Abstract
Exposure to ionizing irradiation may affect brain functions directly, but may also change tissue sensitivity to a secondary insult such as trauma, stroke or degenerative disease. To determine if a low dose of particulate irradiation sensitizes the brain to a subsequent injury, C56BL6 mice were exposed to brain only irradiation with 0.5 Gy of 56Fe ions. Two months later, unilateral traumatic brain injury was induced using a controlled cortical impact system. Three weeks after trauma animals received multiple BrdU injections and 30 days later were tested for cognitive performance in the Morris water maze. All animals where able to locate the visible and hidden platform during training; however, treatment effects were seen when spatial memory retention was assessed in the probe trial (no platform). While sham and irradiated animals showed spatial memory retention, mice that received trauma alone did not. When trauma was preceded by irradiation, performance in the water maze was not different from sham-treated animals, suggesting that low dose irradiation had a protective effect in the context of a subsequent traumatic injury. Measures of hippocampal neurogenesis showed that combined injury did not induce any changes greater that those seen after trauma or radiation alone. After trauma there was a significant decrease in the percentage of neurons expressing the behaviorally-induced immediate early gene Arc in both hemispheres, without associated neuronal loss. After combined injury there were no differences relative to sham-treated mice. Our results suggest that combined injury resulted in decreased alterations of our endpoints compared to trauma alone. While the underlying mechanisms are not yet known, these results resemble a preconditioning, adaptive, or inducible-like protective response, where a sublethal or potentially injurious stimulus (i.e. irradiation) induces tolerance to a subsequent and potentially more damaging insult (trauma).
Keywords: hippocampus, traumatic brain injury, immediate early gene, radiation
INTRODUCTION
The human brain can encounter ionizing irradiation under a variety of circumstances, ranging from therapeutic management of tumors, industrial or battlefield situations, to exposure in the space environment. While changes involving macroscopic tissue destruction generally occur only after high radiation doses such as that seen during radiation therapy (Tofilon and Fike, 2000), less severe morphologic changes can occur after much lower doses. This can result in variable degrees of cognitive impairment that often manifest as deficits in hippocampal dependent learning and spatial information processing (Abayomi, 1996; Crossen et al., 1994; Roman and Sperduto, 1995). The underlying mechanisms associated with radiation-induced cognitive impairments have remained elusive. They are likely to be multifactorial, but important possibilities include alterations in the neurogenic cell populations in the dentate gyrus (DG) (Mizumatsu et al., 2003; Raber et al., 2004a; Rola et al., 2004b), loss of mature neurons in the DG (Fan et al., 2007), alterations in NMDA subunits (Shi et al., 2006), genetic risk factors (Villasana et al., 2006), and reductions in the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) (Rosi et al., 2008). Additionally, radiation-induced changes in the hippocampal formation, and presumably the associated cognitive impairments, are strongly linked to changes in the neurogenic microenvironment (Fike et al., 2007; Rola et al., 2004b). Recent studies have also shown that the effectiveness of irradiation on neurogenic cells can vary depending upon the nature of the microenvironment prior to exposure, e.g. the presence of oxidative stress (Fike et al., 2007; Rola et al., 2008; Rola et al., 2007). Injury and disease, such as traumatic brain injury, share specific pathologic characteristics with ionizing irradiation. Neurogenesis is impacted by traumatic brain injury, and like irradiation, alterations in this process are associated with cognitive impairments (Chirumamilla et al., 2002; Sun et al., 2007).
While data are starting to appear with respect to how an altered microenvironment may influence radiation response, few data are available regarding how a previous radiation exposure may affect other types of injury. Given that radiation itself can induce environmental changes such as inflammation and oxidative stress (Rola et al., 2008; Rola et al., 2007; Rosi et al., 2008), a low dose of ionizing irradiation might predispose the brain to either a heightened or reduced vulnerability upon a second insult. Traumatic brain injury could interact with the radiation challenge resulting in either less or more injury than would be expected based on additive effects. This could be relevant to individuals who undergo cranial radiotherapy, or are exposed to relatively low radiation doses in an uncontrolled situation (e.g. space environment, radiologic terrorism), and then experience a subsequent injury.
The behaviorally-induced immediate early gene Arc and its protein product are induced in the hippocampus by spatial exploration in the same percentage of neurons observed with electrophysiological recording (Guzowski et al., 1999; Rosi et al., 2005; Ramirez-Amaya et al., 2005; Rosi et al., 2009). The expression of Arc maps neuronal function that underlies information processing (Rosi et al., 2009) and may be applicable to adult born neurons (Ramirez-Amaya et al., 2006). It is unclear whether Arc expression changes in response to challenges like trauma and irradiation and correlates with cognitive injury.
In the present study, using measures of cognition, Arc expression and neurogenesis, we assessed whether a relatively low dose of irradiation would predispose the brain to a reduced or heightened vulnerability after a subsequent traumatic injury. We chose the heavy ion, 56Fe, because it induces changes in the central nervous system (CNS) at low doses (Rola et al., 2008) and it constitutes a potential risk for people exposed in the space environment. Our findings demonstrate that radiation prior to trauma results in less injurious consequences relative to either single insult alone.
MATERIALS AND METHODS
Animals
Fifty seven male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), 8 week old, were used in these experiments (Fig. 1). Mice were housed and cared for in compliance with the United States Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and institutional IACUCs. Animals were shipped to Brookhaven National Laboratory (BNL) and allowed to acclimatize for 3–5 days prior to irradiation with 56Fe ions. After irradiation, the animals were housed at BNL for 1–2 days prior to shipment to the University of California, San Francisco by courier.
Figure 1.
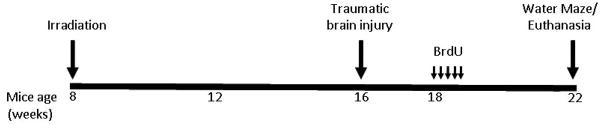
Experimental design time line. Eight week old C57BL/6 mice were irradiated with 56Fe (0.5 Gy), and 8 weeks later animals received either focal traumatic brain injury or sham craniotomy. Two weeks after trauma animals were injected daily for 5 days with BrdU (100 mg/Kg). Four weeks after BrdU, animals were either euthanized for measurements of neurogenesis or underwent Morris water maze testing for 5 days. Animals that underwent Morris water maze were sacrificed 30 minutes after the last probe trial for measurement of Arc.
One cohort of animals (n=37: 0Gy/sham (Sham), n=9; 0.5Gy/sham (Radiation), n=10; 0Gy/trauma (Trauma), n=8; 0.5Gy/trauma (Combined Injury), n=10) was used for the behavioral study and the immunohistochemical assessment of Arc expression.
Because cognitive training and testing could potentially constitute an enriched environment and impact measures of neurogenesis (van Praag et al., 2000), a second cohort of mice (n=20: 0Gy/sham (Sham), n=5; 0.5Gy/sham (Radiation), n=5; 0Gy/trauma (Trauma), n=5; 0.5Gy/trauma (Combined Injury), n=5) was used for the determination of neurogenesis and did not undergo behavioral training and testing.
Irradiation
Irradiation was done using a collimated beam of 600 MeV/nucleon 56Fe particles produced by the AGS Booster Accelerator at Brookhaven National Laboratory (BNL) as previously described in detail (Villasana et al., 2008). Up to 4 mice were simultaneously exposed to brain-only irradiation. Briefly, the delivered beam was restricted to 1cm diameter circular apertures that covered the brain areas of the mice. For dose calibration, a NIST-traceable 1 cm3 Far West™ thimble chamber with an air-filled bulb and tissue equivalent walls was placed at the target position behind the collimator apertures. The 56Fe26+ ion beam was extracted at 600 MeV/n, and had an energy at the target surface of 585.1 MeV/n, a residual range of 12.3 cm in water, and an LET of 175.2 keV/mm. The beam was delivered as twenty 300 msec pulses per minute for an average dose rate of ~ 5 Gy/min. Delivered doses were +/− 0.5% of the requested value. A description of dose composition and fragmentation behind various target materials for similar iron ion beams (1087 MeV/n and 555 MeV/n) produced at the BNL AGS accelerator has been reported previously (Zeitlin et al., 1998).
Mice were anesthetized with 4% isoflurane, and placed in custom bite-bar cradles to stabilize the head position. The cradles were then placed in a clear acrylic anesthesia box pre-aligned with the collimator and the beam line. Sedation was maintained with 2.5% isoflurane administered throughout the irradiation procedure. A single fraction of 0.5 Gy was delivered to the brain of each animal. For the entire irradiation procedure, animals were under isoflurane anesthesia for an average of 10 min. Non-irradiated control mice were treated identically without being exposed to radiation.
Traumatic Brain Injury
Traumatic brain injury or sham surgery was induced 7–8 weeks after irradiation (Fig. 1). Each mouse was placed in a stereotaxic frame (Kopf, Tujunga, CA), and after a midline skin scalp incision, soft tissues were removed and the skull exposed. A circular craniotomy, 3.5 mm in diameter, was made in the left parietal skull between the bregma and lambda, 0.5 mm lateral to the midline; the dura was not incised. Traumatic injury was induced using a controlled cortical impact as previously described (Rola et al., 2006). Briefly, a 3 mm diameter convex impact tip was used, and the impact was set to a 1.0 mm deformation delivered at a velocity of 4.5 m/sec. Sham mice and those that received irradiation only underwent the same surgical procedure but were not injured. After this procedure the scalp was sutured and each animal received a subcutaneous injection of warm physiologic saline (1 ml) to prevent dehydration. During surgery and recovery periods body temperature was maintained with a circulating-water heating pad.
BrdU Injection
Fourteen - 17 days following traumatic brain injury or sham injury, all mice received daily injections of BrdU (100 mg/kg) for 5 consecutive days. Four weeks after the first BrdU injection mice were either perfused with ice-cold saline followed by ice-cold 4% paraformaldehyde for measurements of neurogenesis or underwent Morris water maze testing and tissue collection for analysis of Arc expression (Fig. 1).
Morris Water Maze
Assessment of hippocampus-dependent cognitive performance was started 14 weeks after irradiation using the Morris water maze test (Morris, 1984) as previously described (Benice et al., 2006). Briefly, a circular pool (diameter 140 cm) was filled with opaque water (24°C) and mice were trained to locate a submerged platform (luminescence: 200 lux). To determine if treatment affected the ability to swim or learn the water maze task, mice were first trained to locate a clearly marked platform (visible platform, Days 1 and 2). Mice were subsequently trained to locate the platform when it was hidden beneath the surface of the opaque water (Days 3–5). Training during the hidden platform session (acquisition) required the mice to learn the location of the hidden platform based on extra-maze cues. For both visible and hidden sessions, there were 2 daily sessions which were 2 hr apart. Each session consisted of 3 trials (with 10-min inter-trial intervals). A trial ended when the mice located the platform. Mice that failed to locate the platform within 60 sec were lead to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they were physically on the platform for a minimum of 3 sec. During visible platform training, the platform was moved to a different quadrant of the pool for each session. For the hidden platform training, the platform location was kept constant. Mice were placed into the water facing the edge of the pool in one of 9 randomized locations. The start location was changed for each trial. Swimming patterns were recorded with the Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands) set at 6 samples/second. The time to locate the platform (latency) was used as a measure of performance for the visible and hidden sessions.
To measure spatial memory retention, probe trials (platform removed) were conducted 1 hr after the last hidden trial of each mouse on each day of hidden platform training (i.e. 3 separate probe trials). The time spent in the target quadrant, the quadrant where the platform was previously located during hidden platform training, was compared to the time spent in the 3 non-target quadrants. In addition, for each group, the percentage of mice that spent more time in the target quadrant than any other quadrant was calculated. For the probe trials, mice were placed into the water in the quadrant opposite from the target quadrant.
After the last probe trial, mice were returned to their own cages. Thirty min after the last probe trial, mice were killed by cervical dislocation and decapitated. Brains were removed quickly (within 60 sec) and frozen in −70°C isopentane.
Histological Procedures and Analysis
Neurogenesis
Studies of neurogenesis were performed as previously reported (Mizumatsu et al., 2003; Rola et al., 2007). To determine the effects of single or combined treatment on the survival of newly born cells in the dentate subgranular zone (SGZ), fixed brains were sectioned using a sliding microtome and 50 μm floating sections were immunostained as previously described (Mizumatsu et al., 2003; Rola et al., 2007). Newly born cells were stained using rat anti-BrdU (1:50; Oxford Biotechnology, Kidlington, Oxford, UK), neuronal cells were stained with biotinylated mouse anti-NeuN (1:200; Chemicon, Temecula, CA, USA) and astrocytes were stained with GFAP (Pharmigen, San Diego, CA, USA); cell specific staining was revealed respectively with Texas red (Vector) and Alexa Fluor 499 or 594 (Molecular Probes, Eugene, OR, USA).
To calculate the numbers of BrdU-positive (BrdU+) cells in the dentate gyrus, 12 sections of a one-in-six series were scored per animal (Rola et al., 2007). All counts were limited to the dentate granule cell layer and a 50 μm border along the hilar margin that included the dentate SGZ; cell counts were obtained for both hemispheres. Total numbers were obtained by multiplying the measured value by 6. BrdU+ cells were counted in representative sections from each animal using confocal microscopy.
Confocal procedures were performed using a Nikon C-1 confocal microscope (Melville, New York), using techniques previously described {Mizumatsu, 2003 #1077; Rola, 2007 #1287}. Appropriate gain and black-level settings were obtained on control tissues stained with secondary antibodies alone. Upper and lower thresholds were always set using a range indicator function to minimize data loss due to saturation. Each cell was manually examined in its full ‘z’ dimension with use of split panel analysis, and only those cells for which the BrdU+ nucleus was unambiguously associated with the neuronal marker were scored as positive.
Arc protein
The brains from multiple animals were blocked together and cryosectioned (Rosi et al., 2005) such that each slide contained sections from one animal from each of the experimental conditions: 0Gy/sham surgery; 0.5Gy/sham surgery; 0Gy/trauma; 0.5Gy/trauma. All slides were stored at −70°C until processed for immunocytochemical analysis. Brain sections were taken from the medial portion of the dorsal hippocampus (anteroposterior ~ 2.92 – 4.0 mm from bregma). Quantitative assessment of Arc protein was done using methods previously reported by us in detail (Rosi et al., 2008; Rosi et al., 2005). Tissue sections were fixed in 2% paraformaldehyde, and after blocking with a tyramide signal amplification kit (TSA; PerkinElmer Life Science, Emeryville, CA), were incubated in polyclonal rabbit anti-Arc antibody for 48 h at 4°C, (1:500; rabbit anti-Arc a gift from P.F. Worley, Johns Hopkins University School of Medicine, Baltimore, MD). Sections were then incubated with an anti-rabbit biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 2 h at room temperature, followed by amplification with an avidin-biotin system for 45 minutes. Staining was reveled using a cyanine-3 (CY3) TSA fluorescence system (PerkinElmer).
To determine if single or combined treatment affected the ability of newly born cells to become actively integrated in the dentate gyrus (DG) triple immune staining for NeuN, Arc and BrdU was performed in four slides from each conditions previously reported (Fike et al., 2009). Biotinylated mouse anti-NeuN (1:200; Chemicon, Temecula, CA) was applied first and revealed with CY5 (PerkinElmer), followed by Arc and BrdU as described above. Nuclei were counterstained with DAPI (Molecular Probes).
Microscopic imaging for Arc protein, and NeuN/Arc/BrdU was performed at 20X magnification using a Zeiss AXIO IMAGER Z1 microscope with motorized z-drive for transmitted light and epi-fluorescence (Rosi et al., 2008). For each endpoint, two coronal sections per mouse were randomly selected from the dorsal hippocampus (1.7 to 2.3 mm posterior from Bregma) and used to reconstruct mosaics of the ipsilateral and contralateral DG (Rosi et al., 2008). Each mosaic was formed from 6–8 z-stack (1.0 μm optical thickness/plane) images.
Manual counts of granule cells neurons (NeuN), cells expressing Arc protein, and Arc/NeuN/BrdU were performed by an experimenter blind to the experimental conditions.
To determine if traumatic brain injury and/or combined injury resulted in variations in granule cell density, the numbers of cells per mm2 were counted in upper and lower blades from selected regions of the ipsilateral DG in one representative section from each animal in the study.
Positive neurons for Arc protein had perinuclear-cytoplasmic staining surrounding the cell and visible in at least three planes together with the cell nucleus across the z-stack (Rosi et al., 2008; Rosi et al., 2005). To avoid classification errors, we carefully verified that the staining belonged to the cell of interest by nuclear counterstaining. The numbers of Arc positive neurons/mm2 characterized by these criteria were determined in the upper and lower blades of the DG (Rosi et al., 2008).
To analyze if single or combined injury influenced the migration of newly born neurons, the dentate granular cell layer was partitioned into 3 regions of interest representing the inner, intermediate, and outer third. The proportion was adjusted throughout the whole length of the DG, assuring that each layer always represented ~ 33% of the whole thickness. The numbers of BrdU labeled neurons (BrdU+/NeuN+) within the various layers of the DG granular layer were then determined for each experimental group.
Statistics
Analysis of variance (ANOVA) was used to analyze the effects of treatment (radiation, trauma or combined injury) on water maze performance using SPSS software. Repeated measure ANOVAs were used to compare visible and hidden water maze learning curves (radiation and trauma as between subjects factors and session as within subjects factor) and Tukey-Kramer post hoc tests were used when appropriate. For analysis of performance in the water maze probe trials, one way ANOVAs were used along with Dunnett’s posthoc tests comparing each non-target quadrant to the target quadrant, when appropriate, using Prism software.
StatView Software (version 5.0.1) and ANOVAs were used for the analysis of immunohistochemistry cell counts. The treatments were the independent variables and the percentages of cells from each category described above were the dependent variables. When an overall ANOVA was significant (p<0.05) individual between-groups comparisons were performed with Bonferroni post hoc to correct for multiple comparisons.
RESULTS
56Fe irradiation and traumatic brain injury was well tolerated by all animals; changes in body weight over time were not different from those seen in sham treated controls (not shown).
Quantitative counts of the granule cell neurons from representative sections, revealed comparable total numbers of neurons/mm2 in all of the treatment groups (p=0.59): Sham = 9,668 ± 414 (mean ± SEM); Trauma = 10,437 ± 399; Radiation = 10,331 ± 368; Combined Injury = 10,314 ± 426.
Cognitive studies
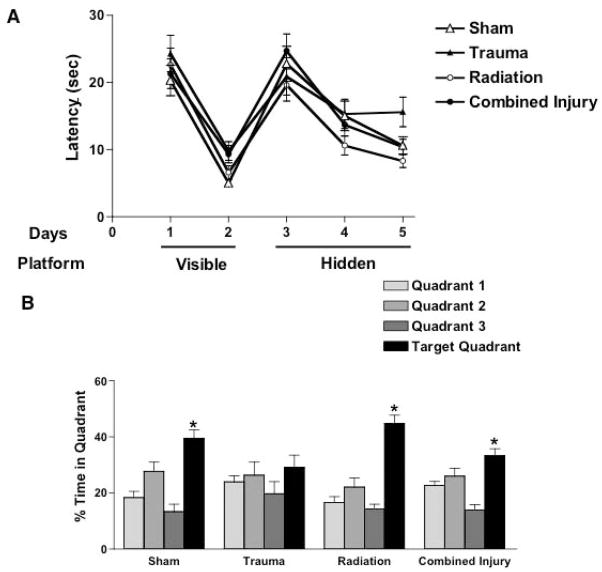
There were no group differences in swim speeds during the visible platform sessions, indicating that there were no deficits in motor or exploratory behavior as result of irradiation, trauma or combined injury (not shown). Therefore, latency (time to locate the platform) was used to compare the water maze learning curves. All groups learned to locate the visible platform (Fig. 2A). There were no significant effects of radiation only or trauma only on the ability of mice to locate the visible platform or a trauma x irradiation interaction. However, there was a trend towards an effect of trauma (F = 3.408, p = 0.074). All groups also learned to locate the hidden platform (Fig. 2A). There were no effects of radiation only or trauma only on ability to locate the hidden platform or a trauma radiation interaction. In contrast to the water maze learning curves, group differences were detected when spatial memory retention was assessed in the probe trial following the first day of hidden platform training (Fig. 2B). While both sham treated and irradiated mice showed spatial memory retention by spending most of the time in the target quadrant which previously contained the hidden platform than any other quadrant, mice that received trauma alone did not (no effect of quadrant: p = 0.428). However, in the combined injury group, there was some recovery relative to trauma alone (Fig. 2B). The combined injury group also showed spatial memory retention and spent more time in the target quadrant than any other quadrant. The percentage of mice in each group that spent more time in the target quadrant than any other quadrant showed the same pattern. Seventy five percent of sham treated and 90% of irradiated mice spent more time in the target quadrant than any other quadrant; in contrast, only 44% in the trauma group but 60% in the combined injury group spent more time in the target quadrant. Following additional hidden water maze training, all groups showed spatial memory retention and spent more time in the target quadrant than any other quadrant in the probe trials following the second and third day of hidden platform training (not shown).
Figure 2.
Figure 2A: Latency (time) to find a visible (Days 1,2) or a hidden platform (Day 3–5) in the Morris water maze test. Each day consisted of 6 trials each. After single or combined treatments all mice were able to learn the water maze task, as represented by decreases in latency to reach both the visible and the hidden platform. There were no group differences in swim speeds. Each group consisted of 8–10 mice. Each data point represents mean values and standard error of the mean (SEM).
Figure 2B: Spatial memory retention in mice in the Morris water maze probe trial following the first day of hidden platform training. While both sham treated and irradiated mice showed spatial memory retention by spending most of the time in the target quadrant which previously contained the hidden platform, mice that received trauma alone did not (no effect of quadrant: p = 0.428). When trauma occurred 30 days after 0.5 Gy of 56Fe (combined injury), there was some apparent recovery relative to trauma alone. Each group consisted of 8–10 mice. Each data point represents mean values and standard error of the mean (SEM). ANOVA showed an effect of quadrant in the Sham, Radiation only, and Combined Injury groups (*p < 0.0001). For these individual groups, subsequent posthoc tests revealed the following differences between the target and the 3 non-target quadrants. Sham: Target versus zones 1 and 3: p < 0.001; Target versus zone 2: p < 0.05. Irradiation: Target versus zones 1–3: p < 0.001. Combined injury: Target versus zones 1 and 3: p < 0.001; Target versus zone 2: p > 0.05.
Neurogenesis
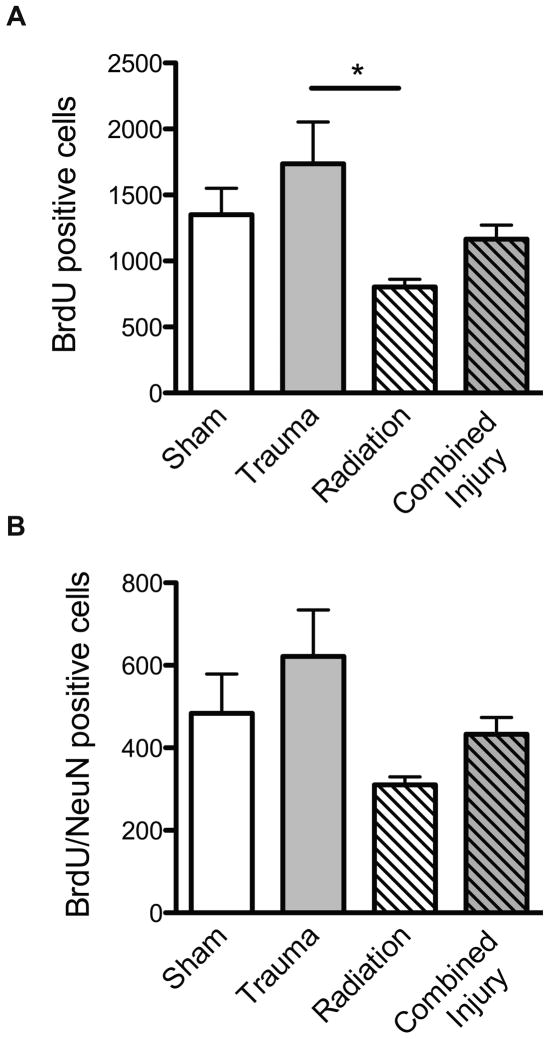
The presence of BrdU+ cells four weeks after BrdU injection represents the long-term survival of newly generated cells, independent of phenotype. There was a significant group difference in this measure (p = 0.029). In the ipsilateral hemisphere of sham treated mice, there were 1,352 ± 199 BrdU+ cells in the dentate SGZ and 803 ± 59 in irradiated mice (p = 0.06, Fig. 3A). Compared to irradiation, the total number of BrdU+ cells in the dentate SGZ was higher following trauma (1,736 ± 317, p =0.004) and combined injury (1,166 ± 105; p =0.057). Changes in the contralateral hemisphere showed the same pattern as seen in the ipsilateral side except after trauma only where there was no increase in total number of BrdU+ cells (not shown). In contrast to the total number of BrdU+ cells, there was only a trend towards a group difference in BrdU+/NeuN+ cells (p = 0.07; Fig. 3B). In sham treated mice, an average of 484 ± 96 newly generated cells differentiated into neurons (BrdU+/NeuN+) in the ipsilateral hemisphere. Radiation alone decreased the number of BrdU+/NeuN+ cells in the ipsilateral hemisphere (310 ± 19), while trauma alone increased the numbers of new neurons (621 ± 112). After combined injury there was an increase in the numbers of newly born neurons (433 ± 41) compared to radiation alone.
Figure 3.
Total number of BrdU positive cells (A) and BrdU positive/NeuN positive cells (B) in the dentate subgranular zone after single or combined treatment. There was a significant group difference for BrdU only (p =0.039, A) and there was a significant difference between animals that received trauma only and radiation only (* p = 0.004). In terms of newly born neurons there was a trend toward a group difference (p =0.07, B). Each treatment group consisted of 4=5 mice. Each bar represents a mean value and error bars are SEM.
Migration of new born neurons across the dentate gyrus
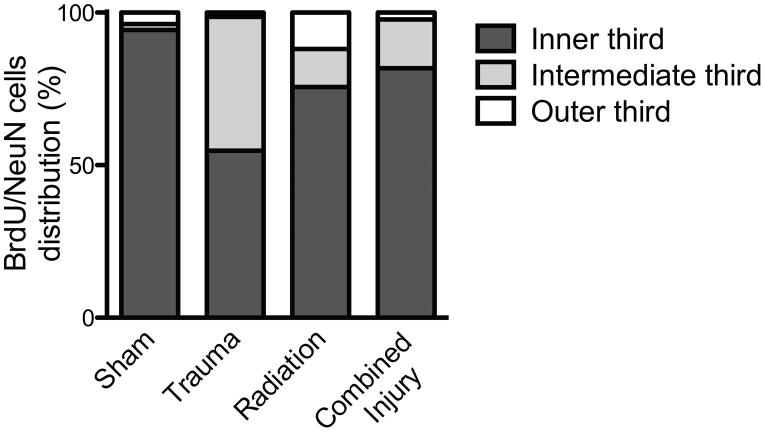
To determine if irradiation, trauma or combined injury influenced the migration of the newly born neurons within the DG, we quantified the number of BrdU+/NeuN+ cells in the inner, intermediate and outer third of the DG granule cell layer. The proportion of newly born neurons found in the DG inner third was significantly different across the groups (Fig. 4; ANOVA: F (3,29) =3.428; p=0.03). In sham treated controls, the majority of newly born neurons were found in the inner third of the granular layer (94.3 ± 3.2%), while in animals that received trauma, only 55.76 ± 16.0% of newly born neurons were found in the same area (p<0.05 vs Sham). In animals that received irradiation only or combined injury, the fractions of newly born neurons in the inner third of the DG were 75.63 ± 8.5% and 81.78 ± 5.7% respectively. The proportion of newly born neurons found in the intermediate third of the DG significantly differed across groups (ANOVA: F (3,29) =5.642; p=0.0036). Post-hoc tests revealed that the percentage of newly born neurons was significantly higher in animals that received trauma only (43.81±16.16%) as compared to sham (1.91±1.33%; p<0.01), irradiation only (12.43 ± 4.8%; p<0.05) or animals that received combined injury (16.00 ± 5.6%; p<0.05). The proportion of newly born neurons in the outer third of the DG did not statistically differ across experimental groups (ANOVA: F (3,29) =2.137; p=0.1171).
Figure 4.
Proportions of newly born neurons (BrdU+/NeuN+) within the inner, intermediate and outer third of the DG granule cell layer. The proportion of newly born neurons found in the inner third was significantly different across the groups (ANOVA; p=0.03). Post Hoc comparisons between groups revealed that: while in sham treated controls the majority of newly born neurons were found in the inner third of the granular layer, this proportion was significantly lowered in trauma only animals (p<0.05 vs Sham). The proportion of newly born neurons found in the intermediate third also significantly varied across groups (ANOVA, p=0.004). The percentage of new born neurons found in the intermediate third layer was significantly higher in animal that received trauma only as compared to sham (p<0.01), irradiation only (p<0.05) or animals that received combined injury animals (p <0.05).
Behaviorally-induced immediate early gene Arc
In our experimental paradigm, the expression of the behaviorally-induced Arc protein in the granule neurons was measured 30 min after the last probe trail of the water maze (Fig. 5). Trauma alone significantly reduced the number of neurons expressing Arc protein relative to all the other treatment conditions in both hemispheres (Fig. 6; ANOVA: ipsilateral hemisphere: F (3, 31) =5.7; p< 0.003; contralateral hemisphere: F (3, 31) =4.341; p< 0.01). After combined injury there was an increase in the number of neurons expressing Arc protein relative to what was seen after trauma alone both in the ipsilateral and contralateral hemisphere, suggesting that irradiation prevented the trauma-related decrease in Arc expression. Measures of Arc mRNA showed the same pattern as Arc protein (not shown).
Figure 5.
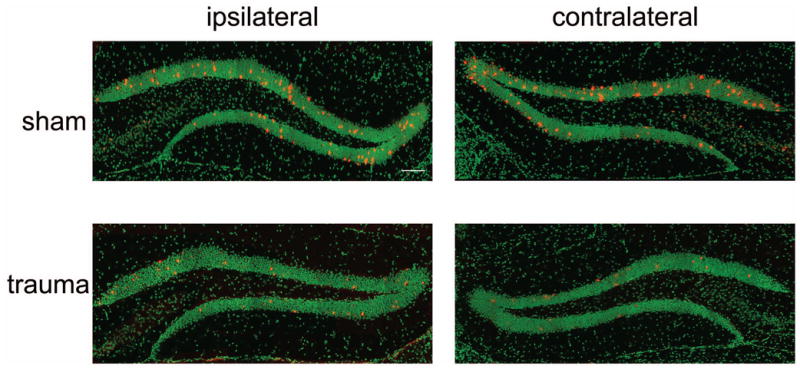
Qualitative characterization of Arc expression (in red) in granule cell neurons of the DG 30 minutes after a probe trial in sham treated controls (A, B) and animals that received traumatic injury (C, D). Nuclei were counterstained with SYTOX Green. Bar, 100 μm.
Figure 6.
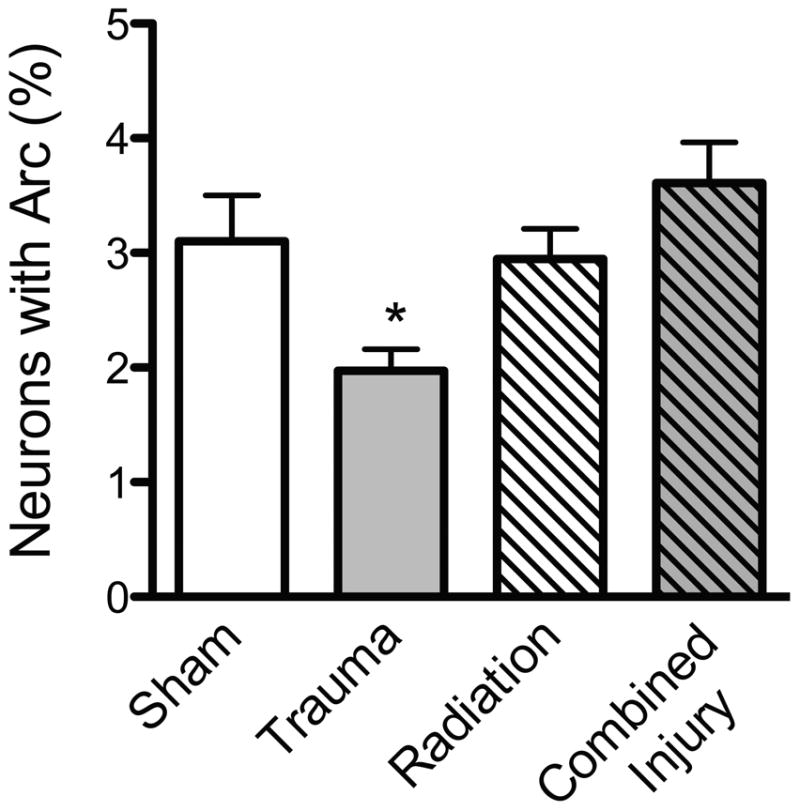
Percentages of granule cells neurons expressing Arc protein 30 min after the final probe trial. Animals that received trauma only showed a significant reduction in the percentage of neurons expressing Arc protein relative to all the other treatment conditions in both hemispheres (*, p< 0.003).
Finally, we quantified the numbers of newly born neurons (BrdU+/NeuN+) that also expressed Arc protein (Fig. 7). While tissue availability and low event frequency precluded a rigorous statistical analyses, we were able to detect low numbers (2–3%) of newly born neurons that co-expressed Arc protein in all treatment groups except trauma alone. Taken together with our other results these data suggest that prior irradiation prevents trauma-induced alterations in the expression of Arc protein in adult and newly born neurons.
Figure 7.
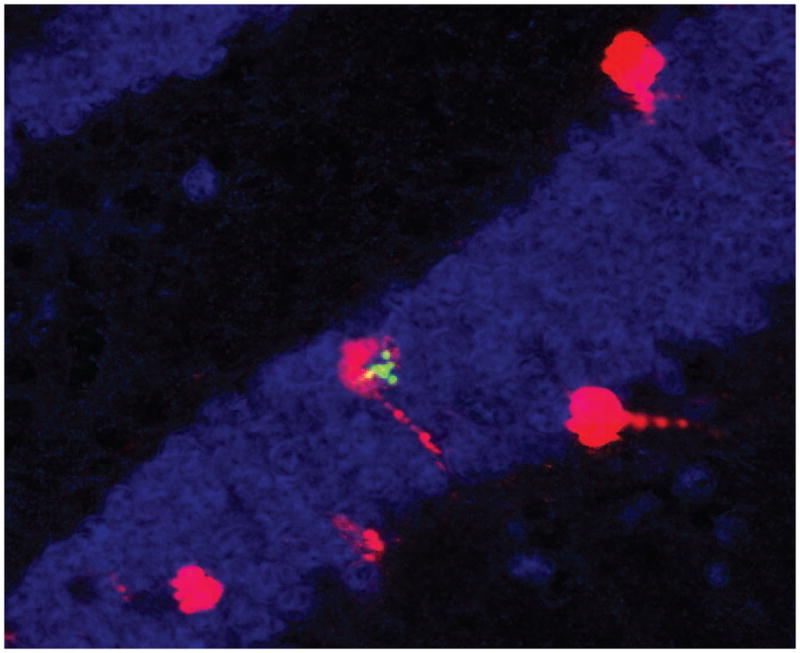
Representative image of a newly born (BrdU+, green) neuron (NeuN+, blue) that is functionally integrated (Arc +, red) into the upper blade of the dentate gyrus.
DISCUSSION
Cognitive impairments develop in response to a variety of insults to the CNS, including disease or injury, and can affect overall function and quality of life. While considerable data exist with respect to the cognitive consequences of neurodegenerative diseases, injury, cancer treatment, etc., few studies have considered if or how a combination of insults may affect the time course or extent of cognitive changes. The present study was done in the context of radiation exposure and was designed to determine if a low dose of particulate irradiation predisposed the brain to a reduced or heightened vulnerability to a secondary traumatic insult.
While the conceptual basis of this study was guided by interest of combined injury in a space environment, the resultant data are also relevant to individuals exposed to irradiation in a therapeutic setting or in a nuclear battlefield or radiologic terrorism scenario. Overall, the data shown here suggest that if a low dose of irradiation precedes a mild/moderate traumatic brain injury by 30 days, there are fewer consequences relative to either of the single insults alone. The underlying mechanism(s) for this effect is (are) not yet known.
Cognitive impairments were quantified using the Morris water maze, a well-described method for determining hippocampus-dependent learning and retention of spatial memory (Morris, 1984). The particular testing paradigm used here employed three probe trials, one of which was performed at the end of each day of multiple hidden training sessions (Raber et al., 2009). A significant impairment was observed after the first probe trial in mice that received trauma only, but that impairment was not apparent if the traumatic injury was preceded by a single low dose of 56Fe (Fig. 2B). This suggests an adaptive type of response where a sublethal or potentially injurious stimulus (i.e. irradiation) induced tolerance to a subsequent and potentially more damaging insult (trauma). It is important to note that the impairment observed in mice that received trauma only (i.e. probe trial 1) was eliminated if animals underwent additional training. This latter finding highlights the strength of including multiple probe trials in the design of the water maze task, a result previously reported in the assessment of radiation-induced cognitive impairments in mice lacking the gene for extracellular superoxide dismutase (Raber et al., 2009). Furthermore our data show that the changes induced by trauma can be obviated by irradiation as well as additional training, suggesting that different mechanisms may be involved in the rescue of hippocampal dependent cognitive functions. Our results are also consistent with a report showing that after severe hippocampal damage, intense training can improve water maze performances (Whishaw and Jarrard, 1996).
The underlying mechanisms associated with cognitive impairments are likely to be multifactorial, but one important possibility involves the process of hippocampal neurogenesis. Neurogenesis has been shown to be important in learning and memory (Dupret et al., 2008; Imayoshi et al., 2008) and may play a major role in hippocampus-associated neurological diseases, such as depression (Drew and Hen, 2007), Alzheimer’s disease (Gouras and Fillit, 2006), and epilepsy (Parent, 2002). Data from our laboratory have shown that cells in the neurogenic zone of the hippocampal DG are extremely sensitive to low/moderate doses of irradiation (Mizumatsu et al., 2003; Monje et al., 2002; Rola et al., 2004a; Rola et al., 2004b; Rola et al., 2005; Rola et al., 2007), and at least for photon irradiation, that such changes are associated with hippocampal dependent cognitive impairments (Raber et al., 2004b; Rola et al., 2004b). Additionally, data from rodent models have shown that focal traumatic brain injury also induces significant changes in neurogenesis (Kleindienst et al., 2005; Lu et al., 2007; Lu et al., 2006; Rola et al., 2006; Sun et al., 2007) that can be associated with altered cognitive outcome (Kleindienst et al., 2005; Lu et al., 2007; Sun et al., 2007). The present results involving irradiation are in general agreement with our previous published data and show a reduction in the number of newly generated neurons after irradiation with 56Fe ions. However this apparent reduction was not associated with cognitive impairment as measured with the Morris water maze. It is possible that the behavioral paradigm used here was not sensitive enough to discriminate subtle changes induced by irradiation, and that such subtle changes could be sufficient to cause protective events to be engaged at the time of traumatic brain injury.
Further complicating this issue is that in those animals showing significant cognitive impairments (trauma alone), numbers of newly born neurons were the same or higher than those seen in sham-treated controls and animals that received irradiation alone (Fig. 3B). Additionally, a single dose of 56Fe irradiation prior to traumatic brain injury resulted in intermediate values relative to either of the single insults alone. Taken together, and in the context of our cognitive data, these findings suggest that there is not a simple relationship between neurogenesis and cognition, and that after injury, changes in neurogenesis are not unequivocal predictors of cognitive outcome.
Increased neurogenesis and migration has been reported in animal models of epilepsy, stroke, trauma, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease (Parent, 2003). While it has been suggested that the increased generation of new neurons might be an adaptive response that could contribute to recovery of learning and memory after trauma (Kernie and Parent, 2010), our data demonstrate that despite elevated numbers of newly generated neurons after trauma, those animals were cognitively impaired. These results suggest that at least for the treatment paradigm and time course used here, increased neurogenesis alone did not provide an effective mechanism of recovery.
It has been previously reported that after ischemic injury, there is increased neurogenesis and that new neurons are found in the outer layers of the dentate gyrus. This contrasts with what is seen in normal animals where most adult-born neurons are generally restricted to the inner third of the DG (Kempermann et al., 2003). Here we demonstrated that after radiation, trauma or combined injury there was a substantial difference in the migration patterns for newly generated neurons when compared to sham treated controls. The injured brain releases numerous extracellular proteins, chemokines, ions and neurotrophic factors that may play roles in regulating neurogenesis and migration of the new neurons (Belmadani et al., 2006). In addition, the injured brain activates both astrocytes and microglia, which are both known to secrete a variety of growth factors as well as immune modulators that may affect progenitor proliferation and survival (Bessis et al., 2007; Myer et al., 2006). While we did not specifically address these issues, it is conceivable that the ‘abnormal’ migration seen here after injury may affect the ability of the new neurons to be properly integrated into the local circuitry. More data are required before such a thesis can be addressed.
The relationship between neurogenesis and cognitive function is complicated and very well could be context-dependent, that is, it may differ depending upon the experimental conditions being investigated. But whether or not the extent of neurogenesis is increased or decreased, the mere measurement of numbers of newly generated neurons, or where they migrate, does not provide definitive information about the functional status of those cells. Along the same lines, it is challenging to identify changes in specific molecular components associated with learning and memory that respond to insults and correlate with cognitive injury (Guzowski et al., 2000; Rosi et al., 2008; Rosi et al., 2009; Rosi et al., 2006). Gene expression associated with learning and memory produces de novo synthesis of proteins that alter synaptic strength and are essential for memory processes. In the present study we used the expression of the plasticity-related immediate early gene Arc to assess the effects of brain irradiation and/or trauma on activity pattern of neurons engaged in hippocampal dependent tasks. The temporal and spatial characteristics of behaviorally-induced Arc expression corroborate neuronal activity profiles obtained using well-accepted electrophysiological recordings (Guzowski et al., 1999; Rosi et al., 2005; Ramirez-Amaya et al., 2005; Rosi et al., 2006; Rosi et al., 2009). Disruption of Arc expression in the dentate granule cells may contribute to deficits in learning and memory after injury (Rosi et al., 2006; Rosi et al., 2009) and we have reported that brain irradiation reduces the expression of Arc (Rosi et al., 2008). To our knowledge no data exist regarding Arc expression after trauma or combined injury.
A number of studies have shown that Arc expression occurs in both mature and newly born neurons (Ramirez-Amaya et al., 2006; Ramirez-Amaya et al., 2005). It is particularly noteworthy that the fraction of newly born neurons that expressed behaviorally-induced Arc, while relatively low (~2.8%) was significantly higher than the proportion of cells expressing Arc in the mature population of granule cells (~1.6%) (Ramirez-Amaya et al., 2006). This may be of considerable importance given that newly born neurons have been reported to be preferentially incorporated into networks associated with spatial memory (Kee et al., 2007). In the present study, we found that in the only treatment group showing cognitive impairments (trauma only) we were unable to find any newly born (BrdU) Arc+ neurons. The other groups, including control, radiation only and combined injury had small numbers of (~2%). However, the limited availability of suitable tissue sections and the low numbers of triple labeled cells limits our ability to make definitive conclusions. Nonetheless, these provocative data suggest that more studies are warranted to address the potential significance of the functional integration of newly born neurons.
While the quantification of newly born Arc+ cells was complicated by relatively low numbers of cells, we were able to detect much higher numbers of mature granule cell neurons that expressed Arc protein. In untreated controls, approximately 3% of the total granule cell population expressed Arc protein. Given that there are approximately 250,000 granule cells in the DG (Kempermann et al., 1997), the values determined here before and after injury constitute significant numbers of cells. In animals that received irradiation only or combined injury, the fractions of neurons expressing Arc protein were the same as controls. In the animals that received trauma there was a significant decrease (30%) in the numbers of neurons expressing Arc (Fig. 6) both in the in the ipsilateral and contralateral hemispheres. Given that Arc expression plays an important role in the neuroplastic mechanisms critical to memory consolidation (Guzowski et al., 2001) and inhibition of Arc protein expression impairs the consolidation of long-term memory during Morris water maze testing (Guzowski et al., 2000), our data showing the reduction of Arc expression after trauma could explain the inability of animals to properly recall the location of the platform during the probe trail (impaired consolidation of long term memory). The Morris water maze task involves bilateral hippocampal integrated activity of multiple systems working in concert for both acquisition and recall of memory. Even if the injury is given ipsilaterally, the consequences can spread throughout both hemispheres. In fact, traumatic brain injury can result in a bilateral denervation of the commissural fibers that connect the two dentate gyri (Swanson et al., 1978), and neuronal degeneration in the contralateral dentate gyrus (Hall et al., 2005). Although our data did not provide direct evidence of this possibility, they support it from a cellular (Arc) and behavioral (impaired probe trail) endpoint. While a definitive cause and effect relationship cannot be established from these endpoints, together with our cognitive data, they suggest that the quantification of Arc protein expression may be a valid predictor for altered hippocampal circuitry resulting in cognitive impairment.
Cells in the CNS can respond to a mild insult such as ischemia, hypoxia, or excitotoxin exposure with activation of endogenous protective mechanisms that enable those cells to survive subsequent insults. The underling mechanisms of this effect, which is similar to a preconditioning response described by others (Dirnagl et al., 2009; Gidday, 2006; Jones et al., 2008; Kirino, 2002; Obrenovitch, 2008) may depend on the activation of several neuroprotective pathways, such as, protease-activated receptors (Cannon et al., 2006), free radical mechanisms, transduction signaling pathways, transcription factors, chaperones, neuroinflammatory responses, trophic factors (for review see: Cadet and Krasnova, 2009). It is not yet known if any of these mechanisms play a role in our combined injury involving irradiation and trauma.
In conclusion, low dose irradiation significantly prevents the consequences of a subsequent traumatic brain injury. Understanding the molecular mechanism(s) responsible for this neuroprotective response may provide guidance for the development strategies/approaches to counter the adverse effects of single or combined injury.
Acknowledgments
Grant Sponsor: NASA, NIH
Grant Number: NNJ05HE33G, NNJ04HC90G, R01CA133216, R21AI080531
we would like to thank Nicole Huth and Genevieve Fiore for their valuable help in counting cell density.
Refernces
- Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26(12):3182–91. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55(3):233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Cellular and molecular neurobiology of brain preconditioning. Mol Neurobiol. 2009;39(1):50–61. doi: 10.1007/s12035-009-8051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Schallert T, Hua Y, Richardson RJ, Xi G. Protease-activated receptor-1 mediates protection elicited by thrombin preconditioning in a rat 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2006;1116(1):177–86. doi: 10.1016/j.brainres.2006.07.094. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19(6):693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8(4):398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6(3):205–18. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25(1):38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18(1):115–27. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19(2):122–32. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gouras G, Fillit H. Neurogenesis as a therapeutic strategy for cognitive aging and Alzheimer’s disease. Curr Alzheimer Res. 2006;3(1):3. doi: 10.2174/156720506775697151. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21(14):5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22(2):252–65. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jones NM, Kardashyan L, Callaway JK, Lee EM, Beart PM. Long-term functional and protective actions of preconditioning with hypoxia, cobalt chloride, and desferrioxamine against hypoxic-ischemic injury in neonatal rats. Pediatr Res. 2008;63(6):620–4. doi: 10.1203/PDR.0b013e31816d9117. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007 doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94(19):10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–74. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22(11):1283–96. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22(6):645–55. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24(7):1132–46. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, de la Pena L, Barker C, Camphausen K, Tofilon PJ. Radiation-induced changes in gene expression involve recruitment of existing messenger RNAs to and away from polysomes. Cancer Res. 2006;66(2):1052–61. doi: 10.1158/0008-5472.CAN-05-3459. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Can Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Morris RJ. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(Pt 10):2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev. 2008;88(1):211–47. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- Parent JM. The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res. 2002;50(1–2):179–89. doi: 10.1016/s0920-1211(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9(4):261–72. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004a;55(3):381–9. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004b;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Raber J, Villasana L, Rosenberg J, Zou Y, Huang TT, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2009 doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25(7):1761–8. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Fishman K, Baure J, Rosi S, Lamborn KR, Obenaus A, Nelson GA, Fike JR. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat Res. 2008;169(6):626–32. doi: 10.1667/RR1263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202(1):189–99. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. Indicators of hippocampal neurogenesis are altered by 56Fe Irradiation in a dose dependent manner. Radiat Res. 2004a;162:442–446. doi: 10.1667/rr3234. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impariment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004b;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rola RVS, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. High LET irradiation induced inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res. 2005;164:556–560. doi: 10.1667/rr3412.1. [DOI] [PubMed] [Google Scholar]
- Rola R, Zou Z, Huang T-T, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Rad, Biol & Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68(23):9763–70. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132(Pt 9):2464–77. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25(3):723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142(4):1303–15. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK. Spatial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Radiat Res. 2006;166(6):892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204(1):264–72. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181(4):681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166(6):883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Villasana L, Poage C, van Meer P, Raber J. Passive avoidance learning and memory of 56Fe sham-irradiated and irradiated human apoE transgenic mice. Radiats Biol Radioecol. 2008;48(2):167–70. [PubMed] [Google Scholar]
- Whishaw IQ, Jarrard LE. Evidence for extrahippocampal involvement in place learning and hippocampal involvement in path integration. Hippocampus. 1996;6(5):513–24. doi: 10.1002/(SICI)1098-1063(1996)6:5<513::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Zeitlin C, Heilbronn L, Miller J. Detailed characterization of the 1087 MeV/nucleon iron-56 beam used for radiobiology at the alternating gradient synchrotron. Radiat Res. 1998;149(6):560–9. [PubMed] [Google Scholar]