Abstract
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study randomized HIV-infected mothers and their infants to receive either maternal lipid-based nutrient supplements (LNS) during lactation or no LNS and then to 1 of 3 antiretroviral drug (ARV) arms (maternal, infant, or no drugs). Assigned interventions were provided from 0 to 28 wk and all infants (n = 1619) were given LNS during (24–28 wk) and following (28–48 wk) weaning. This paper assesses the feasibility of infant LNS as a breastmilk replacement and uses longitudinal random effects models to examine associations of interventions, morbidity, and season with weight-for-age (WAZ), length-for-age (LAZ), and BMI-for-age (BMIZ) Z-scores from 24 to 48 wk. Infant LNS adherence was high (94.1% ate it daily). From 24 to 48 wk, mean WAZ (−0.42 to −0.76 SD; P < 0.001) and LAZ (−0.93 to −1.56 SD; P < 0.001) steadily declined, whereas BMIZ remained >0 throughout. A higher LAZ was associated with assignment to the maternal LNS arm (β=0.19; P < 0.05). Lower WAZ and BMIZ were associated with seasonal food insecurity (β=−0.08 and −0.09, respectively; both P < 0.001), fever (β=−0.07 and −0.13; both P < 0.001), diarrhea (β=−0.19 and −0.23; both P < 0.001), and assignment to the infant ARV arm (β=−0.17 and −0.17; both P < 0.05). The magnitude of the season and morbidity effects was small and BAN infants had higher weights and lengths than their counterparts in the general population. High LNS adherence and the modest impact of morbidity on growth indicate that LNS is a feasible breastmilk replacement for HIV-exposed infants weaned early, but controlled trials are needed to quantify the effects of LNS on growth in this population.
Introduction
In resource-poor settings, breastfeeding is essential to promote optimal infant growth and health. However, for HIV-infected women, breastfeeding is associated with risk of mother-to-child transmission (MTCT)12 (1–4). Early weaning, particularly with poor quality or unhygienically prepared complementary foods, may contribute to growth faltering in HIV-exposed infants (5–11). Thus, it is critical to find strategies that prevent MTCT and at the same time promote healthy growth and development.
In most countries, health workers counsel HIV-infected mothers about infant feeding based on international recommendations, which have changed substantially during the last decade (12–14). In 2003, the WHO advised that HIV-infected mothers exclusively breastfeed for the first months of life and rapidly wean their infants as soon as replacement feeding was acceptable, feasible, affordable, sustainable, and safe (AFASS) (13). Within a few years, it was apparent that early weaning of HIV-exposed infants did not improve survival (15). These data prompted WHO to recommend in 2007 that HIV-infected women exclusively breastfeed for 6 mo and continue breastfeeding after 6 mo unless replacement feeding was AFASS (16). Additional studies showed that HIV-exposed infants weaned early sustained higher rates of diarrheal morbidity and mortality over time and were more likely to be underweight than those who were breastfed longer (5–11, 17, 18). These data, together with evidence that antiretroviral drugs (ARV) prevent HIV transmission through breastmilk (3), led to the recommendation in 2010 that HIV-infected women exclusively breastfeed for 6 mo and continue breastfeeding to 12 mo when ARVs are available (12).
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study was designed in the context of the 2003 HIV and infant feeding recommendations. HIV-infected mothers and their infants were randomized at delivery to receive either maternal nutrition supplementation during lactation or no supplementation and then to 1 of 3 ARV arms (maternal, infant, or no drugs). The randomized interventions were provided from 0 to 28 wk. Mothers in the BAN Study were counseled to exclusively breastfeed for 24 wk then wean their infants within 4 wk (19). To address the possibility of inadequate feeding following weaning (20), all infants were provided with lipid-based nutrient supplements (LNS) from 24 to 48 wk of age. LNS are ready-to-use foods typically composed of peanut butter, milk powder, sugar, vegetable oil, and micronutrients. They have been successfully used for community-based care of children with severe and moderate acute malnutrition (21, 22) and there is evidence that they promote child growth and development (23, 24). LNS are attractive for infant feeding in low-resource settings, because they require no preparation, can be fed to the child directly, and do not support bacterial growth (25). To our knowledge, LNS have not previously been used as a breastmilk replacement for HIV-exposed infants during the second half of infancy.
In many low-income countries, adherence to the current WHO recommendations may be challenging due to inconsistent availability and accessibility of ARVs, and maternal decisions about infant feeding do not always conform to recommendations (26–29). Therefore, it remains important to understand what types of strategies maintain growth and limit morbidity of HIV-exposed infants once semi-solid and solid foods are introduced. The use of LNS as a breastmilk replacement during the second half of infancy could serve this purpose.
The main aims of this research were to: 1) describe infant growth patterns from 24 to 48 wk, when LNS was given as a breastmilk replacement; 2) identify factors that potentially influence growth in weaned, HIV-exposed infants during the second 6 mo of life, with a focus on morbidity, season, and residual effects of interventions provided from 0 to 28 wk; and 3) determine if the use of LNS as a breastmilk replacement is feasible by examining data on adherence and growth. Because all BAN infants received LNS from 24–48 wk, we compared the growth of study infants with that of infants of the same age in the Malawi Demographic and Health Survey (DHS).
Methods
Study population
The BAN Study recruited HIV-1-infected, pregnant women at antenatal clinics in Lilongwe, Malawi. The eligibility criteria have been described in detail elsewhere (3). Briefly, at screening, mothers had CD4+ count ≥250 cells/mm3, hemoglobin ≥70 g/L, and no prior ARV use. Infants had a birth weight ≥2 kg and were excluded if they were HIV-positive within the first 2 wk after delivery [infant HIV testing procedures described in (30)].
Ethical approval for the study was obtained from the Malawi National Health Science Research Committee and the institutional review boards at the University of North Carolina at Chapel Hill and the U.S. CDC.
Study interventions
Following delivery, eligible mother-infant pairs were randomized using a 2 × 3 factorial design to 1 of 6 study arms: maternal LNS/maternal ARV (mLNS-mARV), maternal ARV (mARV), maternal LNS/infant ARV (mLNS-iARV), infant ARV (iARV), maternal LNS (mLNS), or control (C). The maternal nutrition intervention was given in the form of 140 g/d of LNS designed to meet the energy, micronutrient, and protein needs of lactation (Supplemental Table 1). The mARV intervention consisted of a multi-drug, highly active regimen, while infants received daily oral nevirapine. Assigned interventions began at birth and continued through 28 wk.
The present analysis uses data from 24 to 48 wk, during (24–28 wk) and after weaning (28–48 wk). All infants initially received 75 g/d of LNS starting at 24 wk and the quantity increased to 100 g/d as the infants grew. The LNS was supplied by 3 local producers (Rab Processors, Project Peanut Butter, and Valid International). The LNS made by Rab Processors contained sugar, peanut paste, soy milk powder, soy oil, soy protein, vitamins and minerals, and vanilla flavor. A total of ~28 infants (<2%) received this version of LNS for ≥1 mo. The study discontinued use of Rab’s LNS in May 2005. The majority of BAN infants received LNS made by Project Peanut Butter and Valid International using Nutriset’s recipe containing groundnuts, dried full-cream milk, vegetable oil, sugar, and vitamin-mineral premix. A 100 g/d LNS ration provided 2300 kJ, 15 g protein, and at least the recommended daily amount of 22 vitamins and minerals (Supplemental Table 1). Infants were given LNS to replace the energy and nutrients they would have received from breastmilk. Mothers were advised to feed their infants plain LNS from a spoon to ensure adequate intake. They were also counseled to feed the infant enriched porridge in addition to LNS and to increase portion size, feeding frequency, and the variety of foods with age. To offset LNS sharing, all BAN-enrolled women were given 2 kg/wk of maize flour for family consumption throughout their participation in the study.
Study procedures and variable definition
Study visits and data collection took place at the BAN clinic at Bwaila Hospital in Lilongwe, Malawi. Infant weight and recumbent length were measured at 0, 24, 28, 32, 36, 42, and 48 wk using Tanita digital infant scales (0.1 g increment) and length boards made to UNICEF specifications (0.1 cm increment) (31). Z-scores were calculated using the WHO growth standards (32). Faltering was defined as a decrease in Z-score >0.67 from 24 to 48 wk (33, 34).
Infant morbidity data were collected at every study visit. Mothers were asked if the infant had specific symptoms, clinical events, or hospitalizations since the last study visit. If the infant was reported to have been ill, a checklist was used to obtain details of the illness from the mother and all reports of morbidity were recorded regardless of duration. In addition, the infant was examined by a clinician. For the present analysis, we focused on 2 types of infant morbidity: fever and diarrhea. Fever and diarrhea variables were created based on both the maternal report of illness and the clinician’s physical examination, so they represent current illness and illness since the last visit.
Mothers reported on infant adherence to LNS use at 32, 42, and 48 wk. They were asked how often they fed the infant the recommended amount of LNS since their last study visit: never, sometimes (1–3 d/wk), often (4–6 d/wk), and always (7 d/wk). Information on mixed feeding and breastfeeding cessation since the last study visit was obtained by maternal report starting at 8 wk. Using the breastfeeding cessation data, we created a variable with 3 categories: stopped before 24 wk, stopped between 24 and 28 wk, and stopped after 28 wk.
Socioeconomic data were obtained from mothers during screening. The annual period of food insecurity occurs during the rainy season from mid-December to mid-April. Seasonal food insecurity was coded as none (<25%), partial (25–75%), or all (>75%) based on the estimated number of rainy season days between the current and previous study visit.
Statistical analysis
Randomized infants were included in the analysis sample if they were singletons and had at least one anthropometric measurement from 24 to 48 wk. Measurements were included until infants were confirmed to be HIV infected. Of 2369 infants enrolled at birth, 2320 were singletons. Of the 1710 infants remaining in the sample at 24 wk, 1619 had at least one measurement from 24 to 48 wk as well as data on all covariates and were included in the longitudinal analysis of weight-for-age Z-score (WAZ). There were no significant differences in socio-demographic characteristics of the randomized sample of singleton births and the sample of infants included in the WAZ analysis. Slightly fewer participants were included in the length-for-age Z-score (LAZ) (n = 1609) and BMI-for-age Z-score (BMIZ) (n = 1607) models due to missing length values.
Descriptive analyses.
Descriptive data on BAN infants were compared using paired t tests for means and χ2 tests for proportions. Z-scores for infants in the Malawi DHS were recalculated using the WHO growth standard and proportions of infants who were underweight, stunted, or had low BMIZ were calculated using sample weights to account for the survey design. The proportions of BAN and DHS infants were compared and P values were calculated using the Pearson χ2 statistic corrected for the survey design.
The proportion of infants with current or reported fever or diarrhea was calculated for each study visit and for two 12-wk periods (>24–36 and >36–48 wk). We examined sex differences in morbidity across the 12-wk periods as a possible explanation for sex differences in growth. Statistical analyses were carried out using Stata 11.2. Statistical tests were considered significant at the α = 0.05 level. Values in the text are means or proportions.
Modeling of growth patterns.
Longitudinal random effects models were used to quantify the association of potential determinants of growth with infant WAZ, LAZ, and BMIZ, controlling for corresponding birth Z-score and study visit. We considered these time-varying (season, fever, diarrhea, and infant LNS adherence) and non-time–varying (treatment arm, maternal height, maternal education, maternal age, first birth, mother’s CD4+ count at 24 wk, timing of breastfeeding cessation, and infant sex) predictor variables for inclusion in the models. Final models included variables significant at the α = 0.10 level or those shown in previous studies to have an important relationship with growth. We found no significant interaction between treatment arm and study visit. To characterize the impact of season and morbidity variables in the models, we compared the overall means for WAZ, LAZ, and BMIZ that were estimated from the fitted longitudinal models with the means predicted for a hypothetical population with no food insecurity or with no fever or diarrhea.
Results
The background characteristics of mothers and infants included in the analysis are shown in Table 1. Reported infant LNS adherence was high, with 94.1% consuming LNS 7 d/wk and 3.5% eating LNS 4–6 d/wk.
TABLE 1.
Characteristics of mother-infant pairs included in the WAZ analysis sample1
| Characteristic | WAZ analysis sample (n = 1619) |
| Mothers | |
| Age at screening, y | 26.5 ± 5.1 |
| Education beyond primary school, % | 34.9 |
| Married, % | 92.8 |
| First birth, % | 10.4 |
| Height, cm | 156.9 ± 5.5 |
| BMI <18.5, % | 5.0 |
| BMI >25, % | 19.3 |
| Infants | |
| Female sex, % | 48.3 |
| Birth WAZ | −0.55 ± 0.88 |
| Birth LAZ | −0.75 ± 1.01 |
| Birth BMIZ | −0.36 ± 1.08 |
Values are means ± SDs or percent. BMIZ, BMI-for-age Z-score; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score.
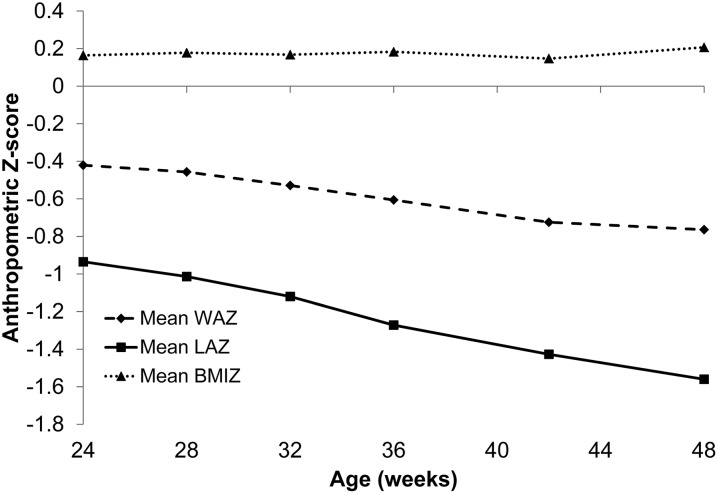
Infants’ WAZ and LAZ were less than the WHO median and decreased throughout the analysis period (Fig. 1). From 24 to 48 wk, mean infant WAZ (−0.42 to −0.76 SD; P < 0.001) and LAZ (−0.93 to −1.56 SD; P < 0.001) declined; 27.1 and 46.6% faltered in WAZ and LAZ, respectively. BMIZ was slightly greater than the WHO median from 24 to 48 wk and did not differ from the beginning to the end of the analysis period (0.16 to 0.21 SD; P = 0.30).
FIGURE 1.
Mean WAZ, LAZ, and BMIZ from 24–48 wk of infants participating in the BAN Study. BMIZ, BMI-for-age Z-score; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score.
The proportion of BAN infants who were underweight or stunted increased at each visit, whereas the proportion with low BMIZ was stable throughout the study period (Table 2). The proportion of BAN infants who were underweight, stunted, or had low BMIZ was significantly lower at all visits than for infants of similar age in the Malawi DHS, with the exception of underweight at 28 and 48 wk.
TABLE 2.
| BAN |
Malawi DHS 2004 |
|||
| n/total | % | n/total | % | |
| Underweight infants (WAZ < −2 SD) | ||||
| 24 wk | 95/1592 | 6.0 | 10/187 | 16.4*** |
| 28 wk | 113/1561 | 7.2 | 20/177 | 10.8 |
| 32 wk | 119/1470 | 8.1 | 29/174 | 19.1*** |
| 36 wk | 150/1437 | 10.4 | 29/156 | 20.9** |
| 42 wk | 159/1400 | 11.4 | 42/186 | 22.3*** |
| 48 wk | 169/1379 | 12.3 | 28/162 | 17.5 |
| Stunted infants (LAZ < −2 SD) | ||||
| 24 wk | 250/1591 | 15.7 | 48/187 | 22.1* |
| 28 wk | 283/1561 | 18.1 | 55/177 | 34.6*** |
| 32 wk | 307/1468 | 20.9 | 75/174 | 40.7*** |
| 36 wk | 351/1437 | 24.4 | 58/156 | 35.0* |
| 42 wk | 442/1400 | 31.6 | 84/186 | 44.1** |
| 48 wk | 473/1379 | 34.3 | 88/162 | 46.2** |
| Infants with low BMIZ (< −2 SD) | ||||
| 24 wk | 42/1591 | 2.6 | 28/187 | 15.4*** |
| 28 wk | 43/1561 | 2.8 | 14/177 | 8.1*** |
| 32 wk | 37/1468 | 2.5 | 20/174 | 12.1*** |
| 36 wk | 43/1437 | 3.0 | 18/156 | 11.2*** |
| 42 wk | 50/1400 | 3.6 | 19/186 | 11.5*** |
| 48 wk | 33/1379 | 2.4 | 21/162 | 13.1*** |
Values are the number of underweight, stunted, or low BMI infants divided by the total sample and the proportion at each time point. Asterisks indicate the level of significance when proportions in BAN and DHS infants were compared: *P < 0.05, **P < 0.01, ***P < 0.001. BAN, Breastfeeding, Antiretrovirals, and Nutrition; BMIZ, BMI-for-age Z-score; DHS, Demographic and Health Survey; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score.
The proportion of infants with fever (13–18%) or diarrhea (7–16%) increased from 24 to 32 wk (Supplemental Fig. 1). Dividing the 24–48 wk period into two 12-wk segments, 37% of infants had fever and 33% had diarrhea from >24 to 36 wk and 32% had fever and 31% had diarrhea from >36 to 48 wk. Diarrhea was more common in boys (35%) than girls (30%) (P < 0.05) from >24 to 36 wk, but there was no significant difference in diarrhea by sex from >36 to 48 wk and boys and girls did not differ in fever during either 12-wk segment.
Longitudinal models indicated that fever, diarrhea, seasonal food insecurity, and assignment to the iARV study arm were negatively associated with WAZ and BMIZ, but not with LAZ (Table 3). Assignment to the mLNS study arm was positively associated with LAZ. Female sex, higher maternal education, and greater maternal height were also positively associated with WAZ and LAZ. Greater maternal height was positively associated with BMIZ.
TABLE 3.
Longitudinal models showing factors associated with infant WAZ, LAZ, and BMIZ from 24–48 wk1
| WAZ |
LAZ |
BMIZ |
||||
|
n = 1619 |
n = 1609 |
n = 1607 |
||||
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| Seasonal food insecurity since last visit (ref: none) | ||||||
| Partial | −0.02 | (−0.04, 0.00) | −0.03 | (−0.07, 0.00) | 0.00 | (−0.04, 0.03) |
| All | −0.08 | (−0.10, −0.05)*** | −0.02 | (−0.06, 0.02) | −0.09 | (−0.13, −0.05)*** |
| Fever | −0.07 | (−0.10, −0.05)*** | 0.03 | (−0.00, 0.07) | −0.13 | (−0.16, −0.09)*** |
| Diarrhea | −0.19 | (−0.21, −0.16)*** | −0.04 | (−0.08, 0.00) | −0.23 | (−0.27, −0.19)*** |
| Treatment arm (ref: C) | ||||||
| mLNS | 0.00 | (−0.18, 0.17) | 0.19 | (0.04, 0.34)* | −0.16 | (−0.35, 0.03) |
| mLNS-mARV | −0.13 | (−0.29, 0.03) | −0.05 | (−0.18, 0.09) | −0.14 | (−0.31, 0.03) |
| mARV | −0.14 | (−0.30, 0.03) | −0.05 | (−0.19, 0.09) | −0.14 | (−0.32, 0.03) |
| mLNS-iARV | −0.09 | (−0.25, 0.07) | −0.07 | (−0.20, 0.07) | −0.09 | (−0.26, 0.08) |
| iARV | −0.17 | (−0.33, −0.01)* | −0.07 | (−0.20, 0.07) | −0.17 | (−0.34, −0.00)* |
| Infant sex (ref: male) | 0.14 | (0.05, 0.23)** | 0.32 | (0.25, 0.40)*** | −0.06 | (−0.16, 0.03) |
| Maternal height | 0.03 | (0.02, 0.03)*** | 0.04 | (0.03, 0.05)*** | 0.00 | (−0.00, 0.01) |
| Maternal education (ref: primary or less) | 0.08 | (0.01, 0.15)* | 0.11 | (0.05, 0.17)*** | 0.02 | (−0.06, 0.09) |
| Maternal age at screening | 0.00 | (−0.01, 0.01) | −0.01 | (−0.01, 0.00) | 0.00 | (−0.01, 0.01) |
| First birth | 0.01 | (–0.15, 0.17) | 0.00 | (−0.14, 0.13) | −0.05 | (−0.22, 0.13) |
| Corresponding anthropometric Z-score at birth | 0.45 | (0.39, 0.50)*** | 0.46 | (0.42, 0.50)*** | 0.19 | (0.14, 0.23)*** |
| Study visit (ref: 24 wk), wk | ||||||
| 28 | −0.03 | (−0.06, −0.01)* | −0.08 | (−0.13, −0.04)*** | 0.02 | (−0.02, 0.06) |
| 32 | −0.10 | (−0.13, −0.08)*** | −0.20 | (−0.24, −0.16)*** | 0.02 | (−0.03, 0.06) |
| 36 | −0.17 | (−0.19, −0.14)*** | −0.34 | (−0.38, −0.29)*** | 0.04 | (−0.00, 0.08) |
| 42 | −0.27 | (−0.30, −0.25)*** | −0.49 | (−0.53, −0.44)*** | 0.02 | (−0.03, 0.06) |
| 48 | −0.33 | (−0.36, −0.31)*** | −0.64 | (−0.68, −0.60)*** | 0.07 | (0.02, 0.11)*** |
| Intercept | −0.69 | (−0.93, −0.46) | −1.66 | (−1.86, −1.46) | 0.38 | (0.14, 0.63) |
Columns represent growth outcomes (WAZ, LAZ, and BMIZ) and rows are covariates included in the model. For continuous covariates (such as age), the coefficients are slopes, while for indicator variables (such as mLNS), the coefficients are intercepts. *P < 0.05, **P < 0.01, ***P < 0.001. BMIZ, BMI-for-age Z-score; C, control; iARV, infant antiretroviral drug; LAZ, length-for-age Z-score; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplements; mLNS-mARV, maternal lipid-based nutrient supplements, maternal antiretroviral drug; mLNS-iARV, maternal lipid-based nutrient supplements, infant antiretroviral drug; WAZ, weight-for-age Z-score.
To put the effects of seasonal food insecurity, fever, and diarrhea on growth in perspective, we compared the overall means estimated from the longitudinal models with those predicted under specified conditions. At any given study visit, infants with no fever or diarrhea would have gained 0.024–0.048 SD for WAZ, 0.0005–0.002 SD for LAZ, and 0.034–0.066 SD for BMIZ when compared with infants in our sample. Likewise, infants with no seasonal food insecurity would have gained 0.008–0.017 SD at any given study visit across all 3 growth outcomes.
Discussion
The BAN Study recommended weaning at 6 mo to protect HIV-exposed, uninfected infants from HIV transmission through breastmilk. In an effort to limit morbidity and ensure adequate dietary intake, infants were provided with LNS as a breastmilk replacement from 24 to 48 wk of age. BAN infants had modest increases in reported cases of diarrhea and fever during and after weaning. Morbidity, particularly diarrhea, and season were negatively associated with WAZ and BMIZ as was assignment to the iARV arm. Assignment to the mLNS arm was positively associated with linear growth. The proportion of BAN infants who were underweight or stunted steadily increased from the time of weaning to the end of the study, but these proportions were consistently lower than those of the general population of Malawian infants (35).
Previous research indicates that early breastfeeding cessation is associated with increased diarrheal morbidity and mortality (5, 10, 11) as well as growth faltering (17) in HIV-exposed infants, but the relationship between morbidity and growth has not been widely studied in this population. Our finding that diarrhea was negatively associated with growth is consistent with studies of children in sub-Saharan Africa who were not HIV exposed (36–40) and with the results of a study in HIV-exposed Tanzanian children showing an association between diarrhea and weight-for-length (41). Despite the negative association of diarrhea with growth, we found the magnitude of these effects was small in our sample. In addition, prevalence of any diarrhea in BAN infants was lower for similar time periods than among Zambian HIV-exposed infants who were weaned at 4 mo (43% at 7–9 mo and 44% at 10–12 mo) as well as those who were still breastfed (38% at 7–9 mo and 39% at 10–12 mo) (5). This could be attributable in part to the provision of LNS (which is resistant to bacteriologic growth) as a breastmilk replacement or to the counseling on replacement feeding practices provided by the study (19, 42).
The general pattern of growth faltering in BAN infants from 24 to 48 wk is similar to studies in mainly HIV-unexposed infants in Malawi (43, 44) and HIV-exposed infants in Malawi (6), Zambia (17), and Kenya (45). BAN infants did not grow as well as another group of HIV-exposed Zambian infants who were breastfed at their mothers’ discretion and who had very little change in WAZ and small declines in LAZ from 6 to 12 mo (18). BAN infants had similar declines in LAZ but smaller declines in WAZ than HIV-exposed cohorts in Malawi and Zambia (6, 17), even compared with infants in these studies who were breastfed for longer. Rapid decreases in LAZ and slower decreases in WAZ from 6 to 18 mo resulting in weight-for-length or BMI-for-age Z-scores above the median have been noted in studies in Malawi (6, 43) as has the more frequent occurrence of stunting in boys (6, 44, 46).
The delayed effects of randomized interventions provided to mothers and infants from 0 to 28 wk on the growth of infants from 24 to 48 wk could potentially have important public health implications. We previously reported that assignment to the iARV and mARV arms was associated with lower weight and BMI in BAN infants starting at 12–18 wk, but that assignment to mLNS did not influence growth from 0 to 24 wk (30). Lower WAZ and BMIZ during the second half of infancy indicate that the effect of the infant ARV on growth continued even after its use ended. To our knowledge, there are no comparable studies examining the effect of ARV exposure from birth to 6 mo on the growth of uninfected infants during the first year of life. A study in Botswana found that infants with exposure to highly active antiretroviral therapy in utero and through breastmilk had lower LAZ than infants exposed to zidovudine up to 6 mo of age (47). The results of the Botswana analysis together with those from the BAN Study suggest that some types of ARVs used for prevention of MTCT may have a small negative effect on growth. As was previously recommended, longer cohort studies are needed to better understand the impact of ARVs on HIV-exposed, uninfected children (48).
The postintervention effect of mLNS provided from 0 to 28 wk on infant LAZ during the second half of infancy is consistent with a study showing that the effects of LNS on WAZ and LAZ continued after the intervention ended (49). The delayed LNS effect could be explained by better infant micronutrient status or fatty acid profile at 24 wk compared with infants whose mothers did not receive the supplement. It is notable that only those infants who were not exposed to maternal or infant ARV benefitted from mLNS, suggesting that the drugs limited the supplements’ impact on growth.
The main limitation of this study was the lack of a comparison group, because all BAN infants received LNS from 24 to 48 wk. Comparing the growth of BAN infants to Malawi DHS or other studies of HIV-exposed children supplies context but does not allow us to determine the causes of the observed growth patterns. There are differences between the BAN and DHS samples. A large proportion of the infants in the Malawi DHS were from rural areas, where growth is typically poorer than in urban areas (35), where BAN infants lived. However, all BAN infants were HIV exposed and fully weaned from breastmilk as opposed to infants in the DHS who were still breastfed and few of whom were HIV exposed. The lower proportion of faltering infants in the BAN Study compared with those in the DHS may be attributed to their participation in the study, where supplementary food, regular health care, and counseling on feeding practices were provided. Large beneficial effects of study participation on the growth of HIV-exposed and -unexposed children have previously been noted (18).
In conclusion, although the growth of BAN infants progressively faltered during the second half of infancy, their levels of morbidity were lower and their growth faltered less than those of other HIV-exposed populations who were weaned early (5) and were provided with milk- or cereal-based weaning supplements (17, 20). These findings, together with high levels of LNS adherence, indicate that use of LNS as a breastmilk replacement was feasible in the full BAN sample, as previously documented in a small subsample (42). The results of the present study are applicable in the current prevention of MTCT environment, because ARVs are not universally available and some HIV-infected mothers continue to wean early even when AFASS conditions are not met. In addition, LNS could potentially contribute to making conditions AFASS in settings with poor hygiene. This role for LNS could be assessed as part of a controlled trial designed to test whether LNS limits morbidity and promotes growth in HIV-exposed children after weaning.
Supplementary Material
Acknowledgments
The authors are grateful to the following: BAN Study Team at University of North Carolina Chapel Hill, CDC, Atlanta, and UNC Project team in Lilongwe including: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba. M.E.B., C.S.C., D.K., M.G.H., K.Z.K., C.C., A.P.K., D.J.J., C.M.v.d.H., and L.S.A. designed and conducted the study; V.L.F. and L.S.A. carried out the analysis; and V.L.F. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AFASS, acceptable, feasible, affordable, sustainable, and safe; ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; BMIZ, BMI-for-age Z-score; C, control; DHS, Demographic and Health Survey; iARV, infant antiretroviral drug; LAZ, length-for-age Z-score; LNS, lipid-based nutrient supplements; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplements; mLNS-iARV, maternal lipid-based nutrient supplements/infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplements/maternal antiretroviral drug MTCT, mother-to-child transmission; WAZ, weight-for-age Z-score.
Literature Cited
- 1.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74 [DOI] [PubMed] [Google Scholar]
- 2.Becquet R, Bland R, Leroy V, Rollins NC, Ekouevi DK, Coutsoudis A, Dabis F, Coovadia HM, Salamon R, Newell ML. Duration, pattern of breastfeeding and postnatal transmission of HIV: pooled analysis of individual data from West and South African cohorts. PLoS ONE. 2009;4:e7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16 [DOI] [PubMed] [Google Scholar]
- 5.Fawzy A, Arpadi S, Kankasa C, Sinkala M, Mwiya M, Thea DM, Aldrovandi GM, Kuhn L. Early weaning increases diarrhea morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis. 2011;203:1222–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taha T, Nour S, Li Q, Kumwenda N, Kafulafula G, Nkhoma C, Broadhead R. The effect of human immunodeficiency virus and breastfeeding on the nutritional status of African children. Pediatr Infect Dis J. 2010;29:514–8 [DOI] [PubMed] [Google Scholar]
- 7.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Kasonde P, Mwiya M, Tsai WY, Thea DM. Differential effects of early weaning for HIV-free survival of children born to HIV-infected mothers by severity of maternal disease. PLoS ONE. 2009;4:e6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, Hu CC, Tsai WY, Thea DM, Aldrovandi GM. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, Fassinou P, Bedikou G, Timite-Konan M, Dabis F, Leroy V. Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Cote d'Ivoire. PLoS Med. 2007;4:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, Kumwenda NI, Nkanaunena K, Mipando L, Mofenson LM. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13 [DOI] [PubMed] [Google Scholar]
- 11.Onyango-Makumbi C, Bagenda D, Mwatha A, Omer SB, Musoke P, Mmiro F, Zwerski SL, Kateera BA, Musisi M, Fowler MG, et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr. Epub 2009 Sep 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO Press; 2010 [PubMed] [Google Scholar]
- 13.WHO, UNICEF, UNFPA, UNAIDS HIV and infant feeding: a guide for health-care managers and supervisors. Geneva: WHO; 2003 [Google Scholar]
- 14.WHO, UNICEF, UNFPA, UNAIDS. HIV and infant feeding: new evidence and programmatic experience: report of a technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and Their Infants. Geneva, 25–27 October 2006. Geneva: WHO; 2007.
- 15. Sinkala ML, Kankasa C, Kasonde P, Vwalika C, Mwiya M, Scott N, Semrau K, Aldrovandi G, Thea D, et al. No benefit of early cessation of breastfeeding at 4 months on HIV-free survival of infants born to HIV-infected mothers in Zambia: The Zambia Exclusive Breastfeeding Study. CROI, session 24, Abstract 74; 2007 [cited 2013 Feb 20]. Available from: http://retroconference.org/2007/Abstracts/30664.htm.
- 16.WHO, UNICEF, UNFPA, UNAIDS. HIV and infant feeding: update based on the technical consultation held on behalf of the Inter-agency Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers, and Their Infants, Geneva, 25–27 October, 2006. Geneva: WHO; 2007.
- 17.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, Thea DM, Kuhn L. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filteau S, Baisley K, Chisenga M, Kasonka L, Gibson RS. Provision of micronutrient-fortified food from 6 months of age does not permit HIV-exposed uninfected Zambian children to catch up in growth to HIV-unexposed children: a randomized controlled trial. J Acquir Immune Defic Syndr. 2011;56:166–75 [DOI] [PubMed] [Google Scholar]
- 19.Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, Piwoz EG, Zulu C, Chasela C, Soko A, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: The Ban Study in Lilongwe, Malawi. AIDS Educ Prev. 2009;21:141–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becquet R, Leroy V, Ekouevi DK, Viho I, Castetbon K, Fassinou P, Dabis F, Timite-Konan M. Complementary feeding adequacy in relation to nutritional status among early weaned breastfed children who are born to HIV-infected mothers: ANRS 1201/1202 Ditrame Plus, Abidjan, Cote d'Ivoire. Pediatrics. 2006;117:e701–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matilsky DK, Maleta K, Castleman T, Manary MJ. Supplementary feeding with fortified spreads results in higher recovery rates than with a corn/soy blend in moderately wasted children. J Nutr. 2009;139:773–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO, World Food Programme, United Nations Standing Committee on Nutrition, UNICEF Community-based management of severe acute malnutrition. Geneva: WHO; 2007 [Google Scholar]
- 23.Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. Am J Clin Nutr. 2008;87:929–38 [DOI] [PubMed] [Google Scholar]
- 24.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Arch Pediatr Adolesc Med. 2008;162:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briend A. Highly nutrient-dense spreads: a new approach to delivering multiple micronutrients to high-risk groups. Br J Nutr. 2001;85 Suppl 2:S175–9 [PubMed] [Google Scholar]
- 26.Gewa CA, Oguttu M, Savaglio L. Determinants of early child-feeding practices among HIV-infected and noninfected mothers in rural Kenya. J Hum Lact. 2011;27:239–49 [DOI] [PubMed] [Google Scholar]
- 27.Maman S, Cathcart R, Burkhardt G, Omba S, Thompson D, Behets F. The infant feeding choices and experiences of women living with HIV in Kinshasa, Democratic Republic of Congo. AIDS Care. 2012;24:259–65 [DOI] [PubMed] [Google Scholar]
- 28.Østergaard LR, Bula A. They call our children "Nevirapine babies?": aqualitative study about exclusive breastfeeding among HIV positive mothers in Malawi. Afr J Reprod Health. 2010;14:213–22 [PubMed] [Google Scholar]
- 29.UNAIDS UNAIDS data tables 2011. Geneva: UNAIDS; 2011 [Google Scholar]
- 30.Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Knight RJ, Soko A, Jamieson DJ, van der Horst CM, Adair LS. Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0–24 weeks. J Nutr. 2012;142:1350–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cogill B. Anthropometric indicators measurement guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2003.
- 32.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: WHO; 2006.
- 33.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampl M, Gotsch F, Kusanovic JP, Espinoza J, Goncalves L, Gomez R, Nien JK, Frongillo EA, Romero R. Downward percentile crossing as an indicator of an adverse prenatal environment. Ann Hum Biol. 2008;35:462–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Statistical Office Malawi, ORC Macro. Malawi demographic and health survey 2004. Calverton (MD): NSO and ORC Macro; 2005.
- 36.Rowland MG, Cole TJ, Whitehead RG. A quantitative study into the role of infection in determining nutritional status in Gambian village children. Br J Nutr. 1977;37:441–50 [DOI] [PubMed] [Google Scholar]
- 37.Kossmann J, Nestel P, Herrera MG, El-Amin A, Fawzi WW. Undernutrition and childhood infections: a prospective study of childhood infections in relation to growth in the Sudan. Acta Paediatr. 2000;89:1122–8 [DOI] [PubMed] [Google Scholar]
- 38.Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. Predictors of growth from 1 to 18 months among breast-fed Ghanaian infants. Eur J Clin Nutr. 2000;54:41–9 [DOI] [PubMed] [Google Scholar]
- 39.Wamani H, Astrom AN, Peterson S, Tumwine JK, Tylleskar T. Predictors of poor anthropometric status among children under 2 years of age in rural Uganda. Public Health Nutr. 2006;9:320–6 [DOI] [PubMed] [Google Scholar]
- 40.Weisz A, Meuli G, Thakwalakwa C, Trehan I, Maleta K, Manary M. The duration of diarrhea and fever is associated with growth faltering in rural Malawian children aged 6–18 months. Nutr J. 2011;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb AL, Manji K, Fawzi WW, Villamor E. Time-independent maternal and infant factors and time-dependent infant morbidities including HIV infection, contribute to infant growth faltering during the first 2 years of life. J Trop Pediatr. 2009;55:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker ME, Bentley ME, Chasela C, Adair L, Piwoz EG, Jamieson DJ, Ellington S, Kayira D, Soko A, Mkhomawanthu C, et al. The acceptance and feasibility of replacement feeding at 6 months as an HIV prevention method in Lilongwe, Malawi: results from the BAN study. AIDS Educ Prev. 2011;23:281–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maleta K, Virtanen SM, Espo M, Kulmala T, Ashorn P. Childhood malnutrition and its predictors in rural Malawi. Paediatr Perinat Epidemiol. 2003;17:384–90 [DOI] [PubMed] [Google Scholar]
- 44.Espo M, Kulmala T, Maleta K, Cullinan T, Salin ML, Ashorn P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002;91:1364–70 [DOI] [PubMed] [Google Scholar]
- 45.McGrath CJ, Nduati R, Richardson BA, Kristal AR, Mbori-Ngacha D, Farquhar C, John-Stewart GC. The prevalence of stunting is high in HIV-1-exposed uninfected infants in Kenya. J Nutr. 2012;142:757–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalanda BF, van Buuren S, Verhoeff FH, Brabin BJ. Catch-up growth in Malawian babies, a longitudinal study of normal and low birthweight babies born in a malarious endemic area. Early Hum Dev. 2005;81:841–50 [DOI] [PubMed] [Google Scholar]
- 47.Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, Leidner J, Makhema J, Essex M, Shapiro RL. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56:131–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. J Acquir Immune Defic Syndr. 2011;57:290–6 [DOI] [PubMed] [Google Scholar]
- 49.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Postintervention growth of Malawian children who received 12-mo dietary complementation with a lipid-based nutrient supplement or maize-soy flour. Am J Clin Nutr. 2009;89:382–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.