr. 2007;14:35–46. doi: 10.3727/000000007783991772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.