EXECUTIVE SUMMARY
Antimicrobial resistance is recognized as one of the greatest threats to human health worldwide [1]. Drug-resistant infections take a staggering toll in the United States (US) and across the globe. Just one organism, methicillin-resistant Staphylococcus aureus (MRSA), kills more Americans every year (∼19,000) than emphysema, HIV/AIDS, Parkinson's disease, and homicide combined [2]. Almost 2 million Americans per year develop hospital-acquired infections (HAIs), resulting in 99,000 deaths [3], the vast majority of which are due to antibacterial (antibiotic)-resistant pathogens. Indeed, two common HAIs alone (sepsis and pneumonia) killed nearly 50,000 Americans and cost the US health care system more than $8 billion in 2006 [4]. In a recent survey, approximately half of patients in more than 1,000 intensive care units in 75 countries suffered from an infection, and infected patients had twice the risk of dying in the hospital as uninfected patients [5]. Based on studies of the costs of infections caused by antibiotic-resistant pathogens versus antibiotic-susceptible pathogens [6–8], the annual cost to the US health care system of antibiotic-resistant infections is $21 billion to $34 billion and more than 8 million additional hospital days.
The discovery of antibiotics in the 1930s fundamentally transformed the way physicians care for patients, shifting their approach from a focus on diagnoses without means to intervene to a treatment-focused approach that saves lives. Seven decades of medical advances enabled by antibiotics are now seriously threatened by the convergence of relentlessly rising antibiotic resistance and the alarming and ongoing withdrawal of most major pharmaceutical companies from the antibiotic market. Without effective antibiotics, diverse fields of medicine will be severely hampered, including surgery, the care of premature infants, cancer chemotherapy, care of the critically ill, and transplantation medicine, all of which are feasible only in the context of effective antibiotic therapy. Our ability to respond to national security threats (e.g., bioterrorism and pandemics) also is in serious jeopardy. Ultimately, the loss of effective antibiotics will result in a great increase in morbidity and mortality from infections. Antimicrobial resistance is of such tremendous global concern that the World Health Organization (WHO) has proclaimed it the central focus of World Health Day 2011 (April 7).
This policy paper summarizes the Infectious Diseases Society of America's (IDSA) recommendations about how best to address the synergistic crises of rising rates of antibiotic resistance and waning approvals of new antibiotics. IDSA's goal is to represent the best interests of patients and health care professionals by recommending public policy strategies and research activities that reverse antibiotics’ decline and save lives. Specific recommendations for Congress related to legislative action and funding needs are summarized in Tables 1 and 2, respectively. A glossary of abbreviations used throughout the document is available in Appendix A.
Table 1.
Summary of Legislative Recommendations for Congress
| Legislative Recommendations | Section(s) |
| The Generating Antibiotic Incentives Now (GAIN) Act (H.R. 6331 in the 111th Congress) should be further strengthened, with additional incentives to stimulate antibiotic and related diagnostics R&D as well as safeguards to ensure approved antibiotics are used appropriately, and quickly enacted in the 112th Congress. | I.1, VII.2 |
| Congressional leaders should discuss incentives with representatives of the European Commission, as the European Union has set a December 2011 deadline for evaluating and developing an action plan of concrete incentives to spur antibiotic R&D. | I.1 |
| Public-private partnerships (PPPs) and the Assistant Secretary for Preparedness and Response's (ASPR) proposed independent strategic investment firm should be established and funded and existing government-supported collaborations (e.g., ASPR's Biomedical Advanced Development and Research Authority [BARDA]) should be further strengthened to supplement traditional industry R&D for critically needed antimicrobial drugs. | I.2 |
| An “Antibiotic Innovation and Conservation (AIC) Fee” should be established, 75% of which should be used to fund new antibiotic development and 25% of which should be used to fund antimicrobial stewardship. | I.2, V.6 |
| Value-based reimbursement strategies that encourage antibiotic and related diagnostics development must be pursued. | I.3 |
| An expert panel should be created to identify priority pathogens/infections for the purpose of targeting incentives, possibly as part of the Public Health Antimicrobial Advisory Board (PHAAB) contained in the Strategies to Address Antimicrobial Resistance (STAAR) Act (H.R. 2400 in the 111th Congress). | I.4, III.1 |
| Congressional leaders should discuss with the Food and Drug Administration (FDA) the need for additional statutory authority to allow for conditional approvals, post-approval approaches or other novel approaches that will lead to approval and appropriate use of antibiotics that treat urgent unmet medical needs. | II.4, V.8 |
| The STAAR Act should be quickly enacted to establish within the US Department of Health and Human Services (HHS) an Antimicrobial Resistance Office (ARO) and a PHAAB, and to strengthen surveillance, data collection, research, and prevention and control efforts, including development of a network of sentinel surveillance and research sites (i.e., the Antimicrobial Resistance Surveillance and Research Network [ARSRN]) and creation of an Antimicrobial Resistance Strategic Research Plan. | III, IV.1 & 2, V.1, VI.1 |
| The STAAR Act should be further strengthened to permit collection of local level antibiotic use data in humans and animals (species-specific). | IV.3 |
| Congressional leaders, including sponsors of the STAAR Act and GAIN Act, should consider novel and innovative ways to strengthen antimicrobial resistance prevention and control efforts including through: 1) the establishment and support of antimicrobial stewardship programs in all health care settings (e.g., hospitals, long-term care facilities, long-term acute care facilities, ambulatory surgical centers, dialysis centers, outpatient clinics, private practices), which should be required as a condition of participation in the federal Medicare and Medicaid programs or through another regulatory mechanism; 2) strengthened public health and research efforts; and 3) creation of the AIC Fee. | V |
| Opportunities to support career development are necessary to reverse the “brain drain” that has occurred in antibiotic and microbiology research in both academia and industry. Incentives to address this problem should be included in legislation. | VI.7 |
| A clinical specimen repository should be established by the National Institute of Allergy and Infectious Diseases and FDA to support R&D of novel molecular diagnostic tests as part of the GAIN Act or other legislation. | VII.3 |
| The Preservation of Antibiotics for Medical Treatment Act (PAMTA) (H.R. 1549/S. 619 in the 111th Congress) should be enacted and other measures (including FDA regulations) adopted to end the use of antibiotics for growth promotion, feed efficiency, and routine disease prevention purposes in animal agriculture. | VIII.1 |
Table 2.
Summary of Funding Recommendations for Congress
| Agency/Program | Funds Needed | Purpose | Section(s) |
| Congressionally-enacted economic incentives most likely overseen by ASPR or FDA | Depends on which economic incentives are enacted and their scope and size | Entice companies to reengage in antibiotic (and related diagnostics) R&D though the use of a combination of “push” and “pull” mechanisms (grants, exclusivity, tax credits, etc.) | I.1, VII.2 |
| *ASPR's BARDA | ≥$1.7 billion annually of multi-year funding | Support development of therapeutics, diagnostics, and vaccines, including antibiotics and diagnostics that specifically target antibiotic-resistant pathogens | I.2 |
| *ASPR's Proposed Independent Strategic Investment Firm | ≥$200 million annually | Smaller innovative companies with promising antibiotics in development would leverage public funds to obtain additional private venture capital | I.2 |
| *Proposed PPP most likely overseen by ASPR or BARDA | Depends on scope of the effort and availability of private capital | Advance development of promising lead compounds toward approved products targeting the highest priority unmet medical needs where market challenges are most extreme | I.2 |
| FDA's Center for Drug Evaluation and Research (CDER) | Additional $40 million annually | Expand staff to develop clinical trial guidance and Critical Path initiatives ($15 million) and new antibiotic R&D under regulatory science initiative ($25 million) | II |
| Existing Interagency Task Force on Antimicrobial Resistance and the STAAR Act's HHS ARO and PHAAB | $30 million in FY2012 to HHS for the task force; and then $44 million in FY2013 and $80 million in FY2014 for the work of all three | Strengthen coordination and expansion of federal antimicrobial resistance efforts and permit ongoing input from non-government medical and public health experts to enhance federal priority-setting and assure greater accountability | III |
| *US Centers for Disease Control and Prevention (CDC) | $50 million annually beginning in FY2012 | Enable enhanced antimicrobial resistance surveillance, data collection and publication, prevention and control strategies, related research, education of providers and patients, and expansion of antimicrobial stewardship* efforts nationally | IV, V, VI, VII |
| National Institute of Allergy and Infectious Diseases | Additional $500 million annually | Support expansion of antibiotic resistance and development (drug, diagnostics, etc.) research portfolios | V.5, VI, VII |
| FDA's Center for Veterinary Medicine (CVM) | Additional $5 million immediately of multi-year funding | Complete/publish safety reviews of antibiotics of importance to human medicine that are approved for non-therapeutic purposes in food-producing animals | VIII |
| National Antimicrobial Resistance Monitoring System (NARMS) Program (US Department of Agriculture [USDA], CDC, FDA's CVM) | Additional $3 million annually | Increase surveillance (additional bacterial species and numbers and/or types of samples); more sensitive methods; farm-level surveillance of antibiotic-resistant bacteria | IV.2 |
| USDA, Agency for Healthcare Research and Quality, US Agency for International Development, US Department of Veterans Affairs and other members of the Interagency Task Force | Increases specific to each agency's antimicrobial resistance programs, as necessary | Support US efforts to limit and control the development and spread of antimicrobial-resistant infections in humans and animals in the US and abroad. | III |
*Funding for these activities could be supported through the establishment of the AIC Fee described in recommendation I.2 and V.6
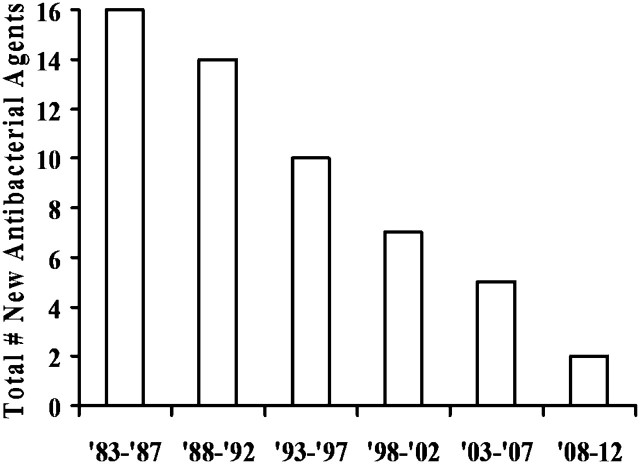
Resistance, which enables microbes to escape being killed by antimicrobial (including antibacterial, antiviral, antifungal, etc.) drugs, undermines physicians’ ability to treat serious and life-threatening infections. The primary focus of this paper is antibiotic resistance because of the extent of the threat posed by antibiotic-resistant infections in health care facilities and communities throughout the world, coupled with a rapidly diminishing antibiotic pipeline (Figure 1) [9–12].
Figure 1.
Number of New Molecular Entity (NME) Systemic Antibiotics Approved by the US FDA Per Five-year Period, Through 3/11.
Where the term “antimicrobial” is used, IDSA's recommendations have applicability to drugs that treat infections caused by most types of microbes. In contrast, the term “antibiotic” is used to denote a recommendation specific to drugs that treat bacterial infections. For simplicity, the term antibiotic is used to encompass both true antibiotics (compounds produced by microbes to kill other microbes) and antibacterial agents that are synthetic.
IDSA RECOMMENDS:
I. Adoption of Economic Incentives and Support for Other Collaborative Mechanisms to Address the Market Failure of Antibiotics
1. Statutorily-defined push and pull economic incentives are urgently needed to correct the current market failure and to motivate companies to reengage in antibiotic (and related diagnostics) research and development (R&D). Such incentives are the focus of the Generating Antibiotic Incentives Now (GAIN) Act (H.R. 6331), bipartisan legislation introduced in the 111th Congress. The GAIN Act should be further strengthened, with additional incentives as well as safeguards to ensure approved antibiotics are used appropriately, and quickly enacted in the 112th Congress.
- 2. New public-private partnerships (PPPs) should be established and existing government-supported collaborative programs (e.g., the Biomedical Advanced Research and Development Authority [BARDA] housed within the US Department of Health and Human Services’ [HHS] Office of the Assistant Secretary for Preparedness and Response [ASPR]) strengthened to supplement (but not replace) traditional industry R&D for critically needed antimicrobial drugs.
- a. PPPs should be funded by a blend of public monies and matching private capital.
- b. Public funding may be from a combination of grants, contracts, and from allocation of 75% of a proposed Antimicrobial Innovation and Conservation (AIC) Fee to a trust fund established under the management of HHS’ ASPR to support the development of promising, high priority candidate antibiotics.
- c. Private capital could be raised through a combination of user fees, license payments, royalty sharing, and/or other methods.
- d. BARDA needs an annual allocation of at least $1.7 billion of multi-year funding to support development of therapeutics, diagnostics, vaccines, and other technologies including new antibiotics and diagnostics that specifically target antibiotic-resistant pathogens.
- e. Funding is needed to support other components of HHS’ revised Public Health Emergency Medical Countermeasures Enterprise (PHEMCE), announced in August 2010. In particular, at least $200 million is needed immediately to support the establishment of an independent strategic investment firm that will assist smaller innovative companies with promising antibiotics in development to leverage public funds to obtain essential private venture capital.
3. Value-based reimbursement strategies that encourage antibiotic and related diagnostics development must be pursued.
4. There is pressing need to establish a panel of experts to document and regularly revise a list of priority pathogens or infections that have resulted or likely will result in an area of significant unmet medical need, and toward which adopted economic incentives should be targeted. The panel should include representatives from government agencies, academic and private infectious disease specialists, and public health experts.
II. New Regulatory Approaches to Facilitate Antimicrobial Development and Approval
1. Clear and feasible regulatory guidelines on clinical trial designs are urgently needed to enable approvals of new antibiotics and other antimicrobials. In setting regulatory guidance, the Food and Drug Administration (FDA) must balance the public health risks of approving a less effective drug with the risk of having no new, critically needed antibiotics available to treat patients infected with resistant pathogens.
2. Already conservative estimates of antimicrobial efficacy relative to placebo/no therapy should not be further “discounted” when setting requirements for non-inferiority margins for clinical trials, as discounting results in excessively large trial requirements.
3. The primary issue in justifying the non-inferiority margin for a clinical trial is determining how much of the clinical benefit of antimicrobial therapy must be preserved, which should be based upon an assessment of the relative merits of the specific experimental drug versus currently available therapy.
- 4. Regulatory guidance is needed to create new pathways to facilitate approval of antibiotics.
- a. Regulatory guidance is needed to allow conduct of organism-specific antibiotic clinical trials, a departure from the approach FDA currently uses to approve new antibiotics (i.e., by infection site and disease).
- b. Regulatory guidance is needed for a variety of other novel antibiotic studies, including acceptable design of superiority clinical trials and/or the use of historically controlled clinical trials.
- c. In areas where urgent unmet medical need exists (e.g., for highly antibiotic-resistant Gram-negative bacteria [GNB]), guidance also is needed on approval of antibiotics based on a relatively small clinical sample size (e.g., <100 patients), possibly by using a conditional approval mechanism buttressed by the establishment of powerful post-approval Risk Evaluation and Mitigation Strategies (REMS)-like safeguards.
- d. Congressional leaders (including GAIN Act co-sponsors) should discuss with FDA officials whether expansion of the agency's existing statutory authority is needed to allow for conditional approvals and post-approval approaches for novel antibiotics that address urgent unmet medical needs. Alternatively, other statutory changes should be identified that agency officials agree would speed the development and approval of priority, novel antibiotics. The GAIN Act already contains promising ideas (e.g., fast-track approval, priority review, deadlines placed on clinical trial guidance development), but additional discussion with FDA is needed.
- 5. Regulatory science must continue to be advanced and developed to make clinical trial designs feasible, clinically relevant, and scientifically rigorous.
- a. Collaborative regulatory science efforts should be encouraged and further expanded. One example is the workgroup recently established by the FDA, National Institute of Allergy and Infectious Diseases (NIAID), and Foundation of the National Institutes of Health (NIH), which includes representation from industry, academia, and IDSA. The Reagan-Udall Foundation, a public-private partnership between FDA and industry, presents additional opportunities. These groups should examine surrogate endpoints for antibiotic clinical trials and pharmacokinetic/pharmacodynamic (PK/PD) parameters that forecast optimal antibiotic dosing, among other topics. These activities will require additional dedicated funding support from the federal government, industry, and other organizations.
- b. Alternatives or surrogates to traditional clinical trial endpoints (e.g., other than survival) should be considered as evidence of clinical benefit to patients.
- c. Cutting-edge statistical methods (e.g., Bayesian statistics) should be used to increase efficiency of clinical trials.
- d. An additional $40 million should be allocated to FDA, including an additional $15 million to expand staff to develop clinical trial guidance and Critical Path initiatives and $25 million to support a strong focus on new antibiotic R&D under FDA's new regulatory science initiative.
III. Greater Coordination of Relevant Federal Agencies’ Efforts
1. The Strategies to Address Antimicrobial Resistance (STAAR) Act (H.R. 2400 in the 111th Congress) should be further strengthened, as outlined in this paper, and enacted to establish within HHS: a) an Antimicrobial Resistance Office (ARO); and b) a Public Health Antimicrobial Advisory Board (PHAAB) composed of non-government experts to support the work of the existing Interagency Task Force on Antimicrobial Resistance, and strengthen coordination, prioritization, and accountability of federal efforts.
2. Sufficient funding must be appropriated for the activities of the existing interagency task force as well as for the ARO and PHAAB once they are established. Specifically, IDSA recommends $30 million in funding in fiscal year (FY) 2012, $44 million in FY2013, and $80 million in FY2014.
IV. Enhancement of Antimicrobial Resistance Surveillance Systems
1. National data on antimicrobial resistance rates, linked to clinical outcomes, should be gathered in real time and made public.
2. A federally funded network of sentinel sites that includes specimen collection linked to clinical data is needed to evaluate rapidly emerging resistance in a variety of clinically important organisms and infections, and to develop, implement, and evaluate prevention strategies.
3. National and local data on antimicrobial, and particularly antibiotic, use across the spectrum (human, veterinary and other agricultural) must be collected and made publicly available. In the animal agriculture context, FDA should collect species-specific (poultry, swine, cattle, etc.) antimicrobial use data directly from local feed mills, where drugs are mixed into animal feed. The STAAR Act should be strengthened to incorporate these elements.
4. The Centers for Disease Control and Prevention's (CDC) antimicrobial resistance funding must be significantly and immediately increased to $50 million to enable critical public health-related objectives, outlined in this paper, to be achieved.
V. Strengthening Activities to Prevent and Control Antimicrobial Resistance
1. Current law should be strengthened to improve antimicrobial resistance prevention and control efforts through novel and innovative mechanisms.
2. Antimicrobial stewardship (i.e., coordinated interventions designed to improve appropriate use of antimicrobial drugs, including preventing inappropriate antimicrobial use and limiting antimicrobial exposure) is a critical tool to protect antibiotics from misuse and overuse. New incentives and requirements must be established for implementation and maintenance of successful antimicrobial stewardship programs across all health care settings (e.g., hospitals, long-term care facilities, long-term acute care facilities, ambulatory surgical centers, dialysis centers, outpatient clinics, private practices), including by requiring stewardship programs as a condition of participation in the federal Medicare and Medicaid programs or through another regulatory mechanism.
3. CDC's educational efforts on appropriate use of antimicrobials, including the Get Smart program, serve as a critical starting point for establishing antimicrobial stewardship programs. These educational efforts must be expanded for providers and patients.
4. Research is needed to define “inappropriate” antimicrobial prescribing and to better understand the primary drivers of such use.
5. Research is needed to define optimal components and goals of antimicrobial stewardship programs in different health care settings, including clinically relevant patient outcomes, and to develop national metrics to monitor program success.
6. An AIC Fee should be established, 25% of which should be used to fund antimicrobial stewardship program implementation and 75% of which should be used to fund new antibiotic development (see recommendation I.2).
7. Rapid molecular diagnostics are urgently needed to support appropriate antimicrobial use (see recommendation VII).
- 8. FDA should study and implement mechanisms to prevent over-prescription of antibiotics.
- a. As discussed in recommendation II.4, new clinical trial pathways should be established by regulatory guidance that enable companies to seek approval for organism-specific, narrow indications (e.g., infections caused by resistant GNB). Current FDA approval processes may be antithetical to antimicrobial stewardship principles. For example, antibiotics with broad activity, including against resistant GNB, have been licensed for the treatment of skin infections that are caused by a narrow spectrum of bacteria for which other effective options are available. By using new approval pathways focused on medical need, FDA can help limit the overuse of newly approved broad spectrum antibiotics (e.g., those that kill resistant GNB) by preventing their use to treat infections caused by a narrow spectrum of bacteria (e.g., skin infections not caused by GNB).
- b. Other strategies to protect antibiotics post-approval should be considered, such as a REMS-like program for antibiotics.
VI. Significant Investments in Antimicrobial-Focused Research
1. The Antimicrobial Resistance Strategic Research Plan called for in the STAAR Act should be developed and implemented with a particular focus on antibiotic resistance.
2. Basic science research should be expanded to further study antimicrobial resistance mechanisms and epidemiology; identify new lead compounds; and develop vaccines, immunotherapies, and other technologies to prevent and treat infections in humans and animals.
3. Support for translation of promising compounds from pre-clinical research into clinical trials should be expanded.
4. Clinical and health outcomes research is needed to: a) define the natural history, outcomes, and magnitude of antimicrobial benefit for treatment of infections; and b) conduct comparative-effectiveness studies to define shorter durations of antimicrobial therapy and clinical and laboratory parameters that support early cessation of therapy.
5. Research is needed to optimize the PK/PD of antimicrobial therapy.
6. A clinical trial network is needed to support studies of antimicrobial therapies and antimicrobial resistance, building on the success of the existing HIV/AIDS clinical trials network.
7. Funding to support career development and faculty retention is necessary to reverse the “brain drain” that continues to occur in antibiotic and microbiology research in both academia and industry.
8. Annual funding for NIAID should be increased by $500 million by direct appropriation to support expansion of its antibiotic resistance and development research portfolio.
VII. Greater Investment in Rapid Diagnostics R&D and Integration into Clinical Practice
Novel molecular diagnostics are needed that improve clinical care and public health. Such diagnostics can rapidly identify which illnesses are due to non-bacterial pathogens (e.g., viruses) and therefore do not need antibiotic therapy, which illnesses are due to bacteria and require antibiotic therapy, and which illnesses are due to drug-resistant bacteria. Ideally, these tests will be inexpensive, rapid, sensitive, specific, and able to be used close to or at point-of-care, and will lead to improved health care outcomes, reduced health care costs, reduced antibiotic resistance, and enhanced novel antibiotic development.
Federally-supported research and economic incentives are necessary to support R&D of novel molecular diagnostic tests and to strongly encourage their integration into clinical practice.
To limit the need for repetitive, expensive clinical trials and support rapid, efficient development and approval of new molecular diagnostic tests, a well-characterized clinical sample repository should be established by NIAID and FDA.
VIII. Eliminating Non-Judicious Antibiotic Use in Animals, Plants, and Marine Environments
The Preservation of Antibiotics for Medical Treatment Act (PAMTA) (H.R. 1549/S. 619 in the 111th Congress) and/or other measures (including FDA regulations) should be adopted to end use of antibiotics for growth promotion, feed efficiency, and routine disease prevention purposes in animal agriculture and to ensure that these precious drugs are being used wisely in all settings. All use of antibiotics in animal agriculture should be carried out under the supervision of a veterinarian using a prescription or other practical mechanism, and over-the-counter purchases must be prohibited.
FDA Guidance #152 (“Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern”) should be revised to re-evaluate the current ranking of drugs according to their importance to human medicine. The guidance's scope should be broadened beyond enteric (food-borne) pathogens.
FDA must complete and publish safety reviews of those antibiotics of importance to human medicine that are approved for non-therapeutic purposes in food-producing animals, examining their role in the selection and dissemination of antibiotic-resistant food-borne pathogens.
CONCLUSIONS
The availability of effective antibiotics is not a “lifestyle” issue, and the lack of availability of these agents is not theoretical. Society worldwide is facing a public health crisis due to stagnation in the antibiotic drug pipeline combined with rapidly spreading, deadly antibiotic-resistant pathogens. The lack of effective antibiotics already is resulting in deaths and maiming of patients and the problem will only continue to worsen until Congress and the Administration act. The time for debate about the problem has passed. Immediate action is critically needed now.
INTRODUCTION
In 2000, Nobel Laureate Dr. Joshua Lederberg wrote in the journal Science that “the future of humanity and microbes will likely evolve as episodes…of our wits versus their genes” [13]. In only 11 years since Dr. Lederberg wrote these prescient words, the world has witnessed an enormous expansion of infections resistant to antibacterial agents (“antibiotics”). For example, methicillin-resistant Staphylococcus aureus (MRSA) infections, which were traditionally only noted among hospitalized patients, have become endemic in community settings [14–19]. Antibiotic-resistant Gram-negative bacteria (GNB) also have spread widely through US and global health care systems. Increasingly they have become resistant to all antibiotics available for treatment: i.e., pan-drug resistant (PDR). Examples of these PDR GNB organisms include Acinetobacter baumannii [20–29], carbapenemase-producing Klebsiella pneumoniae [30, 31], and Pseudomonas aeruginosa [28, 29, 32, 33]. Extended-spectrum beta lactamase (ESBL)-producing Enterobacteriaceae (e.g., Escherichia coli and Enterobacter spp.), often resistant to all orally administered antibiotics, have spread through health care systems and more recently into communities [34–44]. Most recently, a new antibiotic resistance mechanism (New Delhi metallo-β-lactamase 1 or NDM1) emerged in India and spread to communities in the United Kingdom [45] and the US [44]. NDM1 E. coli and Klebsiella strains are resistant to all antibiotics except tigecycline or colistin, and in some cases to these drugs as well [44, 45].
Collectively, highly problematic antibiotic-resistant organisms are summarized by the ESKAPE mnemonic: Enterococcus, Staphylococcus, Klebsiella, Acinetobacter, Pseudomonas, and ESBL (Enterobacter and E. coli). ESKAPE indicates that these bacteria have developed defenses that permit them to escape the actions of available, effective therapies. The ESKAPE pathogens are currently the most important causes of the antibiotic resistance crisis in the US and other developed countries [11, 46]. Such pathogens also are spreading through developing countries, which already are experiencing significant public health problems from extreme drug-resistant (XDR) or PDR Mycobacterium tuberculosis (TB). Collectively, disease caused by the ESKAPE pathogens, TB, and other highly problematic antibiotic-resistant bacterial pathogens, including hypervirulent and fluoroquinolone-resistant Clostridium difficile, and multi-drug resistant (MDR) Streptococcus pneumoniae and Neisseria gonorrhoeae, result in enormous morbidity, mortality, and health care expense in the US and throughout the world [2, 3, 6, 9, 10, 47–49].
Just one organism, methicillin-resistant Staphylococcus aureus (MRSA), kills more Americans every year (∼19,000) than emphysema, HIV/AIDS, Parkinson's disease, and homicide combined [2]. Almost 2 million Americans per year develop hospital-acquired infections (HAIs), resulting in 99,000 deaths [3], the vast majority of which are due to antibiotic-resistant pathogens. Indeed, two common HAIs alone (sepsis and pneumonia) killed nearly 50,000 Americans and cost the US healthcare system more than $8 billion in 2006 [4]. In a recent survey, approximately half of patients in more than 1,000 intensive care units in 75 countries suffered from an infection, and infected patients had twice the risk of dying in the hospital as uninfected patients [5]. Based on studies of the costs of infections caused by antibiotic-resistant pathogens versus antibiotic-susceptible pathogens [6–8], the annual cost to the US health care system of antibiotic-resistant infections is $21 billion to $34 billion and more than 8 million additional hospital days. Antimicrobial resistance was recently recognized as one of the greatest threats to human health on the planet [1], so much so that the World Health Organization (WHO) has proclaimed antimicrobial resistance the focus of World Health Day (April 7) 2011.
The problem of antimicrobial resistance is not specific to bacteria—medically important viruses (e.g., HIV, influenza), fungi (e.g., Candida, Aspergillus), and parasites (e.g., malaria) also develop antimicrobial resistance. However, a unique convergence of overuse and misuse of antibiotics, the remarkable genetic plasticity of bacteria, the acquisition of resistant bacterial infections in both community and hospital settings, and a market failure of antibiotic development has created an enormous public health concern regarding antibiotic resistance in bacteria. For this reason, antibiotic resistance is the primary focus of this policy paper.
Paradoxically, concomitant with the rise of antibiotic-resistant bacteria, US Food and Drug Administration (FDA) approval of critically needed new antibiotics has dramatically slowed (Figure 1) [9–12]. Of great significance, nearly all major pharmaceutical companies have withdrawn from or greatly downsized their antibiotic research and development (R&D) programs over the past two decades, and the egress from the market is actively continuing. The combined threat of increasing numbers of drug-resistant bacteria and the diminishing antibiotic pipeline places us at risk not only from health care-associated and community-acquired infections, but from threats (bioterrorism, pandemics) that could affect our nation’s security.
To reverse this trajectory and call policymakers’ attention to the growing crisis, IDSA launched its Bad Bugs, No Drugs advocacy campaign in 2004 [49]. Unfortunately, antibiotic resistance and the waning approvals of new antibiotics have only worsened since 2004. Since then, IDSA has undertaken many clinical, scientific and public policy activities, including: 1) published practice guidelines on the development of antimicrobial stewardship programs for hospitals [50] and on the prevention and management of C. difficile infections [51], along with the Society for Healthcare Epidemiology of America; 2) co-sponsored, along with FDA's Center for Drug Evaluation and Research (CDER), workshops on the development of new antibiotics for Community-Acquired Bacterial Pneumonia (CABP) [52, 53] and Hospital-Acquired Bacterial Pneumonia/Ventilator-Associated Bacterial Pneumonia (HABP/VABP) [54]; 3) published data in support of new antibiotic development for skin and soft tissue infections [55]; 4) co-sponsored, along with FDA's Center for Devices and Radiological Health (CDRH), a workshop on diagnostics for respiratory infections; 5) proposed new research protocols on optimizing antibiotic effectiveness and antimicrobial stewardship for federal support; 6) testified at FDA Anti-Infective Drug Advisory Committee meetings and other FDA hearings and at Congressional briefings and hearings; 7) supported the Institute of Medicine Forum on Microbial Threats’ 2010 Workshop on Antimicrobial Resistance [56]; and 8) assisted members of Congress in drafting legislation introduced in the 110th and 111th sessions of Congress designed to directly address antimicrobial resistance issues [9].
In 2010, in recognition of the need for creative, new ideas to address the antibiotic pipeline problem and a measurable goal by which to gauge progress, IDSA launched the “10 × ’20 initiative” [57]. The 10 × ’20 initiative calls for the development of 10 novel, safe and effective, systemic antibiotics by 2020. Forty-five public health organizations and professional societies across the spectrum of medicine, including the American Medical Association and American Academy of Pediatrics, have endorsed the 10 × ’20 initiative [58]. Aside from the short term goal of increasing availability of critically needed new antibiotics, the underlying theme of 10 × ’20—akin to Dr. Lederberg's warning about the future of human-microbe relations—is the need to establish an infrastructure that recognizes and responds to ongoing changes in antibiotic resistance and facilitates antibiotic R&D in perpetuity.
In August 2010, the Administration, via the US Department of Health and Human Services (HHS), announced a broad plan as part of HHS's Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Review: Transforming the Enterprise to Meet Long-Range National Needs [59]. The plan focused on advancing the development of new countermeasures, including antibiotics, to address public health emergencies and national security threats. The Administration should be commended for this effort and its support, but antibiotics are in stiff competition for the limited resources necessary to support all aspects of the plan, and Congress has yet to advance new funding to support the initiative.
Members of the US Congress have begun to respond in other ways to the highly complex, interrelated public health and antibiotic research and pipeline problems. Although some legislation has been enacted over the past decade, more substantive legislation is needed. Recently, legislation has been introduced containing incentives to spur industry to develop new, priority antibiotics (and related diagnostics) and to press FDA to resolve multiple disincentives that are contributing to the market failure of antibiotic development (H.R. 6331, the Generating Antibiotic Incentives Now [GAIN] Act, introduced by Rep. Phil Gingrey [R GA-11], an obstetrician, in the 111th Congress). Other legislation has been introduced to strengthen the federal response to antimicrobial resistance, and antibiotic resistance in particular, through better coordination of efforts and enhanced surveillance, research, and prevention and control efforts (H.R. 2400, the Strategies to Address Antimicrobial Resistance [STAAR] Act, introduced by Rep. Jim Matheson [D UT-2] in the 111th Congress). Finally, legislation has been introduced to prevent non-judicious uses of antibiotics in animal agriculture (H.R. 1549/S. 619, the Preservation of Antibiotics for Medical Treatment Act [PAMTA], introduced by Rep. Louise Slaughter [D NY-28] and the late Sen. Edward Kennedy [D-MA] in the 111th Congress).
In July 2010, the Senate Appropriation Committee voiced its concern (see Appendix B) calling antibacterial resistance and the resulting failure of antimicrobial therapies in humans “a mounting public health concern,” and highlighting the “unresolved scientific issues regarding clinical development in the antibacterial drug arena, which has been identified as a serious impediment to new antibacterial development” [60]). The Senate Committee directed the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), HHS’ Office of the Assistant Secretary for Preparedness and Response (ASPR), and ASPR's Biomedical Advanced Research and Development Authority (BARDA) to strengthen funding and make more seamless efforts to develop new antibiotics, particularly to treat problematic GNB, as well as much needed diagnostics. FDA was urged to issue clinical trial guidance documents that provide a clear approval pathway to drug companies. Further, FDA was asked to identify ways to promote the development and/or appropriate use of priority antibiotics for humans as current market incentives are inadequate. The agency was asked to report back to the Senate Committee on each of these requests by December 2010 [61]. As of March 2011, this has not yet occurred.
To continue momentum in this area, advance a broad public policy response, and accelerate significant new investment in research to address antibiotic resistance, on July 26–27, 2010, the IDSA, FDA, and NIAID co-sponsored a public workshop on “Antibacterial Resistance and Diagnostic Device and Drug Development Research for Bacterial Diseases.” The workshop's goals were:
“To discuss the scientific data addressing key issues in the following areas: the scale of the current bacterial resistance problem including extent, trajectory, and cost; the science and mechanisms of bacterial resistance; the science of development of rapid diagnostic devices; the science of antibacterial drug development.” (Videos, slides, and transcripts of the workshop are available on the IDSA website at http://www.idsociety.org/arworkshop.html.)
This policy paper summarizes IDSA's recommendations about how to address the discovery and development of new antibiotics, prevention of antibiotic resistance, and the development of rapid diagnostics that will enable more directed therapy. IDSA's goal is to represent the best interests of patients and health care professionals by recommending public policy strategies and research activities to address antibiotic resistance and save lives. These recommendations are derived from discussions and conferences (including the FDA/NIAID/IDSA July 2010 workshop) encompassing experts from academia, industry, and government in the fields of antimicrobial resistance, pathogen diagnosis, and drug development.
IDSA's eight broad areas of focus for its policy recommendations include the need to: 1) adopt economic incentives and support other collaborative mechanisms to address the antibiotic market failure by rekindling antibiotic R&D; 2) create new regulatory approaches to facilitate the clinical development of antimicrobials; 3) more effectively coordinate federal antimicrobial resistance efforts; 4) enhance antimicrobial resistance surveillance and data collection; 5) strengthen activities to prevent and control antimicrobial resistance; 6) strengthen investments in antimicrobial-focused research; 7) strengthen investment in development and utilization of rapid molecular diagnostics for infectious diseases; and 8) eliminate non-judicious antibiotic use in agriculture and other settings. Specific recommendations for Congress related to legislative action and funding needs are summarized in Tables 1 and 2, respectively.
IDSA RECOMMENDS:
I. Adoption of Economic Incentives and Support for Other Collaborative Mechanisms to Address the Market Failure of Antibiotics
1. Statutorily-defined Push/Pull Incentives are Urgently Needed to Correct the Current Market Failure and to Motivate Companies to Reengage in Antibiotic (and Related Diagnostics) R&D.
Within pharmaceutical companies’ internal deliberations about how best to invest R&D resources, antibiotics are at a distinct disadvantage compared with most other drug categories. The return-on-investment potential (known as Net Present Value [NPV] in industry parlance) of antibiotics, which are normally taken for one to two weeks, cannot compete with drugs that treat chronic diseases, which are taken for months or years [9, 62–67]. A combination of factors has resulted in a market failure of new antibiotic development, including the ability of antibiotics to cure most infections in just a few days, antibiotic resistance which makes the drugs less effective over time, and deliberate and essential measures taken by physicians to limit antibiotics’ use to protect their effectiveness over time [9, 11, 48, 62, 67].
IDSA and others have extensively published on the need for statutorily-defined economic incentives to improve the return-on-investment/NPV calculation of antibiotics and make them more competitive with other therapeutic products as candidates for development [47–49, 57, 62]. To fix the broken antibiotic pipeline and create a sustainable R&D enterprise, it is necessary to determine the right combination of economic incentives (“push” and “pull” mechanisms) to entice companies to reengage in antibiotic R&D [9, 10, 48, 66]. Examples of push incentives are grants, contracts, and tax credits. Examples of the pull incentives are guaranteed markets, liability protection, patent extensions, data and market exclusivity, and prizes.
Such incentives are the focus of important bipartisan legislation, the GAIN Act (H.R. 6331 in the 111th Congress), which was first introduced in the US House of Representatives on September 29, 2010. The GAIN Act provides an excellent starting point for discussing the right combination of incentives needed to jumpstart novel antibiotic (and related diagnostic) R&D. As discussed in recommendation VII, the availability and clinical application of diagnostic tests are incredibly important to appropriately treat antibiotic-resistant infections and to support new antibiotic R&D. Recommendation VII.2 includes specific economic incentives targeting diagnostic development for Congressional leaders’ consideration. The GAIN Act's co-sponsors should be commended for their efforts to date. IDSA is working with them to strengthen the bill for its reintroduction and enactment in the 112th Congress. As deliberations move forward, Congressional leaders, including the GAIN Act co-sponsors, should discuss incentives with representatives of the European Commission, as the European Union has set a December 2011 deadline for evaluating and developing an action plan of concrete incentives to spur antibiotic R&D [68].
2. New Public-private Partnerships Should be Established and Existing Government-Supported Collaborations Strengthened to Supplement (But Not Replace) Traditional Industry R&D for Critically Needed Antimicrobial Drugs.
To address infections caused by MDR/XDR/PDR bacteria, for which market challenges are extreme, new, non-profit public-private partnerships (PPPs) should be established and government-supported collaborative programs (ASPR's BARDA and proposed independent strategic investment firm, and NIAID-supported Cooperative Research and Development Agreements [CRADA]) should be further strengthened. The intent of such public-private collaborations is to advance the development of promising lead compounds toward approved products.
Since a PPP focused on antibiotic development would not be profit-driven, it could focus on developing critically needed drugs for indications in which current markets are very small (e.g., drugs to treat XDR/PDR Acinetobacter and Klebsiella). Removing profit motive from the equation also will help to limit the marketing of “priority” antibiotics to more serious and life-threatening indications. Focusing sponsor's marketing programs will enhance stewardship (see recommendation V.2) of these drugs and will prolong their effectiveness. Thus, the advantage of the PPP is that it could merge antibiotic conservation efforts with new antibiotic R&D efforts. Examples of successful PPPs that are already underway targeting tuberculosis drug development and resistance include the World Health Organization's Stop TB Partnership [69], the Global Alliance for TB Drug Development [70], and the recently announced Critical Path to TB Drug Regimens (CPTR) [71].
It is important to note that PPPs are not meant to replace the essential activities of private companies in drug discovery and development. Rather, PPPs are intended to complement efforts to reinvigorate market-driven, for-profit antibiotic development. Private companies’ R&D activities must still be strengthened through powerful economic incentives, and additional companies must be lured back into this field. We cannot rely on an unproven PPP model to fix the current situation.
PPPs primarily target larger companies for which risk and insufficient return on investment are the primary barriers to antibiotic R&D, not resource availability. Government-supported collaborative programs, on the other hand, provide direct funding to companies to assist them in bridging what has come to be known as the “valley of death,” i.e., the financial chasm between conducting phase I clinical drug trials and much more expensive phase II clinical trials. Such programs include BARDA, established by Congress in 2006 as part of the Pandemic and All-Hazards Preparedness Act, and the independent strategic investment firm announced in August 2010 as part of HHS’ PHEMCE review [59]. BARDA is intended to provide an integrated, systematic approach to the development and purchase of the necessary vaccines, drugs, therapies, and diagnostic tools for public health medical emergencies with the potential to impact national security. The strategic investment firm is intended to spur the development of new antimicrobial drugs and other high priority products by sharing the risk of development with companies and will help these companies leverage additional private investment in these important products.
In contrast to the PPP model, BARDA and the strategic investment firm likely will be most attractive to small and mid-sized companies, for which resource availability is a primary barrier to completion of clinical development. Congress’ support for BARDA's antibiotic efforts and for establishing and funding the strategic investment firm are essential. BARDA and the strategic investment firm will fund companies to “push” promising products from pre-clinical into clinical trials. BARDA then can use larger amounts of funding to “pull” critically needed products across the gap between phase I and phase II clinical trials.
The PPP and government agency support can be funded by both public monies and private capital. Government funding could be provided by the agency in the form of grants or contracts, including through matching funds, for example at a 2:1 ratio of private capital from the applicant company to government funding. With respect to public monies, IDSA proposes creation of an Antimicrobial Innovation and Conservation (AIC) Fee. The AIC Fee would be a flat fee (e.g., ∼$3 per daily dose, inflated by the consumer price index annually) charged against the wholesale purchase of every daily dose unit of antibiotics (both branded and generic) in the US, including for human, animal and plant agriculture, and aquaculture use. The fee would be paid by the dispensing entity (e.g., pharmacy, animal feed mill, aquaculture company, etc.) at the time of wholesale purchase from the supplier.
The rationale for such a fee is that effective antibiotics represent a “shared societal benefit,” and every antibiotic manufacturer, prescriber, and user must share the responsibility to maintain this benefit. Antibiotic resistance resulting from antibiotic use (both appropriate and inappropriate) is an example of the “tragedy of the commons” [72]. A prescription may help the individual patient, plant, or animal, but such use also causes collective erosion of the benefit (effectiveness of antibiotics) for society as a whole. Analogously, use of highways by a vehicle has a cost to all users. Tolls (and differential rates) are means to have users pay their fair share of societal costs for establishing and maintaining a shared benefit. Because of the emergence of resistance, use of antibiotics differs from use of all other drugs that affect only the individual patients taking them. Hence, an AIC Fee would be charged to maintain the “shared societal benefit” of effective antibiotic therapy. Obviously, safeguards need to be incorporated into the AIC Fee structure to ensure that any costs passed on to consumers will not negatively impact vulnerable populations’ access to these important drugs.
As described in recommendation V.6, 25% of the AIC Fee would be allocated to a CDC antimicrobial stewardship fund. The remaining 75% of the AIC Fee could be allocated to a trust fund established under the management of ASPR within HHS, to support the development of promising, high priority candidate antibiotics. This can occur through BARDA, the strategic investment firm, and a PPP. Other sources of public funding include appropriations and transfer of other federal agency funds.
Government agency funds would be augmented by allocation of matching private capital from application companies. The PPP would raise private capital through user fees, license payments, royalty sharing, and/or other methods. The PPP could develop its own drugs internally, and also would partner with industry to develop drugs. Industry would use the PPP to develop promising molecules with very limited market potential, such as a drug that could only target bacteria causing relatively small numbers of infections per year, or for drugs with high risk but high potential payoff if development was successful. In such cases, industry would license the drug to the PPP, which then would take charge of developing the molecule from pre-clinical through phase II trials, with a plan to partner back with the licensing company to co-develop for phase III trials, if the drug made it that far. If the drug was successful in phase III trials, the partnering company would manufacture, distribute, and market the drug and would share royalties with the PPP based on pre-agreed terms. If after completion of phase II trials, the originating company decided not to participate in phase III trials, the PPP would be free to seek alternate private partners to complete clinical development, manufacturing, marketing, and distribution of the drug. The PPP also could raise private money in other ways.
Federal funding agencies, such as BARDA, and the proposed federal strategic investment firm must have the capacity and dedicated funding to create financial grants, contracts, venture capital investments, and partnerships with industry to stimulate the discovery and development of antibiotic and related diagnostics. In addition to supporting an annual commitment of $500 million at NIAID to strengthen the agency's antibiotic resistance and antibiotic discovery research portfolios (see recommendation VI.8), IDSA calls for: 1) an annual allocation of at least $1.7 billion of multi-year funding to BARDA to facilitate development of therapeutics, diagnostics, vaccines, and other technologies, including new antibiotics and diagnostics to treat and detect infections caused by ESKAPE and other serious and life-threatening pathogens; and 2) at least $200 million to support the new strategic investment firm's antibiotic venture capital investments.
Such funding would facilitate creation of entire drug and diagnostics portfolios within sponsors, evolving away from funding a single program that is high risk for the funder and provides poor flexibility for the company.
3. Value-based Reimbursement Strategies that Encourage Antibiotics and Related Diagnostics Development Must be Pursued.
Adopting reimbursement rates that are more aligned with antibiotics’ and related diagnostics’ true value is another critical way to stimulate new antibiotic and rapid diagnostics development. Antibiotics, in particular, often are undervalued when one considers the benefits they bring in terms of numbers of lives saved, increased disability-adjusted life years (DALYs), increased productivity, and reduced health care costs [66, 73, 74]. Policymakers should rethink current reimbursement strategies to reward sponsors of innovative products, particularly those products that address areas of unmet medical need. Specific criteria to consider in appropriately valuing an antibiotic is whether the drug possesses a broader spectrum of antibacterial activity or a better safety profile than existing drugs, or whether it treats highly drug-resistant pathogens or employs a new mechanism of action. Also to consider are whether the drug was approved based on superiority trials and the drugs’ potential for reducing health care expenditures (e.g., lengths of hospital stays, etc.).
Finally, novel reimbursement strategies must be considered that strongly reward antibiotic drug pioneers who agree to forgo broad (and more profitable) FDA-approved label indications for indications that narrowly target high priority public health needs (see related recommendation V.8). Such strategies could help to avoid rapid depletion of a priority antibiotic's effectiveness by limiting its overall marketing potential.
4. A Panel of Experts Should be Established to Document and Regularly Revise a List of Priority Pathogens or Infections Against Which Incentives Should be Targeted.
A panel of experts, envisioned as a qualifying antimicrobial product committee (QAPC), comprised of representatives from government agencies such as FDA, CDC, NIAID, BARDA and ASPR, as well as academic or private infectious diseases specialists and public health experts, should be established under the GAIN Act or similar legislation to document and regularly revise a list of those priority pathogens or infections that have created or likely will create an area of unmet medical need and toward which adopted economic incentives should be targeted. The QAPC perhaps could be established as a subgroup of the STAAR Act's advisory board (see recommendation III) to streamline efforts.
II. New Regulatory Approaches to Facilitate Antimicrobial Development and Approval
1. Clear and Feasible Regulatory Guidelines on Clinical Trial Designs are Urgently Needed to Enable Approvals of New Antibiotics and Other Antimicrobials.
Clinical development of promising antimicrobial agents cannot proceed in the absence of clarity regarding the requirements for licensure of the drugs. Considering together the economic disincentives antibiotic developers currently are facing (see recommendation I) and the lack of a clear regulatory approval pathway for these drugs over the past decade, one can easily understand why antibiotic approvals have decreased so markedly and companies have withdrawn from antibiotic R&D to pursue more lucrative areas of drug development. To correct this imbalance, FDA must quickly assure clear and feasible regulatory pathways for the development of antibiotics by issuing clinical trial guidance documents for industry that contain designs the agency will find acceptable. Such guidelines should recognize the importance of making pivotal studies clinically relevant, and should strike a balance between clinical reality and statistical desirability. Guidance is needed both for non-inferiority and superiority studies (see Appendix C for an overview on both types of trials); currently no clear and feasible path exists for conduct of superiority trials. In setting regulatory guidance for antibiotic development, FDA must balance the public health risks of approving a potentially less effective drug with the risk of having no new, critically needed antibiotics available to treat patients infected with resistant pathogens.
2. Already Conservative Estimates of Antimicrobial Efficacy Relative to Placebo/no Therapy Should Not be Further “Discounted” When Setting Requirements for Non-inferiority Margins for Clinical Trials, as Discounting Results in Excessively Large Trial Requirements.
FDA should cease the practice of “discounting” already conservative estimates of antibiotic efficacy when setting requirements for non-inferiority margins, and hence trial size (i.e., the number of patients who have to be studied) for pivotal trials [55, 75]. The purpose of discounting is to account for limitations in the quality of historical data used to provide an estimate of how effective antibiotic therapy is versus placebo or no therapy. However, when the estimate of antibiotic effect size is already highly conservative, discounting results in an overly conservative, arbitrary mathematical calculation of non-inferiority margins [62, 75]. There is no logical basis for selecting how much of antibiotic efficacy to first “discount” and second “preserve” when setting non-inferiority margins. As a result, discounting results in arbitrary, subjective, and unjustified requirements to conduct very large clinical trials which are not feasible to execute. Such requirements have greatly contributed to the lack of new antibiotic R&D and the egress of industry from the antibiotic market [9, 12, 55, 62–64, 75, 76].
3. The Primary Issue in Justifying the Non-inferiority Margin for a Clinical Trial is Determining How Much of the Clinical Benefit of Antimicrobial Therapy Must be Preserved, Which Should be Based Upon an Assessment of the Relative Merits of the Specific Experimental Drug Versus Currently Available Therapy.
The issue of how much of antimicrobial efficacy to “preserve” when setting non-inferiority margins is not a statistical question, it is a clinical question. Qualified experts in clinical medicine, who care for patients and know the current challenges and needs for improving treatment, possess the expertise required to define how much of a potential decrease in treatment benefit can be justified as a trade-off against the critical need to develop new efficacious and safe drugs and have them available for clinical use.
The treatment effect of antibiotic therapy for serious and life-threatening infections is very large (Table 3). Thus, for clinical trials of new antibiotics, the primary issue in justifying the non-inferiority margin is determining how much of that clinical benefit must be preserved. This decision should be justified based upon an assessment of the relative merits of the specific experimental drug. Regulators and physicians, as experts in public health needs, should be willing to accept a small increase in statistical imprecision regarding treatment effect size in return for facilitating development of critically needed new drugs, particularly if the experimental drug offers other substantive advantages over existing therapy. Factors to be considered include relative advantages of the experimental drug versus existing agents in antibiotic spectrum of activity (particularly activity against XDR and PDR pathogens), safety, or dosing, or the advantage of a novel mechanism of action. Wider non-inferiority margins should be tolerated for drugs with substantive advantages in these areas, whereas narrower margins should be required for drugs with little to no advantages in these areas.
Table 3.
Antibiotic-Mediated Mortality Reductions for Specific Infections
| Disease | Pre-Antibiotic Mortality Rate | Antibiotic Mortality Rate | Change in Mortality |
| Community Pneumonia [53] | ∼ 23% | ∼ 7% | −16% |
| Nosocomial Pneumonia [54] | ∼ 60% | ∼ 30% | −30% |
| Bacterial Endocarditis [112–115] | ∼ 100% | ∼ 25% | −75% |
| Bacterial Meningitis [116–117] | >80% | <20% | −60% |
| Skin Infection [55, 118] | ∼ 11% | <.5% | −10% |
| By comparison, treatment of myocardial infarction (i.e., heart attack) with aspirin or streptokinase [119] | −3% | ||
In short, as new antibiotics are critically needed, we must balance feasibility of conducting studies (and the resultant public health benefit of facilitating approval of effective new antibiotics) against a desire to narrow the non-inferiority margin. While patients may be harmed if less effective drugs are allowed to reach the market, they also may be harmed if they have an infection for which no effective antibiotics have been developed. Furthermore, if the criteria for study conduct are so strict that it is infeasible to enroll meaningful numbers of patients in the US, or the trial results are not generalizable post-approval, we run the risk that the observed safety and efficacy of the drug in its pivotal studies will not be informative regarding the safety and efficacy of the drug for patients in the US who are exposed to the drug. The key is to create a regulatory path that balances these competing risks.
4. Regulatory Guidance is Needed to Create New Pathways to Facilitate Approval of Antibiotics.
Development of drugs for the treatment of infections caused by specific, problematic pathogens (e.g., ESKAPE pathogens) is stymied by: 1) lack of guidance on such development programs; 2) small market sizes, which provide insufficient financial incentive for companies to move into this area; and 3) the difficulty of identifying and enrolling patients with such infections.
Organism-specific studies, in which patients with multiple disease types are enrolled in a single study, similar to the path taken for studies of invasive fungal infections, can help mitigate these concerns. For example, the enrollment of patients with infections caused by resistant GNB causing a variety of serious or life-threatening infections, rather than a single type of infection, would greatly expand the target population for enrollment, making it more feasible to enroll the required number of subjects in studies. Furthermore, the market size of the resulting indication would be larger since multiple diseases would be studied from one trial, increasing the financial return on incentive for companies. Yet, all of the infections would be caused by antibiotic-resistant GNB, so marketing of the drug would be concordant with public health need, and the drug would not be wasted for treating less resistant organisms. For these reasons, regulatory guidance should be made available on conduct of organism-specific studies.
Regulatory guidance also is needed for other novel antibiotic studies, including acceptable design of superiority clinical trials and/or the use of historically controlled clinical trials. Finally, regulatory guidance is needed that permits FDA approval based on a relatively small clinical sample size (<100 patients) for infections caused by XDR/PDR GNB that occur in critically ill patients as well as to address future, potential areas of urgent unmet medical need.
Members of Congress (including the GAIN Act co-sponsors) should discuss with FDA officials whether expansion of the agency's existing statutory authority is needed to allow for conditional approvals and powerful post-approval approaches (e.g., Risk Evaluation Management Strategies [REMS]-like safeguards) for novel antibiotics that address urgent unmet medical needs (e.g., highly antibiotic-resistant XDR/PDR GNB). Alternatively, other statutory changes should be identified that agency officials agree would speed the development and approval of high priority, novel antibiotics. The GAIN Act already contains several promising ideas (e.g., fast-track approval, priority review, deadlines placed on clinical trial guidance development), but additional discussion with FDA is warranted specific to areas of urgent unmet medical needs.
5. Regulatory Science Must Continue to be Advanced and Developed to Make Clinical Trial Designs Feasible, Clinically Relevant, and Scientifically Rigorous.
IDSA strongly supports the collaborative regulatory science effort recently initiated by FDA, NIAID, and the Foundation of the NIH (FNIH) along with industry, academia, and IDSA to examine surrogate endpoints for antibiotic clinical trials, as well as pharmacokinetic/pharmacodynamic (PK/PD) parameters that forecast optimal antibiotic dosing. Such antibiotic-focused R&D activities should be encouraged and further expanded. The Reagan-Udall Foundation, a public-private partnership established in 2007 between FDA and industry, provides another avenue for potential support. However, moving these critical activities forward will require dedicated funding from the federal government, industry, and other funding organizations.
To reiterate, FDA must balance the risk of approving a potentially less effective drug with the benefit of making a potentially life-saving therapy available sooner for patients who desperately need it. Therefore, FDA should consider alternatives or surrogates to traditional clinical trial endpoints (for example, other than survival) that are acceptable for regulatory approval as evidence of clinical benefit to patients. The use of novel statistical approaches, such as Bayesian methods, as a means to increase efficiency of clinical trials of antibiotic therapy should be encouraged. FDA should consider the pre-test probability of a drug's efficacy based on the totality of pre-clinical and phase I and II clinical trial data when setting parameters for planned pivotal phase III clinical trials, and when interpreting results of those trials.
FDA has been underfunded and understaffed to meet its many critical functions. IDSA calls for an additional $40 million annually for FDA's antibiotic resistance and antibiotic drug review programs. Specifically, IDSA supports an additional $15 million annually to allow the agency to hire more staff to develop much-needed clinical trial guidance documents and to fund Critical Path initiatives specific to antibiotic drug development. IDSA also requests more than $25 million annually to support a strong focus on new antibiotics R&D within FDA's new regulatory science initiative. This initiative involves the development and use of new tools, standards and approaches to more efficiently develop products and more effectively evaluate product safety, efficacy, and quality.
III. Greater Coordination of Relevant Federal Agencies’ Efforts
1. The STAAR Act (H.R. 2400 in the 111th Congress) Should be Further Strengthened, as Outlined in this Paper, and Enacted.
Federal agencies with programs related to antibiotic resistance, stewardship, and product R&D include: HHS's Centers for Disease Control and Prevention (CDC), FDA, NIH, BARDA, Centers for Medicare and Medicaid Services (CMS), Agency for Healthcare Research and Quality (AHRQ), Health Resources Services Administration (HRSA), and the US Departments of Agriculture (USDA), Defense (DoD), Veterans Affairs, Homeland Security, State (including US Agency for International Development), and Education. Currently, there is inadequate coordination of activities among these federal agencies regarding antimicrobial resistance efforts. Further, there is woefully insufficient funding dedicated to federal antimicrobial resistance efforts and to addressing the market failure of antibiotics.
An Interagency Task Force on Antimicrobial Resistance, co-chaired by CDC, FDA and NIAID, was authorized under Section 319E (51) of the Public Health Service Act, but this authorization expired September 30, 2006. Although many dedicated federal officials sit on the interagency task force, no centralized office exists to facilitate the coordination of the task force activities, prioritize the federal response, establish benchmarks by which to measure progress, and provide a platform for ongoing discussion and action across agencies. There also is no established process for engaging outside experts to provide input into federal policymaking in this area. As a result, the task force has had limited accomplishments, lacks sufficient public transparency of its activities, and has failed to carry out most of the 84 action elements, including 13 key action items, in the original Action Plan of 2001. An effort to update the Action Plan, initiated in December 2007, has been delayed; despite promises to publish a draft updated plan in 2008, 2009, and 2010, none has been published to date. Federal agencies need a coordinating mechanism to determine and continue to update priorities in a timely manner and to ensure the coordination of goals and activities in the federal response to antimicrobial resistance.
The STAAR Act will bring coordination, vitality, and accountability to federal efforts through the establishment of an Antimicrobial Resistance Office (ARO) within the HHS's Office of the Secretary, and by the reauthorization of the interagency task force. The Director of ARO will serve as the director of the existing interagency task force. The STAAR Act also would establish a Public Health Antimicrobial Advisory Board (PHAAB) composed of infectious diseases and public health experts. This panel will provide much-needed advice to the ARO director and interagency task force about antimicrobial resistance on an ongoing basis.
Prompt passage of the STAAR Act will enable a coordinated, effective response that spans multiple federal departments and agencies and allows them to work together to mitigate inappropriate use of antibiotics, strengthen research efforts, and enhance federal surveillance, prevention and control, and data collection efforts.
2. Sufficient Funding must be Appropriated for the Activities of the Existing Interagency Task Force as Well as for the ARO and PHAAB Once They are Established.
Congressional appropriators should sufficiently fund the activities of the existing interagency task force as well as the ARO and PHAAB once they are established. Specifically, IDSA recommends $30 million in funding be provided to HHS in fiscal (FY) 2012 for the work of the task force and that this funding be increased to $44 million in fiscal year FY2013, and $80 million in FY2014 to support the task force, the ARO, and PHAAB.
IV. Enhancement of Antimicrobial Resistance Surveillance Systems
1. National Data on Antimicrobial Resistance Rates, Linked to Clinical Outcomes, Should be Gathered in Real Time and Made Publicly Available on a Regular Basis.
Currently, antimicrobial resistance rates are made public only sporadically. The STAAR Act includes provisions for strengthening surveillance on a national level for antimicrobial resistance and antimicrobial use. The systematic collection of data on antimicrobial, and particularly antibiotic, resistance is necessary for a variety of infections and pathogens. Specific data on type and quantity of antimicrobials used throughout the spectrum of patient care are needed to define the overuse and misuse of antimicrobial agents; only by understanding the scope and severity of the problem can interventions be developed to reverse the problem.
The European Union (EU) has successfully implemented systems across all 27 member countries to track antimicrobial resistance trends for public health purposes and to collect antimicrobial use data. The European Antimicrobial Resistance Surveillance Network (EARS-Net) [77] and the European Surveillance Antimicrobial Consumption (ESAC) [78], respectively, are funded by the European Centre for Disease Prevention and Control (ECDC). No system comparable to EARS-Net and ESAC exists in the United States. Just as in Europe, the capacity to analyze and disseminate such resistance trends and antibiotic use data must become a cornerstone of the US health care system.
IDSA recommends that national antimicrobial resistance rates be published annually or biannually. Furthermore, akin to the comprehensive CMS databases on health economics that are posted online to facilitate health economics research, the full linked database of susceptibility profiles, molecular epidemiology, and clinical outcomes should be available via the internet for research and public policy purposes.
2. A Federally Funded Network of Sentinel Sites That Collects Both Clinical Specimens and Clinical Data is Necessary to Detect and Evaluate Rapidly Emerging Resistance in a Variety of Organisms and to Develop, Implement, and Evaluate Prevention Strategies.
To respond to current resistance trends, and to plan for emerging trends, it is necessary to understand the frequency of resistance to antimicrobial agents, and particularly antibiotics, among medically important pathogens across geographical areas. Data must be current to ensure correct intervention decisions. In addition, specimen collection is needed for the evaluation of emerging resistance mechanisms in pathogens of clinical importance. In short, an integrated network of sentinel sites with diverse geographic representation is required.
The STAAR Act requires the CDC and NIAID to establish and maintain a network of specialized sites: the Antimicrobial Resistance Surveillance and Research Network (ARSRN), to ensure ongoing accurate data and pathogen collection as well as to conduct relevant research (see recommendation VI). ARSRN sites would track cultures obtained from both inpatients and outpatients, assess resistance patterns, and report in real-time to a central antimicrobial resistance data management center. The ARSRN, and its component sites, also would conduct studies to assess resistance risks, develop interventions specific to those documented risks, and implement strategies, in collaboration with the CDC and NIAID, designed to mitigate the impact of resistant pathogens.
Current national surveillance systems lack the flexibility to rapidly establish surveillance for newly emerging resistant pathogens, collect specimens, identify the mechanisms of resistance in each pathogen, and identify the risk factors associated with acquisition of the pathogen by patients. While current national data collection efforts provide useful data on a variety of HAIs and on resistance rates of selected invasive pathogens, the US does not rapidly monitor and assess newly emerging resistance trends for many pathogens of medical importance.
For example, the existing National Healthcare Safety Network (NHSN) is a CDC-managed internet-based surveillance system that has defined modules of data collection in which health care facilities participate. Currently, more than 3000 facilities from all 50 states submit data to NHSN. Recently, CMS proposed a national requirement for submission of HAIs data to NHSN. However, NHSN does not encompass collection of microbial isolates, and the types of infection under surveillance are limited to those pre-specified by the data collection module.
Active Bacterial Core surveillance (ABCs) of the CDC Emerging Infections Program is an important population-based surveillance system that includes pathogen collection and can assess risk factors for infection and population-based impact of interventions, such as vaccines. However, ABCs is defined for selected pathogens from infections encompassing sterile body sites. As it is currently operating, ABCs cannot easily be modified to assess for the ongoing emergence of resistance among a changing variety of medically important pathogens, particularly infections involving non-sterile clinical sites (e.g., GI tract) where emerging resistance is frequently first evident.
In short, national surveillance efforts need to be strengthened and modified to encompass the rapid detection of emerging resistant infections. The development of the ARSRN will build upon the existing surveillance systems and provide an early warning system for evolving resistance in medically important pathogens, and a platform to rapidly assess control strategies that includes developing, implementing, and evaluating novel interventions that prevent the spread of resistant pathogens. The ARSRN will greatly enhance and dovetail with the overall national surveillance capacity.
Another current national surveillance system in which isolates are obtained is the National Antimicrobial Resistance Monitoring System (NARMS). NARMS was established in 1996 as a collaborative effort between FDA's Center for Veterinary Medicine (CVM), USDA, and CDC. NARMS monitors changes in antibiotic susceptibilities of selected enteric bacterial organisms in humans, animals, and retail meats using a susceptibility panel of antibiotics important in both human and animal medicine. Food-borne pathogen isolates are collected from ill humans, and are sent to CDC from all 50 states. Specimens from sick animals are sent to the USDA via the Veterinary Diagnostic Laboratories. Isolates from retail chicken, pork, and beef are sent to FDA. A limited number of animal specimens are obtained from USDA inspected slaughter and processing facilities and from healthy animals on farms. Animal and human isolates currently monitored in NARMS are non-typhoid salmonella, Campylobacter spp., E. coli O157:H7 stool isolates, and enterococci. CDC also tests additional human isolates including Salmonella spp., Listeria monocytogenes, Shigella spp., and E. coli O157:H7. Current funding levels have made it impossible to sufficiently monitor other life-threatening pathogens, such as MRSA and extra-intestinal E. coli. An increase of $3 million annually for NARMS would enable increased surveillance, to include additional bacterial species and numbers and/or types of samples, and allow researchers to utilize more sensitive detection methods. Additional funding also would allow NARMS to initiate farm-level surveillance of antibiotic-resistant bacteria.
Establishment of the ARSRN and expansion of NARMS will create complementary core facilities for repository of isolates from health care, veterinary, and retail settings to provide investigators with isolates for evaluation of resistance mechanisms, molecular characterization of strains and planning for vaccine development. Establishment of such a repository will create an extremely valuable resource for academia and industry, particularly because epidemiological data can be included with the isolates. Furthermore, the central data repository will be in a unique position to gather widespread data on susceptibility to antimicrobial agents and link the resistance data to clinical outcomes for patients infected with these organisms. These data would be extremely useful to the US FDA and other regulatory agencies as well as to the Clinical and Laboratory Standards Institute (CLSI) in their deliberations of in vitro antimicrobial susceptibility breakpoints (i.e., indicators that predict if a particular antibiotic will be effective to treat infection caused by a particular microbe).
3. National and Local Data on Antimicrobial, and Particularly Antibiotic, Use Across the Spectrum (Human, Veterinary and Other Agricultural) Must be Collected and Made Publicly Available.
Use of antimicrobial agents is the primary driver of the spread of antimicrobial resistance. In contrast to EU member nations, the US does not systematically gather data on antimicrobial prescriptions and use. Antimicrobial use data collected from hospitals, pharmacies and pharmacy benefit managers, outpatient clinics, surgical centers, long-term care facilities, and other settings, across the country and through all age groups, is needed to accurately understand how antimicrobials, and particularly antibiotics, are used. Furthermore, the data would allow assessment of the impact of appropriate use interventions that are the cornerstone of antimicrobial stewardship activities. Although FDA currently receives some use data from a private vendor, such data, by terms of the contract, are not shared publicly.
The federal government should collect and share antimicrobial use data, possibly by contracting with private vendors, as other countries do. The data should include the amount and type of agents used in both humans and animals. Currently, antibiotic use data is collected only at the national level. Additional data should evaluate drug use by agent, geographical area (local, state, and national), age group (human only), type of care, disease indication, etc. Animal data should be collected from feed mills and manufacturers, and should be reported by species (i.e., cattle, swine, poultry, and aquaculture). Feed mills, where antibiotics are mixed into food animals’ feed across the US, are the best place to collect species-specific, local drug use data. The STAAR Act should be strengthened to incorporate these elements.
4. CDC's Antimicrobial Resistance Funding Must be Significantly and Immediately Increased to $50 Million to Enable Critical Public Health-related Objectives, Outlined in This Paper, to be Achieved.
Substantial increases in funding are necessary to strengthen existing US antimicrobial resistance surveillance, data collection, prevention and control, and related research efforts. The expansion of CDC's antimicrobial resistance activities is crucial to protect Americans from these serious and life-threatening infections. Funding for these activities at CDC should be significantly and immediately increased to at least $50 million.
V. Strengthening Activities to Prevent and Control Antimicrobial Resistance
1. Current Law Should be Strengthened to Improve Antimicrobial Resistance Prevention and Control Efforts Through Novel and Innovative Mechanisms.
Current STAAR Act provisions would strengthen antimicrobial resistance prevention and control efforts nationwide. However, Congressional leaders, including sponsors of the STAAR Act and GAIN Act, should consider several novel and innovative ways, outlined below, to strengthen antimicrobial resistance prevention and control efforts including through the adoption of: antimicrobial stewardship programs in all of our nation's health care facilities; strengthened public health and research efforts; a fair and novel mechanism to pay for antimicrobial stewardship programs and antibiotic development (see also recommendation I.2); and novel, FDA-initiated mechanisms to prevent over-prescription of newly approved antibiotics.
2. New Requirements and Incentives Must be Implemented to Encourage Implementation and Maintenance of Successful Antimicrobial Stewardship Programs.
Antimicrobial stewardship refers to coordinated interventions designed to improve and measure the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy, and route of administration. The major objectives of antimicrobial stewardship are to achieve optimal clinical outcomes related to antimicrobial use, to minimize toxicity and other adverse events, to reduce the costs of health care for infections, and to limit the selection for antimicrobial resistant strains [50]. Currently, there are no national or coordinated legislative or regulatory mandates designed to optimize use of antimicrobial therapy through antimicrobial stewardship. Given the societal value of these diminishing precious resources, IDSA supports broad implementation of comprehensive antimicrobial stewardship programs across all health care settings (e.g., hospitals, long-term care facilities, long-term acute care facilities, ambulatory surgical centers, dialysis centers, and private practices), recognizing that flexibility in program requirements must be allowed based on facility size. CMS should be directed to require participating health care institutions to develop and implement antimicrobial stewardship programs. This can be achieved either through development of a new condition of participation in the Medicare and Medicaid programs or through incorporation into existing regulations.
Federally funded incentives should be made available to increase the successful implementation and maintenance of antimicrobial stewardship programs. First, institutions introducing antimicrobial stewardship programs should receive financial support on a “pay for implementation” basis to limit the financial burden on individual institutions as well as follow-on payments to maintain these programs. Such reimbursements are necessary to pay the infrastructure costs required to implement stewardship programs (e.g., pay salaries to personnel staffing the program, provide infrastructure to monitor antibiotic prescriptions, educate staff). Ultimately, such extra hospital payments would result in substantial societal benefits and cost savings by reducing inappropriate use of broad-spectrum antibiotics and diminishing antimicrobial resistance. A new funding mechanism that fairly places the burden on all users of antibiotics is proposed to support implementation and maintenance of these programs (see recommendation V.6).
Moreover, the rates of prescription of antibiotics per patient day in the hospital, or per clinic visit for outpatients, should be monitored and publicly reported. Such publicly reported data should be benchmarked across institutions, and should form the basis for a CMS quality measure that would pressure hospitals to control inappropriate antibiotic prescriptions.
3. CDC's Educational Efforts for Providers and Patients Must be Expanded.
The CDC's Get Smart program serves as an important starting point for implementation of antimicrobial stewardship programs. Tools available through the Get Smart program target health care providers and patients. Increased resources are needed so that CDC can: 1) conduct primary research to define effective communications strategies to inform the public and physicians about inappropriate antimicrobial use; and 2) expand Get Smart, improve CDC's educational tools, and allow for a more effective public dissemination campaign.
4. Research is Needed to Define “Inappropriate” Antibiotic Prescription and to Better Understand the Primary Drivers of Such Inappropriate Use.
Except for certain narrowly defined, high-risk situations, administration of antibiotics to patients, animals, or plants that are not infected by bacteria constitutes inappropriate use. There are also more subtle but equally important inappropriate uses. For example, it is inappropriate to prescribe a drug with a broad spectrum of antibiotic activity (e.g., including activity against XDR GNB) to a patient with an infection caused by a narrow spectrum of pathogenic bacteria (although such use is often consistent with the US FDA-approved indications for the drugs). It is equally inappropriate to prescribe a narrow spectrum drug to a critically ill patient for whom a broader spectrum drug is necessary. Yet different sources may use varying definitions of inappropriate antibiotic use. A standardized definition of inappropriate antibiotic use is needed to facilitate harmonized data collection and interpretation by various professional and government agencies.
Antibiotic prescription for non-bacterial infections can result from pressure from patients, physician insecurity about whether a bacteria or virus is causing an infection (or indeed whether an infection or non-infectious disease is causing the patient's illness), or concern about litigation. However, few studies have defined the relative impact of these and other drivers of inappropriate antibiotic prescription, or have investigated how to positively intervene to improve such behaviors. Primary research is needed to better understand the expectations of patients for receiving a prescription of antibiotic therapy at an office visit, and the specific concerns of physicians when they decide whether to write a prescription for an antibiotic agent in this setting. Only by understanding the primary drivers of inappropriate antimicrobial prescriptions can effective interventions be designed to prevent such prescriptions. Such research should be funded by relevant agencies, such as CDC and AHRQ.
5. Research is Needed to Define Optimal Components and Goals of Antimicrobial Stewardship Programs in Different Health Care Settings, to Define Clinically Relevant Patient Outcomes, and to Develop National Metrics to Monitor Program Success.
The goal of antimicrobial stewardship programs is to optimize the outcome of infection while minimizing toxicity, side effects, development of resistance, and health care costs. Clinician training and continuing education in appropriate antimicrobial use in the US is highly variable, non-standardized, infrequent, and highly prone to bias, especially when conveyed or sponsored by pharmaceutical firms or their agents. Apart from initial training and, to a limited extent, in preparation for board recertification examinations, there is little if any compulsory training or education of physicians in antimicrobial stewardship.
IDSA and other US and international organizations develop and disseminate guidelines on appropriate antimicrobial use for a wide variety of infection syndromes based on a synthesis of the extant literature and expert consensus. However, these guidelines may not sufficiently inform best practices locally because of regional, inter-institutional, and temporal variation in pathogen prevalence and susceptibility patterns. Moreover, most physicians do not commit to an in-depth study of these guidelines. Thus, prescribing physicians may possess a fragmentary knowledge base about optimal antimicrobial use. Research and educational programs are needed to improve training of physicians and implementation of antimicrobial stewardship programs.
To date, research on the efficacy of antimicrobial stewardship programs has focused on: 1) the ability of such programs to control pharmacy costs; and 2) single-center experiences. Research is needed to determine the impact of such programs on antimicrobial, and in particular antibiotic, resistance rates in hospitals of varying size and scope, ambulatory surgical centers, and long-term care facilities. Additionally, research is needed to determine how to bring best stewardship practices into community practices, including in rural and urban underserved areas.
In general, stewardship plans that have required limitation of one antibiotic drug class, replacing it with another drug class, have resulted in decreased resistance to the targeted class at the cost of increased resistance to the substitute classes [79–81]. In contrast, one study evaluated a single center's experience with a global stewardship program in which the Infectious Diseases faculty held an antibiotic approval pager 24 hours per day, 7 days per week [82]. All broad spectrum antibiotics required approval from an ID faculty member, rather than restricting one class of drugs and replacing it with another. Comparing the 18 months of intervention with the preceding year, the hospital experienced a 32% decrease in total antibiotic expenditures, and a modest decrease in resistance rates for several GNB, including P. aeruginosa [82]. Comprehensive stewardship programs such as this example provide one way to achieve more appropriate antibiotic use; however, more research is required [50]. Such programs are expensive to maintain. Multi-center studies are needed that demonstrate the optimal structure of such a program, the impact on sustained levels of decreased resistance, and/or the impact on antibiotic resistance across multiple institutions.
Funding is needed for CDC, NIAID, AHRQ, and/or other relevant agencies to support large, multi-centered evaluations of a variety of stewardship interventions and to develop national metrics by which success of such programs can be measured. Research is needed to evaluate: general guidance strategies in which infectious diseases experts implement pathways and/or help select antibiotic therapy for hospitalized patients, use of “automatic stops” of antibiotics to prevent inappropriately long courses of treatment and/or multiple agents, use of shorter durations of therapy for specific infections, use of novel diagnostic strategies to reduce the need for initiating antibiotic therapy, de-escalation (drug withdrawal) strategies, and computerized decision support systems to guide clinicians in implementing these strategies. In addition, research is needed to better define optimal cost-effective staffing of antimicrobial stewardship programs in health care institutions of all sizes and levels of acuity across the continuum of care, including long-term care facilities and ambulatory care facilities.
De-escalation strategies should focus on preserving agents with activity against highly resistant GNB or other ESKAPE pathogens. However, even antibiotics that are often considered “narrow” spectrum (e.g., ampicillin) have broad activity against bacteria from an ecological perspective. Therefore, emphasis should be on strategies to stop antibiotic therapy completely at the earliest effective time point.
6. An “Antibiotic Innovation and Conservation (AIC) Fee” Should be Established to Help Fund Implementation of Antimicrobial Stewardship Programs (In Addition to Antibiotic Development).
As with antibiotic development, antimicrobial stewardship programs need a stable funding mechanism. As discussed more fully in recommendation I.2, an AIC Fee should be established to provide that funding mechanism. Twenty-five percent of the funds collected through the fee would be allocated to establish and maintain a fund, overseen by the CDC, which would be used to: 1) support research to determine the most effective stewardship strategies across the continuum of care; and 2) promote implementation and maintenance of antibiotic stewardship programs in health care facilities across the country. The remaining 75% of the funds collected through the fee would support the discovery and development of new antibiotics.
7. Rapid Molecular Diagnostics are Urgently Needed to Support Appropriate Antimicrobial Use.
The development and availability of novel molecular diagnostic tests is one of the most important pathways toward improving antimicrobial stewardship, as well as supporting antibiotic development. For example, informing physicians in real time that a patient's signs and symptoms are due to a viral pathogen, and not a bacterial pathogen, will provide reassurance to both the physician and patient that no antibiotic therapy is needed. The prominent example of the impact of a molecular diagnostic test on antibiotic prescriptions is the use of rapid tests to demonstrate the presence or absence in the pharynx of group A Streptococcus (S. pyogenes) in a symptomatic patient [83–88]. Rapid diagnostic tests are needed for many other pathogens causing a wide variety of other bacterial infections. (See recommendation VII for specific recommendations related to diagnostics.)
8. FDA Should Study and Implement Mechanisms to Prevent Over-prescription of Newly Approved Antibiotics.
Currently, FDA's approval process may be antithetical to antimicrobial stewardship, because it does not consider the potential for newly approved broad-spectrum antibiotics to be used to treat infections caused by a narrow spectrum of bacterial pathogens. For example, agents with broad GNB activity have been licensed for the treatment of skin infections and CABP, both of which are caused by a narrow spectrum of bacteria for which many other effective antibiotic options are available. Unfortunately use of such antibiotics to treat skin infection contributes to the erosion of their activity against GNB for which few effective antibiotics are available. Companies may market drugs only for their FDA approved indications. Therefore, one means to converge new antibiotic development with stewardship is to change the focus of development to critical public health needs, which in the near-term means specifically targeting drugs for infections caused by MDR/XDR/PDR GNB. If such drugs were indicated only for the treatment of resistant GNB, the drugs could not be marketed for diseases such as skin infections and CABP, which should prolong their effectiveness.
Therefore, new clinical trial pathways should be created to enable companies to seek approval for narrow indications, including for example organism-specific indications (as discussed in recommendation II.4). Economic incentives (as described in recommendation I including increased reimbursements) will be necessary to enable companies to choose to seek narrow indications when such a pathway is available, because while doing so will benefit public health, limiting the marketing potential of the antibiotic will inevitably reduce its sales, further decreasing its return on investment.
FDA should release guidance documents providing clear paths to approval specifically for infections caused by resistant GNB. Such guidance should include “organism-specific” studies, superiority pathways, and specific non-inferiority pathways for diseases commonly caused by GNB (e.g., nosocomial pneumonia, intra-abdominal infections).
Adoption of other strategies to protect antibiotics could be implemented under an FDA REMS-like program for high priority antibiotics. Programs that could effectively curtail overuse and misuse of antibiotics post-approval include limiting the number of providers authorized to prescribe the drug, requiring prospective recording and public reporting of all prescriptions for the drug at the individual provider level, and requiring documentation of the nature of the infection for which each prescription of the drug is required. Federal funding should be made available to study and develop such REMS-like programs to protect critically needed antibiotics.
VI. Significant Investments in Antimicrobial-Focused Research
1. The Antimicrobial Resistance Strategic Research Plan called for in the STAAR Act Should be Developed and Implemented with a Particular Focus on Antibiotic Resistance.
There is a compelling need for more, better funded, and better coordinated federal antimicrobial resistance-related research activities. Since there is no strategic plan on resistance research and product development (including new drugs, diagnostics, biologics, vaccines, and devices), key research areas remain unaddressed. IDSA strongly supports the STAAR Act's proposal that the federal government, led by NIAID and CDC, develop an Antimicrobial Resistance Strategic Research Plan. The Strategic Research Plan should result in a robust, well-directed, and targeted antimicrobial resistance program, define high-priority research needs, and address scientific challenges. Such a plan would clarify goals and set benchmarks for evaluating progress, particularly in the areas discussed below.
2. Basic Science Research Should be Expanded to Further Study Antimicrobial Resistance Mechanisms and Epidemiology; Identify New Lead Compounds; and Develop Vaccines, Immunotherapies, and Other Technologies to Prevent and Treat Infections in Humans and Animals.
a. Mechanisms of resistance and epidemiology
Research into mechanisms by which antimicrobial resistance occurs, including molecular mechanisms causing resistance, genetic regulation of expression of resistance, and molecular epidemiology of resistant strains and resistance genes, is in urgent need of expansion. Included in molecular epidemiology studies are those in which the genomes of large numbers of clinical isolates are sequenced and analyzed to define which genetic changes, including mutations, cause resistance in the clinical setting. Having sequential isolates from the same patient over time, with tracking of changes in susceptibilities of those strains to antimicrobial agents, is a powerful way to identify mutations that cause antimicrobial resistance. Other technologies, such as transcriptomics, are capable of defining mutations leading to resistance phenotypes. Also needed are studies defining means to overcome resistance by blocking or down-regulating expression of resistance mechanisms. Finally, studies are needed that explore the role of sessile bacterial growth, growth within biofilms, and “persister” bacteria in mediating relapse and resistance during and after antibiotic therapy.
b. Lead antibiotic compound identification
The limiting step in preclinical antibiotic development is not the identification of novel microbial targets (i.e., protein or other cellular components to poison). A large number of potential targets for antibiotic development are known. The limiting step is identifying novel lead molecules that can kill bacteria while remaining (relatively) non-toxic to the patient. Novel screening techniques and technologies are critically needed to facilitate discovery of new lead molecules. Such technologies may include (but are not limited to) use of cutting-edge bioinformatics to facilitate more accurate high throughput screens, and increasingly sophisticated spectroscopy, imaging, and molecular binding technologies to identify lead compounds that interact with known targets.
Another promising area of research is the expansion of the study of natural sources of antibiotics to include “uncultureable” bacteria. Since most bacterial species have never been successfully cultured, these organisms represent a rich and completely untapped source of novel antibiotic scaffolds. New technologies to enable culturing of these organisms are needed, which will then facilitate screening of those organisms for production of new antibiotic compounds.
Also emphasized is the need for superior high throughput screens to test for efficacy in animal models of infection (“in vivo”). Since the initial discovery of sulfonamides, it has been known that agents that are not active in the test tube (“in vitro”) may have potent antibiotic efficacy in vivo [89]. Use of invertebrate models for high throughput in vivo screening of antibiotic efficacy may be a promising avenue of research. Species studied in infectious diseases models include Caenorhabditis elegans, fruit flies, moth larvae, etc. Expansion of use of such models, and development of new invertebrate models, could be useful to improve the rapidity of pre-clinical development. Such models require validation against vertebrate models as predictors of in vivo efficacy.
A greater emphasis on basic science and lead molecule identification is critical to support antibiotic development. To achieve the 10 × ’20 goal of 10 new antibiotics by 2020, IDSA encourages NIAID (and other agencies, e.g., DoD) to make it a high priority to fund research focused on the discovery of promising antibiotic lead scaffolds that can be optimized into candidate compounds.
It is important to recognize and support the complexity of the work required to progress lead scaffolds into candidate molecules. Discovery of a lead is only the first step and substantial further support is required in many related disciplines (e.g., synthetic chemistry, toxicology, and formulation development). As antibiotic discovery is an iterative, high-risk enterprise, it is necessary to support and monitor this work over an extended period of time. The goal is the identification of lead, “drug-able” compound series, which via chemical optimization will yield candidate molecules suitable for progression into phase I clinical trials. Thus, new lead compounds identified with NIAID funding should be followed prospectively to define which molecular series and candidate molecules progress into phase I trials. Characteristics of the molecules and of the assays and methods used to identify the molecules and used to complete pre-clinical development also should be monitored and compared with success rate at phase I translation. Such data will help select accurate screening methods and development tools, and thereby facilitate future success in identifying important lead scaffolds.
c. Vaccines, immunotherapies, and infection prevention technologies
Expansion of NIAID funding of both innate and adaptive immune strategies to prevent and treat antimicrobial resistant infections, and particularly bacterial infections, is needed. Strategies with promise include: active vaccination, passive immunization with polyclonal or monoclonal antibodies, and other immune enhancing therapies. Understanding the fundamental immunology of bacterial infections and host susceptibility to these infections is a key foundation of basic knowledge required for technology to be successfully translated into prevention and treatment strategies. Investment in effective animal vaccines also will benefit public health by decreasing the need for the use of antibiotics in agriculture.
Other technologies also may be used to prevent infections. For example, enhanced environmental disinfection, to reduce the burden of antibiotic-resistant pathogens in health-care environments and on the equipment, clothing, and skin of health-care providers, could diminish transmission of antibiotic-resistant pathogens to patients. New technologies that more effectively and rapidly disinfect health care environments, personal items, and skin should be developed, studied, and implemented if effective at preventing infections. Finally, the human microbiome is complex and provides resistance against colonization by pathogens. Understanding colonization resistance can lead to probiotic and pre-biotic approaches to preventing and controlling infection.
3. Support for Translation of Promising Compounds From Pre-clinical Research into Clinical Trials Should Be Expanded.
A growing array of services is available at NIAID to support translation of promising molecules from pre-clinical into clinical research. Aside from small business grants, NIAID has developed an extensive infrastructure to support pre-clinical toxicity studies, good manufacturing practices (GMP) compliance, regulatory support for filing Investigational New Drug (IND) applications, business plan development, and planning and conduct of phase I clinical trials. Such services are available for small molecule antibiotics and vaccines. IDSA strongly supports NIH's continued expansion and utilization of such services by academicians, start-up companies, and biotechnology and pharmaceutical companies. The efficiency (turn-around time) of such services should be evaluated to ensure grants, development expenses, and other facets of candidate drug development are optimally impacted.
4. Clinical and Health Outcomes Research is Needed to Study Infections and Interventions to Improve Outcomes and Reduce Antibiotic Exposure.
a. Studies of natural history, outcomes, and magnitude of therapeutic effect for specific infections
To support development of antimicrobial agents (as for other drug classes), it is critical to understand the natural history of their target diseases. For example, studies of well-defined cohorts of patients with highly antibiotic-resistant GNB are critically needed to inform the design and conduct of clinical trials for new antibiotics for these infections. Other needed information deriving from such studies includes: epidemiology (such as which patients are at risk for these infections and which health care sites have a high incidence of highly resistant infections), outcomes with currently available therapy (for study power planning purposes), demographics, and co-morbidities. Furthermore, biomarkers, including vital signs, standard clinical laboratory tests, and cutting-edge molecular assays measuring cytokines, immunogenetics, acute phase reactants, general proteomics, and metabolomics, are needed to identify surrogate markers for antibiotic primary efficacy endpoints, and factors that predict antibiotic success or failure.
It also is critical to understand the outcome of antibiotic-resistant infections with early versus delayed initiation of effective therapy. Such data provide an estimate of the overall efficacy of antibiotics for such resistant infections. Efficacy estimates are required to justify non-inferiority margins and selection of endpoints for future studies of novel antibiotics [90–92]. Finally, for non-life threatening infections, knowledge of the outcome of untreated, placebo-treated, or ineffectively treated patients is critical for defining placebo-response rates. Again, such data are important for power calculations and establishment of defensible non-inferiority margins for future clinical trials.
b. Comparative effectiveness studies of shorter durations and early cessation of therapy
A key means to reduce inappropriate antibiotic use is to shorten duration of antibiotic therapy for infections. The shortest durations of therapy needed for optimal clinical cure are unknown for virtually all infections. Recent studies of VABP, CABP, and urinary tract infections all support the concept that treatment durations can be shortened [93–95]. Since shortening duration of therapy further reduces antimicrobial sales, industry is unlikely to fund such studies. Therefore, it is necessary for public funding to be made available to conduct such studies, which will result in less public exposure to antimicrobial agents, thereby slowing the spread of antimicrobial resistance. NIAID has expanded its portfolio of clinical trial funding, and this expansion should be encouraged and continued. Participation of other granting agencies, such as AHRQ and CDC, also should be encouraged in this area.
Another promising area of study to reduce duration of antibiotic therapy is the use of biomarkers to determine when patients have resolved their infections. For example, when levels of procalcitonin in the blood return to normal on therapy, patients have been taken off treatment early and safely for respiratory tract infections [96–98]. Similarly, following Clinical Pulmonary Infection Scores (CPIS) over time has been used to stop therapy early for nosocomial pneumonia [99]. Further study of these and other tools to support shorter durations of effective therapy for infections is needed.
5. Research is Needed to Optimize PK/PD of Antimicrobial Therapy.
The dose of a drug used in its pivotal efficacy study should be the optimal dose most likely to achieve efficacy while minimizing the risk of toxicity. IDSA strongly supports continued funding by FDA, NIAID, or other relevant agencies, of studies of PK/PD parameters that forecast optimal drug dosing. Needed research includes: 1) continuing to refine and define the capability and limitations of PK/PD to predict efficacy and dosing; 2) definition of candidate drugs’ optimal killing parameters and hence dosing; and 3) the ability of various dosing strategies to prevent emergence of resistance. Such studies should be conducted in both pre-clinical models, and, critically, in clinical trials.
6. A Clinical Trial Network is Needed to Support Studies of Antimicrobial Therapies and Antimicrobial Resistance, Building on the Success of the Existing HIV/AIDS Clinical Trials Network.
NIAID has proposed to establish a new infectious disease clinical trials infrastructure in parallel with the successful HIV/AIDS clinical trials network. At the time of this paper’s drafting, it appears the central foci of the new network will be antibiotic-resistant bacterial infections and emerging infections. Given current, urgent, unmet medical needs, IDSA strongly supports these foci. There is a critical need for a federally funded clinical trial network infrastructure for the purposes of: studying surrogate markers for antibiotic primary efficacy endpoints, pivotal trials of new antimicrobial drugs, post-licensure comparative studies, validation of molecular diagnostics, antimicrobial stewardship studies, and perhaps assessment of the clinical equivalency of generic drugs. For example, a variety of possible interventions have been suggested to improve the outcomes of invasive MRSA infections (e.g., higher-dose vancomycin, higher-dose daptomycin, use of adjunctive antibiotics). Similarly, despite the growing prevalence of infections caused by XDR GNB, there is an almost complete lack of randomized trials of possible therapeutic agents for these infections (e.g., colistin, tigecycline, fosfomycin).
Moreover, regulatory standards for conduct of pivotal studies of novel antibiotics are clearly increasing [53, 54, 100]. For example, future studies of novel antibiotics will need to enroll patients before any, or virtually any, non-study antibiotic is administered. Because most MDR infections occur in the setting of hospitalized patients with much antecedent history and treatment (including antibiotics), it will not be possible to conduct such studies without highly trained core study sites that are capable of the highest level of clinical trial conduct.
Similarly, the requisite quality of microbial samples to support an etiologic diagnosis will be higher in future studies (e.g., samples obtained by invasive, medically sophisticated procedures, such as bronchoalveolar lavage may be necessary to support enrollment into a nosocomial pneumonia trial). A microbial diagnosis will be required in most or all evaluable patients in future clinical trials, which will place a tremendous burden on investigators to use sophisticated molecular diagnostic assays to attempt to achieve a higher frequency of microbial confirmation of infection. Increasingly sophisticated biomarker assays likely are to be studied. Such diagnostic methods may be particularly challenging in vulnerable populations such as seriously ill premature newborns where the risk of invasive procedures to establish a microbiologic diagnosis for a research study may not be ethically justifiable. Hence, conduct of such studies will require highly trained, sophisticated clinical investigators and study coordinators, and appropriate resources.
An established network of clinical trial sites would improve the quality of study data, enable more timely enrollment of patients, and result in a significantly higher proportion of patients being enrolled in the US, thereby ensuring that study results are relevant to the US population. The need and reasons for such a clinical trial network have been previously emphasized [46].
It is critical that an overly bureaucratic approach not stifle innovation or study conduct within the network [101, 102]. The network must be flexible and agile, with the ability to rapidly respond to new or re-emerging infections as they arise. Further, it must balance both pediatric and adult unmet infectious diseases needs. Hence, an efficient network structure and operating procedure should be instituted to support protocol selection, development, implementation, and conduct. It is envisioned that the proposed network would be part of the ARSRN, as specified by the STAAR Act.
7. Funding to Support Career Development and Faculty Retention is Necessary to Reverse the “Brain Drain” That Continues to Occur in Antibiotic and Microbiology Research in Both Academia and Industry.
The previous several decades have witnessed a steady erosion and loss of talent from antibiotic and microbiology research in both academia and industry. It is essential that federal funding be made available to support development of young investigators to reinvigorate the field, and to bring more senior investigators back into the field. Expansion of funding of basic, clinical, and epidemiological research will enable investigators to build careers in antibiotic research, which is crucial to reviving the basic foundation of future antibiotic discovery and resistance prevention. Furthermore, career development awards for young investigators should be encouraged in this area. Strengthening efforts to recruit, train, and retain young investigators is not yet a part of the STAAR Act or GAIN Act. Congress should consider this as an essential element of antimicrobial resistance legislation as these bills move forward.
8. Annual Funding for NIAID Should be Increased by $500 Million by Direct Appropriation to Support Expansion of its Antibiotic Resistance and Development Research Portfolio.
IDSA is calling for a $500 million annual increase in Congressional appropriations to support an expansion of NIAID's budget in the area of antibiotic resistance and antibiotic discovery research. As discussed previously [46], NIAID is aware of the need for additional research to address the antimicrobial, and particularly antibiotic, resistance problem. However, the overall flat budget of NIH and NIAID limits the Institute's ability to sufficiently increase funding for critically needed new research in antibiotic resistance and development, [103] as elaborated above.
VII. Greater Investment in Rapid Diagnostics R&D and Integration into Clinical Practice
1. Novel Molecular Diagnostics are Needed that Improve Clinical Care and Public Health.
In a policy paper on molecular diagnostics for respiratory tract infections, IDSA called for the development and clinical use of novel, molecular diagnostic tests to improve rapidity, sensitivity, and specificity of making a microbial diagnosis of infection [104]. These efforts need to be expanded to cover the full spectrum of sites of human infection and the potentially different approaches needed based on the patient's condition (e.g., solid-organ or bone marrow transplant, HIV-infected, cancer chemotherapy, and premature neonates).
There are multiple benefits of such tests, as discussed below. Tests ideally should be: inexpensive, rapid, close to or at point-of-care, sensitive, and specific. Furthermore, use of such tests should ideally improve health care outcomes, reduce overall health care costs, serve a population benefit (e.g., reduce antibiotic resistance) by supporting antimicrobial stewardship efforts, and/or support novel antibiotic development by facilitating patient enrollment in pivotal clinical trials.
2. Federally-supported Research and Economic Incentives are Necessary to Support R&D of Novel Molecular Diagnostic Tests and to Strongly Encourage Their Integration Into Clinical Practice.
To optimize use of novel molecular diagnostic tests, it is important that the tests be capable of detecting pathogenic bacteria, in addition to viruses. Diagnostic test panels must be established to support capability of the tests to identify bacterial pathogens. Tests ideally should detect pathogenic organisms from patient samples, in addition to pure cultures. The devices should facilitate detection of small numbers of organisms, rather than the larger number available in pure culture. Quantitative standards are needed to assist in the distinction between colonizing organisms and invasive pathogens, particularly in respiratory specimens. Tests should be validated in large numbers of samples to determine appropriate quantitative thresholds.
As discussed in further detail in IDSA’s policy paper on rapid diagnostic tests for respiratory tract infections, clinical validation should not include use of the test to determine whether disease is present, only whether or not a specified organism is present in the sample [104]. Clinicians and clinical investigators should determine whether disease is present by integrating the results of diagnostic tests with all other clinical information.
IDSA strongly supports the use of novel molecular diagnostic tests to enrich the microbiologically confirmed, evaluable population in antibiotic pivotal studies. This issue is discussed at length in the IDSA policy paper on molecular diagnostics [104]. Studies of molecular diagnostic tests should focus on their ability to facilitate prevention of antibiotic prescription for diseases that may be viral or non-infectious in etiology, to target initial antibiotic therapy to the appropriate bacterial pathogen in hospitalized patients, and to de-escalate or stop antibiotic therapy in patients in whom it has been empirically initiated.
As Congressional leaders consider potential incentives to stimulate antibiotic development and ensure the appropriate use of these precious drugs, it is essential that incentives supporting the development and utilization of new, related diagnostic tests also be adopted. Such incentives may include: 1) defining improvements to the process for determining payment rates for qualifying diagnostic tests and developing a system for assigning temporary Healthcare Common Procedure Coding System (HCPCS) codes to new tests until a permanent code is established; 2) encouraging the development of companion diagnostics by extending the period of data exclusivity for an antibiotic for which the manufacturer has developed a companion diagnostic test, and/or creating a market exclusivity period for qualifying medical devices, if the manufacturer of such device co-develops a companion diagnostic test with an antibiotic; 3) requiring FDA to provide expedited review for qualifying devices; and 4) reimbursing relevant diagnostics appropriately based on their value to society (see recommendation I.3)
3. A Well-characterized Clinical Sample Repository Should be Established by Federal Agencies to Speed Validation of Molecular Diagnostic Tests.
In principle, the simplest clinical validation study for a molecular diagnostic test would be comparison of the test to the “truth standard,” using prospectively characterized clinical specimens stored in a repository. The ability to access samples without conducting new and parallel clinical trials to obtain specimens would make validation much less expensive and faster. Such well-characterized clinical samples could be obtained, for example, from phase II or III clinical trials of drugs or devices. The samples should be collected in a prospective manner with a protocol defining inclusion and exclusion criteria, and linked to all clinical data available from the source patient, in addition to results of reference diagnostic tests. Sponsors would likely have to do bridging studies to show that fresh samples and frozen samples performed similarly with their assay. A repository would represent a significant advance over current sample collection performed by a pharmaceutical company for drug or device approval, in which each company exclusively owns all samples collected, and no cross-validation of samples for different agents all treating or diagnosing one clinical indication (e.g., pneumonia) is possible. One reasonable option would be to increase NIAID funding to support establishment of such a clinical specimen repository at FDA's CDRH, similar to the clinical cancer specimen repository that the National Cancer Institute supports. Furthermore, establishment of the ARSRN as part of the STAAR Act would enable collection of specimens outside of clinical trials in collaboration with highly proficient clinical microbiology laboratories.
VIII. Eliminating Non-Judicious Antibiotic Use in Animals, Plants, and Marine Environments
1. PAMTA (H.R. 1549/S. 619 in the 111th Congress) Should be Enacted and Other Measures Adopted to End the Use of Antibiotics for Growth Promotion, Feed Efficiency, and Routine Disease Prevention Purposes in Animal Agriculture and to Ensure That These Precious Drugs are Being Used Wisely in All Settings.
IDSA strongly supports enactment of PAMTA and the adoption of comparable measures (including FDA Center for Veterinary Medicine [CVM] regulations) to stop the use of antibiotics for growth promotion, feed efficiency, and routine disease prevention purposes in animal agriculture. IDSA also supports requiring prescriptions and veterinary oversight of all antibiotics given to animals. Antibiotic use in agriculture, similar to human medicine, should be carried out under the supervision of a veterinarian, within the boundaries of a valid veterinarian-client-patient relationship.
In addition, FDA/CVM should: 1) define procedures for antibiotic administration in animals that will permit short-term antibiotic use for those animals that have a current therapeutic need or an immediate prophylactic need due to an infectious outbreak in surrounding animals where such animal has been exposed or is highly at risk for exposure to disease; and 2) work with CDC and USDA to expand post-approval surveillance under NARMS (see recommendation IV.2). As discussed in recommendation IV.3, the amount and type of antibiotics used in animal feed should be tracked and made publicly available on an annual basis. In addition, ongoing risk analysis is needed to better understand the impact of the remaining uses of antibiotics in animal agriculture on human and animal health (see recommendation VIII.3).
2. FDA/CVM Guidance #152 (“Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern”) Should be Revised to Re-evaluate the Current Ranking of Drugs According to Their Importance to Human Medicine.
IDSA urges a reassessment of existing FDA/CVM Guidance #152 [105], which is the framework by which the agency approves new antibiotic products for use in animals. FDA must reevaluate the current ranking of drugs according to their importance to human medicine. In particular, the agency should reconsider the criteria used to categorize antibiotics as “critically important” and “highly important” to human health. The scope of Guidance #152 criteria should be broadened beyond enteric pathogens. The current focus on enteric-only pathogens fails to consider the human health risk posed by horizontal gene transfer or clonal spread of resistant strains of bacteria, including such species as Enterococcus and E. coli, which are a normal part of the bacteria living in intestines of food animals, but cause infections outside the intestines in humans.
3. FDA Must Complete and Publish Risk Assessments of Those Antibiotics of Importance to Human Medicine that are Approved for Non-therapeutic Purposes in Food-producing Animals, Examining Their Role in the Selection and Dissemination of Antibiotic-resistant Food-borne Pathogens.
FDA must complete, update, and publish risk assessments for antibiotics of importance to human medicine, which currently are approved for non-therapeutic purposes in food-producing animals. These reviews are necessary to ascertain the role of such use of antibiotics in the selection and dissemination of antibiotic-resistant food-borne pathogens. Since 2003, FDA/CVM has required that the pre-approval safety reviews for all new antibiotic veterinary drugs include an evaluation of the likelihood that the proposed drug use in animals will lead to resistant infections in humans. Because almost all antibiotics being used for growth promotion and other non-therapeutic purposes in livestock production were approved by FDA before 2003, most have either not undergone reviews with respect to antibiotic resistance or have undergone reviews that are inconsistent with current standards. To ensure that these drugs meet current safety standards, it is important that post-market safety reviews be done for those classes of antibiotics important to human medicine that also are being used for routine non-therapeutic purposes in animal agriculture. These would include penicillins, tetracyclines, macrolides, lincosamides, streptogramins, aminoglycosides, and sulfonamides. Such reviews are expensive: $5 million should be appropriated immediately to enable FDA to carry out this important work.
Antibiotics are commonly used outside of humans and animals in aquaculture, horticulture, and even in marine paint to limit barnacle growth. Antibiotic use in aquaculture is essentially unregulated and may result in significant environmental contamination, although data are lacking and additional research is needed. The use of antibiotics such as tetracycline, streptomycin, and gentamicin (which are used on plants and fruit to prevent fire blight), may be justified in limited circumstances, but monitoring of use and the development of resistance in target bacterial pathogens should be established. It is reasonable and prudent to minimize or prohibit the non-human use of any antimicrobial that has current or potential application for the treatment of infections in humans.
CONCLUSIONS
It is difficult to accurately convey the enormous impact effective antibiotics have had in saving patients’ lives and eliminating tremendous suffering in the US and throughout the world. The most fundamental impact of the introduction of antibiotics was a dramatic decline in death from bacterial infections of all types. For example, the overall mortality rate from infectious diseases in the US fell by ∼220 per 100,000 population (75%) over the first 15 years of the antibiotic era [106]. Almost overnight, mortality rates for diseases such as pneumonia, endocarditis, and meningitis dropped substantially after the introduction of new antibiotics (Table 3). Indeed, so enormous were the mortality benefits of antibiotics that all subsequent medical advances since the early 1950s—including the advent of critical care medicine—have resulted in only minor further reductions in death from infections. Specifically, during the second half of the 20th century, despite all intervening advances in medical care, mortality rates from infections declined only by an additional 20 per 100,000, less than 10% of the decline achieved immediately following the availability of antibiotics [106]. The US federal government recognized this plateau effect in reduction of mortality from infections through the 1950s and 1960s, and understood [107] that it was due to the remarkable power of antibiotics [108–109].
Beyond saving lives of infected patients, today the enormous efficacy of antibiotics enables conduct of complicated and deeply invasive surgery, aggressive chemotherapy for treatment of cancer, fundamental elements of critical care such as central venous catheter placement and mechanical ventilation, supportive care for premature infants, and solid and liquid organ transplantation. None of these medical advances would be feasible without effective antibiotics to prevent and treat the infections that occur as a side effect of the advances themselves. Indeed, one of the leading physicians of the 20th century, Dr. Walsh McDermott, a Lasker Award winner who served as first president of the Medical Board of the National Academy of Sciences (precursor to the Institute of Medicine), commented that:
“It is not too much to state that the introduction of [antibiotics] has represented a force for change in the 20th century of the same general kind as James Watt's modification of the steam engine did in the 18th.” [110]
In short, as described by both Dr. McDermott and Dr. Lewis Thomas [111], the power of antibiotic therapy resulted in nothing less than a total revolution in the practice of medicine. Antibiotics fundamentally transformed the profession from a diagnostic, non-interventional field to a therapeutic, interventional profession.
The loss of effective antibiotic therapy due to antimicrobial resistance and the withering antibiotic R&D pipeline will result in a great increase in deaths from infections. This issue—the availability of effective antibiotics—is not a “lifestyle” issue, and the loss of such agents is not theoretical. We are facing a worldwide health crisis that already is resulting in deaths and maiming of patients, and will increasingly do so in the coming decades unless urgent action is taken. The time for debating the problem has passed. Immediate action is critically needed, as outlined in this policy paper.
Acknowledgments
This supplement is sponsored by IDSA and is dedicated to John G. Bartlett, MD, FIDSA for his tireless commitment to the work of the Society and toward combating antimicrobial resistance.
This policy paper stems from the critical, ongoing efforts of member experts who serve on IDSA's Antimicrobial Resistance Work Group, Research on Resistance Work Group, and Antimicrobial Availability Task Force. The paper's research recommendations stem, in particular, from discussions with and presentations by the planners and participants of a public workshop that occurred July 26-July 27, 2010, which was co-sponsored by FDA, NIAID and IDSA. The workshop's executive committee included Drs. Edward Cox (FDA's co-chair), Michael Kurilla (NIAID co-chair), Martin Blaser (IDSA's co-chair), Dennis Dixon (NIAID), David Gilbert (IDSA), and Louis Rice (IDSA). The workshop program committee also included: 1) for IDSA, Drs. John G. Bartlett, Henry F. Chambers, Neil O. Fishman, Anthony Harris, John H. Powers, III, L. Barth Reller, Lisa Saiman, and Brad Spellberg (also rapporteur); 2) for CDC, Drs. Lesley McGee, Arjun Srinivasan, and Cynthia G. Whitney; 3) for FDA, Drs. John Farley, Steven Gitterman, Sally Hojvat, Joseph Toerner, and Katherine Laessig; 4) for NIAID, Drs. Rose Aurigemma, Maureen Beanan, and Jane Knisely; and 5) pharmaceutical and diagnostics industry representatives, including Drs. Barry I. Eisenstein, Steve Gilman, Steven J. Projan, John H. Rex, Joyce Sutcliffe, Fred C. Tenover, and Barbara Zimmer.
IDSA's Board of Directors wishes to thank each of the individuals mentioned above as well as the paper's authors for their tireless commitment to find solutions to the antibiotic resistance and antibiotic pipeline problems. In particular, the Board wishes to acknowledge the efforts of Brad Spellberg and Robert Guidos in the development of this policy paper.
The authors would like to extend special thanks to Drs. Louis Rice and George Talbot for their careful review of the manuscript.
Potential conflicts of interest. B. S. has consulted for Pfizer, Basilea, The Medicines Company, Achaogen, Novartis, Cerexa, Trius, Nektar, Theravance, Meiji, Eisai, Anacor, and GlaxoSmithKline. He has received grant funding from the NIH, clinical trial grant support from Novartis, Astellas, Gilead, and Cubist, and owns equity in NovaDigm Therapeutics Inc.
M. B. is on Scientific Advisory Boards for Avidbiotics, Danon, Procter & Gamble, Adamas, Puretech, and has current research support from NIH, Dow Chemical, L'Oreal, Gates Foundation, and the Diane Belfer Program for Human Microbial Ecology. He serves on the Advisory Board for Clinical Research for NIH.
R. G. is an IDSA employee.
H. B. provided consultation for Basilea, Cerexa, Cubist, Durata, Merck (adjudication committee), Methylgene, J&J, Nabriva, Optimer, Rib-X, Targanta/TMC, Theravance, and Wyeth/Pfizer (data safety monitoring board) in the last 12 months.
J. B. employer, the University of California, has contracts for consulting with Johnson & Johnson, Trius, Bayer, Nabriva, Pfizer and Cerexa/Forest, and contracts for clinical trials with Johnson & Johnson, Cubist, and Trius.
B. I. E. is a full time employee of Cubist and also holds stock in Eli Lilly.
D. Gerding holds patents for the treatment and prevention of Clostridium difficile infection that are licensed to ViroPharma, is a consultant for ViroPharma, Optimer, Cubist, Merck, Pfizer, Hospira, Medicines Co, Astellas and Actelion, and has received research grants from GOJO, Merck, Optimer, Sanofi-Pasteur, Eurofins Medinet and ViroPharma.
R. L. has received grant funding through the US CDC.
L. B. R. has no conflicts to disclose.
J. R. is an employee and shareholder of AstraZeneca Pharmaceuticals.
D. S. has received grant funding from the US CDC.
E. S. is employed by Hospital Corporation of America (HCA, Inc.), is on the speaker bureau for Cubicin, Ethicon and Sage, and has received grant funding from AHRQ and CDC.
F. T. is an employee and shareholder of Cepheid, a molecular diagnostics company, and has received honoraria from the Association of Public Health Laboratories and the Washington Infectious Diseases Society.
D. Gilbert has consulted for Achaogen, Pfizer, Merck, and Advanced Life Sciences.
The paper's drafters possessed broad professional expertise across the spectrum of infectious diseases medicine. The pharmaceutical and diagnostics industry representatives, Drs. Eisenstein, Rex, and Tenover, are IDSA members, and their input was essential for understanding the broader implications of the paper's policy recommendations and warranted their being listed among the paper's drafters. The final policy recommendations adopted by IDSA's Board of Directors do not necessarily represent the opinions of the drafters or the organizations for which they work.
Appendix A: GLOSSARY OF ABBREVIATIONS (Alphabetical)
| ABCs = Active Bacterial Core surveillance |
| AHRQ = Agency for Healthcare Research and Quality |
| AIC Fee = Antibiotic Innovation and Conservation Fee |
| ARO = Antimicrobial Resistance Office |
| ARSRN = Antimicrobial Resistance Surveillance and Research Network |
| ASPR = Assistant Secretary for Preparedness and Response |
| BARDA = Biomedical Advances Research and Development Authority |
| CABP = community-acquired bacterial pneumonia |
| CDC = Centers for Disease Control and Prevention |
| CDER = Center for Drug Evaluation and Research |
| CDRH = Center for Devices and Radiological Health |
| CLSI = Clinical and Laboratory Standards Institute |
| CMS = Centers for Medicare and Medicaid Services |
| CPIS = clinical pulmonary infection score |
| CRADA = Cooperative Research and Development Agreements |
| DHHS = Department of Health and Human Services |
| EARS-Net = European Antimicrobial Resistance Surveillance Network |
| ESAC = European Surveillance of Antimicrobial Consumption |
| ESBL = extended-spectrum beta lactamase |
| ESKAPE = Enterococcus, Staphylococcus, Klebsiella, Acinetobacter, Pseudomonas, and ESBL (Enterobacter and E. coli) |
| ECDC = European Centre for Disease Prevention and Control |
| EU = European Union |
| FDA = Food and Drug Administration |
| GAIN Act = Generating Antibiotic Incentives Now Act |
| GMP = Good Manufacturing Practices |
| GNB = Gram-negative bacilli (bacteria) |
| HABP = hospital-acquired bacterial pneumonia |
| HAIs = hospital-acquired infections |
| IDSA = Infectious Diseases Society of America |
| IND = investigational new drug |
| MRSA = methicillin resistant Staphylococcus aureus |
| NARMS = National Antimicrobial Resistance Monitoring System |
| NHSN = National Health Safety Network |
| NIAID = National Institute of Allergy and Infectious Diseases |
| NIH = National Institutes of Health |
| NPV = net present value |
| PAMTA = Preservation of Antibiotics for Medical Treatment Act |
| PDR = pan-drug resistant |
| PHAAB = Public Health Antimicrobial Advisory Board |
| PHEMCE = Public Health Emergency Medical Countermeasure Enterprise |
| PK/PD = pharmacokinetic/pharmacodynamic |
| PPP = public-private partnership |
| R&D = research and development |
| REMS = Risk Evaluation and Mitigation Strategies |
| STAAR Act = Strategies to Address Antimicrobial Resistance Act |
| USDA = US Department of Agriculture |
| VABP = ventilator-associated bacterial pneumonia |
| XDR = extremely drug resistant |
Appendix B: RELEVANT STATEMENTS BY THE US SENATE APPROPRIATIONS COMMITTEE
The US Senate Appropriations Committee Stated in the 111th Congress:
“Antimicrobial Resistance—The Committee strongly urges the National Institute of Allergy and Infectious Diseases to devote additional resources to developing new antibacterial drugs. Priority bacteria include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and ESBL positive bacteria such as E. coli and Enterobacter species, which cause the majority of healthcare-associated infections. Rapid diagnostic tests that support antibacterial clinical trials and antibiotics' appropriate use are also needed.
…Antibacterial resistance and the diminishing antibacterial pipeline are complex problems. Multi-pronged solutions are required to sufficiently limit the impact of antibacterial resistance on patients and the public and to spur the development of products to address antibacterial resistant infections. The Committee encourages the Assistant Secretary for Preparedness and Response and the National Institute for Allergy and Infectious Diseases to create a seamless approach to the research and development of new antibacterial drugs, particularly those designed to combat Gram-negative infections, which will help the transition across the spectrum of enterprise from basic research to product development and procurement.” [60]
With Respect to FDA Funding, the US Senate Agriculture Appropriations Subcommittee Stated:
“Antimicrobial Resistance—Antimicrobial resistance, and the resulting failure of antimicrobial therapies in humans, is a mounting public health concern.
Antibiotic Development—The Committee continues to be concerned about unresolved scientific issues regarding clinical development in the antibacterial drug arena, which has been identified as a serious impediment to new antibacterial development. In its report last year, the Committee directed FDA to issue clinical trial guidance for several serious indications. The Committee directs FDA to report [back to the Committee] by December 3, 2010, on its progress, including the status of FDA's work toward a final guidance on community-acquired bacterial pneumonia as well as how FDA plans to address guidance for multi-drug and pan-resistant organisms.
The Committee last year also encouraged FDA to identify ways to promote the development and/or appropriate use of priority antibacterial drugs for humans for which current market incentives are inadequate, by working with other governmental entities and interested parties to begin this work. The Committee directs FDA to address these issues in its December 2010 report, as well.” [61]
Appendix C: AN OVERVIEW OF SUPERIORITY AND NON-INFERIORITY TRIALS OF ANTIBIOTICS
I. Background
There are two broad categories of clinical trials used to determine if a new drug should be approved by FDA: superiority trials and non-inferiority trials [100, 120, 121]. Complexities exist for execution and interpretation of both types of studies, particularly when applied to antibiotics for the treatment of serious or life-threatening infections.
II. Superiority Trials of Antibiotics
There are two major categories of superiority clinical trials [120, 121]. In one form, the experimental drug is compared with placebo (“placebo-controlled trial”). In the second form, the experimental drug is compared with another drug (“active-controlled trial”). A third study design, “historically-controlled” trials, may compare the experimental drug to either background medical care or to an active control, and are only acceptable for registrational trials (i.e., to support drug approval) under very strict conditions [98]. Historically-controlled trials are discussed briefly with active-controlled trials below.
A. Placebo-Controlled Superiority Trials of Antibiotics
Placebo-controlled superiority trials are ethical to conduct if: 1) there is no available therapy that is known to be effective for the disease being studied (i.e., there is “equipoise” regarding the benefit of any available therapy); or 2) if the disease being studied is unlikely to cause harm to the patient before effective rescue therapy can be provided if the patient's disease progresses while being treated with placebo [120]. The clear and substantial efficacy of antibiotics for serious and life-threatening bacterial infections, both in terms of lives saved and prevention of morbidity, precludes conduct of placebo-controlled trials for these infections (Table 3 and [53–55, 62, 118, 119, 122]). Furthermore, the rapidity of progression of typical bacterial infections precludes the use of effective escape therapy to prevent harm from patients being treated with placebo in most settings. One example of a setting in which placebo-controlled studies may be ethical to conduct for antibiotics is uncomplicated urinary tract infection. However, the established efficacy of antibiotic therapy for such infections makes enrollment of patients into such studies very difficult from a practical perspective, even if ethically acceptable. Thus, for most serious and life-threatening bacterial infections, placebo-controlled trials cannot be conducted.
B. Active-Controlled Superiority Trials of Antibiotics.
An experimental antibiotic that can kill bacteria resistant to a comparator antibiotic should have superior efficacy to that comparator antibiotic when treating patients infected with those resistant bacteria. However, in conducting an active-controlled clinical trial, the comparator antibiotic(s) must be selected so as to not deprive patients of available effective therapy. Therefore, active-controlled superiority trials of antibiotics are ethical to conduct only if: 1) the control (i.e., the comparator antibiotic) is active against most, or all, of the bacterial strains likely to be encountered in the study; OR 2) all available antibiotics that could be used as comparators for the study are inadequately active against the strains likely to be encountered, such that effective therapy is not being denied to patients; OR 3) effective rescue therapy can be instituted rapidly enough to preclude serious illness upon recognition that the strain causing the infection is resistant to the comparator drug (e.g., uncomplicated urinary tract infections).
The susceptibility (ability of antibiotics to kill the bacteria) of the disease-causing bacteria is almost never known at the time an infected patient is enrolled in a clinical trial evaluating initial antibiotic treatment. Therefore, the comparator drugs chosen for study in antibiotic clinical trials are selected because they are anticipated to be effective against all, or almost all, strains likely to be encountered during conduct of the study. Because antibiotic therapy is generally so effective when treating infections caused by susceptible bacteria, it is unlikely that an experimental antibiotic can achieve superiority to a marketed comparator antibiotic when the bacteria causing the infections under study are susceptible to both antibiotics. In most circumstances, such studies pose an unacceptable risk to the study sponsor of failing to show that that the experimental antibiotic is superior to the comparator antibiotic, even if the experimental antibiotic is, in fact, highly effective.
One scenario in which active-controlled superiority clinical trials of antibiotics are intuitively both ethical to conduct and can be reasonably expected to achieve superiority is the study of an experimental antibiotic with efficacy against PDR bacteria. Since no antibiotic is available that is effective to treat PDR bacteria, the experimental antibiotic would have a reasonable chance to show superiority if it was active against the target bacteria. The possible efficacy of the experimental antibiotic for treating infections caused by PDR pathogens raises questions about the ethics of randomizing patients in a pivotal study to the chance of treatment with an ineffective standard comparator regimen. However, a superiority study is ethical in this situation because: 1) the safety profile of the experimental drug is not established, while the safety profile of the comparator regimen is established; and 2) the efficacy of the experimental regimen is possible, but not yet definitively established.
It should also be possible to study infections caused by PDR bacteria in historically-controlled trials, such that all patients under active investigation in a trial are receiving experimental therapy, with objective outcomes compared to those achieved in historical controls treated with standard therapy in compliance with regulatory standards [98]. There are many practical barriers to conduct of superiority trials for antibiotics, underscoring IDSA's call for FDA to establish guidance on the conduct of such studies to standardize and clarify their appropriate design.
III. Non-Inferiority Clinical Trials of Antibiotics
Since superiority studies cannot be conducted for most serious infections, the only possible pathway to approval for many new antibiotics is the conduct of a “non-inferiority” clinical trial, which seeks to determine if the experimental antibiotic is similar in efficacy to a standard drug already on the market. For the last few years, FDA has been reconsidering the standards it uses to judge non-inferiority clinical trials. This re-evaluation of the regulatory standards for non-inferiority trials is the result of both a greater understanding of the statistical complexities underpinning the interpretation of results from non-inferiority trials [100, 120, 123–126], as well as intense public scrutiny in the aftermath of highly publicized post-approved drug failures, such as that of telithromycin [76, 127, 128], for which questions of safety and appropriateness of non-inferiority trial conduct were raised.
The fundamental statistical dilemma regarding interpretation of the results of non-inferiority trials relates to the fact that experimental drugs are not directly compared with placebo/no therapy in a non-inferiority study [98, 121]. Therefore, if the experimental drug is found to be “non-inferior” to the comparator drug, there are two possible statistical interpretations: 1) both drugs are superior to placebo for the disease under study, and the experimental drug should be approved by the regulatory agency; OR 2) neither drug is superior to placebo for the disease under study, and the reason why the drugs appear to have similar efficacy is that a similar placebo-effect is seen in both arms. Approval of the experimental drug under the latter scenario would result in marketing of an ineffective drug to the public.
The following is a simple logic flow that can be used to ensure that ineffective drugs are not approved as a result of successful non-inferiority studies:
1) If the comparator drug is known to be superior in efficacy to placebo from prior studies, AND
2) the experimental drug is similar in efficacy to the comparator drug, THEN
3) the experimental drug also must be superior in efficacy to placebo.
By this logic, non-inferiority clinical trials should only be used when the comparator drug has been previously shown to be superior to placebo. Unfortunately, this desire for previous randomized placebo-controlled trials, while logical, is also the fundamental underpinning for why antibiotic development, out of proportion to other drugs, has been so severely impacted by the current regulatory environment. Antibiotics were among the first effective drugs, and became available in the US in late 1936, fully two decades before randomized placebo-controlled trials came into widespread use [119, 129]. Thus, for virtually all serious infections, there are no randomized, placebo-controlled studies to precisely define how effective comparator antibiotics are, which makes problematic the design of modern non-inferiority studies for these diseases.
Nevertheless, as discussed in this policy paper, less sophisticated studies from the 1930s–1940s unequivocally document a massive survival benefit of antibiotics for serious bacterial infections (Table 3). Overall, the rate of death from infections in the US fell by ∼220 per 100,000 population during the first 15 years of the antibiotic era; that rate of death then fell only by a further ∼20 per 100,000 over the following 45 years, during which time all other advances in modern medicine (including critical care medicine) were achieved [62, 113]. There is no question that antibiotics are life-saving for serious bacterial infections.
It has also been argued that non-inferiority trials should not be conducted because what society needs are “better” drugs, not “non-inferior” drugs, to treat antibiotic-resistant infections. However, as discussed, patients in whom a new antibiotic is likely to be superior to an old antibiotic are those infected by bacteria resistant to the old drug. Such patients cannot ethically be enrolled in the clinical trial, since they cannot be randomized to a chance of receiving ineffective treatment. For example, when studying a new antibiotic with efficacy against MRSA, one cannot randomize patients infected with MRSA to a 50% chance of being treated with methicillin. Instead one has to compare a new antibiotic to an old antibiotic for the treatment of infections susceptible to both drugs. In these comparisons, the new drug is very unlikely to be superior to an effective old drug, making such studies impractical.
Sufficient data are available to ensure that comparator antibiotics used in non-inferiority studies for new antibiotics are massively more effective than placebo. Because antibiotics do have very large treatment effects for serious bacterial infections and because superiority studies of antibiotics are impractical in most cases, non-inferiority studies are relevant and necessary to support development and approvals of new antibiotics.
References
- 1.Walker B, Barrett S, Polasky S, et al. Environment. Looming global-scale failures and missing institutions. Science. 2009;325:1345–6. doi: 10.1126/science.1175325. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170:347–53. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–84. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 7.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54:109–15. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31:365–73. doi: 10.1086/651094. [DOI] [PubMed] [Google Scholar]
- 9.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 10.The bacterial challenge: time to react a call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. Available at: http://www.ema.europa.eu/pdfs/human/antimicrobial_resistance/53394009en.pdf. Accessed 1 August 2010. [Google Scholar]
- 11.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 12.Shlaes DM, Moellering RC., Jr The United States Food and Drug Administration and the end of antibiotics. Clin Infect Dis. 2002;34:420–2. doi: 10.1086/338976. [DOI] [PubMed] [Google Scholar]
- 13.Lederberg J. Infectious history. Science. 2000;288:287–93. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 14.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 15.Moellering RC., Jr The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:368–70. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- 16.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 17.Chambers HF. Community-associated MRSA–resistance and virulence converge. N Engl J Med. 2005;352:1485–7. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 18.Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–90. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 20.Wadl M, Heckenbach K, Noll I, et al. Increasing occurrence of multidrug-resistance in Acinetobacter baumannii isolates from four German University Hospitals, 2002–2006. Infection. 2010;38:47–51. doi: 10.1007/s15010-009-9225-x. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann MS, Eber MR, Laxminarayan R. Increasing resistance of acinetobacter species to imipenem in United States hospitals, 1999–2006. Infect Control Hosp Epidemiol. 2010;31:196–7. doi: 10.1086/650379. [DOI] [PubMed] [Google Scholar]
- 22.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–8. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 23.Dizbay M, Tunccan OG, Sezer BE, Hizel K. Nosocomial imipenem-resistant Acinetobacter baumannii infections: epidemiology and risk factors. Scand J Infect Dis. 2010;42:741–6. doi: 10.3109/00365548.2010.489568. [DOI] [PubMed] [Google Scholar]
- 24.Valencia R, Arroyo LA, Conde M, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30:257–63. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 25.Park YK, Jung SI, Park KH, et al. Independent emergence of colistin-resistant Acinetobacter spp. isolates from Korea. Diagn Microbiol Infect Dis. 2009;64:43–51. doi: 10.1016/j.diagmicrobio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Lautenbach E, Synnestvedt M, Weiner MG, et al. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2009;30:1186–92. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 27.Adams MD, Nickel GC, Bajaksouzian S, et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009;53:3628–34. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32:450–4. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 29.McGowan JE., Jr Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. Am J Med. 2006;119(6 Suppl 1):S29–36. doi: 10.1016/j.amjmed.2006.03.014. discussion S62–70. [DOI] [PubMed] [Google Scholar]
- 30.Bradford PA, Bratu S, Urban C, et al. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis. 2004;39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 31.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–5. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 32.Mastoraki A, Douka E, Kriaras I, Stravopodis G, Manoli H, Geroulanos S. Pseudomonas aeruginosa susceptible only to colistin in intensive care unit patients. Surg Infect (Larchmt) 2008;9:153–60. doi: 10.1089/sur.2007.004. [DOI] [PubMed] [Google Scholar]
- 33.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. Imipenem resistance among pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2006;27:893–900. doi: 10.1086/507274. [DOI] [PubMed] [Google Scholar]
- 34.Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Infect Control. 2006;34(5 Suppl 1):S20–8. doi: 10.1016/j.ajic.2006.05.238. discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 35.Muratani T, Matsumoto T. Urinary tract infection caused by fluoroquinolone- and cephem-resistant Enterobacteriaceae. Int J Antimicrob Agents. 2006;28(Suppl. 1):S10–3. doi: 10.1016/j.ijantimicag.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Kang CI, Kim SH, Park WB, et al. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 2004;48:4574–81. doi: 10.1128/AAC.48.12.4574-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang CI, Kim SH, Kim DM, et al. Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2004;25:860–7. doi: 10.1086/502310. [DOI] [PubMed] [Google Scholar]
- 38.Woerther PL, Angebault C, Lescat M, et al. Emergence and dissemination of extended-spectrum beta-lactamase-producing Escherichia coli in the community: lessons from the study of a remote and controlled population. J Infect Dis. 2010;202:515–23. doi: 10.1086/654883. [DOI] [PubMed] [Google Scholar]
- 39.Kang CI, Song JH, Chung DR, et al. Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2010;36:284–7. doi: 10.1016/j.ijantimicag.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Bano J, Picon E, Gijon P, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50:40–8. doi: 10.1086/649537. [DOI] [PubMed] [Google Scholar]
- 41.Lee MY, Choi HJ, Choi JY, et al. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J Infect. 2010;60:146–53. doi: 10.1016/j.jinf.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Azap OK, Arslan H, Serefhanoglu K, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147–51. doi: 10.1111/j.1469-0691.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750. [PubMed] [Google Scholar]
- 45.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–81. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 47.Testimony of the Infectious Diseases Society of America (IDSA) Presented by Brad Spellberg, MD, FIDSA before the house committee on energy and commerce subcommittee on health. June 9th. Antibiotic resistance: promoting critically needed antibiotic research and development and appropriate use ("Stewardship") of these precious drugs. Available at: http://www.idsociety.org/10x20.htm. Accessed 12 February 2011. [Google Scholar]
- 48.Morel CM, Mossialos E. Stoking the Antibiotic Pipeline. BMJ. 2010;340:1115–18. doi: 10.1136/bmj.c2115. [DOI] [PubMed] [Google Scholar]
- 49.Bad Bugs, No drugs: as antibiotic discovery stagnates, a public health crisis brews. Available at: http://www.idsociety.org/BBNDWhitePaper04.htm. Accessed 12 February 2011. [Google Scholar]
- 50.Dellit TH, Owens RC, McGowan JE, Jr., et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 51.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 52.Spellberg B, Fleming TR, Gilbert DN. Executive summary: workshop on issues in the design and conduct of clinical trials of antibacterial drugs in the treatment of community-acquired pneumonia. Clin Infect Dis. 2008;47(Suppl. 3):S105–7. doi: 10.1086/591389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spellberg B, Talbot GH, Brass EP, Bradley JS, Boucher HW, Gilbert D. Position paper design features of future clinical trials of Anti-bacterial agents for community-acquired pneumonia. Clin Infect Dis. 2008;47:S249–65. [PMC free article] [PubMed] [Google Scholar]
- 54.Spellberg B, Talbot GH For the Infectious Diseases Society of America (IDSA), American College of Chest Physicians (ACCP), American Thoracic Society (ATS), and Society of Critical Care Medicine (SCCM) Recommended design features of future clinical trials of anti-bacterial agents for Hospital-Acquired Bacterial Pneumonia (HABP) and Ventilator-Associated Bacterial Pneumonia (VABP) Clin Infect Dis. 2010;51(Suppl 1):S150–70. doi: 10.1086/653065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spellberg B, Talbot GH, Boucher HW, et al. Antimicrobial agents for complicated skin and skin structure infections: justification of non-inferiority margins in the absence of placebo-controlled trials. Clin Infect Dis. 2009;49:383–91. doi: 10.1086/600296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choffnes ER, Relman DA, Mack A. Antibiotic resistance: implications for global health and novel intervention strategies: workshop summary. For the institute of medicine, forum on antimicrobial threats. Washington, DC: The National Academies Press; 2010. Available at: http://www.iom.edu/Home/Reports/2010/Antibiotic-Resistance-Implications-for-Global-Health-and-Novel-Intervention-Strategies.aspx. Accessed 12 February 2011. [PubMed] [Google Scholar]
- 57.Infectious Diseases Society of America. The 10 × '20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–83. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 58.Organizations Endorsing IDSA's 10 × ’20 initiative as of January 26, 2011. Available at: http://www.idsociety.org/10x20endorsers.htm. Accessed 26 January 2011. [Google Scholar]
- 59.The public health emergency medical countermeasures enterprise review: transforming the enterprise to meet long-Range national needs. Available at: http://www.hhs.gov/nvpo/nvac/meetings/upcomingmeetings/korch_presentation.pdf. Accessed 12 February 2011. [Google Scholar]
- 60.Senate report 111–243, departments of Labor, health and human services, and education, and related agencies appropriation bill, 2011; 111th congress, 2nd session. Available at: http://www.gpo.gov/fdsys/pkg/CRPT-111srpt243/pdf/CRPT-111srpt243.pdf. Accessed 12 February 2011. [Google Scholar]
- 61.Senate report 111–221, report on agriculture, rural development, food and drug administration and relate agencies appropriation bill, 2011; 111th congress, 2nd session 2011, pg. 111–221. Available at: http://www.gpo.gov/fdsys/pkg/CRPT-111srpt221/pdf/CRPT-111srpt221.pdf. Accessed 12 February 2011. [Google Scholar]
- 62.Spellberg B. The antibacterial pipeline: why is it drying up, and what must be done about it? In: Choffnes ER, Relman DA, Mack A, editors. Antibiotic resistance: implications for global health and novel intervention strategies: workshop summary for the institute of medicine, forum on antimicrobial threats. Washington, DC: The National Academies Press; 2010. pp. 299–332. [PubMed] [Google Scholar]
- 63.Shlaes DM. The abandonment of antibacterials: why and wherefore? Curr Opin Pharmacol. 2003;3:470–3. doi: 10.1016/j.coph.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6:427–30. doi: 10.1016/j.mib.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Projan SJ, Shlaes DM. Antibacterial drug discovery: is it all downhill from here? Clin Microbiol Infect. 2004;10(Suppl. 4):18–22. doi: 10.1111/j.1465-0691.2004.1006.x. [DOI] [PubMed] [Google Scholar]
- 66.Tillotson G. Stimulating antibiotic development. Lancet Infect Dis. 2010;10:2–3. doi: 10.1016/S1473-3099(09)70333-0. [DOI] [PubMed] [Google Scholar]
- 67.Tillotson GS. Where does novel antibiotics R&D stand among other pharmaceutical products: an industrial perspective? Expert Rev Anti Infect Ther. 2008;6:551–2. doi: 10.1586/14787210.6.5.551. [DOI] [PubMed] [Google Scholar]
- 68.Council of the European Union: council conclusions (see#16) on innovative incentives for effective antibiotics. Available at: http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/111608.pdf. Accessed 31 January 2011. [Google Scholar]
- 69.Stop TB Partnership. Available at: http://www.stoptb.org. Accessed 26 January 2011. [Google Scholar]
- 70.TB Alliance: global alliance for TB drug development. Available at: http://www.tballiance.org/home/home.php. Accessed 26 January 2011. [Google Scholar]
- 71.Critical path to TB drug regimens. Available at: http://cptrinitiative.org/. Accessed 26 January 2011. [Google Scholar]
- 72.Hardin G. The tragedy of the commons. Science. 1968;162:1243–8. [PubMed] [Google Scholar]
- 73.Tillotson GS. Development of new antibacterials: a laudable aim, but what is the value? Clin Infect Dis. 2010;51:752–3. doi: 10.1086/655956. ; author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 74.Falagas ME, Fragoulis KN, Karydis I. A comparative study on the cost of new antibiotics and drugs of other therapeutic categories. PLoS One. 2006;1:e11. doi: 10.1371/journal.pone.0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spellberg B, Lewis RJ, Boucher HW, Brass EP. Design of clinical trials of antibacterial agents for community acquired bacterial pneumonia (CABP) Clin Invest. 2011;1:19–32. doi: 10.4155/cli.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shlaes DM, Moellering RC. Telithromycin and the FDA: implications for the future. Lancet Infect Dis. 2008;8:83–5. doi: 10.1016/S1473-3099(08)70002-1. [DOI] [PubMed] [Google Scholar]
- 77.European Antimicrobial Resistance Surveillance Network (EARS-Net) Available at: http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/Pages/index.aspx. Accessed 26 January 2011. [Google Scholar]
- 78.European surveillance of antimicrobial consumption. Available at: http://app.esac.ua.ac.be/public/. Accessed 26 January 2011. [Google Scholar]
- 79.Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–7. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 80.Young EJ, Sewell CM, Koza MA, Clarridge JE. Antibiotic resistance patterns during aminoglycoside restriction. Am J Med Sci. 1985;290:223–7. doi: 10.1097/00000441-198512000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Lipworth AD, Hyle EP, Fishman NO, et al. Limiting the emergence of extended-spectrum Beta-lactamase-producing enterobacteriaceae: influence of patient population characteristics on the response to antimicrobial formulary interventions. Infect Control Hosp Epidemiol. 2006;27:279–86. doi: 10.1086/503016. [DOI] [PubMed] [Google Scholar]
- 82.White AC, Jr., Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis. 1997;25:230–9. doi: 10.1086/514545. [DOI] [PubMed] [Google Scholar]
- 83.Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009;6:e1000084. doi: 10.1371/journal.pmed.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62:1407–12. doi: 10.1093/jac/dkn376. [DOI] [PubMed] [Google Scholar]
- 85.Meier FA, Howland J, Johnson J, Poisson R. Effects of a rapid antigen test for group A streptococcal pharyngitis on physician prescribing and antibiotic costs. Arch Intern Med. 1990;150:1696–700. [PubMed] [Google Scholar]
- 86.Worrall G, Hutchinson J, Sherman G, Griffiths J. Diagnosing streptococcal sore throat in adults: randomized controlled trial of in-office aids. Can Fam Physician. 2007;53:667–71. [PMC free article] [PubMed] [Google Scholar]
- 87.Humair JP, Revaz SA, Bovier P, Stalder H. Management of acute pharyngitis in adults: reliability of rapid streptococcal tests and clinical findings. Arch Intern Med. 2006;166:640–4. doi: 10.1001/archinte.166.6.640. [DOI] [PubMed] [Google Scholar]
- 88.Ayanruoh S, Waseem M, Quee F, Humphrey A, Reynolds T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Pediatr Emerg Care. 2009;25:748–50. doi: 10.1097/PEC.0b013e3181bec88c. [DOI] [PubMed] [Google Scholar]
- 89.Northey EH. The sulfonamides and allied compounds. New York: Reinhold Publishing, Inc.; 1948. [Google Scholar]
- 90.International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Guidance for industry. E10, choice of control group and related issues in clinical trials. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm125912.pdf. Accessed 15 June 2009. [Google Scholar]
- 91.Guidance for industry: non-inferiority trials. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed 28 July 2010. [Google Scholar]
- 92.Temple RJ. Active control non-inferiority studies: theory, assay sensitivity, choice of margin. Available at: http://www.fda.gov/ohrms/dockets/ac/02/slides/3837s1_02_Temple.ppt. Accessed August 1, 2010. [Google Scholar]
- 93.Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–60. doi: 10.1086/377539. [DOI] [PubMed] [Google Scholar]
- 94.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 95.Milo G, Katchman E, Paul M, Christiaens T, Baerheim A, Leibovici L. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004682.pub2. 2005 Apr 18;(2):CD004682. [DOI] [PubMed] [Google Scholar]
- 96.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 97.Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in Ventilator Associated Pneumonia - a randomized study. Eur Respir J. 2009;34:1364–75. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 98.Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010;48:2325–9. doi: 10.1128/JCM.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 100.Guidance for Industry. Community-acquired bacterial pneumonia: developing drugs for treatment. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm123686.pdf. Accessed 15 June 2009. [Google Scholar]
- 101.Institute of Medicine. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2010/A-National-Cancer-Clinical-Trials-System-for-the-21st-Century-Reinvigorating-the-NCI-Cooperative/NCI%20Cancer%20Clinical%20Trials%202010%20%20Report%20Brief.pdf. Accessed 1 December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Young RC. Cancer clinical trials—a chronic but curable crisis. N Engl J Med. 2010;363:306–9. doi: 10.1056/NEJMp1005843. [DOI] [PubMed] [Google Scholar]
- 103.Peters NK, Dixon DM, Holland SM, Fauci AS. The research agenda of the National Institute of Allergy and Infectious Diseases for antimicrobial resistance. J Infect Dis. 2008;197:1087–93. doi: 10.1086/533451. [DOI] [PubMed] [Google Scholar]
- 104.Gilbert DN, Spellberg B, Bartlett JG. For the infectious diseases society of America. Position paper. An Unment medical need: rapid molecular diagnostic tests for respiratory tract infections. Clin Infect Dis. 2011 doi: 10.1093/cid/cir055. In Press. [DOI] [PubMed] [Google Scholar]
- 105.Guidance for Industry #152: evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. Available at: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM052519.pdf. Accessed 13 Aug 2010. [Google Scholar]
- 106.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 107.Shmeck H. Health of nation lags behind scientific gains. Available at: http://spiderbites.nytimes.com/pay_1971/articles_1971_07_00001.html. Accessed 28 April 2009. [Google Scholar]
- 108.Stewart WH. Symposium schools of public health: changing institutions in a changing world three papers presented on the occasion of the dedication of the Ernest Lyman Stebbins Building September 18, 1968 The Johns Hopkins University School of Hygiene Public Health. Baltimore, MD: Johns Hopkins University; 1968. Areas of challenge for the future. [Google Scholar]
- 109.DHEW Publication No. (HSM) 73-1265; PHS Publication No. 1000-Series 4-No. 7, March, 1968. Page 2. Use of vital and health records in epidemiologic research: A report of the United states National Committee on vital and health Statistics. Available at: http://www.cdc.gov/nchs/data/series/sr_04/sr04_007.pdf. Accessed 28 April 2009. [PubMed] [Google Scholar]
- 110.McDermott W, Rogers DE. Social ramifications of control of microbial disease. Johns Hopkins Med J. 1982;151:302–12. [PubMed] [Google Scholar]
- 111.Thomas L. Notes of a medicine-w atcher. New York: The Viking Press; 1983. The youngest science. [Google Scholar]
- 112.Is endocarditis lenta always fatal? Lancet. 1935;226:383–4. [Google Scholar]
- 113.Kerr AJ. Subacute bacterial endocarditis. Springfield, IL: Charles C Thomas; 1955. [Google Scholar]
- 114.Guest CM, Harrison FF. Acute endocarditis due to Staphylococcus aureus successfully treated with penicillin. Am J Med. 1948;5:908–11. doi: 10.1016/0002-9343(48)90170-3. [DOI] [PubMed] [Google Scholar]
- 115.Christie RV. Penicillin in subacute bacterial endocarditis. Br Med J. 1949;2:950. doi: 10.1136/bmj.2.4634.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chemotherapy of meningitis. Lancet. 1938;231:733–4. [Google Scholar]
- 117.Waring GW, Weinstein L. The treatment of pneumococcal meningitis. Am J Med. 1948;5:402–18. doi: 10.1016/0002-9343(48)90090-4. [DOI] [PubMed] [Google Scholar]
- 118.Madsen ST. Scarlet fever and erysipelas in Norway during the last hundred years. Infection. 1973;1:76–81. doi: 10.1007/BF01638479. [DOI] [PubMed] [Google Scholar]
- 119.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–60. [PubMed] [Google Scholar]
- 120.Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455–63. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 121.International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Guidance for Industry. E9, statistical principles for clinical trials. Available at: http://www.emea.europa.eu/pdfs/human/ich/036396en.pdf. Accessed 15 June 2009. [Google Scholar]
- 122.McDermott W, Deuschle K, Adair J, Fulmer H, Loughlin B. Introducing modern medicine in a Najavo community. Science. 1960;131:197–205. doi: 10.1126/science.131.3395.197. [DOI] [PubMed] [Google Scholar]
- 123.Fleming TR. Current issues in non-inferiority trials. Stat Med. 2008;27:317–32. doi: 10.1002/sim.2855. [DOI] [PubMed] [Google Scholar]
- 124.Powers JH, Ross DB, Brittain E, Albrecht R, Goldberger MJ. The United States Food and Drug Administration and noninferiority margins in clinical trials of antimicrobial agents. Clin Infect Dis. 2002;34:879–81. doi: 10.1086/339803. [DOI] [PubMed] [Google Scholar]
- 125.Powers JH. Noninferiority and equivalence trials: deciphering ‘similarity’ of medical interventions. Stat Med. 2008;27:343–52. doi: 10.1002/sim.3138. [DOI] [PubMed] [Google Scholar]
- 126.Guidance for Industry: non-inferiority clinical trials. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf Accessed. [Google Scholar]
- 127.Ross DB. The FDA and the case of Ketek. N Engl J Med. 2007;356:1601–4. doi: 10.1056/NEJMp078032. [DOI] [PubMed] [Google Scholar]
- 128.Soreth J, Cox E, Kweder S, Jenkins J, Galson S. Ketek–the FDA perspective. N Engl J Med. 2007;356:1675–6. doi: 10.1056/NEJMc076135. [DOI] [PubMed] [Google Scholar]
- 129.Doll R. Controlled trials: the 1948 watershed. BMJ. 1998;317:1217–20. doi: 10.1136/bmj.317.7167.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]