Abstract
Purpose.
To assess and compare changes in the biomechanical properties of the cornea following different corneal collagen cross-linking protocols using scanning acoustic microscopy (SAM).
Methods.
Ten donor human corneal pairs were divided into two groups consisting of five corneal pairs in each group. In group A, five corneas were treated with low-fluence (370 nm, 3 mW/cm2) cross-linking (CXL) for 30 minutes. In group B, five corneas were treated with high-fluence (370 nm, 9 mW/cm2) CXL for 10 minutes. The contralateral control corneas in both groups had similar treatment but without ultraviolet A. The biomechanical properties of all corneas were tested using SAM.
Results.
In group A, the mean speed of sound in the treated corneas was 1677.38 ± 10.70 ms−1 anteriorly and 1603.90 ± 9.82 ms−1 posteriorly, while it was 1595.23 ± 9.66 ms−1 anteriorly and 1577.13 ± 8.16 ms−1 posteriorly in the control corneas. In group B, the mean speed of sound of the treated corneas was 1665.06 ± 9.54 ms−1 anteriorly and 1589.89 ± 9.73 ms−1 posteriorly, while it was 1583.55 ± 8.22 ms−1 anteriorly and 1565.46 ± 8.13 ms−1 posteriorly in the untreated control corneas. The increase in stiffness between the cross-linked and control corneas in both groups was by a factor of 1.051×.
Conclusions.
SAM successfully detected changes in the corneal stiffness after application of collagen cross-linking. A higher speed-of-sound value was found in the treated corneas when compared with the controls. No significant difference was found in corneal stiffness between the corneas cross-linked with low- and high-intensity protocols.
Keywords: cornea, collagen cross-linking, riboflavin, UVA, scanning acoustic microscopy (SAM), biomechanics
The study showed similar increase of speed of sound in low- and high-intensity collagen cross-linking on human corneas in vitro.
Introduction
Decreased corneal mechanical stability is one of the key features of keratoconus.1 It has been reported that the stiffness of the keratoconic cornea is reduced by 40% compared with that of the normal cornea.1 A loss of corneal rigidity leads to a reduced resistance to intraocular pressure, which causes bulging of the cornea and reduction of vision.2 Therefore, treatment of keratoconus should focus on strengthening the corneal matrix in order to preserve corneal integrity and obviate the visual impairment problem.
Corneal collagen cross-linking using ultraviolet A (UVA) irradiation and the photosensitizer riboflavin has emerged in the last decade to treat corneal ectasia such as keratoconus3,4 and other corneal pathologies such as bullous keratopathy.5 This treatment aims to increase the biomechanical properties of the compromised keratoconic cornea by inducing new cross-links within the collagen fiber network.4,6,7 Different cross-linking protocols such as high-intensity and transepithelial8,9 cross-linking have been developed recently to improve the cross-linking delivery to patients, reduce the UVA exposure time, and avoid the epithelial removal that is associated with some postoperative complications.10
Several studies have demonstrated that keratoconic corneas have improved visual acuity, refraction, keratometry readings, aberrations, and corneal thickness11–13 after collagen cross-linking treatment. Additionally, collagen cross-linking induces several changes to the physicochemical properties of the corneal collagens, such as increased stiffness,14–16 collagen fiber diameter,17,18 and resistance to enzymatic digestion19 and heat degradation.20 The changes in stiffness caused by corneal collagen cross-linking have been examined by several research groups, and different methodologies have been used to measure the induced stiffness in human and animal corneas after collagen cross-linking.7 An increase in the Young's modulus by a factor of 1.5× in human corneas,21 1.8× in porcine corneas,14 and 1.6× in rabbit corneas22 has been reported after application of collagen cross-linking in vitro. Furthermore, the stiffening effect of collagen cross-linking was found to be concentrated in the anterior cornea.15
The most frequently used technique to measure stiffness of the cornea is the strip extensometry technique, due to its simplicity.23 However, strip extensometery is a destructive method as it involves cutting the stromal lamellae24; and only one strip can be taken from each cornea, so only one measurement can be obtained from each cornea. Additionally, the accuracy and the reliability of this method are limited25 because the technique flattens the naturally curved strips taken from the corneas. The strip extensometry technique neglects this natural thickness variation between the central and peripheral parts of the cornea.24
A technique that has been widely used to detect micromechanical variations in engineering materials26 is scanning acoustic microscopy (SAM). SAM has also been applied to biologic tissues and has been used to examine micromechanical changes in various tissues, including bone, teeth,27,28 and blood vessels.29 Furthermore, we have recently used the technique on human corneal samples and have shown its effectiveness in detecting mechanical variations between cross-linked and control corneas.30 SAM provides an evaluation of the biomechanical properties of tissues quantitatively and qualitatively. It allows pixel-by-pixel analysis of tissue properties with a high spatial resolution (approximately 1 μm at 1 GHz).29,31,32
The components and principle of SAM are discussed in a previous publication.30 In brief, the SAM consists of an ultrasonic transducer that emits and receives acoustic waves. The transducer transforms the electrical pulses into ultrasound waves (100 MHz–1 GHz),29 which are focused by an acoustic lens and which then pass through a coupling fluid (usually distilled water) . The ultrasound waves then propagate within the tissue and reflect back with different wave speeds according to the different stiffness values of the tissue tested.29,31,32 SAM resolves many of the problems associated with strip extensometry and provides localized (micrometer-scale) stiffness information as compared to the bulk mechanical response determined with strip testing.
In this study we applied the SAM technique to study the mechanical properties of the cornea after cross-linking treatment on eye-banked human corneas. To the best of our knowledge, this study presents for the first time SAM data to map the mechanical properties of human corneas that have undergone collagen cross-linking, and the results are compared with those in non-cross-linked corneas. In addition, this study explored the stiffening effect of a high-intensity cross-linking protocol and compared it with low-intensity cross-linking using the SAM technique.
Materials and Methods
Corneas
Twenty postmortem human corneas (10 pairs, from 8 male and 2 female donors) were used in this study. The corneal pairs were divided into two treatment groups. The age range of the donors was 61 to 86 years. The corneas with a 3-mm scleral rim were removed from the eyes within 24 hours of death and placed in organ culture media at 34°C.33 During this period the corneas swell due to changes in the extracellular matrix. After 10 days, the corneas were assessed macroscopically and microscopically (using endothelial cell counts). The corneas were then placed in fresh media containing 5% dextran and incubated at 34°C for a further 24 hours to allow the corneas to shrink back to their physiological thickness.33
This study was approved by the Committee on the Ethics of Research on Human Beings of The University of Manchester and followed the terms of the Declaration of Helsinki. Corneas were obtained from Manchester Eye Bank. These corneas were contraindicated for corneal transplantation (for causes that did not involve the eye directly, e.g., insufficient medical history). All the corneas had research permission from the donors' relatives if transplantation was not possible.
Treatment Groups
In group A (low-intensity cross-linking group), five pairs of corneas were epithelium debrided (8 mm) by gentle scraping with a blade and exposed to one or two drops of isotonic riboflavin 0.1% solution (10 mg riboflavin-5-phosphate in 20% dextran T-500 10 mL solution) at 3-minute intervals for 30 minutes; one cornea from each pair was then exposed to UVA 370 nm, 3 mW/cm2 for 30 minutes while riboflavin was instilled at 5-minute intervals using a VEGA CBM X-Linker (CSO, Florence, Italy). The contralateral cornea was subject to riboflavin instillation for a further 30 minutes without exposure to UVA irradiation (60 minutes in total). The cornea was then rinsed with saline and placed back in a bottle of fresh media containing 5% dextran, to limit the corneal swelling, and incubated at 34°C for 24 hours prior to assessment.
In group B (high-fluence CXL group), the five corneal pairs were 8-mm epithelium debrided by gentle scraping with a blade and were exposed to 0.1% riboflavin (10 mg riboflavin-5-phosphate in 20% dextran T-500 10 mL solution) instillation for 30 minutes. Five corneas were then exposed to high-intensity UVA irradiation (365 nm, 9 mW/cm2) for 10 minutes using an IROC AG X-Linker (IROC, Zurich, Switzerland) while the riboflavin was being applied, and the contralateral corneas were exposed to riboflavin only for 10 minutes and served as controls (40 minutes in total for each cornea). The cornea was then rinsed with saline and placed back in a bottle of fresh media containing 5% dextran, to limit the corneal swelling, and incubated at 34°C for 24 hours prior to assessment.
Each cornea was bisected and embedded in optimal cutting temperature (OCT, Tissue-Tek; CellPath, Powys, UK). The OCT-embedded corneas were then sectioned on a cryostat (Leica CM-3050-S Cryostat; Leica Microsystems, Milton Keynes, UK) to a thickness of 7 μm. The sections were mounted on glass slides and stored at −20°C until usage. Sections of the central cornea were then imaged using SAM.
Scanning Acoustic Microscopy Imaging
The principle of SAM has been described previously by our group.30 SAM evaluation was conducted with a SAM 2000 microscope (PVA TePla Analytical Systems GmbH, Herborn, Germany) improved with a custom data acquisition and control system.30 The SAM data were collected with multilayer phase analysis (MLPA).30 In summary, using the MLPA method, the acoustic focal point of the lens was initially set 4 μm above the substrate surface of the corneal tissue. Then, a series of 200 × 200 μm C-scan images were taken at different z-positions commencing from this height and moved slightly in small steps toward the substrate surface (0.1-μm steps over a range of 5 μm in this study). This process was repeated in a systematic pattern until the central part of the cornea was imaged. For corneas, the optimal acoustic frequency was found at 761 MHz; hence all of the samples were scanned at this frequency. At this frequency, a spatial resolution of 1.3 μm was achieved. Distilled water was used as the coupling fluid, and the experiments were conducted at room temperature.
The images were processed offline with custom software using the phase analysis method developed by Zhao et al.34 The grayscale value for every pixel (x, y position) was extracted from all the images at each z position to form a V(z) curve. The V(z) data were then filtered and processed by fast Fourier transformation (FFT). A two-dimensional phase array was then obtained for the dataset, which was processed and converted to a speed-of-sound map.
Statistical Analysis
All data are reported as mean ± SE. Kolmogrov-Smirnov test was used to determine if the data were normally distributed. Data were found to be normally distributed (P > 0.05). A two-sample t-test was used to test whether the mean values of the measurements were significantly different from each other. Data analysis was conducted using SPSS 19.0 (SPSS, Chicago, IL), and a P value of <0.05 was considered to be statistically significant.
Results
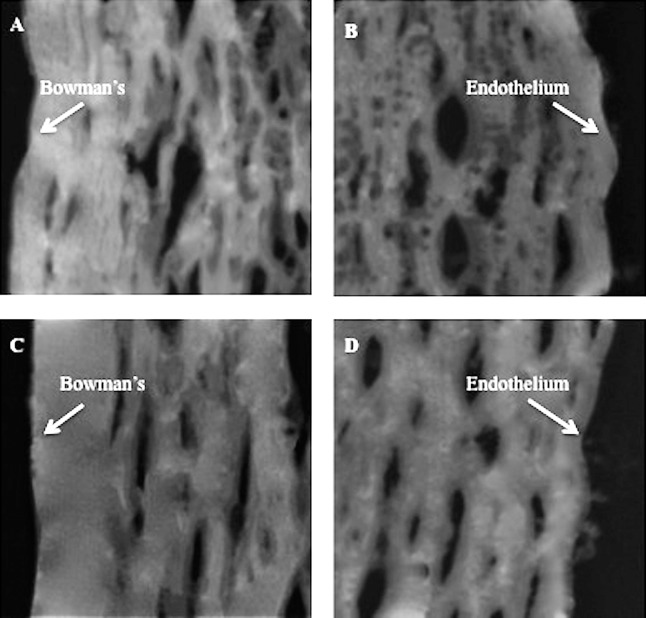
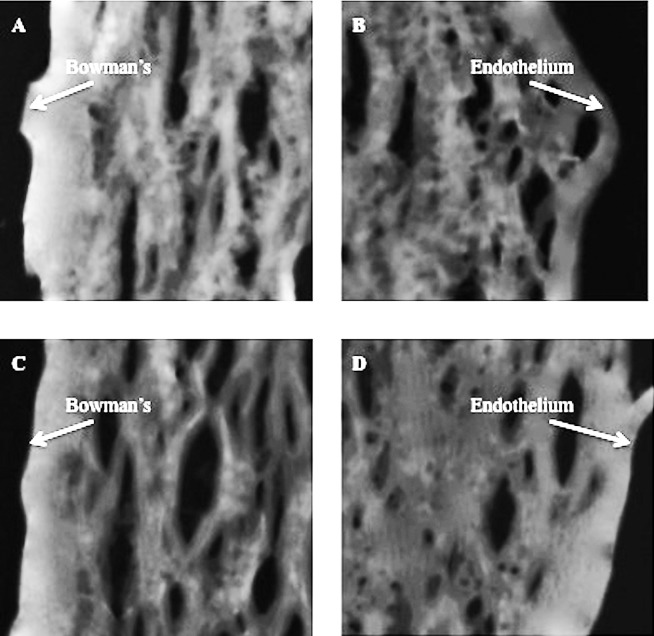
SAM images of corneal tissue are shown in Figures 1 and 2. These images are taken from corneas that underwent low-fluence CXL treatment (Figs. 1A, 1B) and their contralateral controls (Figs. 1C, 1D) and from corneas that underwent high-fluence CXL treatment (Figs. 2A, 2B) and their contralateral controls (Figs. 2C, 2D). The cross-linked corneal sections of both groups (Figs. 1A, 2A) showed brighter images anteriorly than posteriorly (Figs. 1B, 2B). Furthermore, the anterior (200 × 200 μm) region of the cross-linked corneas (Figs. 1A, 2A) was found to be brighter than that from the control group (Figs. 1C, 2C).
Figure 1.
The anterior and posterior 200 × 200 μm portion of the low-fluence CXL group ([A, B], respectively) and untreated (C, D) cornea tissue sections.
Figure 2.
The anterior and posterior 200 × 200 μm portion of the high-fluence CXL group (A, B) and untreated (C, D) cornea tissue sections.
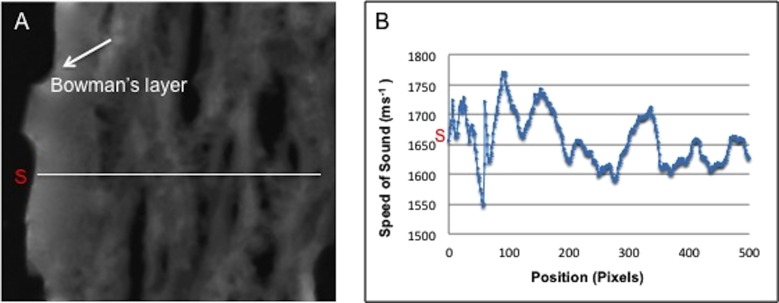
Variations of the speed-of-sound values in any selected region were quantitatively measured by averaging the values within a region of the cornea excluding the artificial gaps (caused by the cryosectioning procedure). Additionally, local variations in stiffness can be measured using line profile mapping as shown in Figure 3.
Figure 3.
Line profile (A) across the corneal tissue and (B) the corresponding speed-of-sound variations.
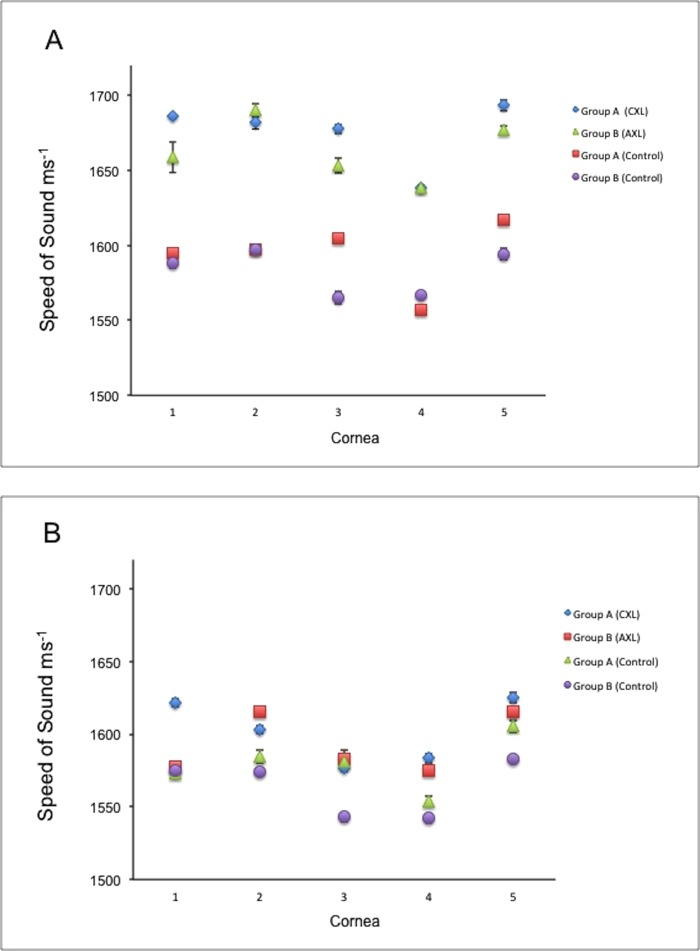
SAM imaging of the cross-linked and control corneas was done on the central part of the corneal section in both groups. The speed of sound of each sample was then compared between the anterior (200 × 200 μm) region and the posterior (200 × 200 μm) region (Figs. 4A, 4B). In the low-fluence CXL treatment group A, the mean ± SE of speed of sound of the cross-linked corneas was 1677.38 ± 10.70 ms−1 anteriorly and 1603.90 ± 9.82 ms−1 posteriorly, which represents an increase of 1.046×. This increase was found to be statistically significant (P = 0.001). The speed of sound in the control corneas of this group was 1595.23 ± 9.66 ms−1 anteriorly and 1577.13 ± 8.16 ms−1 posteriorly. This difference was not significant (P = 0.190). The increase in stiffness between the cross-linked and control corneas in the anterior 200 × 200 μm region was significant (P = 0.005); this stiffness increased by a factor of 1.051×. In the high-fluence CXL treatment group B, the mean ± SE of speed of sound of the cross-linked corneas was higher anteriorly (1665.06 ± 9.54 ms−1) than posteriorly (1589.89 ± 9.73 ms−1), representing a 1.047× increase. This difference was statistically significant (P = 0.001). In the contralateral corneas, the speed of sound was 1583.55 ± 8.22 ms−1 anteriorly and 1565.46 ± 8.13 ms−1 posteriorly, showing no significant difference (P = 0.157). The increase in stiffness between the cross-linked and control corneas in the anterior 200 × 200 μm region was by a factor of 1.051×, showing a significant change (P = 0.005). Furthermore, no significant differences were found in the speed of sound between the two protocols anteriorly (P = 0.415) or posteriorly (P = 0.340).
Figure 4.
The speed-of-sound measurements of the anterior 200 × 200 μm region (A) and the posterior 200 × 200 μm region (B) of the low-fluence CXL (CXL) and high-fluence CXL (AXL) groups. Higher values were detected from the anterior regions of the cross-linked corneas of both groups when compared with the posterior regions of same cornea and with the controls.
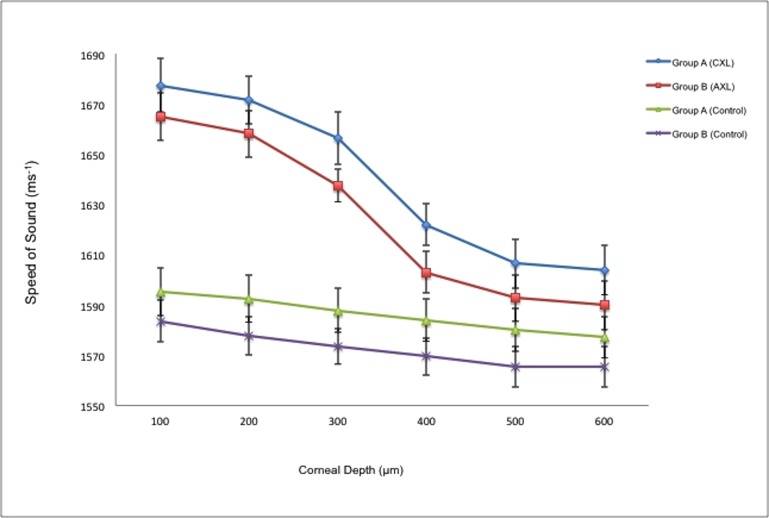
Additionally, the speed of sound across the stromal regions of the cornea was assessed for the low-fluence and high-fluence cross-linked groups (Fig. 5). In the cross-linked groups, there was a steady decrease in the magnitude of speed of sound from the anterior region through to the posterior regions of the stroma. This decrease in the speed of sound was found to fit a cubic function for both the low-fluence CXL group (y = 2.0947x3 − 21.728x2 + 47.47x + 1649; R2 = 0.9938) and the high-fluence CXL group (y = 1.8965x3 − 19.693x2 + 41.357x + 1641; R2 = 0.9972).
Figure 5.
Speed-of-sound measurements of the cross-linked corneas in groups A and B and their contralateral controls from the anterior to the posterior regions of the stroma. A similar decrease in speed of sound was noted in both cross-linked groups.
Discussion
Measurement of the changes in stiffness of the cornea is often used as indirect evidence of UVA/riboflavin collagen cross-linking, as the chemical bonds induced by collagen cross-linking cannot be directly visualized by staining methods.15 An increase in the corneal biomechanical rigidity after application of collagen cross-linking has been reported for animal14,22,35 and human corneas.15 Most standard techniques measure the bulk biomechanical properties of corneal tissue.7 However, the corneal stroma is heterogeneous, and its stiffness depends on its microstructure. The stroma is packed with collagen fibers and proteoglycans anteriorly, which contribute to the stiffness of this tissue.36,37 Additionally, the collagen cross-linking effect is depth dependent and is concentrated in the anterior one-third of the cornea.15 Therefore, a technique that can measure the stiffness of the corneal tissue at the microscopic level is essential to assess the effect of this treatment comprehensively.
This study has demonstrated that stiffness, via measurement of the speed of sound, can be measured for thin histologic eye-banked human corneal sections after application of collagen cross-linking treatment using SAM. Moreover, the study compares for the first time the induced stiffness resulting from low- versus high-intensity cross-linking treatment on postmortem human corneas at different depths.
We chose SAM as it can measure the speed of sound, which is directly proportional to the square root of the stiffness (Young's modulus) in the tissue, pixel by pixel.29,32 SAM thus provides a more accurate measure of the induced stiffness effect after cross-linking.30 Additionally, several measurements can be taken from the same sample, and the same tissue sections can be used for subsequent staining or histologic tests.30
The outcomes of this study showed that the low-intensity collagen cross-linking (30-minute UVA exposure) increased the stiffness of the corneal tissue by 1.051×. This confirms our previous feasibility study findings30 for application of SAM to frozen corneal tissue sections. Additionally, this study showed that high-intensity collagen cross-linking (10-minute UVA exposure) induced similar corneal stiffness changes (increase of 1.051×). The similarity of these results can be explained by the fact that the two protocols deliver the same radiant exposure (5.4 J/cm2)38; and according to the Bunsen-Roscoe law, the induced photochemical reaction depends on the total irradiation dose regardless of the irradiation time.39 However, it has been proven that the Bunsen-Roscoe law is not valid for application of very high-intensity cross-linking (40–50 up to 90 mW/cm2 for less than 2 minutes) on porcine corneas ex vivo.40 Our results confirm the findings of Schumacher et al.41 using stress–strain measurements on porcine corneas; they found an increase in stiffness by a factor of 1.33× in the “standard treatment” corneas and by a factor of 1.32× in the high-intensity cross-linked corneas when compared with controls. Again there was no significant difference between treatments. When comparing the speed-of-sound measurement of the low-fluence CXL corneas with the high-fluence CXL corneas, we found a higher value in the low-fluence CXL group (1675.65 ± 21.37 ms−1) than in the high-fluence CXL group (1663.56 ± 20.17 ms−1). However, it should be noted that in the present study, the speed of sound was also relatively lower in the control corneas of the high-fluence CXL group, and the magnitude of change between controls and treatment groups was similar in both low- and high-fluence CXL groups. It has been suggested that shorter irradiation time in the case of higher-intensity cross-linking might limit its efficacy in generating chemical bonds. This observation has been reported previously by Hammer et al. (Hammer A, et al. IOVS 2013;54:ARVO E-Abstract 4073) and in the study by Wernli et al.40 of porcine eyes and is consistent with a recent modeling study predicting that higher-intensity protocols for shorter times will lead to a decrease in the rate of cross-link generation.42 The present study does not demonstrate this effect on human corneas with the 10 mW/cm2 intensity of high-fluence CXL, although the difference might be more apparent with higher-intensity cross-linkers. The previous study40 used a range of cross-linking intensities up to 90 mW/cm2, and this might have led to the differences in results between the present study and those of Hammer et al. and Wernli et al.40 A larger sample (more than 50 corneas) is also needed to demonstrate equivalence of the two protocols statistically on human corneas.41 This would require a significant amount of human corneal tissue, which will have both ethical and practical implications.
The safety of low- and high-intensity collagen cross-linking protocols with regard to the stromal keratocytes and endothelium has been studied by our group in vitro, and similar results were observed with the two protocols (Beshtawi I, Hillarby H, O'Donnell C, submitted for publication, 2013). The findings of the present study and the previous histologic study (Beshtawi I, Hillarby H, O'Donnell C, submitted for publication, 2013) suggest the two treatment types have similar efficacy and safety profiles, respectively.
Previous in vitro studies have shown an improvement in the biomechanical properties after application of collagen cross-linking in human corneas.14,15 Young's modulus was reported to increase by a factor of 1.5×21 and 1.6×.15 Our results are slightly lower than those reported previously, which may be due to the different experimental procedures. Previous studies used a whole strip of cornea from which to take the measurements (often using strip extensometery), whereas in this study we obtained the speed-of-sound measurements from the anterior one-third of the cornea, where the cross-linking effect is suggested to occur,15 as well as from the middle and posterior parts of the cornea.
We also assessed the changes in the speed of sound from the anterior part to the posterior parts of the cross-linked corneas in the low- and high-fluence CXL groups and their contralateral controls. Our results confirm the concentration of the cross-linking biomechanical effect in the anterior 200 μm of the corneal stroma. A higher speed of sound was obtained from the anterior 200 μm versus the posterior 200 μm of the treated corneas, indicating that the cross-linking effect mainly occurs in the anterior cornea, which contributes most to the induced change in the mechanical properties. This was suggested previously by Kohlhaas et al.15 using stress–strain measurements on porcine and human corneas and in other studies.17,19,20 This is explained by the fact that riboflavin and the UVA irradiance are mostly absorbed in the anterior 200 μm of the stroma, which is likely to prevent damage to the deeper layers of the cornea, especially the endothelial layer,15 and the deeper structures of the eye such as the crystalline lens and the retina. Additionally, the increase in the speed of sound was found to be depth dependent; it decreases from the anterior part of the corneal stroma to the posterior part in the cross-linked corneas in both groups. SAM showed an increase in the speed of sound in the midstroma of the cross-linked corneas in both groups when compared with the controls. This is in agreement with Schumacher et al.42 and Scarcelli el al.,43 who also showed an increase of cross-links in the midstroma. Furthermore, mathematical modeling of the speed-of-sound data for the low- and high-fluence CXL corneas demonstrated that the optimal fit of both curves followed a cubic function (R2 > 0.99); therefore it gives us the ability to calculate the relative speed-of-sound value and estimate the cross-linking effect at any corneal depth point.
A higher speed-of-sound value was observed in the anterior versus the posterior stroma in the control corneas of both groups. This is due to normal structural differences between the anterior and posterior stroma.37 The collagen fibers, for example, are more tightly packed in the anterior stroma; thus it is stiffer than the posterior parts of the stroma.36
To the best of our knowledge, the principle of SAM cannot be used clinically to obtain measurements of corneal speed of sound in vivo. However, ultrasound biomicroscopy is often used clinically (utilizing 50- to 100-MHz transducers) to obtain qualitative and quantitative measurements for the anterior segment of the human eye.44 Applications include assessing the morphologic changes in the anterior eye segment in health44 and disease45 and after surgeries,46 assessing the anterior chamber angle,47 and diagnosing anterior segment tumors.48 Although the ultrasound measurements provide very good depth measurements, they cannot be used for grading the biomechanical properties of the structures in the eye. The characteristics of the transducers used for the SAM technique make it suitable for measuring the micromechanical properties of tissues.
In conclusion, the stiffness induced by collagen cross-linking is depth dependent (changing from the anterior part toward the posterior part of the corneal stroma) and mainly occurs in the anterior one-third of the stroma. SAM was used successfully here to assess corneal stiffness after application of different protocols of collagen cross-linking. Similar results were observed in corneas treated with low-fluence CXL versus high-fluence CXL, the latter with three times shorter irradiation time. This technique can be used to assess the induced mechanical properties after cross-linking treatment, compare the effect of different protocols, and assess the effect of repeated cross-linking treatment in vitro. The small sample size and the age of the corneas used are the limitations of this study.
Acknowledgments
The authors thank Isaac Zambrano and the Manchester Eye Bank for providing the corneas for this study, Sebastian Brand (Fraunhofer Institute of Material Mechanics, Germany), and Kay Raum (Julius Wolff Institut and Berlin-Brandenburg School for Regenerative Therapies, Germany), who developed the MATSAM software utilized in this study.
Supported by Wellcome Trust (WT085981AIA) for development of the SAM; and An-Najah National University, Nablus, Palestine (IMB).
Disclosure: I.M. Beshtawi, None; R. Akhtar, None; M.C. Hillarby, None; C. O'Donnell, None; X. Zhao, None; A. Brahma, None; F. Carley, None; B. Derby, None; H. Radhakrishnan, None
References
- 1. Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980; 31: 435–441 [DOI] [PubMed] [Google Scholar]
- 2. Ambekar R, Toussaint KC Jr. Wagoner Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011; 4: 223–236 [DOI] [PubMed] [Google Scholar]
- 3. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135: 620–627 [DOI] [PubMed] [Google Scholar]
- 4. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006; 17: 356–360 [DOI] [PubMed] [Google Scholar]
- 5. Krueger RR, Ramos-Esteban JC, Kanellopoulos AJ. Staged intrastromal delivery of riboflavin with UVA cross-linking in advanced bullous keratopathy: laboratory investigation and first clinical case. J Refract Surg. 2008; 24: S730–S736 [DOI] [PubMed] [Google Scholar]
- 6. Raiskup-Wolf F, Hoyer A, Spoerl E, et al. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008; 34: 796–801 [DOI] [PubMed] [Google Scholar]
- 7. Beshtawi IM, O'Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin crosslinking. J Cataract Refract Surg. 2013; 39: 451–462 [DOI] [PubMed] [Google Scholar]
- 8. Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010; 26: 942–948 [DOI] [PubMed] [Google Scholar]
- 9. Filippello M, Stagni E, O'Brart D. Transepithelial corneal collagen crosslinking: bilateral study. J Cataract Refract Surg. 2012; 38: 283–291 [DOI] [PubMed] [Google Scholar]
- 10. Mazzotta C, Balestrazzi A, Baiocchi S, et al. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Exp Ophthalmol. 2007; 35: 580–582 [DOI] [PubMed] [Google Scholar]
- 11. Vinciguerra P, Albe E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009; 116: 369–378 [DOI] [PubMed] [Google Scholar]
- 12. Henriquez MA, Izquierdo L Jr, Bernilla C, et al. Riboflavin/ultraviolet A corneal collagen cross-linking for the treatment of keratoconus: visual outcomes and Scheimpflug analysis. Cornea. 2011; 30: 281–286 [DOI] [PubMed] [Google Scholar]
- 13. O'Brart DP, Chan E, Samaras K, et al. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br J Ophthalmol. 2011; 95: 1519–1524 [DOI] [PubMed] [Google Scholar]
- 14. Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003; 29: 1780–1785 [DOI] [PubMed] [Google Scholar]
- 15. Kohlhaas M, Spoerl E, Schilde T, et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006; 32: 279–283 [DOI] [PubMed] [Google Scholar]
- 16. Lanchares E, Del Buey MA, Cristobal JA, et al. Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 1223–1227 [DOI] [PubMed] [Google Scholar]
- 17. Wollensak G, Wilsch M, Spoerl E, et al. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004; 23: 503–507 [DOI] [PubMed] [Google Scholar]
- 18. Mencucci R, Marini M, Paladini I, et al. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clin Experiment Ophthalmol. 2010; 38: 49–56 [DOI] [PubMed] [Google Scholar]
- 19. Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004; 29: 35–40 [DOI] [PubMed] [Google Scholar]
- 20. Spoerl E, Wollensak G, Dittert DD, et al. Thermomechanical behavior of collagen-cross-linked porcine cornea. Ophthalmologica. 2004; 218: 136–140 [DOI] [PubMed] [Google Scholar]
- 21. Spoerl E. Biomechanical stiffness of keratoconic cornea, crosslinking and correlation to demarcation line. In: 7th International Congress of Corneal Cross-Linking. Zurich, Switzerland; 2011. [Google Scholar]
- 22. Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009; 87: 48–51 [DOI] [PubMed] [Google Scholar]
- 23. Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998; 66: 97–103 [DOI] [PubMed] [Google Scholar]
- 24. Ahearne M, Yang Y, Then KY, et al. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br J Ophthalmol. 2008; 92: 268–271 [DOI] [PubMed] [Google Scholar]
- 25. Elsheikh A, Anderson K. Comparative study of corneal strip extensometry and inflation tests. J R Soc Interface. 2005; 2: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khuriyakub BT. Scanning acoustic microscopy. Ultrasonics. 1993; 31: 361–372 [Google Scholar]
- 27. Eckardt I, Hein HJ. Quantitative measurements of the mechanical properties of human bone tissues by scanning acoustic microscopy. Ann Biol Eng. 2001; 29: 1043–1047 [DOI] [PubMed] [Google Scholar]
- 28. Raum K, Kempf K, Hein HJ, et al. Preservation of microelastic properties of dentin and tooth enamel in vitro—a scanning acoustic microscopy study. Dent Mater. 2007; 23: 1221–1228 [DOI] [PubMed] [Google Scholar]
- 29. Akhtar R, Sherratt MJ, Watson RE, et al. Mapping the micromechanical properties of cryo-sectioned aortic tissue with scanning acoustic microscopy. Mater Res Soc Symp Proc. 2009; 1132E: ukpmcpa27262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beshtawi IM, Akhtar R, Hillarby MC, et al. Scanning acoustic microscopy for mapping the microelastic properties of human corneal tissue. Curr Eye Res. 2013; 38: 437–444 [DOI] [PubMed] [Google Scholar]
- 31. Briggs A. ed An Introduction to Scanning Acoustic Microscopy. Oxford: Oxford University Press; 1985. [Google Scholar]
- 32. Briggs A, Kolosov O. eds Acoustic Microscopy. Oxford: Oxford University Press; 2010. [Google Scholar]
- 33. Armitage WJ, Easty DL. Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci. 1997; 38: 16–24 [PubMed] [Google Scholar]
- 34. Zhao XG, Akhtar R, Nijenhuis N, et al. Multi-layer phase analysis: quantifying the elastic properties of soft tissues and live cells with ultra-high-frequency scanning acoustic microscopy. IEEE Trans Ultrason Ferroelectr Freq Control. 2012; 59: 610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCall AS, Kraft S, Edelhauser HF, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest Ophthalmol Vis Sci. 2010; 51: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991; 32: 2244–2258 [PubMed] [Google Scholar]
- 37. Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001; 85: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caporossi A, Baiocchi S, Mazzotta C, et al. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen—preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006; 32: 837–845 [DOI] [PubMed] [Google Scholar]
- 39. Bunsen RW, Roscoe HE. Photochemical researches, part V: on the measurement of the chemical action of direct and diffuse sunlight. Proc R Soc Lond. 1862; 12: 306–312 [Google Scholar]
- 40. Wernli J, Schumacher S, Spoerl E, et al. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013; 54: 1176–1180 [DOI] [PubMed] [Google Scholar]
- 41. Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011; 52: 9048–9052 [DOI] [PubMed] [Google Scholar]
- 42. Schumacher S, Mrochen M, Wernli J, et al. Optimization model for UV-riboflavin corneal cross-linking. Invest Ophthalmol Vis Sci. 2012; 53: 762–769 [DOI] [PubMed] [Google Scholar]
- 43. Scarcelli G, Kling S, Quijano E, et al. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013; 54: 1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishikawa H, Schuman JS. Anterior segment imaging: ultrasound biomicroscopy. Ophthalmol Clin North Am. 2004; 17: 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roters S, Engels BF, Szurman P, et al. Typical ultrasound biomicroscopic findings seen in ocular hypotony. Ophthalmologica. 2002; 216: 90–95 [DOI] [PubMed] [Google Scholar]
- 46. Trindade F, Pereira F, Cronemberger S. Ultrasound biomicroscopic imaging of posterior chamber phakic intraocular lens. J Refract Surg. 1998; 14: 497–503 [DOI] [PubMed] [Google Scholar]
- 47. Ishikawa H, Esaki K, Liebmann JM, et al. Ultrasound biomicroscopy dark room provocative testing: a quantitative method for estimating anterior chamber angle width. Jpn J Ophthalmol. 1999; 43: 526–534 [DOI] [PubMed] [Google Scholar]
- 48. Reminick LR, Finger PT, Ritch R, et al. Ultrasound biomicroscopy in the diagnosis and management of anterior segment tumors. J Am Optom Assoc. 1998; 69: 575–582 [PubMed] [Google Scholar]