Abstract
High-quality proteins such as soy, whey, and casein are all capable of promoting muscle protein synthesis postexercise by activating the mammalian target of rapamycin (mTORC1) signaling pathway. We hypothesized that a protein blend of soy and dairy proteins would capitalize on the unique properties of each individual protein and allow for optimal delivery of amino acids to prolong the fractional synthetic rate (FSR) following resistance exercise (RE). In this double-blind, randomized, clinical trial, 19 young adults were studied before and after ingestion of ∼19 g of protein blend (PB) or ∼18 g whey protein (WP) consumed 1 h after high-intensity leg RE. We examined mixed-muscle protein FSR by stable isotopic methods and mTORC1 signaling with western blotting. Muscle biopsies from the vastus lateralis were collected at rest (before RE) and at 3 postexercise time points during an early (0–2 h) and late (2–4 h) postingestion period. WP ingestion resulted in higher and earlier amplitude of blood branched-chain amino acid (BCAA) concentrations. PB ingestion created a lower initial rise in blood BCAA but sustained elevated levels of blood amino acids later into recovery (P < 0.05). Postexercise FSR increased equivalently in both groups during the early period (WP, 0.078 ± 0.009%; PB, 0.088 ± 0.007%); however, FSR remained elevated only in the PB group during the late period (WP, 0.074 ± 0.010%; PB, 0.087 ± 0.003%) (P < 0.05). mTORC1 signaling similarly increased between groups, except for no increase in S6K1 phosphorylation in the WP group at 5 h postexercise (P < 0.05). We conclude that a soy-dairy PB ingested following exercise is capable of prolonging blood aminoacidemia, mTORC1 signaling, and protein synthesis in human skeletal muscle and is an effective postexercise nutritional supplement.
Introduction
An increase in amino acid availability following an acute bout of resistance exercise (RE)11 enhances skeletal muscle protein synthesis in humans (1–7). In addition, intact protein ingestion in the form of soy, casein, whey, egg, or beef increases the amino acid supply to muscle, which further promotes muscle protein synthesis during postexercise recovery (8–19). However, there is some disagreement about whether different protein sources produce superior effects on muscle protein synthesis. The primary points of contention include the overall protein quality (i.e., amino acid composition) of the protein source and its digestion rate (i.e., fast, intermediate, or slow).
High-quality dairy (whey and casein) and plant (soy) protein sources contain all of the EAAs and they each have distinct traits thought to offer an advantage for stimulating muscle protein synthesis (20, 21). On average, ∼20–25 g of high-quality protein contains ∼8–10 g EAAs, which are critical for the regulation of muscle protein synthesis (22). Whey contains a higher BCAA content, primarily leucine, compared with other high-quality proteins (20), and its rapid digestion increases blood amino acid concentrations shortly following ingestion (9, 23–25). This effect is transient and returns to resting levels within 2–3 h (23, 24) when consumed independently or following a bout of exercise (9, 25). For these reasons, whey protein (WP) has been considered to be superior compared with other isolated protein sources (13, 14, 25–27). The hyperaminoacidemia occurring with whey ingestion stimulates additional amino acid oxidation, which could contribute to reduced nitrogen retention (i.e., whole-body protein synthesis) (23, 24). When a slowly digested protein such as casein is ingested, it produces a slower but more prolonged (∼6 h) aminoacidemia that results in higher nitrogen retention and less oxidation (23, 24) and is effective in stimulating postexercise muscle protein fractional synthetic rate (FSR) (12, 16). When these milk proteins (whey and casein) are co-ingested, the slowly digested protein, casein, not the whey, contributes the amino acids for a prolonged protein synthetic effect across the leg (28). Meanwhile, soy protein has an “intermediate” digestion rate (11, 29), contains key properties not associated with dairy proteins such as antioxidant/inflammatory activity (30, 31), and effectively stimulates postexercise FSR (11, 13) and overall muscle accretion (32, 33).
We recently demonstrated in a rodent model that a protein blend (PB) is effective at prolonging the FSR response compared with single-source proteins like whey (34). Therefore, we hypothesized that a PB consisting of soy and dairy proteins would capitalize on the unique properties of each individual protein and would optimally deliver amino acids to promote muscle protein synthesis following RE. To address our hypothesis, we conducted a randomized, double-blind study in young adults to compare the effect of a PB (soy, casein, and whey) with a single protein isolate (WP) ingested following a bout of high-intensity exercise on BCAA blood concentrations, mammalian target of rapamycin complex 1 (mTORC1) signaling, and FSR during postexercise recovery.
Participants and Methods
Screening of participants.
We recruited 19 healthy, young participants (17 male, 2 female; age range: 18–30 y) for this double-blind, randomized, clinical trial. Participant characteristics are shown in Table 1. The participants were recruited through locally posted flyers, newspaper advertisements, and by word of mouth. The participants were healthy and recreationally active but were not engaged in any regular exercise training program (<2 sessions high-intensity aerobic or RE/wk) at the time of enrollment. Screening of the participants was performed on 2 separate days (>7 d apart) at the Institute for Translational Sciences-Clinical Research Center (ITS-CRC). The first screening day included 1 repetition maximum (1RM) testing, a clinical history, physical exam, and laboratory tests (complete blood count with differential, liver, and kidney function tests, coagulation profile, fasting blood glucose, hepatitis B and C screening, HIV test, thyroid stimulating hormone, lipid profile, urinalysis, and drug screening). The second screening day included a second 1RM test and a DXA scan (Hologic QDR 4500W) to measure lean and fat mass. 1RM testing was performed on a leg extension machine (Cybex-VR2) and recorded as the highest weight lifted for a single repetition from the 2 testing days. All participants gave written informed consent before enrollment in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and was in compliance with the Declaration of Helsinki as revised in 1983.
TABLE 1.
Participant characteristics1
| n | Age | BMI | Fat | FFM | Lean mass | |
| y | kg/m2 | % | kg | kg | ||
| PB | 10 | 23.1 ± 1.0 | 25.9 ± 0.8 | 24.3 ± 1.7 | 60.3 ± 3.5 | 57.3 ± 3.3 |
| WP | 9 | 25.1 ± 1.2 | 25.5 ± 1.0 | 24.1 ± 2.5 | 61.2 ± 3.0 | 57.9 ± 2.8 |
Data are mean ± SEM. FFM, fat-free mass; PB, protein blend; WP, whey protein.
Study design.
Enrolled participants checked into the ITS-CRC at ∼1700 h the day prior to the study. Participants refrained from exercise at least 72 h before admission. The participants were given a standardized meal at 1900 h prepared by the Bionutrition Division of the ITS-CRC with a macronutrient distribution of 20% protein, 60% carbohydrate, and 20% fat at 12 kcal·kg body weight−1. Participants consumed water ad libitum. The participants were randomized to ingest either the protein blend (n = 10 PB) or whey protein (n = 9 WP) at 1 h following a bout of high-intensity leg RE. The leucine content in the protein beverages was matched by adjusting the total amount given to control for its protein anabolic effect.
Experimental protocol.
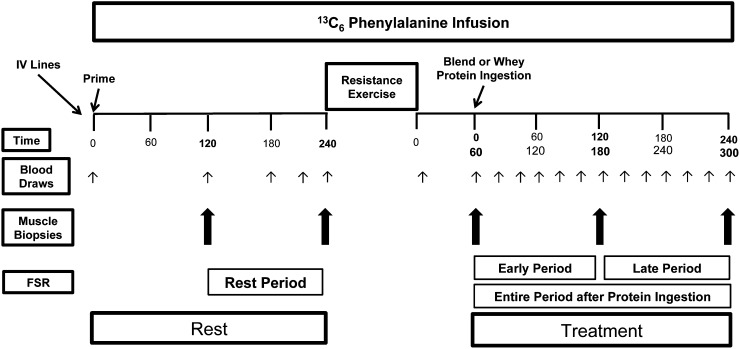
All participants underwent the stable isotope infusion protocol (Fig. 1) at the same time of day (0600–1600 h) on the day following admission. After an overnight fast (∼10 h), an 18G polyethylene catheter was inserted into the antecubital vein from which background blood draws were taken, followed by initiation of a primed, constant infusion (∼10 h) of l-[ring-13C6] phenylalanine (Sigma-Aldrich). The priming dose for the labeled phenylalanine was 2 μmol · kg−1 and the infusion rate was 0.05 μmol · kg−1 · min−1. A retrograde catheter was inserted (0700–0800 h) into a hand vein on the contralateral arm and arterialized blood was extracted with the use of a heating pad prior to sampling. At 2 and 4 h following initiation of the infusion, muscle biopsies were taken from the lateral aspect of the vastus lateralis for the determination of resting mixed muscle FSR. All biopsies were taken with a 5-mm Bergström biopsy needle under sterile procedure and local anesthesia (1% lidocaine). Following the second biopsy, the participants were moved to a leg extension machine for high-intensity RE consisting of 8 sets of 10 repetitions at 55% (set 1), 60% (set 2), 65% (set 3), and ∼70% (sets 4–8) of the participants’ previously determined 1RM with a 3-min rest between sets. Three additional muscle biopsies were taken 1, 3, and 5 h after the completion of exercise. The nutritional supplements were ingested immediately following the 1-h biopsy. The first and second, the third and fourth, and the fifth muscle biopsies were sampled from 3 separate incisions on the same leg, respectively. To minimize multiple sampling, in a given area, skin incisions were separated by ∼7 cm and biopsies taken from the same incision were angled ∼5 cm from each other. This method was previously utilized in our laboratory (35–37) and others (12, 18, 38). Muscle tissue was immediately blotted, frozen in liquid nitrogen, and stored at −80°C until analysis. Blood samples were collected during the resting (0, 120, 180, 200, and 240 min) and postingestion (−60, 0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, and 240 min) periods (Fig. 1) for the determination of blood l-[ring-13C6] phenylalanine enrichment (see below) and amino acid concentration. The infusion study ended following the fifth muscle biopsy and participants were then given a standard meal.
FIGURE 1.
Schematic of randomized, double-blinded experimental protocol. Participants ingested either the PB or WP 1 h following the completion of 8 sets of knee extension RE. The small arrows represent blood draws whereas the large arrows represent biopsies. FSR, fractional synthesis rate; PB, protein blend; RE, resistance exercise; WP, whey protein.
Protein beverage intervention.
The protein beverages (WP or PB) were consumed 1 h following exercise. The beverages were dissolved in 300 mL of water and enriched (8%) with l-[ring-13C6] phenylalanine to maintain isotopic steady state in arterialized blood. The compositions of the beverages are shown in Supplemental Table 1. To match leucine contents between the interventions, participants were given 0.30 or 0.35 g total protein · kg lean mass−1 for WP and PB, respectively. The PB consisted of 19.3 ± 1.1 g total protein (providing 1.8 ± 0.1 g leucine and 8.7 ± 0.5 g EAAs) composed of 50% protein from sodium caseinate, 25% protein from WP isolate, and 25% protein from soy protein isolate (SUPRO). The WP consisted of 17.7 ± 0.9 g protein (providing ∼1.9 ± 0.1 g leucine and 8.9 ± 0.4 g EAAs). The amount of protein given in each group was based on the 8.6 g of EAA in intact protein demonstrated to maximize the FSR response following RE (19).
Free blood amino acid concentration and plasma glucose, lactate, and serum insulin.
Concentrations of phenylalanine and the BCAAs (leucine, isoleucine, and valine) were measured in deproteinized whole blood using GC-MS as previously described using an internal standard solution (39, 40). Serum concentrations of insulin were determined with an ELISA (Millipore) according to the manufacturer’s instructions at rest and before and for several time points following beverage ingestion. Also, plasma glucose and lactate concentrations were measured using an automated glucose and lactate analyzer (YSI).
Muscle protein synthesis and enrichment.
Muscle proteins and muscle intracellular free amino acids were extracted from biopsy samples as previously described (35). GC-MS (GCMS, 6890 Plus CG, 5973N MSD, 7683 autosampler, Agilent Technologies) measurements were made to determine muscle-bound and intracellular free concentrations with the internal standard method through the use of tracer enrichments for l-[ring-13C6] phenylalanine and appropriate internal standards (l-[15N] phenylalanine). Measurements were determined as previously described (40). Mixed-muscle, protein-bound phenylalanine enrichment was analyzed by GC-MS after protein hydrolysis and amino acid extraction (22, 35) using the external standard curve approach (41). We calculated muscle protein synthesis as FSR by measuring the incorporation rate of the phenylalanine tracer into the proteins (Δ protein-bound enrichment over time) and using the precursor-product model to calculate the synthesis rate:
where ΔΕp is the increment in protein-bound phenylalanine enrichment between 2 sequential biopsies, t is the time between the 2 sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the 2 sequential biopsies. Data are expressed as percent per hour.
SDS-PAGE and western-blot analysis.
Immunoblotting was performed as previously described (35). In brief, 20–50 mg frozen muscle tissue was processed and assayed for total protein content. After further processing, each sample (50 μg total protein) was loaded in duplicate onto a 7.5 or 15% polyacrylamide gel (Criterion; Bio-Rad) and subjected to electrophoresis at 150 V for 70 min. Following electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) that was then blocked in 5% nonfat dried milk. Membranes (blots) were then incubated with a single, primary antibody overnight at 4°C. Rabbit polyclonal primary antibodies (Cell Signaling) used were the following: Akt (Ser308), mTOR (Ser2448), S6K1 (Thr389), 4E-BP1 (Thr37/46), and ribosomal protein S6 (Ser240/244). Blots were incubated with secondary antibody (Amersham Bioscience), washed, and then a chemiluminescent solution (ECL plus; Amersham BioSciences) was applied. OD measurements were then immediately obtained with a digital imager (Bio-Rad) and densitometric analysis (Quantity One software, version 4.5.2; Bio-Rad) was performed. Following detection of phosphorylated proteins, blots were stripped of primary and secondary antibodies and then reprobed for total protein, which was determined for each blot. Data were normalized to an internal control and expressed as phosphorylated:total protein.
Statistical analysis.
All values are expressed as mean ± SEM. Data were transformed using the Box-Cox set of transformations to stabilize the variance and make the data approximately normally distributed. To test differences between groups, the data were modeled using an ANCOVA model with resting values as a covariate. Testing differences was thus accomplished through a t test of the variable indicating the difference between groups. Comparisons with resting values were based on inference of the intercept in the ANCOVA model after centering the response and resting variables. Each time point was modeled separately. Significance was set at P < 0.05. All calculations were done in R (42).
Results
Subject characteristics.
Descriptive characteristics for all participants are shown in Table 1. The participants had similar 1RM values of 119 ± 10 and 130 ± 10 kg and their total mean weight lifted was 63 ± 2 and 62 ± 2% of their 1RM for the PB and WP groups, respectively. There were no differences between groups.
Insulin, glucose, and lactate.
Serum insulin concentrations were elevated (P < 0.05) above rest until 40 and 60 min following ingestion in the PB and WP groups, respectively (Supplemental Table 2). There were no differences between groups. Plasma glucose concentrations were unchanged following protein ingestion. Plasma lactate concentrations were elevated (P < 0.05) above rest until 60 min following ingestion in the PB group and 80 min in the WP group. Further, lactate concentrations tended to be lower at 60 min (P = 0.07) and were lower 80 min (P < 0.05) postingestion in the PB group compared with the WP group.
Blood amino acid concentrations.
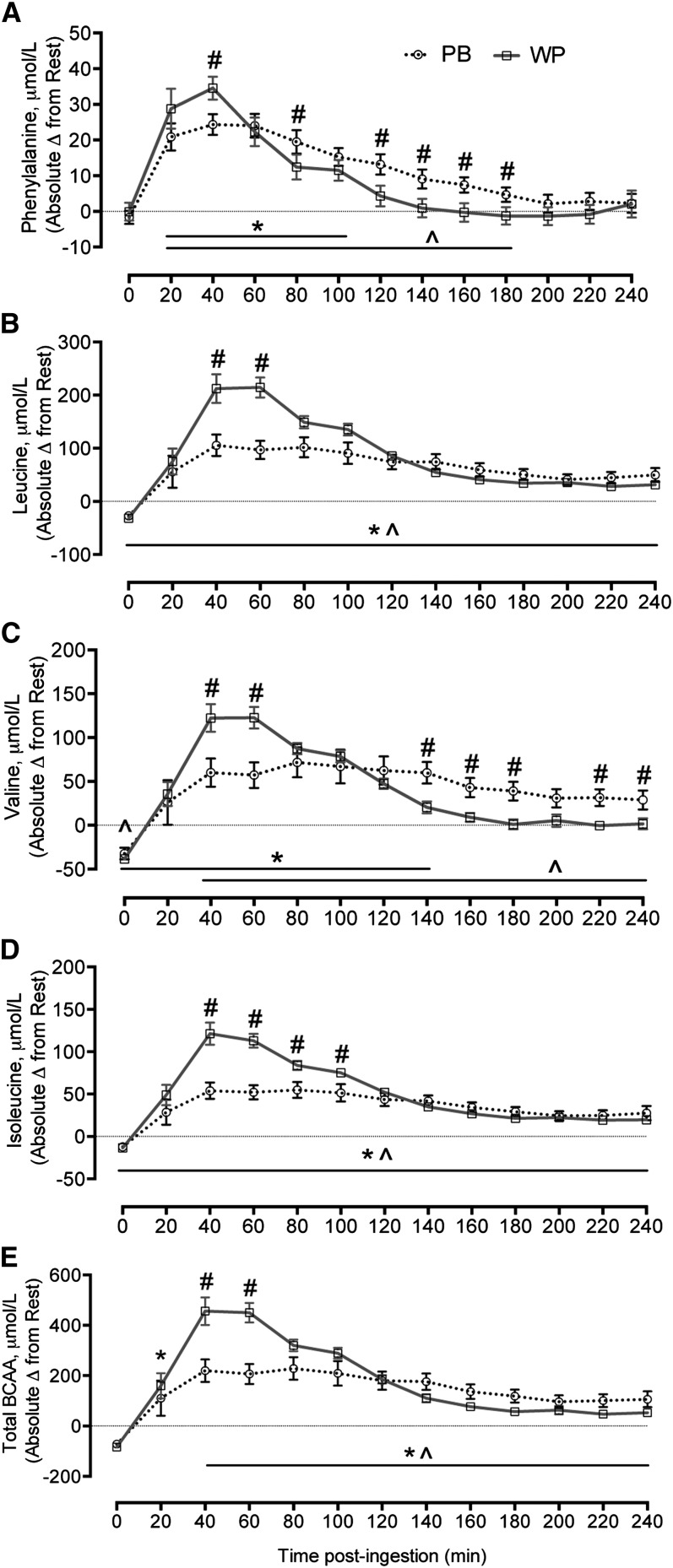
Phenylalanine concentrations were elevated (P < 0.05) from rest in the WP group until 100 min following ingestion, whereas they remained elevated in the PB group to 180 min (P < 0.05) (Fig. 2). Phenylalanine concentrations were significantly greater in the WP group at 40 min and in the PB group at 80, 120, 140, 160, and 180 min after ingestion (P < 0.05). Leucine and isoleucine concentrations were elevated from rest in both groups for the duration of postexercise recovery (P < 0.05). The WP group had higher peak leucine concentrations at 40 and 60 min after ingestion and higher isoleucine concentrations at 40, 60, 80, and 100 min after ingestion compared with the PB group (P < 0.05). Valine concentrations were elevated from rest in the WP group until 140 min following ingestion (P < 0.05), whereas in the PB group, valine concentrations remained elevated for the duration of postexercise recovery (P < 0.05). Valine concentrations where higher in the WP group at 40 and 60 min and the PB group had higher concentrations at 140, 160, 180, 220, and 240 min after ingestion (P < 0.05). Total BCAA concentrations were elevated from rest in both groups for the duration of postexercise recovery (P < 0.05). Total BCAA concentrations were higher in the WP group at 40 and 60 min compared with the PB group (P < 0.05), whereas BCAA tended to be higher in the PB group at 180 min after ingestion (P = 0.06).
FIGURE 2.
Changes from rest in blood phenylalanine (A), leucine (B), valine (C), isoleucine (D), and total BCAA (E) concentrations in young adults during the postexercise recovery period following ingestion of the PB or WP 1 h after completion of RE. Data are mean ± SEM, n = 9 (WP) or 10 (PB). #Different from PB at that time, P < 0.05; *different from resting values for WP, P < 0.05; ^different from resting values for PB, P < 0.05. PB, protein blend; RE, resistance exercise; WP, whey protein.
Blood and muscle intracellular enrichments.
Blood phenylalanine enrichments did not change over time (P > 0.10). However, the enrichments at 180 min postingestion were higher in the WP than in the PB group (P < 0.05). Muscle intracellular phenylalanine enrichments were steady state during the treatment period, but during the resting period, the enrichments increased over time in both groups (P < 0.05). The muscle intracellular phenylalanine enrichments did not differ during the treatment period (P > 0.10) but tended to be lower in PB than in WP at −120 min (P = 0.07) (Supplemental Fig. 1).
Muscle mTORC1 signaling.
There were no group effects for the phosphorylation status of mTORC1 (Ser 2448), Akt (Thr308), 4E-BP1 (Thr37/42), rpS6 (Ser240/244), and S6K1 (Thr389) at rest (data not shown) or postexercise (P < 0.05) (Table 2; representative blots in Supplemental Fig. 2). Compared with rest, phosphorylation increased for mTORC1, rpS6, and 4E-BP1 at 2 and 4 h postingestion in both groups (P < 0.05). In the PB group, the phosphorylation of S6K1 increased at 2 and 4 h postingestion (P < 0.05), whereas the S6K1 phosphorylation in the WP group only tended to increase at 2 h postingestion (P = 0.07). Akt phosphorylation increased at 2 h postingestion in both groups (P < 0.05).
TABLE 2.
Western-blot analyses of synthesis-associated signaling proteins in young adults during the postexercise recovery period following ingestion of the PB or WP 1 h after completion of RE1
| 2 h postingestion |
4 h postingestion |
|||
| Protein | PB | WP | PB | WP |
| phosphorylated/total, fold of rest | ||||
| Akt Ser308 | 1.17 ± 0.14* | 1.27 ± 0.16* | 1.06 ± 0.15 | 0.87 ± 0.18 |
| mTORC1 Ser2448 | 3.51 ± 1.48* | 3.01 ± 0.46* | 2.78 ± 0.68* | 2.83 ± 0.47* |
| p70S6K1 Ser389 | 21.3 ± 7.25* | 12.7 ± 3.12** | 11.9 ± 3.76* | 6.20 ± 1.30 |
| rpS6 Ser240/244 | 3.32 ± 1.33* | 2.38 ± 0.85* | 1.95 ± 0.43* | 1.55 ± 0.55* |
| 4E-BP1 Thr37/42 | 1.27 ± 0.09* | 1.34 ± 0.17* | 1.27 ± 0.10* | 1.17 ± 0.10* |
Data are mean ± SEM, n = 9 (WP) or 10 (PB). *Different from resting values for that group, P < 0.05; **different from resting values for that group, P = 0.07. mTORC1, mammalian target of rapamycin complex 1; PB, protein blend; RE, resistance exercise; WP, whey protein.
FSR.
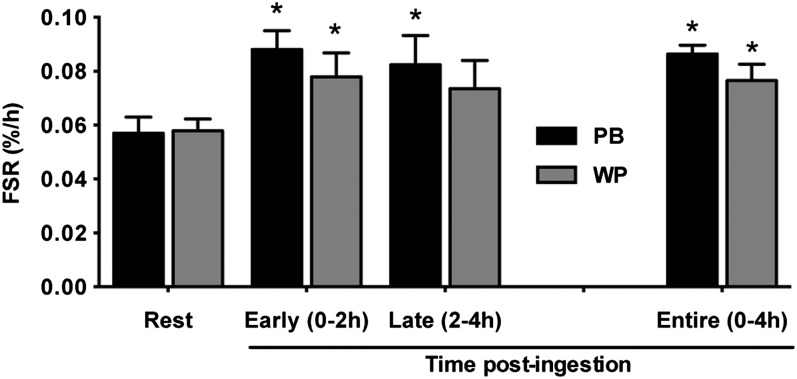
Resting muscle protein synthesis (mixed-muscle FSR) did not differ between the PB and WP groups (P > 0.10) (Fig. 3). The postexercise FSR was elevated from resting values for the early (0–2 h) (P = 0.001), late (2–4 h) (P = 0.030), and entire (0–4 h) (P < 0.001) post-protein ingestion periods in the PB group. In the WP group, postexercise FSR was elevated from resting values during only the early (P = 0.026) and entire (P = 0.002) periods, but not the late (P > 0.10) period. There were no group effects at any time point (P > 0.10).
FIGURE 3.
FSR of the vastus lateralis in young adults during the postexercise recovery period following ingestion of the PB or WP 1 h after completion of RE. Data are presented at rest, early (0–2 h), late (2–4 h), and entire (0–4 h) postingestion periods. Data are mean ± SEM, n = 9 (WP) or 10 (PB). *Different from resting values for that group, P < 0.05. FSR, fractional synthesis rate; PB, protein blend; RE, resistance exercise; WP, whey protein.
Discussion
Proteins from milk (casein and whey), soy, beef, and egg are effective in stimulating postexercise muscle protein synthesis (8–17, 19, 20). Several studies have focused on WP and its effects on promoting lean mass gain (20, 26) due to its suggested superiority to other isolated protein sources (11). Our data are novel in that they utilize the proteins from soy, whey, and casein with different digestion rates (amino acid release profiles) after an acute bout of RE. We show for the first time, to our knowledge, that a soy-dairy PB (25% soy, 25% whey, and 50% casein) is capable of stimulating muscle growth to a similar extent as WP through a marked elevation in muscle protein synthesis and skeletal muscle mTORC1 signaling. We compared this novel intervention with WP as the single source of protein (single digestion rate) while maintaining a similar absolute leucine concentration between the PB and WP. Previous research has compared ingested proteins with only a single digestion rate (“fast” vs. “slow”) following RE (9, 11–13, 15, 16, 25, 43, 44). Further, the comparisons in these reports did not match for the potent anabolic effect of leucine. Additionally, the soy-dairy PB stimulated FSR into the late postexercise period, whereas WP increased FSR from rest into only the early recovery period. In our hands, a soy-dairy PB ingestion following exercise was capable of prolonging blood aminoacidemia, mTORC1 signaling, and protein synthesis in human skeletal muscle.
Our data agree with previous work suggesting that milk (a blend of casein and whey) offers advantages over a single source of protein, such as soy, to supplement RE (10, 45), yet no study until now, to our knowledge, has compared a blended protein source with isolated WP for muscle protein synthesis while matching the leucine content. To date, the protein anabolic effect of WP ingestion following RE has been tested only against interventions examining other macronutrients (17, 46–48), supplemental amino acids (43, 49, 50), or other isolated protein sources (11, 12, 16, 44). Compared with other high-quality protein sources following RE, whey has been suggested to be superior to isolated soy protein (11, 13) and micellar casein (11, 44), which is the least soluble, most slowly digested form of casein (51). However, in these studies, the leucine content, a key anabolic agent, was not matched between interventions, which may skew the results, especially in an aging population (52) where adequate leucine may be especially needed. However, a slightly more soluble form of casein, caseinate, can initiate a comparable anabolic response to WP when ingested following RE (12, 16). Our data further suggest the efficacy of co-ingesting rapidly and slowly digested proteins as a PB for promoting muscle protein synthesis following exercise.
High-quality dairy (whey and casein) and plant (soy) protein sources contain all the EAAs and have individual traits thought to offer a unique advantage for muscle growth (20, 21, 53). One of the most supportive tenets favoring WP has been its higher BCAA content (26, 54), particularly leucine (20, 26). Yet the rapid hyperaminoacidemia of WP is short lived (9, 23–25), as we demonstrated for phenylalanine and valine. Both protein supplements demonstrated a prolonged aminoacidemia as shown with leucine and isoleucine. The WP group had a peak in blood amino acid concentrations at 40 min postingestion greater than that observed in the PB group. Interestingly, this spike in substrate had no additional effect on the muscle FSR compared with the PB group and did not further prolong the WP group’s FSR response into the late period. The PB group had a smaller initial peak than the WP group but demonstrated proof of concept in that it remained elevated above resting values for up to 3 h postexercise for phenylalanine and 4 h postexercise for valine. It is possible that the prolonged substrate availability observed in the proteins with slower release, casein (23, 24) and soy (11, 29), may explain the prolonged FSR response in the PB group. The prolonged aminoacidemia may be attributed to the slower digestion of caseinate and soy protein isolate compared with WP isolate. It is important to note that although we utilized a form of casein protein (i.e., caseinate) with a more rapid digestion than micellar casein, we were still able to prolong the amino acid response as proof of concept.
The mechanisms of the prolonged FSR following exercise and nutrition (protein or amino acids) are unclear. One suggestion is that this could occur through early hyperactivation of the “leucine threshold” during a short time frame. West et al. (25) gave 25 g of WP to demonstrate that the rapid digestion rate of WP in a single bolus was more beneficial for stimulating muscle protein synthesis than repeated small boluses. This theory is supported by the work from our laboratory demonstrating that excess leucine provides further stimulation in the anabolic machinery (55). Further, extra leucine given with a large bolus of amino acids was capable of stimulating FSR into the late (3–6 h) period following RE (1). Given that enough substrate is present (43), the increased late (3–5 h) FSR response with WP can occur without concomitant hyperaminoacidemia (25) in the later time periods, which suggests a strong early signal as a mechanism. Similar to the trend shown elsewhere (12), we did not see this pattern following ingestion of whey as demonstrated in other studies (19, 25). However, we were able to demonstrate a prolonged effect with the PB similar to that seen with caseinate ingestion (12). The discrepancy in the literature regarding the prolonged effect of whey may be a factor of the total protein or the leucine content. Previous studies gave 25 g of WP (3 g leucine, 11.5 g EAAs) (25, 43), whereas we and others (12) gave ∼17.7 g of WP containing (∼1.9 g leucine and ∼8.8 g EAAs) a dose previously demonstrated to produce a maximal response following exercise (19).
The prolonged FSR response with casein or our PB may occur through a continuous and prolonged signal stimulating the mTORC1 pathway and translation initiation. Certainly, we saw similar patterns overall in mTORC1 cell signaling, yet only PB was able to prolong S6K1 phosphorylation, possibly because the WP would have had a maximal signal ∼1 h following ingestion (25).
Regarding chronic exposure to supplementation of isolated protein sources following RE training, WP has tended to demonstrate advantages for muscle accretion in young healthy males (20, 26, 32, 56, 57). The few studies with other protein sources have demonstrated that soy (32, 33, 58) or casein protein (59) is effective in stimulating muscle accretion in a variety of populations. There is a need for future research to test the efficacy of PBs against WP supplementation in promoting muscle growth during exercise training.
In summary, our data and that of others (10) further support the use of a blended protein supplement following RE compared with an isolated protein. A blended protein supplement containing sufficient EAA content, several digestion rates, and a prolonged aminoacidemia clearly promotes muscle protein synthesis during postexercise recovery. Future applications of utilizing PBs to promote or maintain muscle mass may include studies in aging and other muscle-wasting clinical populations such as cancer patients where the use of blended protein supplement has demonstrated a positive effect (60).
Supplementary Material
Acknowledgments
The authors thank Shaheen Dhanani for assistance with recruitment and also Ming Zheng and Shelley Medina for technical assistance. The authors also thank Glenna Hughes for editing the manuscript. B.B.R., E.V., P.T.R., D.K.W., J.M.D., D.M.G., K.L.T., C.S.F., M.J.D., M.B.C., and R.M. designed the research; P.T.R., D.K.W., J.M.D., D.M.G., K.L.T., C.S.F., M.S.B., and M.J.D. conducted research; B.B.R., E.V., P.T.R., D.K.W., J.M.D., D.M.G., K.L.T., C.S.F., M.J.D., M.B.C., and R.M. reviewed the manuscript; P.T.R., D.K.W., J.M.D., D.M.G., M.S.B., K.J., E.V., and B.B.R. analyzed data; and P.T.R. and B.B.R. wrote the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: EAA, essential amino acid; FSR, fractional synthesis rate; ITS-CRC, Institute for Translational Sciences-Clinical Research Center; mTORC1, mammalian target of rapamycin complex 1; PB, protein blend; RE, resistance exercise; 1RM, 1 repetition maximum; WP, whey protein.
Literature Cited
- 1.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106:1730–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–9 [DOI] [PubMed] [Google Scholar]
- 4.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–34 [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–92 [DOI] [PubMed] [Google Scholar]
- 6.Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–57 [DOI] [PubMed] [Google Scholar]
- 7.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging. 2011;15:376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36:2073–81 [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–40 [DOI] [PubMed] [Google Scholar]
- 11.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–92 [DOI] [PubMed] [Google Scholar]
- 12.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. Whey and casein labeled with L-[1–13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab. 2011;300:E231–42 [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond). 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YC, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. Epub 2012 Sep 6 [DOI] [PubMed] [Google Scholar]
- 15.Burke LM, Hawley JA, Ross ML, Moore DR, Phillips SM, Slater GR, Stellingwerff T, Tipton KD, Garnham AP, Coffey VG. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Med Sci Sports Exerc. 2012;44:1968–77 [DOI] [PubMed] [Google Scholar]
- 16.Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. 2011;21:e372–83 [DOI] [PubMed] [Google Scholar]
- 17.Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab. 2008;295:E70–7 [DOI] [PubMed] [Google Scholar]
- 18.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1254–62 [DOI] [PubMed] [Google Scholar]
- 19.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–8 [DOI] [PubMed] [Google Scholar]
- 20.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12:66–71 [DOI] [PubMed] [Google Scholar]
- 21.Paul GL. The rationale for consuming protein blends in sports nutrition. J Am Coll Nutr. 2009;28 Suppl:S464–72 [DOI] [PubMed] [Google Scholar]
- 22.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–8 [DOI] [PubMed] [Google Scholar]
- 25.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803 [DOI] [PubMed] [Google Scholar]
- 26.Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab (Lond). 2010;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–9 [DOI] [PubMed] [Google Scholar]
- 28.Soop M, Nehra V, Henderson GC, Boirie Y, Ford GC, Nair KS. Co-ingestion of whey protein and casein in a mixed meal demonstration of a more sustained anabolic effect of casein. Am J Physiol Endocrinol Metab. 2012;303:E152–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, Everwand J, Benamouzig R, Tome D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr. 2003;133:1308–15 [DOI] [PubMed] [Google Scholar]
- 30.Noakes M. The role of protein in weight management. Asia Pac J Clin Nutr. 2008;17 Suppl 1:169–71 [PubMed] [Google Scholar]
- 31.Ren MQ, Kuhn G, Wegner J, Chen J. Isoflavones, substances with multi-biological and clinical properties. Eur J Nutr. 2001;40:135–46 [DOI] [PubMed] [Google Scholar]
- 32.Candow DG, Burke NC, Smith-Palmer T, Burke DG. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int J Sport Nutr Exerc Metab. 2006;16:233–44 [DOI] [PubMed] [Google Scholar]
- 33.Brown EC, DiSilvestro RA, Babaknia A, Devor ST. Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutr J. 2004;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butteiger DN, Cope M, Liu P, Mukherjea R, Volpi E, Rasmussen BB, Krul ES. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin Nutr. Epub 2012 Aug 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E–BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn EL, Volpi E, Rasmussen BB. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr. 2008;27:447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed Hoboken (NJ): Wiley-Liss; 2005 [Google Scholar]
- 41.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–4 [DOI] [PubMed] [Google Scholar]
- 42.Team RC. R version 13.2. Vienna: R Foundation for Statistical Computing; 2012 [Google Scholar]
- 43.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. 2012;108:958–62 [DOI] [PubMed] [Google Scholar]
- 45.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–81 [DOI] [PubMed] [Google Scholar]
- 46.Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, Rennie MJ, Macdonald MJ, Baker SK, Phillips SM. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med Sci Sports Exerc. 2011;43:1154–61 [DOI] [PubMed] [Google Scholar]
- 47.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–8 [DOI] [PubMed] [Google Scholar]
- 48.Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab. 2004;14:255–71 [DOI] [PubMed] [Google Scholar]
- 49.Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab. 2009;34:151–61 [DOI] [PubMed] [Google Scholar]
- 50.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–53 [DOI] [PubMed] [Google Scholar]
- 51.Driskell JA. Sports nutrition: fats and proteins. Boca Raton (FL): CRC Press; 2007 [Google Scholar]
- 52.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7 [DOI] [PubMed] [Google Scholar]
- 53.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28:343–54 [DOI] [PubMed] [Google Scholar]
- 54.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care. 2008;11:40–4 [DOI] [PubMed] [Google Scholar]
- 55.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cribb PJ, Williams AD, Carey MF, Hayes A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. 2006;16:494–509 [DOI] [PubMed] [Google Scholar]
- 57.Cribb PJ, Williams AD, Stathis CG, Carey MF, Hayes A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med Sci Sports Exerc. 2007;39:298–307 [DOI] [PubMed] [Google Scholar]
- 58.Denysschen CA, Burton HW, Horvath PJ, Leddy JJ, Browne RW. Resistance training with soy vs whey protein supplements in hyperlipidemic males. J Int Soc Sports Nutr. 2009;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demling RH, DeSanti L. Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann Nutr Metab. 2000;44:21–9 [DOI] [PubMed] [Google Scholar]
- 60.Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, van Helvoort A, Wolfe RR. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.