Abstract
A remarkable amount of information has emerged in the past decade regarding sweet taste physiology. This article reviews these data, with a particular focus on the elucidation of the sweet taste receptor, its location and actions in taste transduction in the mouth, its nontaste functions in the gastrointestinal tract (e.g., in enteroendocrine cells), and the brain circuitry involved in the sensory processing of sweet taste. Complications in the use of rodents to model human sweet taste perception and responses are also considered. In addition, information relating to low-calorie sweeteners (LCS) is discussed in the context of these issues. Particular consideration is given to the known effects of LCS on enteroendocrine cell function.
Introduction
The tasting of sweetness is a complex physiologic event. Sweetness is 1 of 5 basic taste qualities. It is historically associated with the mouth (tongue, soft palate), which contains taste buds, the sensory organs of taste (1). Subpopulations of sensory cells in the taste bud respond to sweet molecules by activating local sensory neurons that project to the brain areas that process and interpret sensory information (e.g., brainstem, thalamus, cerebral cortex, and amygdala) (1). Recently, knowledge about sweet taste has grown tremendously, thanks in considerable part to new experimental technologies (e.g., molecular biological tools). This article reviews key aspects of this new information, including discoveries about the sweet taste receptor, and of brain pathways associated with sweet taste perception. In addition, this review discusses how low-calorie sweeteners (LCS)10 and nutritive sweeteners both activate sweet taste receptors to trigger taste perception in the brain, as well as the strengths and limitations associated with the use of rodents as surrogates for humans in the study of sweet taste. Finally, we discuss the recent discovery of sweet taste receptors on intestinal enteroendocrine cells, which release paracrine and endocrine signals that influence glucose homeostasis, along with the effects of LCS and nutritive sweeteners on the activity of these cells.
Mechanisms of Sweetener Detection
Sucrose, saccharin, sucralose, cyclamate, aspartame, and thaumatin all taste sweet to humans. However, the chemical diversity of these natural and synthetic compounds begs the question: Why do they all taste sweet? Research during the past decade has greatly increased our understanding of the molecular, genetic, and cellular mechanisms of sweetener detection. These advances provide important insights into how we interact with sweeteners.
The gustatory system recognizes chemical stimuli that elicit 1 of 5 distinct perceptual qualities: sweet, sour, salty, bitter, and umami (the savory taste of glutamate) (1). Stimulus detection occurs through specialized taste cells, clustered together in small groups (taste buds) found predominantly on the dorsal surface of the tongue and soft palate. Activation of these cells by taste stimuli releases neurotransmitters onto afferent cranial nerve fibers, causing transmission of taste information to the brain. The brain then processes this taste information, along with other sensory information (including olfactory, thermal, and textural), to elicit the perception of flavor and in the context of experience, motivation, preference, and hedonic valence to promote an appropriate ingestive response.
Individual taste cells express only one of several taste receptor types (1). Taste receptors are responsible for initial stimulus detection and selectivity. Type 1 taste receptor (T1R) and T2R are members of the large family of G protein-coupled receptors (2–4). T1R are heterodimers; the umami receptor is composed of the T1R1 and T1R3 subunits (5, 6), whereas the sweet receptor contains T1R2 and T1R3 (6, 7). The larger family of T2R (25 genes in humans) recognizes many diverse compounds that taste bitter (8, 9). Most salty and sour-tasting stimuli are detected by ion channels (10). However, with the exception of the sodium-specific epithelial sodium channel (11), the molecular identities of these channels remain unknown.
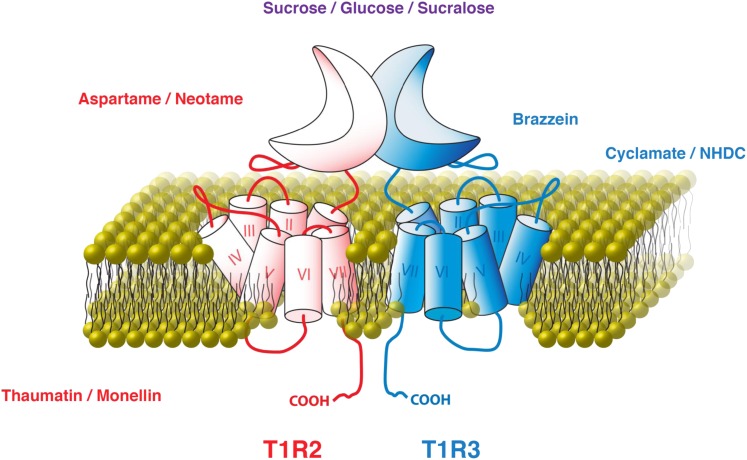
All compounds that elicit a sweet taste bind to and activate the T1R2+T1R3 receptor. However, not all sweeteners bind to the same sites on the receptor (Fig. 1). The sweet taste receptor contains several binding sites for sweeteners and sweet taste inhibitors (12). Each T1R subunit is composed of 3 principal domains: an extracellular venus-flytrap (VFT) domain at the N terminus, a seven transmembrane-spanning domain at the C terminus, and a cysteine-rich linker joining them (12). Natural and artificial sugars (e.g., sucrose, glucose, and sucralose) bind to the VFT domains of both T1R2 and T1R3 (13), whereas dipeptide sweeteners (e.g., aspartame and neotame) bind only to the T1R2 VFT domain (14). The cyclamate binding pocket lies within the seven transmembrane-spanning domain of T1R3 (14, 15) and closely overlaps the binding site for the sweet taste inhibitor lactisole (14, 16). Sweet proteins such as thaumatin and monellin interact across a larger binding surface that can include both subunits and the cysteine-rich linkers (12). Incredibly, each of these distinct sweetener-binding events leads to receptor activation; if they did not, there would be no accompanying perception of sweetness.
FIGURE 1.
T1R2 and T1R3 and the compounds that can activate them. Font colors indicate sweet compounds that bind T1R2 (red), T1R3 (blue), or both subunits (purple). Modified from Vigues et al. (12) with permission. T1R, type 1 taste receptor.
Genetic variation in the T1R genes explains many observed differences in the ability to detect sweeteners across and within species. For example, whereas humans find aspartame to be sweet, rodents are indifferent to it (17). This species disparity in the ability to taste aspartame results from small differences in the gene encoding T1R2 (14). Indeed, a chimeric rodent sweet taste receptor incorporating the human variant of the T1R2 VFT domain is responsive to aspartame, whereas the fully rodent receptor is not (14). Even single amino acid changes can affect the ability of the sweet taste receptor to bind its ligand; a common variant found in some mouse strains (18) markedly reduces the affinity of the T1R3 subunit for sugars (13). Genetic differences also affect sweetener side-tastes. For example, certain variants of 2 T2R bitter receptors respond to saccharin (19), providing a molecular explanation for the observation that some people find that saccharin tastes both sweet and bitter.
Although ligand selectivity of the sweet taste receptor dictates which compounds elicit sweet taste, the taste cell type determines the taste quality elicited (20). A particularly illustrative experiment expressed the human T2R16 receptor, which is responsive to the compound phenyl-β-D-glucopyranoside, in 2 different populations of mouse taste cells: one that normally expresses T2R (“bitter” cells) and one that normally expresses T1R2 and T1R3 (“sweet” cells) (21). Mice do not have a functional T2R16 ortholog and are indifferent to the taste of phenyl-β-D-glucopyranoside, which humans find bitter. As predicted, humanizing the mouse by expressing T2R16 in “bitter” cells resulted in a mouse that found the taste of phenyl-β-D-glucopyranoside to be aversive (i.e., bitter). However, mice in which T2R16 was transgenically expressed in the “sweet” cells found phenyl-β-D-glucopyranoside quite appetitive. Therefore, T1R2+T1R3 determines what is sweet but not why it is sweet.
Although T1R were first identified in the mouth and they function in taste, it is now known that these receptors are expressed throughout the body. Of note is the observation that both T1R2 and T1R3 are expressed in endocrine cells of the gastrointestinal tract, where they may contribute to luminal glucose sensing, the release of satiety hormones such as glucagon-like peptide-1 (GLP-1), the expression of glucose transporters, and the maintenance of glucose homeostasis (22–24). The potential role of T1R in the assimilation of and postingestive response to sugars in the digestive system highlights an interesting parallel in the mouth, where receptors for a number of peptide hormones (e.g., GLP-1, glucagon, cholecystokinin, leptin) are expressed by taste cells, often with their cognate ligands (25). Although the contributions of these mechanisms remain somewhat unclear, many may act to modulate sweet taste (26–28). Such studies suggest that the efficacy of sweetener detection may be modulated in the context of metabolic signals.
What Animal Models of Conditioning Tell Us
Animal models have been extensively used to study energy and LCS preferences, flavor conditioning, and the impact of sweeteners on energy intake and body weight. The rat and mouse are the most commonly studied species because of their wide use in nutritional and behavioral research and their well-characterized “sweet tooth.” The availability of inbred and selected strains that vary in their sweet taste sensitivity or predisposition to diet-induced obesity is another major advantage of rodent models.
The limitations of rodents as models for human sweetener research must be recognized. Rodents differ from humans in their preferences for specific sweeteners. For example, whereas humans report fructose as tasting sweeter than sucrose at equienergy concentrations, rats and mice seem to taste sucrose as the sweeter sugar (29). Rodents are also more limited than humans in their attraction to LCS. Unlike humans, rats and mice do not prefer the taste of aspartame and many rats avoid, rather than prefer, sucralose (17, 30, 31).
The most commonly used LCS in rodent experiments is saccharin, which at best is a weak surrogate for sucrose (32). The most optimal saccharin solutions (0.2–0.4%) for rats are preferred in comparison only to dilute sucrose solutions (2–4%). The bitter aftertaste of saccharin may limit its palatability, but it also seems that saccharin is simply not as sweet to rats as concentrated sugar solutions (33). The current lack of a LCS that matches the palatability of concentrated sugar solutions (≥10% sucrose) limits the ability to use rats to model human consumption patterns of sugar and diet drinks.
Although rodents show a more restricted preference to LCS compared with humans, they exhibit a more expansive preference for carbohydrates in general. Unlike humans, rats and mice are very attracted to starch and starch-derived maltodextrins and seem to “taste” them as distinct from sugars (34). The rodent taste sensitivity to starch and maltodextrin has important implications for the interpretation of studies in which these carbohydrates are used to discretely vary the energy density of foods without changing their taste. In addition, the maltodextrin taste preference of rodents can determine their preference for commercial sweetener products. For example, rats that avoided sucralose solutions displayed strong preferences for solutions containing Splenda (McNeil Nutritionals), which is a blend of sucralose and maltodextrin (35).
Post-oral sugar conditioning.
The palatability of sugar is determined not only by its sweet taste but also by its post-oral nutritive effects. This is demonstrated by the conditioned preference and increased acceptance that rodents develop for flavored solutions that are paired with i.g. sugar infusions (36). Although sweet taste receptors (T1R2+T1R3) are found in the gut (37), they do not mediate this post-oral action of sugars. This is indicated by the finding that genetically modified mice missing the T1R3 receptor subunit are similar to normal mice in their flavor conditioning response to i.g. sucrose infusions (38). In addition, i.g. infusion of the LCS sucralose, which activates gut sweet receptors, does not condition a flavor preference (38). Other findings indicate that glucose-specific sensors mediate sugar conditioning and do so partly by activating the brain dopamine (DA) reward system (39–41).
Humans also develop preferences for flavored foods or drinks that are associated with post-oral sugar effects (42). In a 2010 study, humans increased their liking, compared with participants given placebo capsules, for novel unsweetened teas after they ingested glucose capsules (43). In addition to activating post-oral nutrient sensors linked to brain reward systems, sugars in the gut generate negative feedback signals that lead to meal termination (satiation) and delay subsequent feeding (satiety). These negative feedback signals also serve as unconditioned stimuli that become associated with flavor cues. Animals use these flavor cues to predict the energy content of food (conditioned satiety) and adjust meal size. Conditioned satiety has been studied by training rats to consume low- and high-energy foods labeled with distinctive flavor cues and testing their intake response to the different flavors when presented in a food of intermediate energy density (44). Sham-drinking studies, in which rats with an open or closed gastric cannula are trained to drink concentrated sugar solutions, have demonstrated that sweet taste can serve as a conditioned satiety cue (45).
In contrast to concentrated sugar solutions, the taste of LCS is not associated with potent satiety signals. It has been hypothesized, therefore, that animals consuming both sugars and LCS would learn that sweet taste does not reliably predict energy content and become relatively insensitive to the satiating effects of sweet foods (46). Some supporting evidence was obtained in rats offered foods or drinks sweetened with saccharin or glucose. A provocative extension of this hypothesis suggests that the widespread availability of sweetened, low-energy foods in the human diet may promote, rather than retard, overeating and obesity by conditioning people to disregard sweet taste as a predictor of energy content. In fact, the effect of LCS on appetite and food intake is a subject of considerable discussion (47). Nevertheless, results obtained with rats fed saccharin- and sugar-sweetened foods must be interpreted with caution. Saccharin is a poor sugar substitute for rats and may only match the sweetness of diluted sugar solutions. If this is the case, then rats may learn that sweet taste intensity predicts energy density; slightly sweet foods are low in energy, whereas sweeter foods are energy dense. It is important, therefore, to match the sweetness level of low- and high-energy foods in rodent studies investigating the role of learning in energy regulation and dysregulation. This may not be possible in rats given saccharin or other common LCS. Alternative methods are available, such as training animals to real- and sham-drink sugar solutions to break the association between sweet taste and post-oral nutrition. Although it is more demanding, this procedure may more closely match the human situation in which equally sweet low- and high-energy foods and drinks are consumed.
The Neuroscience of Sweet Taste
The gustatory system allows the brain to monitor the presence of chemicals in the mouth and initiate appropriate acceptance or rejection responses. A sensory system has thus evolved in which membrane receptors convey information on the presence of metabolic fuels in the mouth to brain circuits controlling the initiation of ingestive behaviors. However, the formation of long-term sugar preferences does not exclusively rely on sweetness perception but also requires reinforcing postingestive effects. How does the brain control sugar intake in such a way that previous associations between sensory properties and postingestive effects become a regulating factor during nutrient choice? One possibility is that a brain circuit exists in which sensory and metabolic information converges through independent pathways. Indeed, the midbrain DA system seems to be one such candidate circuit.
The peripheral gustatory system provides the anatomic link between the oral sensory epithelium and the motivational circuits of the brain. As noted, sweet taste signaling is mediated by T1R2+T1R3 receptors and associated intracellular effectors, including phospholipase Cβ2 and transient receptor potential M5 taste channel (TRPM5). Deletion of either of these effectors induces severe impairments in, if not taste blindness for, sweet, umami, and bitter taste transduction (48).
Upon T1R2+T1R3, phospholipase Cβ2, and TRPM5 activation, and ensuing taste cell depolarization and neurotransmitter release, neural afferents of cranial nerves convey gustatory information to the rostral division of the rostral division of the nucleus tractus solitarius (rNTS) of the medulla (49). In rodents, axonal fibers originating in rNTS ascend ipsilaterally to the parabrachial nucleus (PBN), establishing this pontine structure as the second-order gustatory relay (Fig. 2) (50). From the PBN, a dorsal pathway projects to the parvicellular part of the ventroposteromedial nucleus of the thalamus (VPMpc, the taste thalamic nucleus) and a ventral pathway to the amygdalar and lateral hypothalamic areas. Thalamic afferents then project to the primary gustatory cortex, which is defined as the VPMpc cortical target located within the insular cortex. The primate counterpart has been established with the notable exception that rNTS projections seem to bypass PBN and proceed directly to VPMpc (51). Gustatory responses to sugar sweetness have been amply demonstrated in the insular and overlaying opercular cortices of monkeys using electrophysiological methods (52, 53) and in humans using functional neuroimaging (54, 55). Moreover, responses to sugars in the orbitofrontal cortex (an insular target accordingly denominated “secondary taste cortex”) seem to depend primarily on their perceived pleasantness (55), including those situations in which changes in motivational value derive from satiation (56). In contrast, responses to sugars in amygdalar regions have been associated instead with perceived stimulus intensity (55). However, it was recently proposed that the human amygdala may be specifically sensitive to the energy content of sweet compounds, as it seems to be preferentially responsive to beverages sweetened with LCS compared with sugars (57).
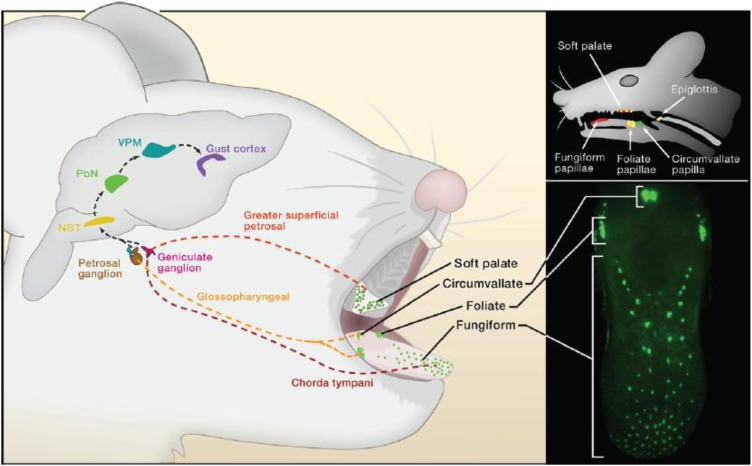
FIGURE 2.
Neuroanatomy of the rodent taste pathways. Taste buds in the mouth are innervated by afferent (chorda tympani, greater superficial petrosal, and glossopharyngeal) cranial nerves. Taste signals are conveyed to the nucleus of the solitary tract (NST) in the medulla and from the NST to the taste portion of the PBN in rodents but seemingly not in primates. From the PBN, parallel projections reach the VPM of the thalamus and forebrain limbic areas, including the amygdala and hypothalamus (not shown). Taste VPM projections define the gustatory aspect of the cortex within the insula, from which taste information is conveyed to higher order regions, including the orbital cortex. Reproduced from Yarmolinsky et al. (1) with permission. NST, nucleus of the solitary tract; PBN, parabrachial nucleus; VPM, ventral posterior medial thalamic nucleus.
Among the neurotransmitter systems known to be involved in regulating the ingestion of sugars, a preponderant role has been assigned to central DA systems. DA antagonists attenuate the hedonic value of sweet-tasting nutrients; animals pretreated with either D1 or D2 DA receptor antagonists behave toward high-concentration sucrose solutions as if they were weaker than usual (58–61). Conversely, tasting palatable foods elevates DA levels in the nucleus accumbens (NAcc) of the ventral striatum (62), a brain region implicated in food reinforcement (63). Taste-elicited stimulation of DA systems seems to take place even in the absence of intestinal nutrient absorption. In sham-feeding studies in which a cannula is implanted in the stomach wall to prevent nutrients from reaching the intestinal tract, NAcc DA levels increase in proportion to the concentration of the sucrose solution used to stimulate the intraoral cavity (64).
In summary, the perceived sweetness associated with sugar ingestion seems to act as a strong inducer of feeding and elicits responses in reward-related brain areas. However, is sweetness perception necessary for animals to develop a behavioral attraction to sugar? One way to address this question involves genetically engineered animals lacking functional taste transduction. De Araujo et al. (41) recently designed a conditioning protocol in which normal and Trpm5 knockout mice were allowed to form nutrient-specific preferences for sipper locations previously associated with a specific substance. The notion was that sweet-blind Trpm5 knockout mice would develop a preference for spouts associated with sucrose solutions when allowed to detect the solutions’ rewarding postingestive effects. In fact, during conditioning sessions, both wild-type and sweet-indifferent Trpm5 knockout mice consumed significantly larger amounts of sucrose than water. In addition, during postconditioning (water vs. water) 2-bottle tests, both wild-type and knockout animals consumed during conditioning sessions significantly more water from the sipper that contained nutritive sweeteners. When the same experiments were run using the LCS sucralose, unlike the case with sucrose, only wild-type animals consumed more sucralose than water during the conditioning sessions. Furthermore, during the 2-bottle test sessions, knockout mice, like their wild-type counterparts, showed no preferences for sippers associated with the delivery of sucralose. Overall, these results provide evidence favoring the hypothesis that postingestive effects exert positive control on ingestive (licking/swallowing) behaviors even in the absence of taste signaling or detection of distinct flavors.
Consistent with these findings, sugar intake by Trpm5 knockout mice, which cannot taste sweetness, increased extracellular DA levels in the NAcc (41). Moreover, sucralose produced significantly higher increases in DA levels in wild-type compared with knockout animals. However, when the same comparison was made for sucrose, no differences were found between the DA release levels in wild-type and knockout mice, i.e., sucralose produced significant DA stimulation only in wild-type mice, whereas sucrose evoked the same DA increase in both wild-type and knockout mice. Consistent with the above, rats given a local NAcc infusion of a DA-1 receptor antagonist did not learn a preference for a flavor paired with i.g. infusions of glucose (65). These results therefore strongly suggest that even in the absence of taste transduction and/or palatability, nutrient intake has the ability to induce measurable tonic increases in NAcc DA.
By what mechanism might DA neurons sense changes in nutritional state? Ren et al. (66) examined whether midbrain DA neurons are influenced by glucose utilization rate in the body. The hypothesis originated from the observations that: 1) Trpm5 knockout mice, although insensitive to both the taste of sugars and sweet L-amino acids, displayed a greater preference for glucose over the nongluconeogenic (but isoenergetic) amino acid L-serine (66); 2) this higher preference for (and intake of) glucose was associated with a higher glucose oxidation rate; and 3) the mice increased glucose intake after direct inhibition of glucose oxidation [using i.v. 2-deoxyglucose (2-DG)] (67, 66). Wild-type mice were fitted with a striatal microdialysis probe and a jugular venous catheter. Striatal DA release was monitored after i.v. 2-DG infusion followed by glucose infusion. The notion was that if glucose metabolism drives the rise in striatal DA release following glucose ingestion, then 2-DG infusion should reduce it. Thus, any inhibitory effect of 2-DG on DA release should be reversed or attenuated by subsequent glucose infusion (to restore glucose oxidation). I.v. infusion of 2-DG reduced extracellular striatal DA levels and subsequent glucose infusion partially reversed this effect. Importantly, glucose infusion produced a robust increase in striatal DA compared with that observed after 2-DG infusion (66). Therefore, glucose provision following inhibition of glucose utilization elicits a stronger relative increase in DA release than that observed when glucose utilization is not inhibited (66–68).
The above findings suggest 2 tentative conclusions. First, sweet-indifferent mice can develop nutrient-specific preferences based solely on physiological (nontaste) cues. Second, brain DA systems act as metabolic sensors responding to glucose oxidation rates. More generally, sugar-specific behavioral preferences and DA release may operate independently of sweetness and may be regulated by glucose oxidation rates. Such regulation most likely operates in addition to preabsorptive signals (37) that may influence DA release during flavor-nutrient conditioning.
LCS, Gastrointestinal Sweet Receptors, and Glucose Homeostasis: Are LCS Nutritionally Active?
As noted above, major advances have been made in characterizing taste receptors over the past decade. The sweet taste receptor and many downstream signaling molecules that transduce taste stimuli have been defined (1, 12). The idea that a process similar to taste might be involved in nutrient sensing in the intestines was advanced several decades ago (69). Subsequently, the taste-related G protein α-gustducin was identified in rat gut (37) and the expression of T1R family receptors was then demonstrated in the intestines and enteroendocrine cells (22, 70). These receptors have been suggested to function in the luminal sensing of sugars as well as in glucose absorption and metabolism (22, 23, 71). T1R are expressed in gut enteroendocrine cells (e.g., L-cells and K-cells) and promote the release of the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) from enteroendocrine L cell lines (22) or duodenal explants (72). These peptides stimulate the synthesis of sugar transport proteins in enterocytes, thus facilitating the absorption of sugars and ultimately their entry into the circulation (71). GLP-1 and/or GIP also act on the pancreas to enhance insulin and reduce glucagon secretion as well as modulate gastric emptying, gut motility, and food intake (appetite reduction) (73). Thus, one role of sweet taste receptors in enteroendocrine cells may be to bind sugars when they are present in the alimentary canal, facilitating their transfer across the gut lumen into the circulation and ultimately into the cells in the body that use them to generate energy.
Because LCS also interact with sweet taste receptors, might they produce effects on the intestinal cells that express them? If so, might LCS then elicit responses typically seen with sugars (74), namely the release of incretins (e.g., GLP-1 and GIP)? If so, LCS might disrupt glucose homeostasis by promoting glucose uptake into tissues in the absence of ingested sugars and thereby possibly lower blood sugar levels. The result might be to increase appetite and food intake and/or disrupt glucose homeostasis, thereby promoting increased incidence of obesity and/or metabolic syndrome (74). Although this chain of events has not been directly tested, many findings, especially in human participants, suggest that it is unlikely to occur.
First, LCS ingestion by humans and animals does not cause the hypothesized changes in blood glucose or hormone levels. For example, rats given acesulfame-K, saccharin, stevia, or sucralose by gavage showed no change in blood concentrations of GLP-1, GIP, and glucose (75). LCS gavage also did not influence the rise in blood glucose during an oral glucose tolerance test (75). In healthy humans, the ingestion of sucrose, but not acesulfame-K, aspartame, cyclamate, or saccharin (at moderate doses), increased blood glucose and insulin over a 2-h period (76). Sucralose given orally to healthy individuals and participants with diabetes at moderate and high doses did not influence plasma C-peptide or glucose concentrations (C-peptide is part of the proinsulin molecule; its blood level reflects recent insulin secretion) (77) and did not alter the blood glucose response to a large, oral glucose load (78). A single, oral, or i.g. dose of sucralose (79, 80), aspartame (80), or acesulfame-K (80) also did not modify plasma concentrations of GLP-1 and peptide YY (peptide YY is also secreted by L-cells and reduces hunger). Furthermore, the chronic ingestion of sucralose by participants with diabetes did not affect fasting plasma levels of glucose, hemoglobin A1c (an indicator of blood glucose levels over time), and C-peptide (81). A similar absence of effects was also observed when the LCS rebaudioside A was consumed chronically by patients with diabetes (82). Addressing one aspect of metabolic syndrome, blood pressure was unchanged in healthy participants following chronic oral ingestion of rebaudioside A (83). Negative effects were also observed when the mouth was bypassed; the i.g. infusion of sucrose, but not saline or sucralose, in humans increased plasma concentrations of glucose, GLP-1, GIP, and insulin and slowed gastric emptying (a known action of GLP-1) (84, 85). Finally, the intraduodenal infusion of glucose increased plasma concentrations of glucose, GLP-1, insulin, and glucose transport across the gut wall; again, sucralose infusion was without effect (86).
Second, LCS ingestion does not increase food intake or body weight. The most convincing effects have been seen chronically in human studies in which LCS have been substituted covertly for energy-containing sweeteners, using either aspartame (87, 88) or a blend of LCS (89) in typical foods. In general, such studies show that the use of LCS reduces energy intake over a period of weeks and can produce modest weight reduction. Covert LCS substitution for energy-containing sweeteners in the diet is appealing in the present context in which the LCS have been hypothesized to promote weight gain by biochemical and endocrine actions. Clearly, they do not. Studies in animals also indicate that the very long-term ingestion of moderate to high doses of LCS in food is not associated with increased food intake or body weight gain (90–93).
In summary, although the presence of sweet taste receptors on gut enteroendocrine cells is likely to be physiologically important, the biochemical, endocrinological, and behavioral evidence presently available, most notably in humans, does not support the hypothesis that LCS cause a biochemical-endocrinological-behavioral cascade, beginning with the stimulation of sweet receptors in intestinal enteroendocrine cells, that increases food intake and body weight.
Conclusions
The molecular structure of the sweet receptor in the mouth was recently elucidated and was subsequently found to be present on intestinal enteroendocrine cells. Sweet receptors in the mouth function in the perception of sweet taste; stimulating them with either nutritive sweeteners or LCS results in activation of neural pathways to and within the brain that interpret and react to sweet stimuli. Indeed, recent research has greatly increased knowledge regarding such brain circuitry. Intestinal sweet receptors may facilitate sugar uptake from the alimentary canal, release into the circulation, and extraction by cells in the body for use for energy production. Both nutritive sweeteners and LCS bind to sweet receptors. LCS, by definition, activate sweet taste receptors in the mouth (as do nutritive sweeteners) and also bind to sweet receptors on intestinal cells and induce the release of hormones in some experimental models. However, the extent to which LCS induce paracrine/endocrine signaling in vivo is presently unresolved, particularly because human studies do not show evidence of such effects or their postulated sequelae. It is hoped that future studies will resolve this disparity. Some such disparities may derive from differences in rodent and human physiology. Although rodents have proven to be an excellent model system for understanding the molecular basis of sweet taste, rodents and humans differ in their perception of both nutritive sweeteners and LCS. We hope that future studies will expand the knowledge base regarding differences in sweet taste perception across species to aid in understanding when animal findings are or are not predictive of the human condition. Much has thus been learned in recent years regarding the biochemistry and physiology of sweet taste, yet much remains to be revealed.
Acknowledgments
All authors contributed to portions of manuscript writing. All authors read and approved the final manuscript.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the conference Low-Calorie Sweeteners, Appetite and Weight Control: What the Science Tells Us, held in Washington, DC, April 7–8, 2011. The conference was sponsored by the Committee on Low-calorie Sweeteners of the International Life Sciences Institute North America. The views expressed in these papers are not necessarily those of the supplement coordinator or guest editors. The supplement coordinator for this supplement was Adam Drewnowski, University of Washington. Supplement Coordinator disclosures: Adam Drewnowski received travel reimbursement for participation in the Low-Calorie Sweeteners Conference. The supplement is the responsibility of the Guest Editor to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Sibylle Kranz. Guest Editor disclosure: Sibylle Kranz has received funding from The Kellogg Company to conduct research projects unrelated to this supplement material. She has also been an invited speaker at a roundtable discussion funded by The Kellogg Company. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Supported by the Committee on Low-Calorie Sweeteners of the North American Branch of the International Life Sciences Institute. J. D. Fernstrom, S. D. Munger, A. Sclafani, I. de Araujo, and A. Roberts received a modest honorarium for their participation in the workshop and development of the manuscript. All authors received travel funding to attend the workshop.
Author disclosures: J. D. Fernstrom is a scientific consultant to the Ajinomoto Company. Research in S. D. Munger’s laboratory is funded by the NIH/National Institute on Deafness and Other Communication Disorders (DC005633, DC010110) and by Tate and Lyle Americas, LLC. S. D. Munger has previously consulted with McNeil Nutritionals LLC on the biology of sweet taste. A. Sclafani’s laboratory is funded by the NIH/National Institute of Diabetes and Digestive and Kidney Diseases Digestive (DK 031135) and the Ajinomoto Amino Acid Research Program. S. V. Molinary is a scientific consultant to the Tate and Lyle Company. Research in I. E. de Araujo’s laboratory is funded by the NIH/National Institute on Deafness and Other Communication Disorders (DC009997). I. de Araujo and A. Roberts, no conflicts of interest.
Abbreviations used: 2-DG, 2-deoxyglucose; DA, dopamine; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; LCS, low-calorie sweeteners; NAcc, nucleus accumbens; NST, nucleus of the solitary tract; PBN, parabrachial nucleus; rNTS, rostral division of the nucleus tractus solitarius; TRPM5, transient receptor potential M5 taste channel; T1R, type 1 taste receptor; VPMpc, ventral posterior medial thalamic nucleus, parvicellular part; VFT, venus-flytrap.
Literature Cited
- 1.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–51 [DOI] [PubMed] [Google Scholar]
- 3.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702 [DOI] [PubMed] [Google Scholar]
- 4.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–4 [DOI] [PubMed] [Google Scholar]
- 5.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 6.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90 [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–11 [DOI] [PubMed] [Google Scholar]
- 9.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–70 [DOI] [PubMed] [Google Scholar]
- 10.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigues S, Dotson CD, Munger SD. The receptor basis of sweet taste in mammals. Results Probl Cell Differ. 2009;47:187–202 [DOI] [PubMed] [Google Scholar]
- 13.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15:1948–52 [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem. 2005;280:34296–305 [DOI] [PubMed] [Google Scholar]
- 16.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 2005;280:15238–46 [DOI] [PubMed] [Google Scholar]
- 17.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, et al. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pronin AN, Xu H, Tang H, Zhang L, Li Q, Li X. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Curr Biol. 2007;17:1403–8 [DOI] [PubMed] [Google Scholar]
- 20.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66 [DOI] [PubMed] [Google Scholar]
- 21.Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–9 [DOI] [PubMed] [Google Scholar]
- 22.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dotson CD, Vigues S, Steinle NI, Munger SD. T1R and T2R receptors: the modulation of incretin hormones and potential targets for the treatment of type 2 diabetes mellitus. Curr Opin Investig Drugs. 2010;11:447–54 [PMC free article] [PubMed] [Google Scholar]
- 25.Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab. 2009;20:163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 2000;97:11044–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010;24:3960–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glendinning JI, Breinager L, Kyrillou E, Kacuna K, Rocha R, Sclafani A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol Behav. 2010;101:331–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 1986;37:253–6 [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A, Clare R. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–8 [DOI] [PubMed] [Google Scholar]
- 32.Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite. 2002;38:155–60 [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A, Nissenbaum JW. On the role of the mouth and gut in the control of saccharin and sugar intake: A reexamination of the sham-feeding preparation. Brain Res Bull. 1985;14:569–76 [DOI] [PubMed] [Google Scholar]
- 34.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: The contribution of a-gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1504–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dess NK, Chapman CD, Monroe D. Consumption of SC45647 and sucralose by rats selectively bred for high and low saccharin intake. Chem Senses. 2009;34:211–20 [DOI] [PubMed] [Google Scholar]
- 36.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–9 [DOI] [PubMed] [Google Scholar]
- 37.Höfer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci USA. 1996;93:6631–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–34 [DOI] [PubMed] [Google Scholar]
- 40.Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol Biochem Behav. 2010;97:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41 [DOI] [PubMed] [Google Scholar]
- 42.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav. 2008;93:798–806 [DOI] [PubMed] [Google Scholar]
- 43.Pelchat ML, Carfagno GM. GI glucose enhances “mere” exposure in humans. Appetite. 2010;54:669 [Google Scholar]
- 44.Booth DA, Davis JD. Gastrointestinal factors in the acquisition of oral sensory control of satiation. Physiol Behav. 1973;11:23–9 [DOI] [PubMed] [Google Scholar]
- 45.Davis JD, Smith GP. The conditioned satiating effect of orosensory stimuli. Physiol Behav. 2009;97:293–303 [DOI] [PubMed] [Google Scholar]
- 46.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattes RD, Popkin BM. Nonnutritive sweeteners sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301 [DOI] [PubMed] [Google Scholar]
- 49.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–77 [DOI] [PubMed] [Google Scholar]
- 50.Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173:1136–9 [DOI] [PubMed] [Google Scholar]
- 51.Scott TR, Small DM. The role of the parabrachial nucleus in taste processing and feeding. Ann N Y Acad Sci. 2009;1170:372–7 [DOI] [PubMed] [Google Scholar]
- 52.Yaxley S, Rolls ET, Sienkiewicz Z, Scott TR. Gustatory responses of single neurons in the frontal opercular cortex of the macaque monkey. Neurosci Lett Suppl. 1985:S456 [Google Scholar]
- 53.Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700 [DOI] [PubMed] [Google Scholar]
- 54.de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–68 [DOI] [PubMed] [Google Scholar]
- 55.Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11 [DOI] [PubMed] [Google Scholar]
- 56.Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci. 1989;1:53–60 [DOI] [PubMed] [Google Scholar]
- 57.Smeets PA, Weijzen P, de Graaf C, Viergever MA. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage. 2011;54:1367–74 [DOI] [PubMed] [Google Scholar]
- 58.Bailey CS, Hsiao S, King JE. Hedonic reactivity to sucrose in rats: modification by pimozide. Physiol Behav. 1986;38:447–52 [DOI] [PubMed] [Google Scholar]
- 59.Geary N, Smith GP. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav. 1985;22:787–90 [DOI] [PubMed] [Google Scholar]
- 60.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–42 [DOI] [PubMed] [Google Scholar]
- 62.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–12 [DOI] [PubMed] [Google Scholar]
- 63.Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–4 [DOI] [PubMed] [Google Scholar]
- 64.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–7 [DOI] [PubMed] [Google Scholar]
- 65.Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27:1525–33 [DOI] [PubMed] [Google Scholar]
- 66.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welberg L. Chemosensation: tasteless mice prefer sugar. Nat Rev Neurosci. 2010;11:538. [DOI] [PubMed] [Google Scholar]
- 68.de Araujo IE, Ren X, Ferreira JG. Metabolic sensing in brain dopamine systems. Results Probl Cell Differ. 2010;52:69–86 [DOI] [PubMed] [Google Scholar]
- 69.Fujita T. Concept of paraneurons. Arch Histol Jpn. 1977;40 Suppl:1–12 [DOI] [PubMed] [Google Scholar]
- 70.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–5 [DOI] [PubMed] [Google Scholar]
- 71.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–4 [DOI] [PubMed] [Google Scholar]
- 73.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57 [DOI] [PubMed] [Google Scholar]
- 74.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab. 2009;296:E473–9 [DOI] [PubMed] [Google Scholar]
- 76.Härtel B, Graubaum HL, Schneider B. The influence of sweetener solutions on the secretion of insulin and the blood glucose level. Ernährungsumschau. 1993;40:152–5 [Google Scholar]
- 77.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19:1004–5 [DOI] [PubMed] [Google Scholar]
- 78.Roberts A. Sucralose and diabetes. Food Food Ingred Jpn. 1999;182:49–55 [Google Scholar]
- 79.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65:508–13 [DOI] [PubMed] [Google Scholar]
- 80.Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011;105:1320–8 [DOI] [PubMed] [Google Scholar]
- 81.Grotz VL, Henry RF, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–12 [DOI] [PubMed] [Google Scholar]
- 82.Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV, Schwartz SL, Lubin BC, Boileau AC, Dicklin MR, et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food Chem Toxicol. 2008;46 Suppl 7:S47–53 [DOI] [PubMed] [Google Scholar]
- 83.Maki KC, Curry LL, Carakostas MC, Tarka SM, Reeves MS, Farmer MV, McKenney JM, Toth PD, Schwartz SL, Lubin BC, et al. The hemodynamic effects of rebaudioside A in healthy adults with normal and low-normal blood pressure. Food Chem Toxicol. 2008;46 Suppl 7:S40–6 [DOI] [PubMed] [Google Scholar]
- 84.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–8 [DOI] [PubMed] [Google Scholar]
- 85.Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in health subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104:803–6 [DOI] [PubMed] [Google Scholar]
- 87.Porikos KP, Booth G, Van Itallie TB. Effect of covert nutritive dilution on the spontaneous food intake of obese individuals: a pilot study. Am J Clin Nutr. 1977;30:1638–44 [DOI] [PubMed] [Google Scholar]
- 88.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51:963–9 [DOI] [PubMed] [Google Scholar]
- 89.Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–9 [DOI] [PubMed] [Google Scholar]
- 90.Ishii H. Chronic feedings studies with aspartame and its diketopiperazine. : Stegink LD, Filer LJ, Jr, Aspartame: physiology and biochemistry; New York: Marcel Dekker; 1984. p. 307–19 [Google Scholar]
- 91.Mann SW, Yuschak MM, Amyes SJ, Aughton P, Finn JP. A combined chronic toxicity/carcinogenicity study of sucralose in Sprague-Dawley rats. Food Chem Toxicol. 2000;38 Suppl 2:S71–89 [DOI] [PubMed] [Google Scholar]
- 92.Curry LL, Roberts A. Subchronic toxicity of rebaudioside A. Food Chem Toxicol. 2008;46 Suppl 7:S11–20 [DOI] [PubMed] [Google Scholar]
- 93.Curry LL, Roberts A, Brown N. Rebaudioside A: two-generation reproductive toxicity study in rats. Food Chem Toxicol. 2008;46 Suppl 7:S21–30 [DOI] [PubMed] [Google Scholar]