Abstract
Analogously to the fenchyl and adamantyl groups, the bornyl and epimeric isobornyl groups are compact lipophilic substituents that can be incorporated into drug design to improve pharmacological or physicochemical properties. Methods are reported for the synthesis and characterization of 2-substituted norbornanes and bornanes that can serve as novel cannabinergic ligand intermediates.
Keywords: Norbornane, Bornyl, Isobornyl, Chiral HPLC, NMR
1. Introduction
The pharmacological and physicochemical properties of certain drug classes can be modified by incorporating compact lipophilic moieties such as the fenchyl,1,2 adamantyl,3–7 and bornyl8 groups. Examples include the CB2 inverse agonist SR1445289 as well as the CB1 selective agonist AM411,10 a crystalline classical cannabinoid analog with distinct pharmacological profiles.11–14 Analogously, we have explored the incorporation of bornyl as well as the epimeric isobornyl groups into our cannabinergic drug design to further optimize ligand pharmacophoric properties. Here, we report our methods for the synthesis and characterization of 2-substituted norbornanes and bornanes that were prepared and characterized as intermediates in the synthesis of novel cannabinoid analogs.
2. Results and discussion
The exo-2-aryl analog 3 of norbornane was conveniently prepared (Scheme 1) by the same method that we described for the preparation of 1-adamantyl analogs.10 Introduction of the norbornyl group by Friedel–Crafts type alkylation of 2,6-dimethoxyphenol (2) with (±)-exo-norborneol (1) in 70% methanesulfonic acid gave the racemic exo-analog (±)-3 in three steps. However, the Wagner–Meerwein rearrangement racemizes optically active norbornanes and bornanes under acidic conditions and greatly limits the usefulness of this method. Thus, we chose a different approach for the synthesis of optically pure 1,7,7-trimethyl (bornane) derivatives that could also provide us with both exo- and endo-epimers.
Scheme 1.
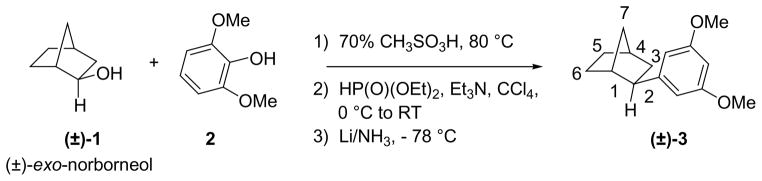
Synthesis of 2-norbornane (exo) analog via Friedel–Crafts type alkylation.
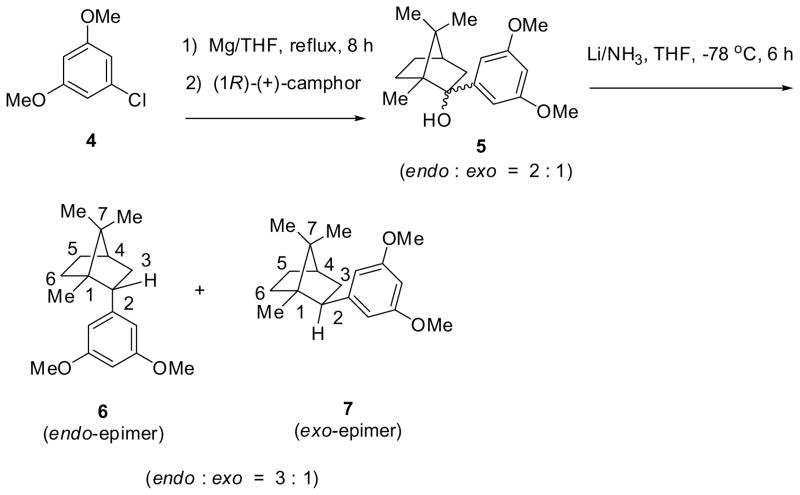
Bornanes were selected for the further exploration of the key cannabinoid side chain pharmacophore as they represented a subtle stereochemical variation of the adamantyl group, both are ten carbon substituents. We now detail a useful method for preparing endo- and exo-epimers of aryl as well as alkyl analogs of 2-bornane with good stereocontrol. This route (Scheme 2) utilized a Grignard reaction with (1R)-(+)-camphor to prepare both endo-(bornyl) and exo-(isobornyl) 2-(3,5-dimethoxyphenyl)-1,7,7-trimethylbicyclo-[2.2.1]heptane analogs, 6 and 7, respectively, that were synthetic intermediates for a new series of classical cannabinoid analogs.15 The Grignard reagent prepared from 1-chloro-3,5-dimethoxybenzene (4) reacted with (1R)-(+)-camphor to give a 2:1 mixture of 3,5-dimethoxyphenyl adducts 5 that was inseparable by both flash column chromatography and chiral HPLC, though we were able to achieve separation after the subsequent step. The reported Grignard reaction of a preformed complex of (1R)-(+)-camphor with cerium(III) chloride16,17 that gives endo-adducts exclusively was not used as both endo- and exo-adducts were desired. The major product of Grignard addition was the endo-adduct as expected from the corresponding reactions of Grignard reagents prepared from bromobenzene,18,19 p-bromoanisole,20 and o-bromoanisole.21 The hydroxyl groups of the mixture of isoborneol and borneol analogs 5 were removed according to the methods reported for the corresponding anisole analog.21 Hydrogenolysis of the 2:1 mixture with palladium on carbon produced some bornyl-skeletal rear-rangement byproduct in addition to the desired reduction products. However, lithium/ammonia reduction cleanly afforded a 3:1 mixture of 5-bornyl-1,3-dimethoxybenzene (6) to 5-isobornyl-1,3-dimethoxybenzene (7), the reduction converting some of the exo-adduct of 5 to both reduction products 6 and 7. A small quantity of the mixture was separated by HPLC on a Chiralpak AD column to unambiguously identify the major product as the endo-epimer 5-bornyl-1,3-dimethoxybenzene (6) that has an exobenzylic proton, and the minor product as the exo-epimer 5-isobornyl-1,3-dimethoxybenzene (7) that has an endo-benzylic proton. The chemical shift differences and coupling patterns of the benzylic protons of 6 and 7 were in agreement with reported values for bornyl and isobornyl analogs.22 The H-2exo proton of 6 was downfield relative to the H-2endo of 7. Also, we observed 4J-coupling23 of the H-2exo benzylic proton of 5-bornyl-1,3-dimethoxybenzene (6) that was confirmed by COSY spectroscopy to be coupling to the coplanar H-6exo. In contrast, the H-2endo benzylic proton of exo-epimer 7 was a characteristic doublet of doublets. The expected ‘W’ couplings of H-3exo to H-5exo for 6 and 7 were also observed. Only couplings of adjacent exo-protons H-3exo and H-5exo were observed to bridgehead H-4 of the bornane derivatives. Observed scalar coupling constants (J) determined by first order analysis of individual proton signals for the endo- and exo-epimers are given in Table 1. All assignments were ultimately confirmed by 2D NOESY experiments (see Supplementary data). It was also found that 2-alkyl substituted analogs, prepared in an analogous manner, 15 also have similar chemical shift effects, especially on H-6exo. Our NMR data should be useful in the assignment of spectra from other bicyclo[2.2.1]heptane derivatives.24,25
Scheme 2.
Synthesis of 2-bornyl (endo) and 2-isobornyl (exo) epimers via a Grignard addition.
Table 1.
Observed coupling constants for endo-(bornyl) and exo-(isobornyl) epimers 6 and 7
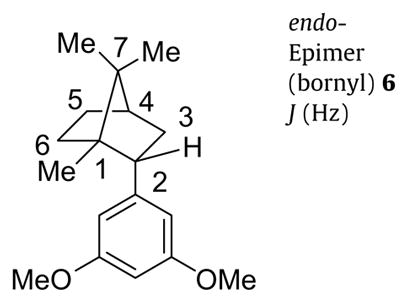
|
endo-Epimer (bornyl) 6 J (Hz) |
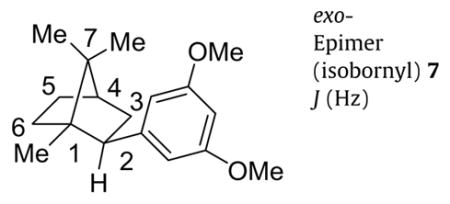
|
exo-Epimer (isobornyl) 7 J (Hz) |
|---|---|---|---|
| J2exo,3exo | 11.5 | J2endo,3exo | 8.0 |
| J2exo,3endo | 5.4 | J2endo,3endo | 9.2 |
| 4J2exo,6exo | 2.5 | 4J2endo,6exo | 0.0 |
| J3exo,3endo | 13.2 | J3exo,3endo | 12.5 |
| J3exo,4 | 4.5 | J3exo,4 | 4.4 |
| 4J3exo,5exo | 3.5 | 4J3exo,5exo | 2.6 |
| J4,5exo | 4.5 | J4,5exo | 4.4 |
| J5exo,5endo | 12.5 | J5exo,5endo | 12a |
| J5exo,6exo | 12.0 | J5exo,6exo | 12a |
| J5exo,6endo | 4.1 | J5exo,6endo | 2.7 |
| J5endo,6exo | 4.6 | J5endo,6exo | 4.4 |
| J5endo,6endo | 9.4 | J5endo,6endo | 9.4 |
| J6exo,6endo | 13.1 | J6exo,6endo | 12a |
Coupling constants determined within 0.5 Hz.
Supplementary Material
Acknowledgments
This work was supported by grants DA-7215, DA-3801, DA-152, and DA-9158 from the National Institute on Drug Abuse.
Abbreviations
- AM411
3-(adamant-1-yl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol
- COSY
correlation spectroscopy
- NMR
nuclear magnetic resonance
- NOESY
nuclear Overhauser enhancement spectroscopy
- SR144528
5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-N-[(1S-endo)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-1H-pyrazole-3-carboxamide
Footnotes
Supplementary data (experimental procedures and characterizations of all compounds. 700 MHz 1H NMR, COSY, and NOESY spectra for endo-epimer 6 and exo-epimer 7 in CDCl3 solutions) associated with this article can be found, in the online version, at doi:10.1016/j.tetlet.2008.07.029.
References and notes
- 1.Janusz JM, Gardlik JM, Young PA, Burkes RV, Stoll SJ, Estelle AF, Riley CM. J Med Chem. 1990;33:1052. doi: 10.1021/jm00165a027. [DOI] [PubMed] [Google Scholar]
- 2.Yuasa Y, Nagakura A, Tsuruta H. J Agric Food Chem. 2001;49:5013. doi: 10.1021/jf010344o. [DOI] [PubMed] [Google Scholar]
- 3.Yu Z, Sawkar AR, Whalen LJ, Wong CH, Kelly JW. J Med Chem. 2007;50:94. doi: 10.1021/jm060677i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohde JJ, Pliushchev MA, Sorensen BK, Wodka D, Shuai Q, Wang J, Fung S, Monzon KM, Chiou WJ, Pan L, Deng X, Chovan LE, Ramaiya A, Mullally M, Henry RF, Stolarik DF, Imade HM, Marsh KC, Beno DWA, Fey TA, Droz BA, Brune ME, Camp HS, Sham HL, Frevert EU, Jacobson PB, Link JT. J Med Chem. 2007;50:149. doi: 10.1021/jm0609364. [DOI] [PubMed] [Google Scholar]
- 5.Webster SP, Ward P, Binnie M, Craigie E, McConnell KMM, Sooy K, Vinter A, Seckl JR, Walker BR. Bioorg Med Chem Lett. 2007;17:2838. doi: 10.1016/j.bmcl.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Cincinelli R, Dallavalle S, Nannei R, Merlini L, Penco S, Giannini G, Pisano C, Vesci L, Ferrara FF, Zuco V, Zanchi C, Zunino F. Bioorg Med Chem. 2007;15:4863. doi: 10.1016/j.bmc.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 7.Brown AD, Bunnage ME, Glossop PA, James K, Jones R, Lane CAL, Lewthwaite RA, Mantell S, Perros-Huguet C, Price DA, Trevethick M, Webster R. Bioorg Med Chem Lett. 2008;18:1280. doi: 10.1016/j.bmcl.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrecher T, Hrenn A, Dormann KL, Merfort I, Labahn A. Bioorg Med Chem. 2008;16:2385. doi: 10.1016/j.bmc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Brelière JC, Le Fur G. J Pharmacol Exp Ther. 1998;284:644. [PubMed] [Google Scholar]
- 10.Lu D, Meng Z, Thakur GA, Fan P, Steed J, Tartal CL, Hurst DP, Reggio PH, Deschamps JR, Parrish DA, George C, Järbe TUC, Lamb RJ, Makriyannis A. J Med Chem. 2005;48:4576. doi: 10.1021/jm058175c. [DOI] [PubMed] [Google Scholar]
- 11.Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, Makriyannis A, Mackie K. Br J Pharmacol. 2004;142:495. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Järbe TUC, DiPatrizio NV, Lu D, Makriyannis A. Behav Pharmacol. 2004;15:517. doi: 10.1097/00008877-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin PJ, Brown CM, Winston KM, Thakur G, Lu D, Makriyannis A, Salamone JD. Behav Pharmacol. 2005;16:477. doi: 10.1097/00008877-200509000-00022. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin PJ, Lu D, Winston KM, Thakur G, Swezey LA, Makriyannis A, Salamone JD. Pharmacol Biochem Behav. 2005;81:78. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Lu D, Guo J, Duclos RI, Jr, Bowman AL, Makriyannis A. J Med Chem. doi: 10.1021/jm8005299. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitrov V, Bratovanov S, Simova S, Kostova K. Tetrahedron Lett. 1994;35:6713. [Google Scholar]
- 17.Pearson AJ, Gontcharov AV. J Org Chem. 1998;63:152. doi: 10.1021/jo971676o. [DOI] [PubMed] [Google Scholar]
- 18.Somfai P, Tanner D, Olsson T. Tetrahedron. 1985;41:5973. [Google Scholar]
- 19.Bergdahl M, Nilsson M, Olsson T, Stern K. Tetrahedron. 1991;47:9691. [Google Scholar]
- 20.Erman WF, Flautt TJ. J Org Chem. 1962;27:1526. [Google Scholar]
- 21.Erman WF. J Am Chem Soc. 1964;86:2887. [Google Scholar]
- 22.Flautt TJ, Erman WF. J Am Chem Soc. 1963;85:3212. [Google Scholar]
- 23.Barfield M, Chakrabarti B. Chem Rev. 1969;69:757. [Google Scholar]
- 24.Koval LI, Dzyuba VI, Ilnitska OL, Pekhnyo VI. Tetrahedron Lett. 2008;49:1645. [Google Scholar]
- 25.Matos RAF, Andrade CKZ. Tetrahedron Lett. 2008;49:1652. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.