Abstract
Motor learning is an essential part of human behavior, but poorly understood in the context of walking control. Here, we discuss our recent work on locomotor adaptation, which is an error driven motor learning process used to alter spatiotemporal elements of walking. Locomotor adaptation can be induced using a split-belt treadmill that controls the speed of each leg independently. Practicing split-belt walking changes the coordination between the legs, resulting in storage of a new walking pattern. Here, we review findings from this experimental paradigm regarding the learning and generalization of locomotor adaptation. First, we discuss how split-belt walking adaptation develops slowly throughout childhood and adolescence. Second, we demonstrate that conscious effort to change the walking pattern during split-belt training can speed up adaptation but worsens retention. In contrast, distraction (i.e., performing a dual task) during training slows adaptation but improves retention. Finally, we show the walking pattern acquired on the split-belt treadmill generalizes to natural walking when vision is removed. This suggests that treadmill learning can be generalized to different contexts if visual cues specific to the treadmill are removed. These findings allow us to highlight the many future questions that will need to be answered in order to develop more rational methods of rehabilitation for walking deficits.
Keywords: locomotion, motor learning, adaptation, generalization of learning, rehabilitation
Walking is a fundamental motor act. As such, it must be flexible enough to accommodate different environments, yet automatic enough so that we do not have to consciously focus on every step. Recently, we, and others, have been exploring the adaptability of locomotion with an eye toward improving rehabilitation of walking for people with brain lesions (e.g., Choi et al., 2009; Reisman et al., 2007, 2009). This review will focus on what we know about adaptive processes for human walking control, and perhaps more importantly, what we do not know.
Adaptive processes allow us to modify our locomotor patterns to suit changing environments. Since this is a critical ability for navigating the world, it is possible that adaptation develops at a very early age in humans. Conversely, the development of adaptation could follow a more protracted time course. This may be particularly true in human children, since they take much longer to learn how to walk independently than most other mammals. While humans typically begin walking 1 year after birth, many other mammals (e.g., horses, elephants) walk on the day that they are born. However, a recent study suggests that the late onset of human walking might be related to large brain mass, which takes extra time to develop (Garwicz et al., 2009). Indeed, if one considers the time from conception (rather than birth) to onset of walking, mammals with large brains relative to their body take longest to walk: humans (~19–25 months) and elephants (~22 months). Both animals have large brains—an adult human brain weighs ~1350 g and an adult elephant brain weighs ~4400 g. However, the percentage of brain mass with respect to the body is larger in humans than in elephants. Thus, brain development seems to be an important influence in dictating the onset of walking in mammals.
Given the dependence of onset of walking on brain development, we wondered if other elements of walking control would follow a protracted developmental time course in humans as the nervous system matures. Specifically, we have been interested in understanding whether children can learn novel walking patterns through adaptive learning mechanisms. Although children are able to walk independently, we predicted that processes to adapt locomotor patterns would not be fully developed since human brain development continues well after birth, through childhood, and even into adulthood (LeBel et al., 2008).
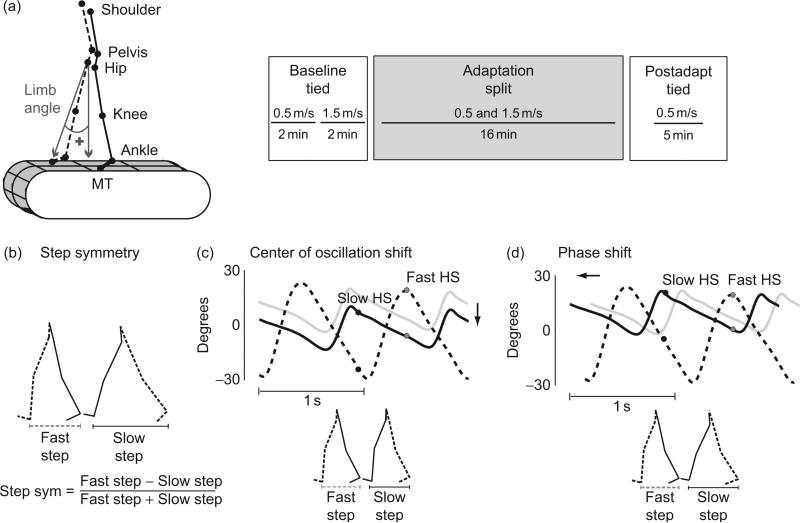
We use a motor learning paradigm to study walking adaptation involving a split-belt treadmill, with independent belts under each leg (Reisman et al., 2005). Using this device, we can study people walking with the belts moving at the same speed, or “tied,” and with the belts moving at different speeds, or “split.” Figure 1a illustrates the general paradigm that is used for these studies. We have previously reported that adults adapt their walking pattern when walking in the split-belt condition over the course of 10–20 min. They specifically change step symmetry (i.e., the normalized difference in step sizes of the two legs; Fig. 1b), using both spatial and temporal strategies as described in Fig. 1c and d. When returning to tied belts, they show aftereffects in both domains, indicating that the nervous system learned and stored a new locomotor pattern that had to be actively unlearned.
Fig. 1.
(a) Diagram of marker locations and an example of the paradigm structure. Limb angle convention is shown on the stick figure (left panel). Panel on the right shows an example experimental paradigm indicating the periods of split and tied-belt walking. The walking pattern is first recorded during a baseline period in which both treadmill belts move at the same speed. Then, changes to the walking pattern are recorded during an adaptation period in which one belt moves two to four times faster than the other. Finally, stored changes to the walking pattern are assessed during a deadaptation period in which the treadmill belts move at the same speed as in the baseline period. (b) An example of kinematic data of two consecutive steps is shown. Kinematic data for every two steps were used to calculate step symmetry, defined as the difference in step lengths normalized by the step lengths sum. (c) Figure adapted from Malone and Bastian (2010). Limb angle trajectories plotted as a function of time in late split-belt adaptation—two cycles are shown. Gray trajectory represents the movement in the slow limb in early adaptation. Positive limb angles are when the limb is in front of the trunk (flexion). Two time points are marked—slow heel strike (HS) in black and fast HS in gray. The spread between the limb angles is directly proportional to the step lengths shown in the bottom. Step lengths can be equalized by changing the position of the foot at landing (i.e., the “spatial” placement of the foot). This spatial strategy is known as a shift in the center of oscillation difference since subjects change midpoint angle around which each leg oscillates, with respect to the other leg. (d) Step lengths can also be equalized by changing the timing of foot landing, as shown by the change in phasing of the slow limb from the gray trajectory (early adaptation) to the black trajectory. This purely temporal strategy is known as phase shift since subjects equalize step lengths by changing the timing of foot landings with respect to each other.
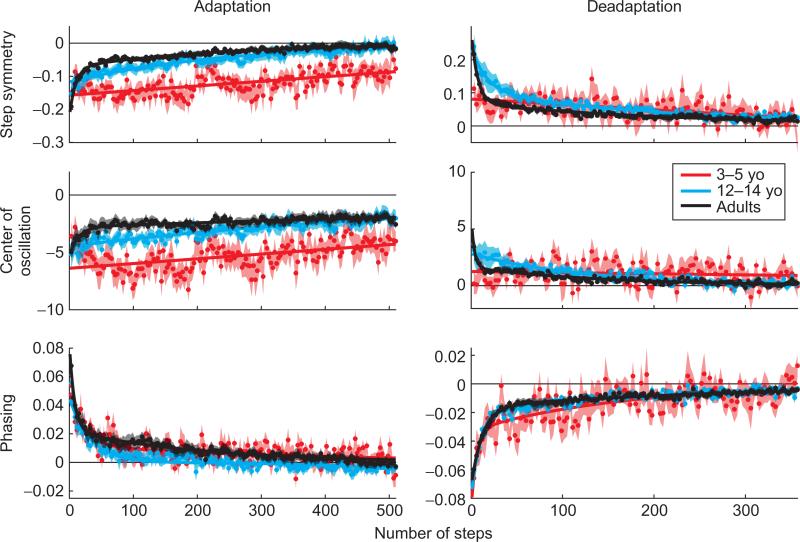
Recent work in our lab suggests that young children can adapt their walking pattern, but appear to show different developmental patterns for spatial versus temporal adaptation of walking (Vasudevan et al., 2011). Our initial intuition was that children might be more flexible in their ability to learn and, therefore, might adapt faster or more completely. Instead, we found that 3- to 5-year-old children adapt step symmetry slowly (Fig. 2a), and this ability does not fully develop until after age 12–14. Similar findings were present for the center of oscillation difference, which is defined as the difference in the midpoint position between heel strike (HS) and toe-off of each leg. Since the center of oscillation is dependent upon where the foot is placed at HS and where it is lifted off at toe-off, this measure reflects spatial locomotor control (Fig. 2b). In contrast, all ages could adapt the temporal parameter of phase at normal rates (Fig. 2c). Our interpretation of this finding is that the ability to adapt spatial control of walking depends on brain functions that are still developing through adolescence. Candidate sites are the cerebellum and motor cortex, though we consider the former to be more likely (Morton and Bastian, 2006).
Fig. 2.
Rates of adaptation (left column) and deadaptation (right column) in 3- to 5-year olds (red; n=10), 12- to 14-year olds (blue; n=10), and adults (black; n=10). Step symmetry data are shown in the top row, center of oscillation difference in the middle and phasing on the bottom. Shaded regions indicate standard error. Data were fit with linear, single-exponential, or double-exponential functions depending on which fit resulted in the highest r2 values. For 3- to 5-year-old step symmetry and center of oscillation difference, linear fits were best; double-exponential fits were best for the phasing data. A single exponential fit was used for 12- to 14-year-old center of oscillation difference adaptation data and all remaining 12- to 14-year-old data were best fit by double-exponential functions. All adult data were fit by double-exponential functions.
This result is interesting and raises many issues about development of movement adaptability. First, it suggests that the nervous system gains some adaptive abilities in late childhood. This is counter to the belief that, because children are developing, they are “more plastic” and should adapt faster. Of course, an important question is whether there are advantages to adapting slower as a child—since children adapt more slowly, do they also deadapt slower and does this make them retain more from day to day, for example? A second issue is whether this result would be observed in adaptation of other kinds of movements, such as finger control. Clearly, there are differences in which brain areas are involved in these different kinds of movements. Walking heavily engages brainstem circuits, which may make its control more unique. Along this line, a third question is what neural substrates are important for adapting temporal versus spatial control of walking and do they control other movements (i.e., reaching)? We are particularly interested in knowing whether spinal circuits are involved in this adaptive process. Previously, we have shown that the cerebellum is necessary for walking adaptation (Morton and Bastian, 2006), but have not been able to probe spinal contributions directly. Finally, do children learn better or faster when trained for longer periods of time (days rather than minutes)? This would obviously be more relevant for rehabilitation, since training is done over days to weeks.
Another set of recent studies from our group has used a similar split-belt treadmill paradigm in healthy adults to explore whether we can change the rate of walking adaptation, and whether we can promote generalization of the adapted pattern to overground walking. These questions are important not only to understand the adaptive process but also to determine how best to leverage this type of learning for rehabilitation. We would like to optimize the amount of adaptation, how long it lasts, and its transfer to more natural walking conditions.
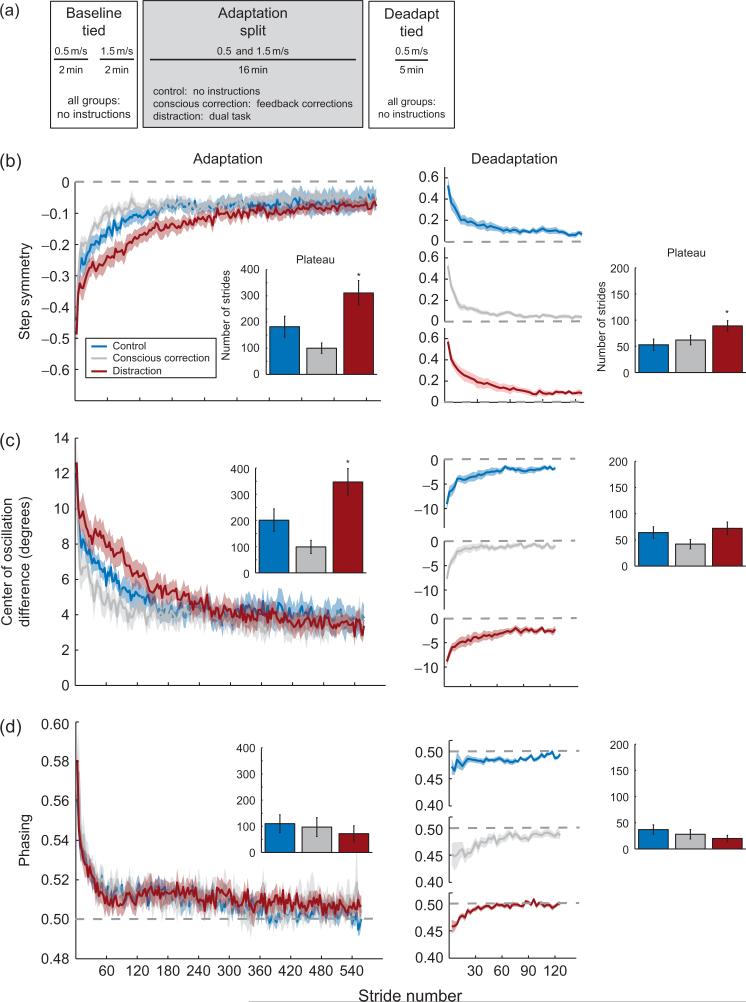
We first tested whether adaptation and deadaptation rates could be altered by (a) asking people to consciously correct their walking pattern to aid adaptation, or (b) distracting them with a dual task during adaptation (Malone and Bastian, 2010). Figure 3a shows the basic paradigm—subjects were tested in baseline tied-belt conditions with no instruction. We then asked each of the three groups to (1) consciously correct their step sizes to be equal by watching their feet on a video screen, (2) perform a secondary task while watching a video, or (3) simply walk with no instructions or distraction. Here, we assessed the adaptation and deadaptation rates. The deadaptation rate is perhaps more interesting in this particular study because all manipulations (e.g., distraction, conscious corrections) were removed in the deadaptation period.
Fig. 3.
(a) Experimental paradigm showing the periods of split-belt walking and conditions. In baseline, tied walking all groups were given no specific instructions. Subjects were divided into three groups for adaptation (split belts). The conscious correction group (N=11) was instructed on how to step more symmetrically and given intermittent visual feedback of their stepping during adaptation. The distraction group (N=11) was given an auditory and visual dual-task they were asked to focus on. The control group (N=11) was given no specific instructions. In deadaptation (tied belts), all groups walked under “Control” conditions, where the visual feedback and distracter were removed. (b) Adaptation and deadaptation curves for step symmetry. Average adaptation curves for the three groups, with standard errors indicated by the shaded area. Baseline values are subtracted out from curves (i.e., symmetry is indicated by a value of 0). Average deadaptation curves for the three groups. Recall that all groups deadapted under the same condition (no feedback or distraction). Curves are shown individually to more clearly illustrate the plateau level. Bar graphs represent group averages for adaptation and deadaptation rate, assessed by the number of strides until plateau is reached (i.e., behavior is level and stable). Note that with step symmetry, the conscious correction group adapted faster, and the Distraction group adapted slower. However, retention was improved with the Distraction group because they took longer to deadapt, despite removal of the distracter. (c) Adaptation and deadaptation curves for the center of oscillation difference. Average adaptation curves for the three groups plotted as in (b). Trends seen in the center of oscillation difference are comparable to those seen in step symmetry. (d) Average adaptation and deadaptation curves for phasing, plotted as similar to (b). Note that our interventions did not significantly affect the rate of adaptation or deadaptation of phasing.
Figure 3b illustrates the main result from this study—adaptation and deadaptation of step symmetry were faster with conscious corrections and slower with distraction (Malone and Bastian, 2010). Thus, conscious corrections during adaptation sped the process up, but this did not lead to better retention in deadaptation. In contrast, distraction slowed the adaptation process, but resulted in better retention since deadaptation was also slower. This demonstrates that the conditions under which the nervous system learns are important, as they strongly influence the pattern of unlearning. In this work, we also found that the conscious correction and distraction effects were due to changes in the rate of adapting the spatial pattern, but not the temporal pattern (Fig. 3c and d). In other words, conscious corrections to change the step size were implemented by changing where the foot was placed, and not when it was moved there. Interestingly, distraction slowed spatial adaptation only, despite the fact that there was no indication of what to change in this condition—subjects could have changed either the spatial or temporal components of walking. These results suggest that adaptation of spatial locomotor control is more flexible and accessible than temporal control.
One interpretation of this finding is that different neural structures are involved in these two control processes, and that spatial control is more easily accessed using conscious cerebral resources. However, timing control may operate at a lower level in the nervous system, such as the brainstem or spinal cord, and is therefore less accessible through cerebral resources. The cerebellum, which is known to influence both spatial and temporal control, has projections to both cerebral motor areas and brainstem regions. Thus, there may be distinct anatomical circuits for these adaptive learning processes.
These results bring up several important questions. First, does distraction lead to better day-to-day retention of newly learned movement patterns? In other words, if a person is distracted during training, will the effects last longer? Second, in rehabilitation, people are often instructed how to move and asked to “try” to move in the desired way. However, our results suggest that patients would retain more of what they learn if they do not use conscious or voluntary resources. Therefore, it is possible that a more effective rehabilitation strategy may be to put patients into a situation that drives the learning of a new pattern without having to use voluntary effort. In other words, perhaps patients would learn better if they were not “trying” so hard. Given our interest in patient rehabilitation, a third interesting question is whether similar effects of conscious correction versus distraction would be observed in patient populations. Can people who have a cerebral stroke, for example, benefit in any way from distraction? Do they even respond in the same way to conscious efforts? In sum these issues have important significance for rehabilitation of walking.
Another important aspect of motor learning is how well the adapted pattern transfers to untrained environments or situations. The amount of transfer, or generalization, indicates how much of the adapted circuit is used in different situations. This question of generalization of device-induced motor learning across different environments has been addressed in recent studies (e.g., Berniker and Kording, 2008; Cothros et al., 2009; Kluzik et al., 2008; McVea and Pearson, 2007; Wolpert et al., 1998). Here, we discuss it in the context of human locomotion. Our prior work has shown that healthy subjects transfer little of the split-belt adaptation to overground walking (Reisman et al., 2009). Instead, it seems that they link the adapted pattern to the context of being on the treadmill. Given our interest in using split-belt treadmills to rehabilitate walking patterns in people with brain lesions, we wanted to understand if we could improve the generalization of split-belt treadmill adaptation to more natural walking situations. We hypothesized that treadmill walking has some unique features that provide very strong contextual cues to people as they walk on it, the main one being the mismatch between vision and proprioception. Specifically, when walking on a treadmill, proprioception tells us that we are moving, but vision tells us that we are not. This is a highly unusual situation, and the nervous system may therefore link the adapted pattern to this particular context.
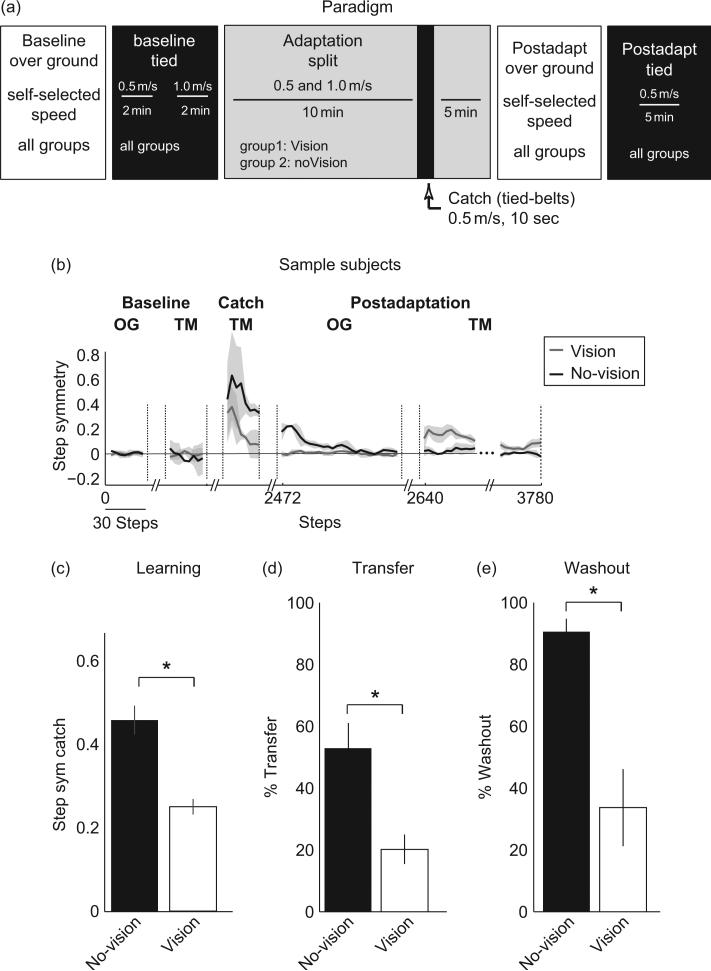
We tested whether removing vision during split-belt treadmill adaptation could improve overground transfer of the new walking pattern (Torres-Oviedo and Bastian, 2010). Subjects walked with or without vision during an adaptation and transfer experiment. Figure 4a illustrates the basic paradigm—subjects walked overground and on the treadmill before and after split-belt adaptation. They were given a “catch” trial of tied-belt walking during adaptation so that we could assess how much they had learned prior to testing the transfer of adaptation effects to overground walking. Figure 4b shows individual subject data for step symmetry from periods of this experiment. Both subjects adapted, though the aftereffects during the catch trial in the subject from the no-vision group were larger than the one from the vision group, indicating that this first subject learned more. Transfer to overground walking was also markedly different between these subjects—the one without vision transferred much more than the one with vision. When subjects returned to the treadmill there was again a striking difference—the subject with no vision showed much greater washout of the adapted pattern compared to the subject with vision. Group data for step symmetry are shown in Fig. 4c–e. Similar changes were observed in phasing (i.e., temporal control).
Fig. 4.
(a) Overall paradigm. In all groups, baseline behavior was recorded overground (OG) and subsequently on the treadmill with the two belts moving at 0.7 m/s. Then subjects were adapted for a total of 15 min, during which one belt was moving at 0.5 m/s and the other belt at 1 m/s. After 10 min of adaptation, a 10-s catch trial was introduced, in which both belts moved at 0.7 m/s. Subjects were readapted (i.e., belts’ ratio at 2:1) for five more minutes before they were asked to walk OG, where we tested the transfer of treadmill adaptation to natural walking. Subjects were transported on a wheelchair to a 6-m walkway where they walked back-and-forward 15 times. All steps on the walkway were recorded except for those when subjects were turning to return to the initial position. Finally, subjects returned to the treadmill where they walked for 5–10 min at 0.7 m/s to determine form the remaining aftereffects the extent to which walking without the device washed out the learning specific to the treadmill. (b) Spatial symmetry (i.e., symmetry in step lengths of the two legs) of sample subjects of the vision and no-vision group when walking on the treadmill (TM) and OG during baseline, catch, and deadaptation periods. Behavior of two sample subjects is shown: one walking with vision (gray trace) and one walking without vision (black trace). Lines represent the running average using a three-step window±SD (shaded area). No differences in step symmetry were observed preadaptation when subjects walked with and without vision on the treadmill or OG. However, the subject that walked without vision had larger aftereffects on the treadmill during the catch trial (i.e., more learning), more transfer of treadmill learning to OG walking, and more washout of learning specific to the treadmill than subject that walked with vision. (c) Aftereffects on treadmill during catch trial for vision and no-vision groups. Subjects that trained without vision had significantly larger aftereffects—greater learning, than subjects that trained with vision. Bars’ height indicates the averaged aftereffects of the first three steps during the catch trial across subjects±SE. (d) Transfer of adaptation effects to OG walking. (e) Washout of treadmill spatial aftereffects following OG walking. Removing vision during training had a significant effect on the washout of step symmetry aftereffects specific to the treadmill. Step symmetry transfer and washout are expressed as a percentage of the aftereffects on the treadmill during catch. Bars’ height indicates the average across subjects±SE of % transfer and % washout for the first three steps OG or when returning to the treadmill. Figures in all panels were adapted from Torres-Oviedo and Bastian (2010). *p<0.01.
This work demonstrates that altering the sensory context can change the extent to which treadmill learning transfers to natural overground walking. We speculate that this could be for a couple of reasons. One possibility is that it changes a person's perception of the source of the error during adaptation (i.e., credit assignment) from the treadmill to the person (Berniker and Kording, 2008). If this were the case, the person would learn to associate the newly learned calibration to one's faulty movements, rather than to being on the treadmill. A second is that closing the eyes may have led to an upweighting of proprioceptive information. It is possible that errors derived from proprioceptive signals encode learning in intrinsic (i.e., body centered) coordinates and thus learning could be more easily carried with the person when they move off of the treadmill.
These results also lead to several questions. First, is it necessary to actually remove vision to improve transfer, or can this be done through other means? For example, if visual and proprioceptive information were congruent during split-belt adaptation, would transfer to overground walking improve? We have started to study this using optic flow patterns displayed to the individuals as they walk. We can manipulate optic flow to match or oppose the proprioceptive signals and would like to be able to understand how these two sources of information are integrated. If it is important to upweight proprioceptive information from the legs to improve transfer to natural walking, adding congruent vision may not help. However, if it is important to remove the sensory mismatch and make the adaptation context more similar to natural walking situations, then adding optic flow may improve it. Another important question is whether individuals with stroke will show a similar effect from changing the sensory context during split-belt treadmill adaptation. Our previous work has shown that people with cerebral lesions caused by stroke (e.g., middle cerebral artery distribution), can adapt their walking pattern and show better transfer to overground walking than controls (Reisman et al., 2009), even with eyes open. Will changing the visual information to match the proprioceptive inputs improve this transfer? We think that it is unrealistic to adapt stroke patients without vision and, therefore, would like to use visual displays to manipulate visual information during this task. Finally, it is not understood whether credit assignment or the ability to assign errors to the environment versus the body is developed throughout childhood. Therefore, we would like to know how children transfer split-belt treadmill adaptation. Does an immature nervous system transfer newly adapted patterns more readily? If so, does this mean that they have difficulty learning context-dependent walking calibrations? These questions are important for reaching our ultimate goal of optimizing this process for long-term training of adults and children with brain damage.
To summarize, we discussed three experiments about walking adaptation. First, humans develop the ability to adapt walking patterns throughout childhood. Children are slower to adapt spatial elements of the walking pattern, and this improves throughout childhood until adolescence. In contrast, temporal adaptation is remarkably conserved even in 3-year olds. This distinction suggests that neural circuits that develop at different times might be involved in spatial versus temporal adaptive processes. Walking adaptation is also a highly automatic process. However, it can be sped up when healthy adults try to consciously modify their walking pattern, but this does not improve retention. Conversely, when distracted during adaptation, healthy adults learn slower, but retain the walking pattern longer. These conscious correction versus distraction effects are due to changes in spatial control of the walking pattern, suggesting that it is accessible through cerebral mechanisms. Finally, we show that transfer of learning from the treadmill to natural overground walking is greatly enhanced by removing visual cues specific to the treadmill context. This may be due to changes in sensory reweighting or to changes in credit assignment. Many other questions remain to be answered, as illustrated throughout this review, which we hope will ultimately lead to more rational bases for walking rehabilitation.
Acknowledgments
This work was supported by NIH Grants F32 NS063642 and R01 HD048741.
References
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nature Neuroscience. 2008;11(12):1454–1461. doi: 10.1038/nn.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: Split-belt treadmill adaptation and feedback control. Brain. 2009;132(Pt 3):722–733. doi: 10.1093/brain/awn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Wong J, Gribble PL. Visual cues signaling object grasp reduce interference in motor learning. Journal of Neurophysiology. 2009;102:2112–2120. doi: 10.1152/jn.00493.2009. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Christensson M, Psouni E. A unifying model for timing of walking onset in humans and other mammals. Proceedings of the National Academy of Sciences USA. 2009;106(51):21889–21893. doi: 10.1073/pnas.0905777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: What determines whether we learn an internal model of the tool or adapt the model of our arm? Journal of Neurophysiology. 2008;100:1455–1464. doi: 10.1152/jn.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. Journal of Neurophysiology. 2010;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea D, Pearson K. Contextual learning and obstacle memory in the walking cat. Integrative and Comparative Biology. 2007;47:457–464. doi: 10.1093/icb/icm053. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. The Journal of Neuroscience. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: What can be adapted and stored? Journal of Neurophysiology. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(Pt. 7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabilitation and Neural Repair. 2009;23(7):735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Seeing is believing: Effects of visual contextual cues on learning and transfer of locomotor adaptation. The Journal of Neuroscience. 2010;30(50):17015–17022. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. Journal of Neuroscience. 2011;31:3055–3065. doi: 10.1523/JNEUROSCI.5781-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Miall R, Kawato M. Internal models in the cerebellum. Trends in Cognitive Sciences. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]