Abstract
Novel antidepressants are needed to enhance the health and quality of life of the hundreds-of-millions of depressed individuals worldwide who remain inadequately treated with today’s approaches. In reality, no new class of antidepressant medication has been introduced in over 50 years. This insufficiency of current drug treatments is evident to those eager to pursue invasive experimental options like that of deep brain stimulation (DBS). Encouragingly, human brain imaging studies and animal work implicate strong relationships between depressive symptoms and patterns of brain activity, which are now open to more empirical assessments using optogenetics. Recent advances in optogenetics permit control over specific subtypes of neurons, or their afferent or efferent projections, and can greatly further our understanding of the neural mechanisms involved in depression and the mechanism of action of DBS and perhaps chemical antidepressants. Here, we discuss how optogenetic tools are being used to answer a broad range of molecular, cellular, and circuit-level questions pertaining to depression which, up until now, have been resistant to other experimental approaches. The emergence of optogenetic technology, when combined with the best-validated animal models of depression, will dramatically increase knowledge about the basic neurobiology of depression as well as facilitate the development of more effective antidepressant treatments.
Keywords: Optogenetics, Depression, Affective Disorders, Deep Brain Stimulation, Prefrontal Cortex, Nucleus Accumbens
Introduction
Clinical depression poses a serious social and economic threat to the United States, with most conservative estimates reporting that at least 30 million adults are affected by this illness (1). Globally, depression accounts for more lost productivity compared to any other disorder (2). Available treatments (including pharmacotherapies, psychotherapies, and several so-called somatic interventions such as electroconvulsive therapy, light therapy, vagus nerve stimulation, and transcranial magnetic stimulation), while effective for many people, leave more than half of affected individuals incompletely treated. More than two thirds of depressed patients remain symptomatic after an initial intervention and twenty percent of these fail to respond to any intervention (3–5). Moreover, all antidepressant medications available today are based on mechanisms of action discovered by serendipity ∼6 decades ago (6).
Novel treatment strategies are long overdue. Prior to new developments, however, a greater understanding of the neurobiological mechanisms of depression is required. Fortunately, structural and functional brain imaging studies, as well as postmortem biochemical analyses, have already been instrumental in identifying neurobiological differences that separate depressed patients from unaffected individuals. This work has taught us that depression involves numerous and converging pathways in the brain, including cerebral cortical, hippocampal, amygdala, striatal, and other subcortical circuitry (6–9). Thus, depression is not a single entity, but rather is comprised of numerous disease states, and it has to date not been possible to parse definitive subtypes of depression based on available approaches.
Despite these limitations, knowledge of the brain circuitry involved in depression has lead to the experimental use of deep brain stimulation (DBS) in treating severely affected patients. Two brain regions, each implicated in depression, have been shown to be effective: subgenual area 25 (Cg25), a region of the anterior cingulate cortex—part of the prefrontal cortex (PFC), and the anterior limb of the internal capsule which is thought to involve the nucleus accumbens (NAc), a region of ventral striatum (10–16). Transcranial magnetic stimulation also shows some efficacy for the treatment of depression (17). However, the mechanism by which stimulation of these regions alleviates symptoms of depression is unknown. For example, it is unclear whether the antidepressant effects of DBS are mediated by activation of neurons in the stimulated region, by activation of passing axons, or even by the inactivation of local neurons through depolarization blockade.
The recent development of optogenetic tools has made it possible for the first time to begin to address some of these questions (18). By combining such tools with animal models of depression, work is beginning to causally relate activity in the brain’s limbic circuitry with depression- and antidepressant-like actions. Here we review the small handful of studies to date, including more preliminary reports, employing optogenetic tools in depression models as well as the tremendous potential of this approach in future years.
How Can Optogenetics Provide Answers?
Most regions of brain contain several subtypes of excitatory and inhibitory neurons. Subsets are projection neurons, while others are local interneurons. Moreover, the brain contains numerous types of glial cells, which play a critical role in modulating neuronal function. Activation or inhibition of each cell-type would be expected to induce a distinct functional, including behavioral, response, although the details in most cases remain poorly understood.
Optogenetics is proving to be uniquely useful in unraveling this information by overexpressing light-sensitive proteins within particular cell-types of interest (Figure 1). This is accomplished by the use of viral vectors that infect only certain types of neurons using cell-type-specific promoters such as CAMKII, which will localize optogenetic proteins to excitatory neurons (19). It is also accomplished by targeted use of viral vectors that express their transgenes in a Cre-dependent manner (20) in combination with mice that express Cre recombinase in specific cell-types, for example, dopamine neurons or striatal medium spiny neurons expressing D1 vs. D2 receptors. (21–24). Additionally, optogenetics can be used to target a particular afferent pathway to a brain region of interest, as just one example, glutamatergic inputs to the NAc coming from the basolateral amygdala as opposed to other afferent regions (25).
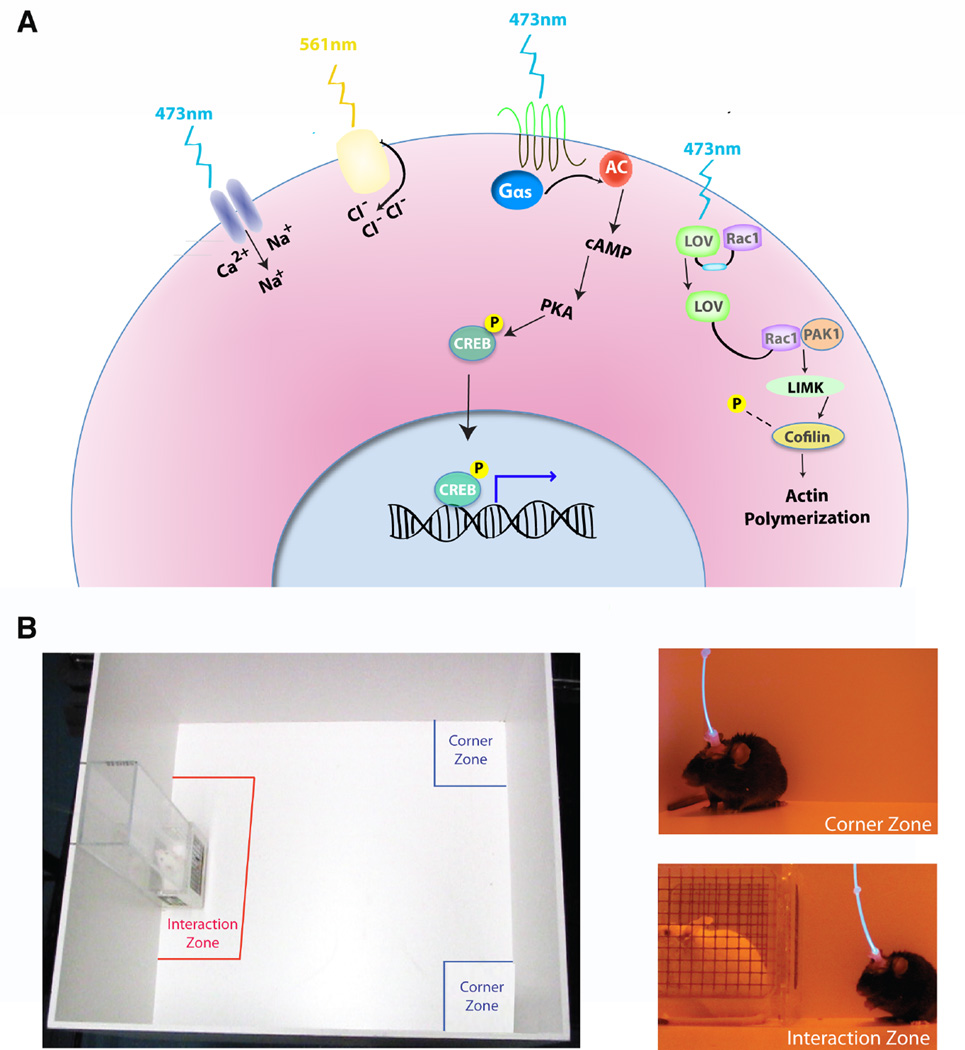
Figure 1.
Optogenetic tools can be used in vivo while assessing rodent affective-like behaviors. (A) Depicted is a cartoon illustrating the current optogenetic expression systems used in vivo in rodent brains. Here we include the many versions of blue light (473 nm) activated channelrhodopins, the cation channels which depolarize neurons when exposed to blue light. Yellow light (561 nm) is used to activate haloprhodopsins, the chloride pumps which inhibit neurons. The OptoXRs, chimeras in which rhodopsin (shown in green) is fused to the intracellular portion of a GPCR (i.e., a Gs-coupled GPCR, shown in black); activation by light activates intracellular signaling cascades, eventually leading to transcriptional regulation in the nucleus (e.g., via increasing the phosphorylation of the transcription factor CREB). Finally, the LOV domain can sterically inhibit a specific protein (e.g., Rac1) to which it is fused; with exposure to blue light, an allosteric confirmation releases LOV from Rac1 and Rac1 signaling occurs eventually resulting in alterations in actin dymamics. (B) Depicted here is an example of the use of optogenetics with a common behavioral assay, social interaction, to evaluate depression-like behavior after chronic social defeat stress. Mice are tested for time spent in the interaction zone (near the novel mouse) or time spent in the corner zone (away from the novel mouse). Panels on the right show examples of a mouse avoiding the novel mouse (top right) or interacting with the novel mouse (bottom right) when neuronal activity is controlled in vivo with light optics (83).
The frequency and temporal pattern of cellular activation can also be tightly controlled using optogenetics. Channelrhodopsins [e.g., channelrhodopsin 2 (ChR2)] are cation channels expressed in the plasma membrane: when activated by ∼470 nm waveforms of light (i.e., blue light), in the case of ChR2, these channels open, followed by a rapid depolarizing current. By utilizing different patterns of light stimulation, it is possible to mimic tonic vs. burst patterns of neuronal firing that are seen in vivo under different physiological circumstances (21). Neuronal inhibition (i.e., hyperpolarizing current) can be readily accomplished as well via the expression of halorhodopsin (NpHR), a chloride pump activated by waveforms of light in the ∼570 nm range (i.e., yellow light) (26,27). By expressing both proteins within the same neurons, it is possible to study the behavioral consequences of activating or inhibiting the same ensembles of neurons (27).
Optogenetics is not limited to the control of neural activity per se, as there are many applications of this technology, which are expanding at an exciting rate (28,29). Some examples that appear applicable to the study of depression include optogenetic tools that control glutamate receptor signaling, G protein-coupled receptor signaling, and intracellular trafficking of signaling molecules (Figure 1) (30–35). Many cellular effectors of these various optogenetic tools have the potential to serve as novel targets for the treatment of depression (36), which emphasizes the great potential of this technology in drug discovery efforts.
Historical Precursors to Optogenetic Studies in Depression
Before the advent of pharmaco- and psychotherapies, psychosurgical methods were used to treat depressive symptoms as well as other neuropsychiatric syndromes. Archaeological evidence (i.e., human skulls with drilled holes) suggests that intracranial surgeries date back at least 5,000 years to Northern Africa and Europe (37–39). Centuries later, the frontal leukotomy (i.e., frontal lobotomy) was introduced. In 1936, Egas Moniz, a Portuguese neurologist, described the PFC as the “psychic center of a person.” He suggested that separating connectivity between cortex and other brain regions would cure psychological pathologies. Moniz, who earned a Nobel Prize in 1949 for introducing leukotomies, initially injected alcohol into the white matter of the anterior frontal lobes to ablate this tissue before he went on to develop more intricate procedures (37,39).
Frontal leukotomies were replaced by more focalized lesions using stereotactic techniques. Areas targeted by these surgical procedures included the cingulate gyrus (i.e., cingulotomy), the subcaudate area to disrupt projections from orbitofrontal cortex to subcortical structures (i.e., subcaudate tractotomy), the anterior limb of the internal capsule to disrupt connections between the frontal lobes and the mediodorsal nucleus of the thalamus (i.e., anterior capsulototomy), and ablative surgeries aimed at combinations of these brain regions (i.e., limbic leucotomy) (40). Interestingly, such focalized ablative surgeries improved some depressed symptoms despite the invasiveness of these approaches.
High frequency DBS was originally used to treat severe motor disorders (41–43), and has emerged as an attractive alternative to the ablative surgeries also once used in the treatment of Parkinson’s disease, essential tremors, and dystonias (44). Given the successes of DBS in treating motor disorders, combined with imaging studies in depression, DBS procedures were postulated as a new strategy in treating depression (12,13). The first report appeared in 2005 (45): chronic high frequency (130 Hz) DBS was applied to the white matter adjacent to the Cg25, based on evidence that this region is metabolically overactive in depressed patients (46,47). Results from this initial study were striking: antidepressant effects were seen in roughly half of the treated patients and were accompanied by diminished activity in Cg25 (45,48). Since that time, DBS has been conducted in many other candidate brain areas, including the NAc, anterior limb of the internal capsule, inferior thalamic peduncle, and habenula (10,15,16,49,50). Many of these studies combined the use of DBS with functional imaging to examine its effects on regional patterns of brain activity. Brain areas outside of the stimulation site were also affected by DBS, consistent with the involvement of multiple circuits in the treatment and pathogenesis of depression (10,15,16, 49,50).
Similar to primate anterior cingulate cortex, the rodent medial PFC is critically involved in the expression of emotional behaviors (51–53). DBS in the ventromedial PFC of rodents also has prominent antidepressant-like effects (54). In fact, rodent DBS studies laid the groundwork for improving future cortical stimulation approaches by identifying many of the stimulus parameters required for producing antidepressant-like effects. High frequency stimulation (∼100 Hz) at an intensity of ∼200 µA into the ventromedial PFC can provide maximal antidepressant-like responses (54). Importantly, the behavioral effect of high frequency stimulation in the medial PFC is likely due to cortically mediated control over other limbic brain regions, including subcortical monoaminergic nuclei, because lesions that deplete serotonin diminish the antidepressant-like effects of medial PFC stimulation (55). Rodent DBS studies will continue to be useful for detecting the antidepressant potential of controlling the activity of additional brain structures. For instance, high frequency DBS in the NAc enhances rhythmic and synchronous inhibition within ascending cortical and other subcortical structures (56).
Optogenetics can now be used to extend these stimulation studies in humans and rodents to elucidate the neurobiological underpinnings of depression-like behavior in animal depression models as well of the antidepressant-like effects of DBS in these models. This approach has already been used to understand DBS action in Parkinson’s disease: optogenetic studies of rodent Parkinson’s models indicated that the therapeutic effect of DBS of the subthalamic nucleus is achieved through activation of axonal inputs to this target region (57), a finding consistent with indirect interpretations from earlier DBS studies in human patients (58).
The ability to apply this technology to depression is derived from at least six major interdisciplinary achievements. First, decades of human brain imaging and postmortem results have identified pertinent brain areas in depression (e.g., subregions of cerebral cortex, NAc, and habenula). Second, the advent of in vivo methods for controlling protein expression in brain, including the use of viral-mediated gene transfer and inducible transgenic animals, has allowed for examining a loss or gain of function for virtually any gene within a defined brain region. Third, ethologically valid animal models of depression, particularly those involving chronic social stress, have high levels of construct, face, and predictive validity which allows for the examination of neural mechanisms involved in depression as well as its treatment. Fourth, improvements in severe cases of depression after DBS confirm a functional role for certain key brain regions in antidepressant responses, and define frequency and patterns of stimulation necessary for these actions. Fifth, techniques to record the functional activity of specific brain areas (or multiple areas simultaneously) in vivo have been incorporated in animal models of psychiatric disease (59). Finally, advancements in optogenetics, as stated earlier, make it possible to manipulate specific cell-types, individual afferent and efferent pathways, and patterns of activity generated within discrete brain regions.
Use of Optogenetics in Animal Models of Depression
The ability to use optogenetics to understand depression and its treatment depends on the availability of useful animal models. In the absence of bona fide genes that cause depression in humans with high penetrance and specificity, animal models of depression have focused on exposing normal rodents to different forms of chronic stress. While the validity of all such models must be evaluated with caution and skepticism (60), there are several rodent models that demonstrate some etiologic and face validity as well as neurobiological abnormalities that are validated in postmortem tissue from depressed humans (61,62). The temporal nature of neural plasticity leading to depressive-like behaviors can be studied in these animal models using in vivo imaging and electrophysiological recording techniques (63–65). The time is ripe, therefore, to use optogenetics to directly control patterns of neural activity to understand circuit-level events related to depression-related behavioral abnormalities and their reversal by antidepressant treatments.
We recently employed the chronic social defeat stress model to explore antidepressant-like actions of optogenetic stimulation of the PFC (66). In this model, 10 days of continuous social stress induces a behavioral syndrome characterized by: social avoidance; anhedonia-like symptoms including reduced preference for sucrose, high fat diet, and sexual behavior; anxiety-like symptoms; disrupted circadian rhythms; a hyperactive hypothalamic-pituitary-adrenal axis; and a metabolic syndrome characterized by increased eating and weight and insulin and leptin resistance (67–70). Many of these symptoms are long-lived and can be reversed by chronic, not acute, administration of antidepressants, such as imipramine or fluoxetine; anxiolytics are ineffective (67, 71). About a third of mice subjected to chronic social defeat stress avoid most of these symptoms; we refer to these mice as “resilient” compared to the majority which are “susceptible” (68). Many of the neurobiological changes that occur in limbic regions of brain in susceptible mice have been validated in the brains of depressed humans (67,68,72–75).
In our optogenetic study with this model, we focused on medial PFC, based on human data for the importance of this region in depression and the antidepressant effects of DBS (see above), and based on prior work that has shown that stress can modify neurons in this brain region in rodent models (76–79). Long-lasting deficits in the functional activity of the ventral portion of the medial PFC have been inferred by the reduced expression of immediate early genes after social defeat stress in rats (79). In line with these results, we found that within postmortem tissue collected from the human anterior cingulate cortex zif268 and arc mRNA expression were significantly downregulated in clinically depressed cases (66). In parallel, we found that chronic social defeat stress in mice causes decreased levels of zif268 and arc mRNA in the ventral medial PFC, one of several regions of the PFC that may have some functional homology with the human anterior cingulate cortex (80). These effects predominate in mice that are susceptible to defeat stress. Our hypothesis is that deficits in immediate early gene expression reflect reduced cortical activity induced by stress; specifically, reduced medial PFC burst firing could result from social stress in susceptible mice (81,82). We hypothesize further that these abnormalities may contribute to the emergence of emotional disturbances. Consistent with this, we found that driving a 100 Hz pulse with an inter-pulse-interval of a few seconds, essentially a burst-like pattern of cortical activity, using in vivo optogenetic stimulation of the medial PFC, induced rapid antidepressant-like responses in susceptible mice (Figure 1) (66). These antidepressant-like effects brought on by optogenetic stimulation did not disrupt other ongoing behaviors, such as locomotor activity, generalized anxiety-like responses, or social memories. These data closely follow the antidepressant effects of direct DBS in medial PFC of rats, mentioned earlier (54). Further work is now needed to confirm that the rapid antidepressant action of optogenetic stimulation of medial PFC is mediated via activation of local pyramidal neurons in the stimulated region as well as to characterize the circuitlevel changes in several other brain regions that result from this action.
Much of our work on the social defeat model has implicated the brain’s reward circuitry, dopaminergic projections from the ventral tegmental area (VTA) to the NAc, as also being crucial in the development of susceptibility vs. resilience (67, 68). We previously demonstrated that susceptibility to social defeat stress is associated with increased firing of VTA dopamine neurons in brain slices and in vivo (68,83). Using optogenetics, we recently demonstrated that activation of these neurons in awake, behaving mice promotes susceptibility (84,85). We are now using optogenetics to selectively activate subpopulations of VTA dopamine neurons that project to distinct limbic brain regions to identify the specific dopaminergic pathway that mediates this effect. It will be interesting to test whether optogenetic suppression of VTA dopamine neuron activity reverses susceptibility and induces a more resilient state. However, recent observations reveal that stimulation of DA neurons in the VTA can also promote antidepressant-like effects in mice in the chronic mild stress paradigm (86). These data suggest plausible differences in the neurobiological mechanisms underlying responses to different forms of stress (e.g., chronic social defeat stress vs. chronic mild stress) and one cannot rule out that different populations of DA neurons projecting to different brain regions (e.g. NAc vs. PFC) may play selective roles in different types of stress. These distinct DA projection pathways can now be examined with optogenetics.
Optogenetic studies of the NAc are also in order, given the promise of DBS to this region in depressed patients. One rationale for pursuing such studies is that DBS and optogenetic activation of the NAc similarly modify the rewarding effect of drugs of abuse, like cocaine (23,87). High rates of comorbidity between addiction and depression, along with the fact that these two syndromes have significantly overlapping mechanisms in the brain’s reward circuitry, further substantiates the need for optogenetic exploration of this region in the context of depression models. Recent optogenetic studies of the NAc in addiction models, for example, have defined very different roles played by the activation of the two major subpopulations of medium spiny neurons—those expressing D1 vs. D2 dopamine receptors, and of cholinergic interneurons, in mediating the addicting properties of cocaine (23,88). Examining the influence of these various celltypes in depression models is now a high priority.
The hippocampus has long been considered to be an important structure in depression (89). Here, treatment with conventional antidepressants such as fluoxetine or imipramine significantly alters cellular activity and the survival of newborn neurons (90– 93), and these events are linked to the behavioral effects produced by these drug treatments (90). The use of advanced high-speed imaging techniques in hippocampal brain slices further highlights the importance of hippocampal subdivisions during both the expression of a stress-induced depressive-like behavior, and subsequent treatment with either fluoxetine or imipramine (63). Specifically, following exposure to chronic mild stress, activity of the dentate gyrus decreases relative to increases in CA1 activity. A reversal of both stress-induced effects in the hippocampus can be achieved with chronic antidepressant treatment (63). This decreased dentate gyrus and increased CA1 activity extends earlier observations whereby depression has been associated with elevated hippocampal output and decreases in hippocampal input (89,90). DBS reduces activity within the stimulated region of anterior cingulate cortex, which receives major excitatory input from the hippocampus (46). Therefore, this pathway is likely critical for the expression of some depressive symptoms, and optogenetics will be useful in directly testing this possibility.
While most of the work using optogenetics in affective-like behavior has pertained to mice, this technique is also applicable to rats. The reason for the current bias towards studies in mice is simple. While the field has moved rapidly toward understanding the roles for specific cell-types involved in behavior, the tools for enabling such cell-type specificity has been sparse for rats. These lack of tools may change as transgenic rat lines further develop, particularly those involving Cre drivers in specific cell-types or those labeling activated neurons (94, 95). Advantages of employing optogenetic approaches in rats include the more sophisticated behavioral assays available in this species and the larger size of the rat brain, which makes parsing neural circuitry more feasible.
The Future of Optogenetics in Depression
Neuroimaging studies are useful for identifying brain areas that potentially contribute to symptoms of depression. It is now essential that we better assess such aberrant patterns of activity in specific cell-types and across neural circuits, as this is feasible in validated animal models of depression. Current high-speed imaging and in vivo imaging technologies like optical microendoscopy or bioluminescence imaging of activity-related genes or Ca2+ probes (63,64,96) can be combined with cell-type specific and circuit specific genetics (20). These examples will be useful for defining changes in neuronal or glial activity that directly control depressive-like behavior as well as mechanisms of antidepressant action.
Using optogenetics to determine molecular correlates of depression is particularly promising. This can be achieved by changing neuronal activity in specific cell-types with optogenetics to modify depression-like behaviors followed by profiling gene expression changes in the manipulated regions or in regions to which they send inputs. For example, we show that loss of BDNF signaling in NAc is correlated with enhanced activity in both the D1 and D2 neuronal subtypes in this region by use of optogenetics (23). We further found that optogenetic stimulation of D1 type neurons decreases levels of the activated form of ERK1/2, a downstream target of BDNF signaling. More recently, we were able to reverse a blunted behavioral response to morphine, seen after increasing BDNF signaling in the VTA, by optogenetic control of VTA projections to the NAc (97). These types of studies in the drug abuse reward circuitry can now be translated to depressionlike behaviors in rodents.
Given our increasing knowledge of the complex molecular events that mediate depression-like behavior in animal models (98), the ability to activate or inhibit a range of signaling proteins with the precise temporal and spatial specificity afforded by optogenetic approaches will be a major boon to the study of the molecular basis of depression. Thus, it is likely that, over the coming years, we will see increased optogenetic dissection not only of neuronal activity, but also of a host of signaling proteins involved in the pathophysiology of depression and the mechanisms of antidepressant action (Figure 1). The OptoXRs, G protein-coupled receptor (GPCR)- opsin chimeras, in which the intracellular loops of rhodopsin are replaced with those of specific GPCRs, represents a viable approach since they have led to insights in GPCR signaling in the NAc in reward behaviors (31). More recently, another group has produced a chimera of rhodopsin and the serotonergic GPCR, Gi/o-coupled 5-HT1A, by tagging rhodopsin with the 5-HT1A C-terminus (99); this light responsive 5-HT1A receptor will be extremely useful given the role of serotonergic signaling in depression-related phenomena. Researchers are also making use of photoactivated nucleotidyl cyclases from bacteria to manipulate cAMP and cGMP levels using light in living cells (99,100).
Additionally, the light-oxygen-voltage (LOV) protein domain, from Avena sativa phototropin, which can allosterically regulate proteins fused to this domain upon exposure to blue light, is a very promising optogenetic tool for controlling molecules. LOV sterically inhibits proteins to which it is fused in the dark and, upon exposure to blue-light, a helix linking LOV to the protein becomes unwound, relinquishing the protein so that it is then able to interact with its binding proteins (100–102). This group fused LOV to Rac1, a small GTPase which plays an important role in regulating actin cytoskeletal dynamics, and demonstrated that photoactivatable-Rac1 (PA-Rac1) in the presence of blue light can produce cell protrusions and ruffling, alter cell motility, and control the direction of cell movement in cell cultures; it was also recently shown to redirect migrating cells in Drosophila (100,102). Our group activated PA-Rac1 in vivo in mouse brain to investigate its role during critical time periods of cocaine exposure and found robust regulation of cocaine-induced behaviors and cytoskeleton regulation when PA-Rac1 was activated in NAc (103); such studies can now be translated to depression-like behaviors. Another group used the LOV domain to target transcriptional regulation by fusing LOV to the Escherichia coli trp repressor, which they term LovTAP, and this fusion protein selectively binds DNA when illuminated with blue light (104,105) The ability to translate this to mammals and create photoswitchable molecules to temporally regulate transcriptional activity is a very powerful prospect, considering the profound role of transcriptional and epigenetic regulation in depression and other neuropsychiatric disorders (8,36,71,73–75,98).
Finally, the promising therapeutic effects of DBS in depression and other neuropsychiatric disorders, and of optogenetics in animal models of these illnesses, raises the question of whether optogenetics is itself a viable treatment in humans. Optogenetics, combined with viral vectors that target particular cell-types, has the potential of exerting more selective stimulatory or inhibitory effects compared to DBS. Technical challenges remain with this approach, which indicates the need for much further research to evaluate the therapeutic potential of optogenetics. Nonetheless, researchers have effectively translated these optogenetic tools to non-human primates (106,107), setting the stage for potential future use in human patients. We will likely see many novel studies using diverse types of optogenetic tools to provide new insights into the neurobiology of depression, which can potentially lead to novel therapeutics that offer more effective treatment in humans.
IN THIS ISSUE Statement.
In this issue we discuss how optogenetic tools are currently being used to answer a broad range of molecular, cellular, and circuit-level questions pertaining to depression which, up until now, have been difficult to answer using other experimental approaches. The emergence of optogenetic technology, when combined with the best-validated animal models of depression, will dramatically increase our knowledge about the basic neurobiology of depression as well as facilitate the development of more effective antidepressant treatments.
Acknowledgments
We thank Dipesh Chaudhury for providing the photos of social interaction behavior combined with in vivo blue light optogenetics in Figure 1. The authors of this review have no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The Global Burden of Disease: 2004 update. Geneva: World Health Organ; 2008. [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 4.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 6.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nature Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 8.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 9.Vaidya VA, Duman RS. Depresssion--emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 10.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn J, Gaebel W, Klosterkoetter J, Woopen C. Deep brain stimulation as a new therapeutic approach in therapy-resistant mental disorders: ethical aspects of investigational treatment. Eur Arch Psychiatry Clin Neurosci. 2009;2(259 Suppl):S135–S141. doi: 10.1007/s00406-009-0055-8. [DOI] [PubMed] [Google Scholar]
- 14.Machado A, Haber S, Sears N, Greenberg B, Malone D, Rezai A. Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clin Neurophysiol. 2009;120:1941–1948. doi: 10.1016/j.clinph.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Malone DA, Jr., Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 17.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 18.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 20.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, et al. Optogenetic Interrogation of Dopaminergic Modulation of the Multiple Phases of Reward-Seeking Behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 32.Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, et al. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci U S A. 2009;106:6814–6819. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn KM, Kuhlman B. Hold me tightly LOV. Nat Methods. 2010;7:595–597. doi: 10.1038/nmeth0810-595. [DOI] [PubMed] [Google Scholar]
- 35.Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem. 2010;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Covington HE, 3rd, Vialou V, Nestler EJ. From synapse to nucleus: novel targets for treating depression. Neuropharmacology. 2010;58:683–693. doi: 10.1016/j.neuropharm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman RP, Goodrich JT. Psychosurgery: a historical overview. Neurosurgery. 2001;48:647–657. doi: 10.1097/00006123-200103000-00041. discussion 657–649. [DOI] [PubMed] [Google Scholar]
- 38.Swayze VW., 2nd Frontal leukotomy and related psychosurgical procedures in the era before antipsychotics (1935–1954): a historical overview. Am J Psychiatry. 1995;152:505–515. doi: 10.1176/ajp.152.4.505. [DOI] [PubMed] [Google Scholar]
- 39.Wind JJ, Anderson DE. From prefrontal leukotomy to deep brain stimulation: the historical transformation of psychosurgery and the emergence of neuroethics. Neurosurg Focus;25:E10. doi: 10.3171/FOC/2008/25/7/E10. [DOI] [PubMed] [Google Scholar]
- 40.Feldman RP, Alterman RL, Goodrich JT. Contemporary psychosurgery and a look to the future. J Neurosurg. 2001;95:944–956. doi: 10.3171/jns.2001.95.6.0944. [DOI] [PubMed] [Google Scholar]
- 41.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 42.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 43.Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010;29:E1. doi: 10.3171/2010.4.FOCUS10106. [DOI] [PubMed] [Google Scholar]
- 44.Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 47.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 49.Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. discussion 585–593. [DOI] [PubMed] [Google Scholar]
- 50.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 52.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 53.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010;44:683–687. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 56.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Dzirasa K, McGarity DL, Bhattacharya A, Kumar S, Takahashi JS, Dunson D, et al. Impaired limbic gamma oscillatory synchrony during anxiety-related behavior in a genetic mouse model of bipolar mania. J Neurosci. 2011;31:6449–6456. doi: 10.1523/JNEUROSCI.6144-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. Highspeed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 64.Barretto RP, Messerschmidt B, Schnitzer MJ. In vivo fluorescence imaging with high-resolution microlenses. Nat Methods. 2009;6:511–512. doi: 10.1038/nmeth.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dzirasa K, Fuentes R, Kumar S, Potes JM, Nicolelis MA. Chronic in vivo multi-circuit neurophysiological recordings in mice. J Neurosci Methods. 2011;195:36–46. doi: 10.1016/j.jneumeth.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, et al. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A Novel Role of the WNT-Dishevelled-GSK3{beta} Signaling Cascade in the Mouse Nucleus Accumbens in a Social Defeat Model of Depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 77.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- 80.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 81.Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- 82.Ono T, Nishino H, Fukuda M, Sasaki K, Nishijo H. Single neuron activity in dorsolateral prefrontal cortex of monkey during operant behavior sustained by food reward. Brain Res. 1984;311:323–332. doi: 10.1016/0006-8993(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 83.Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaudhury D, Juarez B, Tsai HC, Lobo MK, Walsh J, Friedman A, Mouzon E, Mogri M, Deisseroth K, Nestler EJ, Han MH. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Optogenetic manipulation of dopaminergic neurons in brain reward circuit modulates susceptibility to social defeat stress. Online: 907.927. [Google Scholar]
- 85.Walsh J, Friedman A, Lobo MK, Chadhury D, Juarez B, Gradinaru V, Russo S, Deisseroth K, Nestler EJ, Han MH. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Neural circuit mechanisms of behavioral susceptibility and resilience to social defeat. Online: 907.27. [Google Scholar]
- 86.Tye KM, Mirzabekov JJ, Tsai H-C, Warden MRS, Kim S-Y, Thompson KRS, Gunaydin LAS, Finklestein JS, Deisseroth K. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. Online: 306.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- 90.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 91.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 92.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 93.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 94.Guez-Barber D, Fanous S, Golden SA, Schrama R, Koya E, Stern AL, et al. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J Neurosci. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Witten B, Steinberg E, Tye KM, Davidson T, Zalocusky K, Brodsky M, Stuber G, Ramakrishnan C, Gong S, Cho S, Janak PH, Deisseroth K. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Optogenetics in transgenic rats: Dopamine neural activity drives self-stimulation. Online: 306.14. [Google Scholar]
- 96.Welsh DK, Kay SA. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005;16:73–78. doi: 10.1016/j.copbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 97.Koo JW, Mazei-Robison MS, Lobo MK, Dietz DM, LaPlant Q, Ferguson D, Feng J, Sun H, Damez-Werno D, Scobie K, Ohnishi YN, Ohnishi YH, Mouzon E, Dias C, Robison AJ, Vialou V, Neve RL, Russo SJ, Nestler EJ. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Role of BDNF in the VTA in regulating molecular and behavioral responses to morphine. Online: 167.101. [Google Scholar]
- 98.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285:30825–30836. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem. 2010;285:41501–41508. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem. 2011;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu YI, Wang X, He L, Montell D, Hahn KM. Spatiotemporal control of small GTPases with light using the LOV domain. Methods Enzymol. 2009;497:393–407. doi: 10.1016/B978-0-12-385075-1.00016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dietz DM, Sun H, Lobo MK, Gao V, Chadwikc B, Koo JW, Mazei-Robison M, Damez-Werno D, Maze I, Scobie K, Ferguson D, Christoffel D, Hodes GE, Zheng Y, Hahn K, Neve RL, Russo SJ, Nestler EJ. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. The role of Rac1 in mediating cocaine induced structural plasticity; pp. 909–922. Online. [Google Scholar]
- 104.Strickland D, Moffat K, Sosnick TR. Light-activated DNA binding in a designed allosteric protein. Proc Natl Acad Sci USA. 2008;105:10709–10714. doi: 10.1073/pnas.0709610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strickland D, Yao X, Gawlak G, Rosen MK, Gardner KH, Sosnick TR. Rationally improving LOV domain-based photoswitches. Nat Methods. 2010;7:623–626. doi: 10.1038/nmeth.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]