Abstract
Purpose.
We examined the effect of aging on Fas ligand (FasL) function in a mouse model of choroidal neovascularization (CNV).
Methods.
Young and aged mice were laser treated to induce CNV. Bone marrow chimeras were performed between young and aged mice. FasL protein expression was examined in the eye and soluble FasL (sFasL) was measured in the blood. Young and aged mice were treated with a matrix metalloprotease (MMP) inhibitor and systemic sFasL was neutralized by antibody treatment. Macrophages from young and aged mice were tested for sFasL-mediated cytokine production and migration.
Results.
The elevated CNV response observed with aging was dependent on bone marrow–derived cells. FasL expression in the eye was increased with age, but decreased following laser treatment. Aged mice had higher levels of sFasL in the blood compared to young mice. Systemic treatment with an MMP inhibitor decreased bloodborne sFasL, and reduced CNV in young and aged mice. Systemic neutralization of sFasL reduced CNV only in aged mice. sFasL increased cytokine production in aged macrophages and proangiogenic M2 macrophages. Aged M2 macrophages had elevated Fas (CD95) expression and displayed increased migration in response to sFasL compared to M1 macrophages derived from young animals.
Conclusions.
Age modulates FasL function where increased MMP cleavage leads to a loss of function in the eye. The released form of FasL (sFasL) preferentially induces the migration of proangiogenic M2 macrophages into the laser lesions and increases proangiogenic cytokines promoting CNV. FasL may be a viable target for therapeutic intervention in aged-related neovascular disease.
Keywords: macrophages, neovascularization, immune privilege, cytokine, cell migration, age-related macular degeneration
FasL function in the eye decreases with age due to MMP-mediated cleavage, which generates bloodborne sFasL. sFasL preferentially activates proangiogenic M2 macrophages.
Introduction
Age-related macular degeneration (AMD) is a progressive disease that leads to irreversible visual impairment and blindness in millions of people globally. The neovascular form of AMD, which is characterized by choroidal neovascularization (CNV), is the most aggressive form and accounts for the vast majority of blindness associated with this disease. This makes identifying therapies that can control neovascularization a top priority. However, few treatments are available at this time. Anti-VEGF therapy has proved effective at slowing the progression of neovascular AMD, but this treatment does not permanently prevent the progression to blindness.1,2 Consequently, studies exploring the basic mechanisms of disease that might lead to novel therapeutics are an intense area of study.
Age is a significant risk factor in the development of AMD as a number of age-related changes in the eye are associated with the increased incidence of disease.2–4 Aging effects have been noted in animal models5; however, many animal studies designed to explore the mechanisms of AMD are performed on relatively young animals,6–11 which may not take into account age-related factors that might modulate disease, such as changes in the immune system, which may have a significant role in animal models and human AMD.12–14 The laser-induced mouse model of neovascular AMD, which is influenced by macrophages,7,10,11 also is influenced by aging.15–17 However, the effects of aging on the immune mechanisms in this model have not been examined thoroughly to our knowledge. This is in spite of the fact that aging alters immune responses18,19 and macrophage function.16,20
Recently, we described a complex role for macrophages in regulating CNV in this mouse model by demonstrating that macrophages can be pro- or antiangiogenic depending on the conditions of their activation.7,16 In these studies proinflammatory M1 macrophages were inhibitory to the CNV response, while M2 macrophages promoted neovascularization. Furthermore, eyes of aged mice were enriched for M2 macrophages and this accounted for the age-dependent increase in the neovascular response. Thus, the intensity of the neovascularization was the result of a balance between M1 and M2 macrophages, not the absolute number of these cells in the eye.7,17 It still is not clear, however, why age alters macrophage function and what local factors might favor the functions of macrophage subtypes.
Immune privilege is a term applied to organs that have a unique relationship with the immune response. These sites prohibit the spread of inflammation at the expense of immune effector mechanisms, since even minor episodes can threaten organ integrity.21 An important part of immune privilege is the constitutive expression of the death-inducing ligand CD95L (hereafter FasL) on the parenchymal cells of the eye that inhibits inflammation by inducing apoptosis in invading inflammatory cells expressing the Fas receptor (CD95).22–24 Additional studies have extended this concept to pathogenic neovascularization demonstrating an important role for FasL in controlling neovascular responses of the cornea,25 retina,26 and choroid27; an idea that has been extended to tumor biology.28,29 Importantly, the effect of FasL in controlling neovascularization is influenced strongly by the activity of matrix metalloproteases (MMP), which cleave FasL from the cell surface diminishing its proapoptotic function during angiogenic responses. Inhibition of MMP prevents FasL cleavage, and restores its function in the cornea30 and retina.8 The effect of aging on FasL expression and function in the eye currently is unknown and, since this proapoptotic protein is critical to the integrity of the eye,22,31 we thought a better understanding of the changes in FasL with aging should be pursued. Studies presented here revealed that FasL function changes with age due to the increased activity of MMP, which cleave surface FasL, reducing its ability to limit CNV. This resulted in increased levels of soluble FasL (sFasL) release into the blood where bloodborne sFasL impacts the CNV response significantly through differential effects on macrophage subtypes. Specifically, M2 macrophages that dominate the CNV response in aged mice7,16,21,22 produce high levels of VEGF and display enhanced Fas-mediated migration in response to sFasL. M1 macrophages, the dominate macrophage subtype in younger animals, are much less responsive to sFasL in cytokine induction and migration assays. We concluded that increased release of sFasL from the injured eye promotes proangiogenic macrophage infiltration, accounting for elevated CNV responses with age. This also suggests that sFasL might be a therapeutic target in the treatment of blinding eye disorders.
Methods
Animals
C57BL/6J (stock number 000664), Fasflox/flox (stock number 007895), and CreLysm (stock number 004781) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). The Fasflox/flox mice were crossed to the CreLysm to generate the conditional knockout of Fas in myeloid cells.32 The conditional knockout line was verified as congenic with the C57BL/6J strain by microsatellite analysis (Research Animal Diagnostic and Investigative Laboratory [RADIL], University of Missouri, Columbia, MO). Aged mice were generated by purchasing retired breeders (7 months) and maintaining them in our animal facility until the desired age. Young animals typically were 6 to 12 weeks, while aged mice were used when older than 60 weeks. Specific ages are indicated in Figures 1 through 6. All animal experiments were approved by the Animal Studies Committee at Washington University School of Medicine, and conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All experiments contained at least 5 mice per group and were repeated a minimum of 3 times.
Figure 1. .
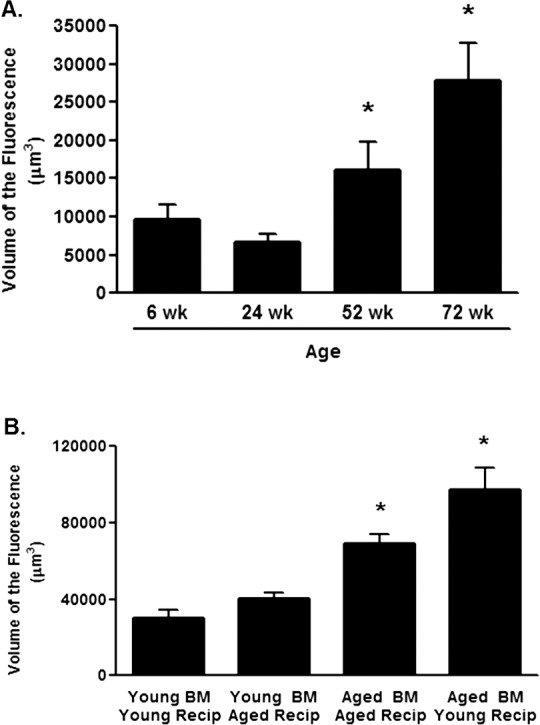
Age-dependent changes in CNV. (A) C57BL/6J mice that were 6, 24, 52, or 72 weeks of age were laser treated and the volume of CNV lesions (expressed as volume of the fluorescence) was assessed on day 7. Asterisk (*) denotes significantly different from the 6-week group (P < 0.01). (B) Bone marrow chimeras were constructed by infusing isolated bone marrow from 6-week young mice (Young BM) or 60-week (Aged BM) into lethally irradiated 6-week (Young Recip) or 60-week recipient (Aged Recip) mice. Mice were rested for 6 weeks before laser-induced CNV was performed. The volume of CNV lesions (expressed as volume of the fluorescence) was assessed on day 7. Asterisk (*) denotes significantly different from the Young BM/Young Recip group (P < 0.01).
Figure 6. .
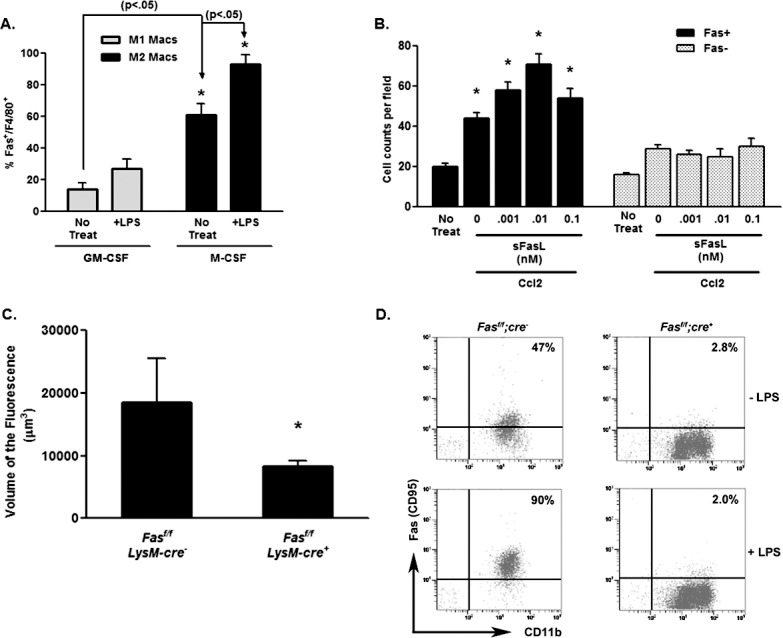
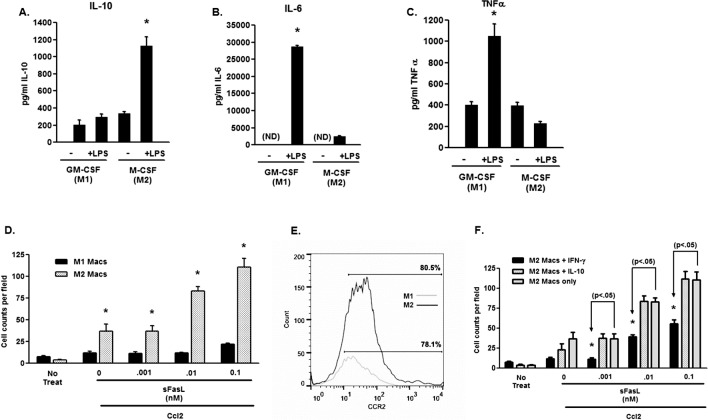
Fas expression on M1 and M2 macrophages. (A) Fas expression on bone marrow–derived M1 (GM-CSF) and M2 (M-CSF) macrophages was determined by flow cytometry gating on the F4/80+ cells. (B) Migration of bone marrow–derived macrophages from control (Fasf/f;LysM-cre−) and Fas-deficient (Fasf/f;LysM-cre+) macrophages was determined in the presence or absence of sFasL and Ccl2. The asterisk (*) denotes significantly different from untreated controls (No Treat). (C) Littermate control Fasf/f;LysM-cre− and Fas-deficient Fasf/f;LysM-cre+ mice were laser treated and the volume of CNV lesions (expressed as volume of the fluorescence) was assessed on day 7. Asterisk (*) denotes significantly different from littermate control (P < 0.01). (D) Bone marrow–derived macrophages from Fasf/f x LysM-cre mice and littermate control mice were examined for Fas expression by flow cytometry. Percentage of the Fas+, CD11b+ cells for each group are shown and were determine before (−LPS) and after (+LPS) treatment with LPS for 24 hours.
Laser-Induced Murine Model of CNV
CNV was induced by rupture of the RPE and underlying Bruch's membrane with a krypton laser in young or aged mice, as described.7,16,27 Mice were anesthetized using intraperitoneal ketamine hydrochloride (86.9 mg/kg) and xylazine (13.4 mg/kg), and their pupils were dilated. Using a krypton red laser, 4 laser burns were placed around the optic nerve (0.05 seconds, 50 μm, 150 mW). After 7 days, the animals were perfused with 3% FITC-conjugated high-molecular weight dextran (2,000 kDa). Eyes were enucleated immediately and fixed in 4% paraformaldehyde for 1 hour. A dissecting microscope was used to remove the cornea and lens, and gently separate the retina from the underlying choroid and sclera. Microscissors were used to make four radial incisions in the sclera-choroidal eyecup to prepare choroidal flat mounts on glass slides. A drop of gel-mount fixative and a glass coverslip were placed on each slide. The choroidal flat mounts were analyzed for presence of CNV by confocal microscopy. The extent of choroidal neovascularization was quantified by Metamorph Imaging software (Universal Imaging Corporation, Downington, PA) and reported as volume of the fluorescence.
Treatment With Doxycycline and Anti-FasL
To neutralize MMP, in vivo doxycycline (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in PBS and administered daily starting on the day of laser treatment by intraperitoneal (IP) injection. Doses were administered on a mg/kg basis and are indicated in Figures 1 through 6. These doses have been used successfully and without side effects in several mouse models.8,33–36 Anti-CD178 (anti-FasL, LEAF) or control hamster IgG (IgG isotype) was injected on the day of laser treatment (day 0), day 1, and day 2 (100 μg per injection per mouse in 150 μL of PBS.
Bone Marrow Chimeras
Bone marrow chimeras were constructed by infusing isolated bone marrow from 6-week young mice into lethally irradiated 60-week aged mice (or vice versa) by established protocols.22,27 In brief, mice received 11 Gy (1 Gy = 100 rads) of radiation before receiving 107 bone marrow cells. Mice were rested for 6 weeks before use in the laser-induced CNV model.
sFasL Levels in Serum
Mice were pre-bled 48 hours before laser treatment. They then were laser treated and injected with PBS or doxycycline. Mice were bled at 48 hours via tail vein. The blood samples were centrifuged at 1800g for 5 minutes, and serum was frozen at −70°C for subsequent sFasL assays. Serum concentrations of sFasL were measured with commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The concentration in serum samples was determined from the standard curve and had a detection limit of 3.6 pg/ml. Levels below the detection limit were coded as 0 pg/mL and were included in the analysis. All assays were conducted in triplicate.
Western Blot Analysis
Mice were killed, and eyes were enucleated and dissected. RPE/choroid/sclera complexes were treated with trypsin (2 mg/mL; Life Technologies, Grand Island, NY) for 30 minutes at 37°C, and the RPE cell layer was peeled off gently using fine forceps. RPE cells were homogenized in ice-cold lysis buffer and centrifuged for 10 minutes at 15,000g at 4°C. Supernatant was collected and protein concentration was determined by bicinchoninic acid (BCA) protein assay. A total of 30 μg of protein was mixed with loading buffer (Sigma-Aldrich, Corp.), boiled for 5 minutes, and loaded onto a 10% Bis-Tris Nu-PAGE gel (Life Technologies). For Western blotting, proteins were transferred to nitrocellulose membranes and then blocked with 5% skim milk in tris-buffered saline, 0.1% Tween-20 (TBST) for 1 hour at room temperature. Blots were incubated with primary antibody overnight at 4°C and followed by corresponding secondary horseradish peroxidase-conjugated antibodies (Cell Signaling, Danvers, MA). Immunoreactive bands were determined by exposing the nitrocellulose blots to chemiluminescence solution and exposing to autoradiography film. Actin was used as the loading control.
Macrophage Isolation
Macrophages were prepared from bone marrow cultures. Bone marrow was isolated from proximal limb bones as described previously.7 Briefly, all muscle tissue was removed from the bones and the bones were washed in 70% alcohol for 5 seconds before two washes in PBS. The ends of the bones were cut with scissors and the marrow harvested by flushing with RPMI-1640 medium using a syringe and 25-gauge needle. Isolated bone marrow cells (2 × 105/mL) were cultured in RPMI-1640 with 10% FBS along with 1000 U/mL granulocyte macrophage colony-stimulating factor (GM-CSF, for M1 macrophages) or macrophage colony-stimulating factor (M-CSF, for M2 macrophages)37,38 for 10 days in 100 mm petri dishes (final volume 10 mL). On days 3 and 6, an additional 500 U/mL and 1000 U/mL GM-CSF (or M-CSF) were added, respectively. On day 10, the nonadherent cells containing dendritic cells were discarded. The adherent cells were removed and harvested mechanically with a cell scraper. Cells were greater than 90% CD11b+ as determined by flow cytometry. M1 and M2 polarization was verified by assessing cytokine profile following lipopolysaccharide (LPS) stimulation (100 ng/mL). Fas expression was tested examined by flow cytometry before and after LPS stimulation (100 ng/mL) for 24 hours.
Macrophage Migration Assay
Tests were conducted using transwell permeable supports (24 well format; Corning/Costar, Corning, NY) with a 5 μm pore size polycarbonate membrane insert separating the upper and lower chambers. Macrophages (1.5 × 105) were seeded in the upper chambers with varying concentrations of recombinant mouse sFasL (0–1; 1 nM = 18.5 ng), while the chemoattractant recombinant mouse Ccl2 (10 ng/mL) was placed in the lower chamber. Cells only in the upper chamber and medium only in the lower chamber served as a negative control. After incubation at 37°C for 2 hours, the inserts were removed carefully, and cells that had migrated into the lower chamber were allowed to settle at 37°C for another 2 hours. Cell numbers in the lower chambers were counted at 100× magnification and at least 10 randomly chosen fields were counted. Duplicate chambers were run for each experiment that was repeated at least 3 times. Data are expressed as mean ± SE.
Cytokine Measurements
Macrophages (young and aged) were treated with varying concentration of sFasL and LPS (200 ng/mL) for 24 hours and the supernatants harvested by centrifugation. In some experiments 2 ng/mL mouse recombinant IFNγ was included. TNFα, IL-10, IL-6, and VEGF were measured by ELISA (R&D Systems) according to the manufacturer's instructions.
Reagents
Recombinant proteins Ccl2, IL-10, M-CSF, GM-CSF, TNFα, and human sFasL were purchased from Peprotech (Rocky Hill, NJ). Mouse IFNγ was purchased from Thermoscientific (Rockford, IL). Anti-CD178 (LEAF), Control Armenian Hamster IgG (LEAF), Anti-CD11b-PE were purchased from Biolegend (San Diego, CA). Anti-FasL (C178) and anti- actin-HRP for Western blots were purchased from Santa Cruz Biotechnology (Dallas, TX). E. coli LPS O55:B4 was purchased from Sigma-Aldrich, Corp. Anti-Fas–biotin was purchased from Life Technologies, Anti-F4/80 FITC and Anti-CD11b-APC from eBioscience (San Diego CA), and Anti-rabbit HRP from Cell Signaling.
Statistical Analysis
Differences between the control and treatment groups in CNV experiments were evaluated on a pairwise basis using a generalized linear modeling approach, adjusting for correlation between the samples taken from each mouse using a repeated measures method. We report P values for these comparisons, unadjusted for multiple comparisons as the comparisons were established a priori. All statistical analyses were conducted using SAS Proc GLM (SAS 9.1.3; SAS Institute, Cary, NC). P < 0.01 was considered significant and these values are indicated in all Figures by an asterisk (*). Differences in macrophage migration and cytokine responses were evaluated by a Student's t-test. P < 0.05 was considered significant and indicated in all Figures by an asterisk (*).
Results
Age-Related Increases in CNV Are Dependent on Bone Marrow–Derived Cells
Previous studies have shown that with increased age the CNV response in the mouse model is elevated following laser treatment,5 and these results are confirmed in Figure 1A, which shows that beginning at 52 weeks of age a consistent and reproducible increase in CNV was observed. That this was related to bone marrow–derived cells was explored by constructing radiation bone marrow chimeras where syngeneic bone marrow cells from young (6 weeks) and mice (60 weeks) aged were transferred to lethally irradiated young (6 weeks) or aged (60 weeks) recipients. Six weeks following bone marrow reconstitution, mice were laser treated and the CNV response was assessed on day 7. Figure 1B shows that when aged mice were reconstituted with young bone marrow CNV responses were similar to what was observed in young animals given young bone marrow. In contrast, when bone marrow from aged mice was present in young or aged mice, CNV responses were elevated consistently. These results suggested that it is age-dependent changes in the bone marrow–derived precursor cells that dictate the effect of aging on CNV.
Age-Dependent Changes in FasL Expression
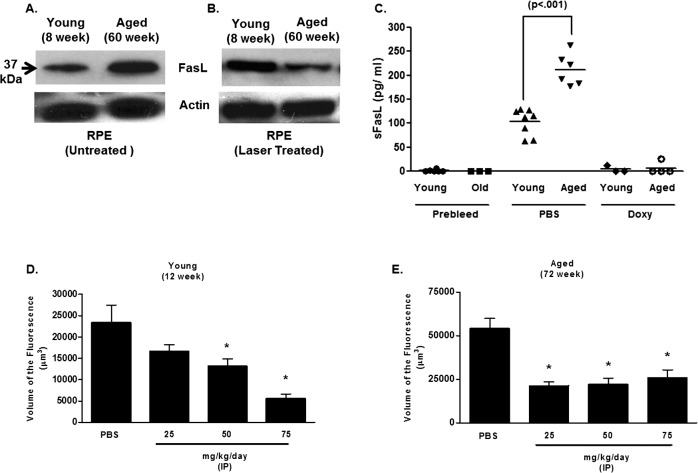
The CNV response in this model is regulated by the expression of FasL on RPE cells, such that loss of FasL function increases the response,8,27 while FasL overexpression can diminish CNV.9 Consequently, we asked whether FasL expression in RPE cells was diminished with age and if this also might contribute to the elevated CNV response. Using an antibody that identifies the extracellular domain of the protein, elevated FasL protein expression in the RPE was detected in the untreated aged eyes compared to younger animals (Fig. 2A, 37 kDa band). However, when expression in the RPE was examined following laser treatment, a reduction in FasL was observed in aged mice (Fig. 2B). Thus, in spite of the elevated FasL expression with aging, laser injury reduced expression significantly compared to young animals, suggesting other factors related to age may be influencing the expression of this protein on RPE cells.
Figure 2.
FasL expression in the eye and blood of young and aged mice. Western blotting for FasL expression in isolated RPE cells from young (8 weeks) and aged (60 weeks) was performed on (A) untreated or (B) laser treated mice. Actin expression was used as the loading control for the Western blots. (C) sFasL levels in serum were determined 48 hours following laser treatment in PBS-treated or doxycycline (Doxy)-treated mice (50 mg/kg/day beginning on the day of laser). Levels were determined using a commercially available ELISA (see Methods). Levels below the detection limit were coded as 0 pg/mL and were included in the analysis. (D) Young (12 weeks) and (E) aged (72 weeks) mice were laser treated and then injected daily by an IP injection of doxycycline at the indicated dosage. The volume of CNV lesions (expressed as volume of the fluorescence) was assessed on day 7. Asterisk (*) denotes significantly different from PBS-treated control.
sFasL Release From Laser Lesions
The expression of FasL on the cell surface is regulated tightly by several factors, including the activity of proteases.8,24,39 In the laser-induced CNV model, FasL is cleaved from the surface of RPE cells by MMP, resulting in the release of a cleavage product (sFasL) that can be detected in the blood maximally at 48 hours post-laser. Neutralizing MMP decreased the blood levels of sFasL, stabilized FasL on the surface of the RPE, and reduced CNV.8 Since increased MMP activity has been linked to AMD and increasing age,40–43 we asked whether the reduction of FasL in the eye following laser treatment of aged mice was the result of cleavage by MMP.8 Initially, we measured the level of bloodborne sFasL in young and aged mice, and found that at 48 hours aged animals had significantly higher levels compared to young animals (Fig. 2C). That elevated sFasL was related to MMP function was demonstrated by treating mice with doxycycline following laser treatment. In young and aged mice doxycycline treatment diminished the level of bloodborne sFasL (Fig. 2C) as well as CNV response (Figs. 2D, 2E). Thus, increased cleavage of FasL by MMP with age can account for the decrease in RPE-associated FasL and the elevated blood levels observed with age.
Neutralization of sFasL in the Blood
The soluble form of FasL (i.e., sFasL) can have potent immunoregulatory properties apart from the induction of apoptosis,31,44–46 including induction of cell migration in macrophages47 and neutrophils.48 Thus, sFasL released from the eye might have similar functions in the laser-induced CNV model. We tested the idea by systemically neutralizing sFasL with an anti-FasL antibody following laser treatment. While anti-FasL treatment had a little effect on CNV in young mice (Fig. 3A), it reduced the CNV response significantly in aged mice (Fig. 3B). Notably, antibody neutralization reduced the response in aged mice, but it did so only to the level of the maximal response observed in young animals. This consistent finding suggested that with aging sFasL may have additional biological properties that were not detectable when the laser model was performed in younger animals.
Figure 3. .
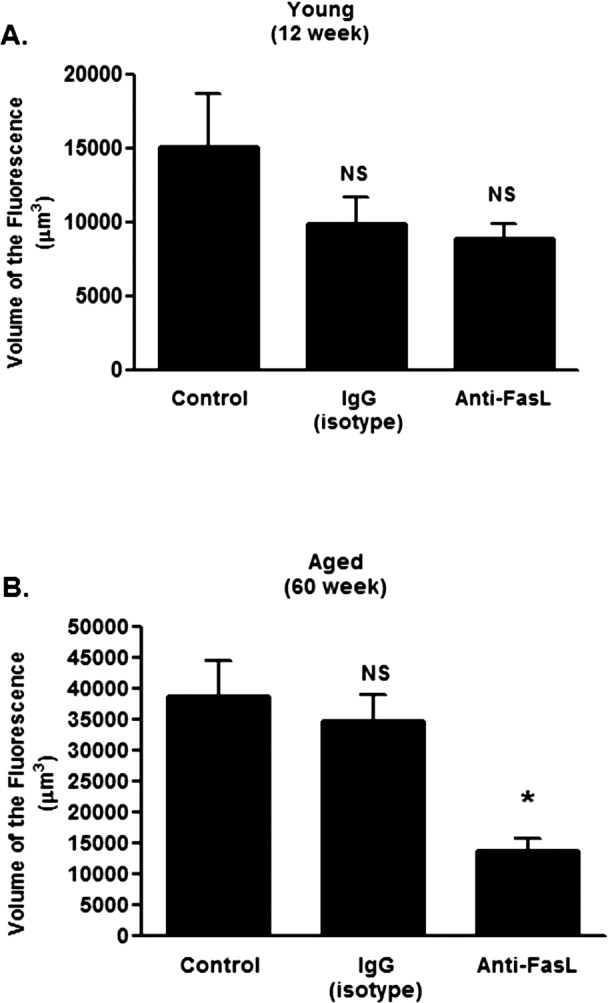
Systemic FasL neutralization in young and aged mice. (A) Young (12 weeks) or (B) aged (60 weeks) mice were laser treated and injected with Anti-CD178 (anti-FasL, LEAF) or IgG isotype control on the day of laser treatment (day 0), day 1, and day 2 (100 μg per injection per mouse in 150 μL of PBS). The volume of CNV lesions (expressed as volume of the fluorescence) was assessed on day 7. Asterisk (*) denotes significantly different from PBS-treated control. NS, not significant.
Effects of sFasL on Macrophage Function
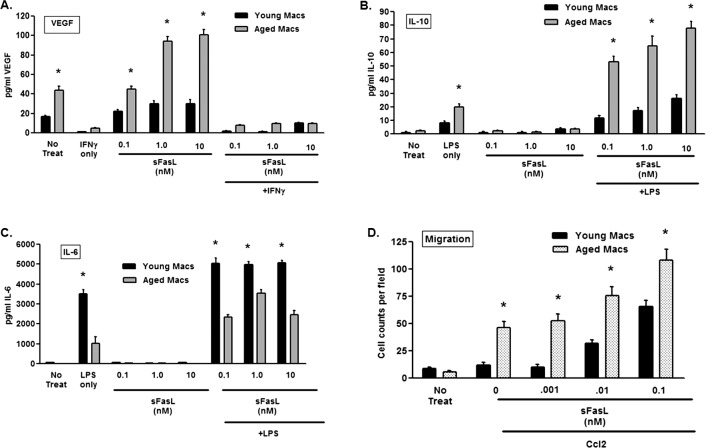
Macrophages regulate the CNV response based on their subtype, such that intensity of neovascularization is dependent on a balance between antiangiogenic M1 macrophages and proangiogenic M2 macrophages.7,16,17 In young animals, M1 macrophages are the predominant subtype in the laser lesions, but with advancing age the M2 macrophages dominate the response elevating CNV.16 An interesting explanation for our results would be that sFasL, which is elevated in aged mice after laser treatment (Fig. 2), has different effects on macrophages derived from young and aged animals. This also would account for the differential effect of neutralizing sFasL in young and aged animals (Fig. 3). We tested this idea by examining 2 parameters of macrophage function, cytokine production and migration, with the idea that macrophages from young and aged mice would response differently to the presence of sFasL, and this would be indicative of the macrophage subtype present. Macrophages were prepared from bone marrow cultures of young and aged animals, and their ability to produce VEGF, IL-10, and IL-6 were determined. sFasL treatment alone induced substantial VEGF production in aged macrophages without the need for an additional stimulus (Fig. 4A). This effect was reversed when IFNγ, an inflammatory cytokine that promotes M1 macrophage function,7,16 was added. In response to sFasL, neither young nor aged macrophages produced IL-10 (Fig. 4B); however, when stimulated with the TLR4 agonist LPS in conjunction with sFasL significant quantities of IL-10 were detected. In contrast, these two cytokines that are important for elevated CNV in aged animals7,16 were produced in much lower quantities by young macrophages. Production of the cytokine IL-6, which is a major cytokine produced by M1 macrophages, was elevated in response to sFasL (+ LPS) in young macrophages, but to a much greater degree compared to aged macrophages (Fig. 4C).
Figure 4. .
Macrophage cytokine and migration in young and aged mice. (A) VEGF production was examined in the supernatants of young and aged bone marrow–derived macrophages following stimulation with sFasL. (B) IL-10 production was assessed in the supernatants of bone marrow macrophages from young and aged mice following stimulation with sFasL or sFasL + LPS (100 ng/mL), and (C) IL-6 production was assessed in the supernatants of bone marrow macrophages from young and aged mice following stimulation with sFasL or sFasL + LPS (100 ng/mL) (D) Migration of bone marrow–derived macrophages to Ccl2 (10 ng/mL) was determined in the presence or absence of sFasL. The asterisk (*) denotes significantly different from untreated controls (No Treat).
In addition to regulating cytokine production, locally produced sFasL has been shown to enter the periphery and enable mononuclear cell migration to sites of injury. This sFasL-mediated tissue infiltration resulted in enhanced injury and cellular damage.47 In keeping with the differential cytokine response in young and aged macrophages, we tested whether sFasL might promote preferentially the migration of macrophages depending upon their age. Bone marrow–derived macrophages were prepared from young and aged bone marrow, and tested in a directed migration assay using Ccl2, a chemokine critical to macrophage function in this model.49,50 As shown in Figure 4D, aged macrophages migrate much more robustly toward Ccl2 and this effect was enhanced significantly by the addition of sFasL. Macrophages from young animals showed minimal migration to Ccl2 at the concentration used; however, with the addition of sFasL migration was increased, but not to same extent as observed with aged macrophages. Thus, sFasL has differential effects on cytokine production and migration depending on age of the mice from which the macrophages were obtained.
The dominance of M1 macrophages in young mice and M2 macrophages in aged mice16 suggested that these differences in the macrophage properties demonstrated in Figure 4 reflect the responses of the majority of cells (i.e., M1 macrophages in young mice versus M2 macrophages in aged mice). Thus, to examine directly the migratory capacity of M1 and M2 macrophages, we performed migration assays on specifically polarized cells. We prepared macrophages by culturing bone marrow cells for 10 days in either GM-CSF or M-CSF, as these treatments have been shown to produce M1 and M2 macrophages, respectively.37,38 As shown in Figure 5, macrophages generated by GM-CSF produced elevated TNFα (Fig. 5A) and IL-6 (Fig. 5B) in response to LPS, with minimal production of IL-10 (Fig. 5C), demonstrating that they are of the M1 type. In contrast, M-CSF–cultured macrophages produced very little TNFα or IL-6, but high amounts of IL-10 (Figs. 5A–C) confirming their M2 nature. When these cells were tested in the migration assay (Fig. 5D), M1 macrophages showed little migratory capacity to Ccl2 in the presence of sFasL, while the addition of sFasL to M2 macrophages significantly increased the directed migration of these cells. This is reflected in the expression levels of Ccr2, where both populations of macrophages expressed Ccr2, M2 macrophages expressing higher levels (Fig. 5E). Interestingly, IFNγ, which promotes a proinflammatory antiangiogenic phenotype in macrophages,7,16 inhibited sFasL-induced migration,while IL-10 treatment had no effect (Fig. 5F). We concluded that sFasL has differential effects on macrophage populations where it enhances preferentially the migration of M2 macrophages, and that the effects of sFasL in aged mice reflect the dominance of M2 macrophages in these animals.
Figure 5. .
Macrophage polarization, cytokine production, and migration. (A) IL-10, (B) IL-6, and (C) TNFα production was determine in the supernatants of M1 (GM-CSF) and M2 (M-CSF) macrophages following stimulation with LPS (100 ng/mL) for 24 hours. (D) Migration of bone marrow–derived M1 (GM-CSF) and M2 (M-CSF) macrophages to Ccl2 (10 ng/mL) was determined in the presence or absence of sFasL. (E) Expression of Ccr2 on M1 (GM-CSF) and M2 (M-CSF) F4/80+ macrophages. (F) Effect of IFNγ and IL-10 on macrophage migration in the presence of sFasL and Ccl2. The asterisk (*) denotes significantly different from untreated controls (No Treat).
Fas (CD95) is the only known receptor for FasL mediating its proapoptotic, cytokine stimulating, and migration promoting properties.44,45,48,51 Macrophages from Fas receptor deficient mice (e.g., in lpr mice) produce copious amounts of proinflammatory cytokines, such as TNFα, IL-12, and IL-1β when stimulated with TLR ligands.32 Thus, without Fas macrophages are more proinflammatory, and polarize toward the M1 subtype and away from the M2 subtype. This also suggests that differential Fas expression might define these macrophage subtypes. We tested this idea by first examining the surface expression of Fas by flow cytometry on polarized M1 and M2 macrophages. Results presented in Figure 6A showed that more F4/80+ M2 macrophages expressed the Fas receptor compared to F4/80+ M1 macrophages, and that this number was enhanced by LPS treatment. In support of the role of Fas in migration M2 macrophages, Fas− macrophages obtained from Fas-deficient Fasf/f x LysM-cre mice did not respond to sFasL in the migration assay (Fig. 6B). Finally, to examine the role of myeloid-derived Fas in the CNV model we tested Fasf/f x LysM-cre mice that are without Fas in the myeloid compartment.32 These results showed that CNV responses were decreased dramatically (Fig. 6C), further implicating the role of Fas expression on myeloid cells in the severity of the CNV lesions. Figure 6D verifies that CD11b+ (which includes the F4/80+ population) from the Fasf/f x LysM-cre mice, expressed minimal Fas receptor.
Discussion
Age is the single greatest risk factor in the development of the blinding eye disease AMD where the prevalence of disease significantly increases as individuals enter their sixth decade.2,3 In spite of this universal observation, other factors that lead to increased disease incidence remain undefined. Increases in damage from environmental stresses, accumulation of damaged proteins and organelles, and loss of function in important biochemical pathways all have been proposed.3,4,13,52 The immune system is also thought to have a role in disease development12–14,53; however, few studies have addressed how age-related changes in the immune response could be involved. The eye also is an immune privileged organ that can have a strong inhibitory influence on inflammatory and neovascular responses via a number of immunosuppressive mediators.21 How age-dependent changes in the immune system are influenced by immune privilege and how age might alter the function of immune privilege is currently unknown. We have examined the effect of age on an important mediator of immune privilege, FasL, and whether such changes might impact a model of neovascular AMD. Our results showed that, while there is an age-dependent increase in FasL expression on RPE cells, laser injury results in a significant decrease in FasL expression that is due to enhanced MMP activity, which cleaves FasL from the cell surface. Furthermore, increased cleavage of FasL results in significant levels of cleaved FasL (sFasL) in the blood that were enhanced greatly in aged animals. Targeting MMP using an MMP inhibitor reduced CNV and diminished bloodborne sFasL, while neutralizing sFasL systemically inhibited CNV, but only in aged mice. This suggested that sFasL has a role in the age-related increase in CNV. In support of this idea, sFasL had a differential effect of macrophages from young and aged mice where it increased cytokine production and migration of the M2-dominated aged macrophage population, but had little effect on M1-dominated young macrophages. We concluded that with aging the release of sFasL from the injured eye promotes the migration of proangiogenic M2 macrophages that can increase tissue damage and the neovascular response. The source of the macrophages in CNV lesion has been attributed to systemic10,15 and local sources.54 Interplay between local and systemic myeloid derived cells also has been suggested.55 We do not know the precise source of the myeloid-derived cells in the current studies; however, our data showed that the release of sFasL likely is involved in attracting proangiogenic M2 macrophages regardless of their source.
Studies have shown that FasL expression is regulated tightly in lymphoid and nonlymphoid cell populations, thereby preventing unwanted damage to Fas+ cells and tissues throughout the body. Cells that use FasL as a barrier to cellular invasion or as an effector molecule typically do not display the protein on their cell surface. It either is stored in secretory vesicles for rapid mobilization to the cell surface56,57 or produced de novo following cellular activation, stress, or injury.7,58 Originally described as a death-inducing ligand, more recent studies have shown the importance of the Fas/FasL interactions in cytokine production and mononuclear cell migration.44,45,48,51 While the membrane form of FasL is known to mediate its proapoptotic function,51 sFasL, which can be released though the activity of proteases,39,56 mediates many of the nonapoptotic functions of the protein.44,46–48,51 Little is known concerning changes in the function of the Fas/FasL interaction with aging, although it has been reported that the proapoptotic functions of FasL can be diminished with age in certain cell types.59 We reported that age decreases FasL-mediated immune privilege locally though increased cleavage by MMP, suggesting an age-related functional decline in the eye. Further, enhanced cleavage and release of sFasL has adverse effects on immune privilege, particularly in aged mice where it can activate and attract preferentially proangiogenic M2 macrophages. These results are consistent with a recent study on spinal cord injury where sFasL release from damaged tissue recruited monocytes from the periphery increasing tissue damage.47 While the macrophage subtype was not examined in that study it is interesting to speculate that M2 macrophages were involved.
Macrophages originate from the bone marrow and migrate through the blood to body tissues. Under the influence of growth factors, such as M-CSF and GM-CSF, as well as chemokines, such as Ccl2, they can migrate to tissue and take up residence in many areas of the body.60,61 There are networks of myeloid-derived cells lining the uveal tract of the eye,62 in the limbal region of the cornea,63 and in the retina.64 Resident macrophages undergo local activation, and help recruit monocytes and precursors from the bone marrow in response to injury and infection. These cells then accumulate and, under the influence of local factors, can change functional phenotypes based on the local environment. Bone marrow–derived cells are critical to the development of CNV in the laser model7,55 and our studies also showed that the increase in the CNV response observed with aging is dictated by the bone marrow, where young mice infused with aged bone marrow showed elevated CNV. In contrast, replacing the bone marrow of aged mice with bone marrow from young animal reduced the response. The exact nature of the bone marrow cells that regulate CNV with age is not known; however, myeloid cells2,7 and/or mesenchymal cells65 could be involved. Further studies are required to define the critical cell populations to understand eventually many of the aging effects on CNV in the mouse model and ultimately in human exudative AMD.
Many subtypes have been described, but a general classification of classically activated (M1) alternatively activated (M2) has been used.61 While this perhaps is an over simplification, it is useful in understanding how macrophages can influence disease states. M1 macrophages are proinflammatory, secreting cytokines, such as TNFα, IL-6, and IL-12. They also are activated by TLR ligands and IFNγ, acquiring the capacity to kill bacteria as well promote immune responses. Importantly, these cells are antiangiogenic. M2 macrophages, which produce much lower amounts of the proinflammatory cytokines, are best known for their production of IL-10, their ability to be anti-inflammatory and antiangiogenic, and their increase in function in environments rich in IL-4, IL-13, and IL-10.7,16,60,61 While the field of ocular angiogenesis has embraced the idea that macrophages promote neovascular AMD,15,49,66 the field has paid little attention to the functional heterogeneity of these cells, assuming that proinflammatory macrophages promote angiogenesis in the eye.67 However, it is clear from the tumor and atherosclerosis literature,20,60,68,69 as well as our own studies with the laser model of CNV,7,16 that macrophage function is influenced by a number of factors related to the microenvironment. In these studies, alternative activated macrophages (M2) are the culprit, promoting angiogenesis and lesion development. In the laser-induced CNV model, we have shown clearly that it is M2 macrophages that promote neovascularization and are responsible for the increased responses observed with aging.16 Importantly, M1 macrophages are antiangiogenic and can inhibit CNV.7,16 Studies reported here further support this idea, where we showed that proinflammatory cytokines, such as IFNγ, diminish the function of proangiogenic M2 macrophages from aged mice, suggesting that conditions that promote proinflammatory, antiangiogenic M1 macrophage function may diminish neovascularization. Furthermore M2 macrophages have much higher levels of Ccr2 compared to M1 (Fig. 5), suggesting that Ccl2, the major chemokines in the neovascular response, preferentially attracts M2 macrophages. It will be interesting to determine if the reported Ccr2 inhibition by pharmacologic agents70 or the loss of the Ccr2 receptor10 involves diminished recruitment of M2 macrophages.
Finally, the levels of sFasL in blood have been examined in a number of models, including cancer,71 arthritis,72 and thyroid disease.73 A recent study demonstrated a correlation between sFasL and AMD,74 suggesting that increased levels of sFasL in the blood of AMD patients might be a biomarker. Interpreting these result in light of our own findings (see the study of Roychoudhury et al.8 and current studies) suggested that targeting the Fas-FasL pathway alone, or in combination with other treatments may be a viable clinical approach to the treatment of neovascular eye disease.
Acknowledgments
Supported by National Institutes of Health Grants EY06765, EY015570, EY02687 (Department of Ophthalmology and Visual Science Core Grant), EY019287 (RSA), a Department of Ophthalmology and Visual Science grant from Research to Prevent Blindness (New York, NY), and The BrightFocus Foundation (Clarksburg, MD).
Disclosure: H. Zhao, None; J. Roychoudhury, None; T.A. Doggett, None; R.S. Apte, None; T.A. Ferguson, None
References
- 1. Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets. 2011; 12: 173–181 [DOI] [PubMed] [Google Scholar]
- 2. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008; 358: 2606–2617 [DOI] [PubMed] [Google Scholar]
- 3. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122: 598–614 [DOI] [PubMed] [Google Scholar]
- 4. Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005; 80: 595–606 [DOI] [PubMed] [Google Scholar]
- 5. Espinosa-Heidmann DG, Suner I, Hernandez EP, Frazier WD, Csaky KG, Cousins SW. Age as an independent risk factor for severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002; 43: 1567–1573 [PubMed] [Google Scholar]
- 6. Bora NS, Kaliappan S, Jha P, et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007; 178: 1783–1790 [DOI] [PubMed] [Google Scholar]
- 7. Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006; 3: e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roychoudhury J, Herndon JM, Yin J, Apte RS, Ferguson TA. Targeting immune privilege to prevent pathogenic neovascularization. Invest Ophthalmol Vis Sci. 2010; 51: 3560–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Semkova I, Fauser S, Lappas A, et al. Overexpression of FasL in retinal pigment epithelial cells reduces choroidal neovascularization. Faseb J. 2006; 20: 1689–1691 [DOI] [PubMed] [Google Scholar]
- 10. Tsutsumi C, Sonoda KH, Egashira K, et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003; 74: 25–32 [DOI] [PubMed] [Google Scholar]
- 11. Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 3578–3585 [DOI] [PubMed] [Google Scholar]
- 12. Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001; 20: 385–414 [DOI] [PubMed] [Google Scholar]
- 13. Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog Retin Eye Res. 2010; 29: 169–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 3586–3592 [DOI] [PubMed] [Google Scholar]
- 16. Kelly J, Khan AA, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007; 117: 3421–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferguson TA, Apte RS. Angiogenesis in eye disease: immunity gained or immunity lost? Semin Immunopathol. 2008; 30: 111–119 [DOI] [PubMed] [Google Scholar]
- 18. Kovacs EJ, Palmer JL, Fortin CF, Fülöp T Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009; 30: 319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rymkiewicz P, Heng Y, Vasudev A, Larbi A. The immune system in the aging human. Immunol Res. 2012; 53: 235–250 [DOI] [PubMed] [Google Scholar]
- 20. Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005; 205: 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev. 2006; 213: 228–238 [DOI] [PubMed] [Google Scholar]
- 22. Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995; 270: 1189–1192 [DOI] [PubMed] [Google Scholar]
- 23. Ferguson TA, Green DR. Fas-ligand and immune privilege: the eyes have it. Cell Death Differ. 2001; 8: 771–772 [DOI] [PubMed] [Google Scholar]
- 24. Stuart PM, Griffith TS, Usui N, Pepose J, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997; 99: 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stuart PM, Pan F, Plambeck S, Ferguson TA. FasL-Fas interactions regulate neovascularization in the cornea. Invest Ophthalmol Vis Sci. 2003; 44: 93–98 [DOI] [PubMed] [Google Scholar]
- 26. Barreiro R, Schadlu R, Herndon J, Kaplan HJ, Ferguson TA. The role of Fas-FasL in the development and treatment of ischemic retinopathy. Invest Ophthalmol Vis Sci. 2003; 44: 1282–1286 [DOI] [PubMed] [Google Scholar]
- 27. Kaplan HJ, Leibole MA, Tezel T, Ferguson TA. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat Med. 1999; 5: 292–297 [DOI] [PubMed] [Google Scholar]
- 28. Bajou K, Peng H, Laug WE, et al. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell. 2008; 14: 324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yap R, Veliceasa D, Emmenegger U, et al. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clin Cancer Res. 2005; 11: 6678–6685 [DOI] [PubMed] [Google Scholar]
- 30. Stuart PM, Pan F, Yin X, Haskova Z, Plambeck S, Ferguson TA. Effect of metalloprotease inhibitors on corneal allograft survival. Invest Ophthalmol Vis Sci. 2004; 45: 1169–1173 [DOI] [PubMed] [Google Scholar]
- 31. Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996; 5: 7–16 [DOI] [PubMed] [Google Scholar]
- 32. Brown NJ, Hutcheson J, Bickel E, et al. Fas death receptor signaling represses monocyte numbers and macrophage activation in vivo. J Immunol. 2004; 173: 7584–7593 [DOI] [PubMed] [Google Scholar]
- 33. Errami M, Galindo CL, Tassa AT, Dimaio JM, Hill JA, Garner HR. Doxycycline attenuates isoproterenol- and transverse aortic banding-induced cardiac hypertrophy in mice. J Pharmacol Exp Ther. 2008; 324: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 34. Fainaru O, Adini I, Benny O, et al. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. Faseb J. 2008; 22: 3728–3735 [DOI] [PubMed] [Google Scholar]
- 35. Rossiter HB, Scadeng M, Tang K, Wagner PD, Breen EC. Doxycycline treatment prevents alveolar destruction in VEGF-deficient mouse lung. J Cell Biochem. 2008; 104: 525–535 [DOI] [PubMed] [Google Scholar]
- 36. Hebda PA, Whaley D, Kim HG, Wells A. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Repair Regen. 2003; 11: 373–379 [DOI] [PubMed] [Google Scholar]
- 37. Verreck FAW, de Boer T, Langenberg DML, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004; 101: 4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sierra-Filardi E, Vega MA, Sánchez-Mateos P, Corbí AL, Puig-Kröger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010; 215: 788–795 [DOI] [PubMed] [Google Scholar]
- 39. Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002; 408: 155–161 [DOI] [PubMed] [Google Scholar]
- 40. Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond). 2007; 21: 1511–1515 [DOI] [PubMed] [Google Scholar]
- 41. Hussain AA, Lee Y, Zhang JJ, Marshall J. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 4459–4466 [DOI] [PubMed] [Google Scholar]
- 42. Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. Faseb J. 2003; 17: 2290–2292 [DOI] [PubMed] [Google Scholar]
- 43. Tatar O, Adam A, Shinoda K, et al. Matrix metalloproteinases in human choroidal neovascular membranes excised following verteporfin photodynamic therapy. Br J Ophthalmol. 2007; 91: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peter ME, Budd RC, Desbarats J, et al. The CD95 receptor: apoptosis revisited. Cell. 2007; 129: 447–450 [DOI] [PubMed] [Google Scholar]
- 45. Nagata S, Golstein P. The Fas death factor. Science. 1995; 267: 1449–1456 [DOI] [PubMed] [Google Scholar]
- 46. Gregory MS, Hackett CG, Abernathy EF, et al. Opposing roles for membrane bound and soluble fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One. 2011; 6: e17659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Letellier E, Kumar S, Sancho-Martinez I, et al. CD95-ligand on peripheral myeloid cells activates Syk kinase to trigger their recruitment to the inflammatory site. Immunity. 2010; 32: 240–252 [DOI] [PubMed] [Google Scholar]
- 48. Seino KI, Iwabuchi K, Kayagaki N., et al. Cutting edge: chemotactic activity of soluble Fas ligand against phagocytes. J Immunol. 1998; 161: 4484–4488 [PubMed] [Google Scholar]
- 49. Raoul W, Auvynet C, Camelo S, et al. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J Neuroinflammation. 2010; 7: 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamada K, Sakurai E, Itaya M, Yamasaki S, Ogura Y. Inhibition of laser-induced choroidal neovascularization by atorvastatin by downregulation of monocyte chemotactic protein-1 synthesis in mice. Invest Ophthalmol Vis Sci. 2007; 48: 1839–1843 [DOI] [PubMed] [Google Scholar]
- 51. O'Reilly LA, Tai L, Lee L, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009; 461: 659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009; 28: 348–368 [DOI] [PubMed] [Google Scholar]
- 53. Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci U S A. 2005; 102: 7053–7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muther PS, Semkova I, Schmidt K, et al. Conditions of retinal glial and inflammatory cell activation after irradiation in a GFP-chimeric mouse model. Invest Ophthalmol Vis Sci. 2010; 51: 4831–4839 [DOI] [PubMed] [Google Scholar]
- 55. Caicedo A, Espinosa-Heidmann DG, Piña Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005; 81: 38–47 [DOI] [PubMed] [Google Scholar]
- 56. Schulte M, Reiss K, Lettau M, et al. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007; 14: 1040–1049 [DOI] [PubMed] [Google Scholar]
- 57. Zuccato E, Blott EJ, Holt O, et al. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci. 2007; 120: 191–199 [DOI] [PubMed] [Google Scholar]
- 58. Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009; 30: 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsu HC, Scott DK, Mountz JD. Impaired apoptosis and immune senescence – cause or effect? Immunol Rev. 2005; 205: 130–146 [DOI] [PubMed] [Google Scholar]
- 60. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005; 23: 344–346 [DOI] [PubMed] [Google Scholar]
- 61. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3: 23–35 [DOI] [PubMed] [Google Scholar]
- 62. McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999; 83: 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niederkorn JY, Peeler JS, Mellon J. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg Immunol. 1989; 2: 83–90 [PubMed] [Google Scholar]
- 64. Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010; 234: 282–304 [DOI] [PubMed] [Google Scholar]
- 65. Lecomte J, Louis K, Detry B, et al. Bone marrow-derived mesenchymal cells and MMP13 contribute to experimental choroidal neovascularization. Cell Mol Life Sci. 2011; 68: 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012; 75: 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011; 61: 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007; 117: 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006; 42: 717–727 [DOI] [PubMed] [Google Scholar]
- 70. Xie P, Kamei M, Suzuki M, et al. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS One. 2011; 6: e28933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Naumnik W, Izycki T, Ossolinska M, Chyczewska E. Serum levels of sFas and sFasL during chemotherapy of lung cancer. Exp Oncol. 2007; 29: 132–136 [PubMed] [Google Scholar]
- 72. Hashimoto H, Tanaka M, Suda T, et al. Soluble Fas ligand in the joints of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1998; 41: 657–662 [DOI] [PubMed] [Google Scholar]
- 73. Owonikoko TK, Hossain MS, Bhimani C, et al. Soluble FAS ligand as a biomarker of disease recurrence in differentiated thyroid cancer. Cancer. 2013; 119: 1503–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang S, Moriarty-Craige SE, Li C, et al. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 1345–1349 [DOI] [PubMed] [Google Scholar]