Abstract
Background: Representative national data of prevalence of anemia and casual factors are missing for population group of reproductive aged non-pregnant females in Serbia. The purpose of the current study was to assess the prevalence and grades of anemia and its association with risk factors among non-pregnant women of childbearing age in Serbia.
Methods: Data were collected as part of the first “National Health Survey”, a cross-sectional, multistage cluster survey, conducted on 677 households in Serbia. A total of 708 females 20-49-year-old were recruited. Socioeconomic, anthropometric, dietary and reproductive data have been collected and hemoglobin levels were determined.
Results: The overall prevalence of anemia was 27.7% (196/708) [95% Confidence Interval (CI), 24.5-31.1%], and more precisely mild (21.9%), moderate (5.1%) and severe (0.7%) anemia. Belgrade residential area [odds ratio 2.11 (95% CI 1.27-3.50), p=0.004], shortage of living space per person (<16m2) [2.18 (1.17-4.03), p=0.014], body mass index (<25) [1.55 (1.04-2.29), p=0.029], alcohol intake [0.52 (0.33-0.81), p=0.004], lack [2.48 (1.31-4.70), p=0.005] or fruit juice consumption 1-2 [2.76 (1.46-5.23), p=0.002] times a week and previously diagnosed, but treated [2.62 (1.29-5.35), p=0.008] or not treated [3.57 (1.71-7.45), p<0.001] anemia were independent predictors of low hemoglobin levels. Deficit of electricity supply and insufficient living space in households, increased risk of moderate anemia, while likelihood of being mild and moderately anemic, augmented with previously diagnosed but, treated or not treated anemia and lack or juice consumption 1-2 times a week.
Conclusions: High prevalence of anemia among non-pregnant women and its association to casual factors needs continuous monitoring and control efforts for anemia in Serbia.
Keywords: anemia, cross-sectional study, non-pregnant women, risk factors
Introduction
Anemia is an important global public health problem affecting the greatest number of females in population group of non-pregnant women (NPW)1. Among NPW of childbearing age in developing countries anemia prevalence ranges from 20.8%2 to 73%3, indicating both, inadequate nutrition and poor health.
The consequences of anemia for reproductive aged women include increased risk of abruptio placentae4, miscarriage5, preterm delivery6, low birth weight7 and perinatal mortality8 for the newborn, as well as increased maternal risk of morbidity9, mortality10, susceptibility to infectious diseases11 and lowered physical and work capacity12. Therefore, anemia is regarded as a public health problem when the frequency of low hemoglobin (Hb) values is more than 5% of the population13.This is why Centers for Disease Control and Prevention (CDC) recommend screening of all non-pregnant women for anemia every 5-10 years throughout their childbearing years14.
It has been clearly confirmed that Hb concentration is a well established index for assessing and monitoring anemia at the population level13. Although there may be many risk factors for low Hb levels in the population, dietary iron deficiency is usually either the main or a major predictor among population group of premenopausal women15. One cross-sectional study that was conducted to investigate the nutritive risk factors for anemia in 1,671 women of childbearing age has shown that iron deficiency (plasma ferritin <15 microgL-1) increased six fold the risk of being anemic16. Likewise, nutritional and socioeconomic underlying contributors such as, low frequency of animal protein17 and juices consumption18, increased rice, wheat fleur and plant based food intake15, and women’ s formal education19, income19, race20, socioeconomic status 21-23, occupation17 and religion affiliation21, were additional risk factors associated with lower Hb values in NPW. Very recent studies evaluating relationship of anemia with nutritional status and type of living area, revealed that non-overweight [body mass index (BMI) <25] NPW24 and females living in rural areas25, were more prone to anemia development than their overweight and obese (BMI ≥25) and urban counterparts.
Several reproductive factors are also considered as possible reasons for higher prevalence of anemia in females20, 22-29. Multiple logistic regression analyses of factors predictive of anemia, performed in cross-sectional surveys have demonstrated high maternal parity (≥2 offsprings) as an independent predictor of anemia in reproductive aged women22,26. In Shobha and colleagues’ study26, multiparous women had almost 2.2 times greater risk of anemia than non-parous females. Other risk factors throughout childbearing years include parasitic infections, although more often reported in females of developing countries3 and concurrent heavy menstrual blood loss27. There is strong evidence that the use of hormonal contraception decreases28, while intra-uterine device which is associated with augmented menstrual blood loss increases29 the odds of being anemic.
Furthermore, particular vulnerability to anemia is nowadays reported in subpopulation groups of refugee women of childbearing age30. Although World Health Organization (WHO) has reported country estimates of anemia of 26.7% for former Yugoslav women including, both, Serbian and Montenegrinian females, representative national data are missing for Serbian population group of NPW1. Up to date, throughout the country, anemia has been reported mainly from a clinical perspective, whereas there has been inadequate information directed from a public health perspective. The need for anemia assessment has been particularly exaggerated since early 1990s when worsening of socioeconomic status (SES) for many families became reality, associated with an intensive refugee movements directed to Serbia. Therefore, we conducted a nationwide cross-sectional study [National Health Survey of Serbia] to investigate the frequency of anemia and its association to risk factors among NPW in Serbia.
Methods
The National Health Survey of Serbia (NHSS), a cross-sectional study conducted by the Ministry of Health of Serbia and the Institute of Public Health of Serbia, covered territory of Serbia with a total population of 7,576,837 people in 1991, which is divided into 3 residential areas - Belgrade, Vojvodina Province and Central Serbia with a total population of 1,552,151, 1,970,195 and 5,606,642 people, respectively. A multistage stratification technique was used for selecting the study sample in which Serbia was grouped in 3 residential areas with 22 districts. The first level of stratification was the selection of 3 residential areas: Belgrade, Vojvodina Province and Central Serbia without Belgrade area. Each area was further stratified into districts and even further into municipal levels. Thereafter, a two-stage stratified sample of clusters has been performed. In the first stage according to the probability approach, 300 municipalities at the level of Serbia were chosen (units of first stage). In the second stage, in each municipality, a cluster has been selected consisting of 15 nearby households (units of second stage). In total, 677 households across the municipalities and 708 non-pregnant females, 20-49 years old were selected to participate in the NHSS.
A standard United Nations Children’s Fund’s questionnaire prepared for Multiple Indicator Cluster Surveys II, has been applied31,32 for collecting information about the socioeconomic, nutritional, anthropometric, reproductive factors and underlying diseases as the following: age, living area, residential status, residential area, subpopulation group, occupation, education level, marital status, living space per person, electricity supply in the household, household wealth index (calculated from household’s ownership of customer items including washing machine, refrigerator, tractor, car, television, telephone, personal computer, flooring material, type of drinking water source, toilet facilities, and other characteristics related to wealth status-characterized into low, medium and high), alcohol intake, household’s food budget, times of breakfast, lunch, dinner, morning and afternoon snack, poultry, fish, pork, beef, meat products, milk, fresh fruits, vegetables and fruit juices consumption, as well as kind of spread used over bread; use of contraception, intrauterine device and hormonal contraceptives and diagnosed and treated/not treated anemia in the last 12 months, presence of obstructive pulmonary disease; diabetes mellitus, infection, duodenal ulcer, rheumatic, renal and cardiovascular diseases. Reference categories were used as the following, for dietary characteristics the normal consumption pattern (6-7 times/day) and the worst possibilities for household’s food budget and kind of spread used over bread, values of allowances >70% and “kajmak”, as a spread, respectively, because, the mean monthly family budget spent for food supply in Serbia accounts 50%33 and “kajmak as typical Serbian creamy, salty, dairy product, rich in fat content of 75% is often over consumed in the households, as self prepared milk product34.
Height and weight were measured using standard anthropometric methodology35: height, barefoot by portable stadiometer (Holtain, Crymmych, Wales) with 0.5 cm of precision and weight without heavy clothing, by digital scale (HANSON, Watford, Hertforshire, England) and precission of 100g. BMI (expressed in kg m-2) was calculated and classified according to previously published criteria36.
Hemocue system (Hemo-cue, Angelholm, Sweden)37 was used to estimate the concentration of Hb in capillary blood. WHO criteria were applied to define anemia (Hb concentration of <120 gL-1) and anemic status as mild (Hb, 100-119.99 gL-1), moderate (Hb, 70-99.99 gL-1) and severe (Hb, < 70 gL-1) and non anemic (Hb, ≥ 120 gL-1) among NPW38. Hb values of less than 50 gL-1 and more than 180 gL-1 were considered spurious and excluded from analysis according to the criteria published elsewhere39.
Data analysis
Results are reported as medians with interquartile range (IQR) or as proportions with 95% Confidence Intervals (CIs).
Statistical tests used included Kolmogorov-Smirnov and Shapiro-Wilks tests and Mann Whitney U test. All risk factors tested in the present study had been previously described for reproductive aged women. Dimensional variables tested for risk factors were converted in categorical attributes with the use of cut-off points that were either consistent with previously published definitions (e.g., non overweight, BMI <25 kgm-2), important for the course of anemia (e.g., multiparious, two or more offsprings) or in accordance with customary epidemiological judgment (e.g., living space per person, <16 m2).
For the univariate analyses χ2 test and binary logistic regression analysis were performed. Factors found to be statistically significant by univariate analysis were further examined by multivariable logistic and polynomial logistic regression analysis. A stepwise backward approach was performed. Odds ratios (ORs) are reported with 95% CIs. A p-value <0.05 was considered to be statistically significant. Statistical analysis was performed by using SPSS software (version 19.0, IBM SPSS Inc, Chicago, IL, USA).
Results Study participants
Seven hundred and forty six reproductive aged women were surveyed in the study. Thirty eight participants were excluded from the final analysis of whom: 37 reported possible pregnancy and in the other one, blood analysis revealed Hb values >180 gL-1. A total of 708 [median age 36 years (IQR, 13 years)] NPW completed the survey. In 196 out of 708 (27.7%) [95% CI 24.5-31.1%] survey participants anemia has been diagnosed compared to 512/708 (72.3%) [95% CI 69-75] in whom normal Hb values were detected. The median Hb values were 110.00 gL-1 (IQR, 10.3 gL-1) in anemic and 130.20 gL-1 (IQR, 19.3 gL-1) in non anemic NPW, (p< 0.001). About 47% of the women originated from urban households and 53% were rural classified. Both anemic and non anemic NPW had same education level [median schooling time of 12 years (IQR, 4 years)]. There was lack of statistically significant difference between anemic and not anemic groups on weight, height, living area, residential status (Table 1) and comorbidities. Most of the NPW had mild (155 of 708) [21.9% (95% CI, 19.0-25.1%)] or moderate (36 of 708) [5.1%, (95% CI, 3.7-5.1%)] anemia, but few of them suffered from severe (5 of 708) [0.7%, (95% CI, 0.3-1.6%)] grade of anemia (Table 1). Prevalence rates of anemia (with associated 95% CI) relating to the evaluated risk factors are summarized at Table 2.
Table 1. General data of non-pregnant women of childbearing age in Serbia.
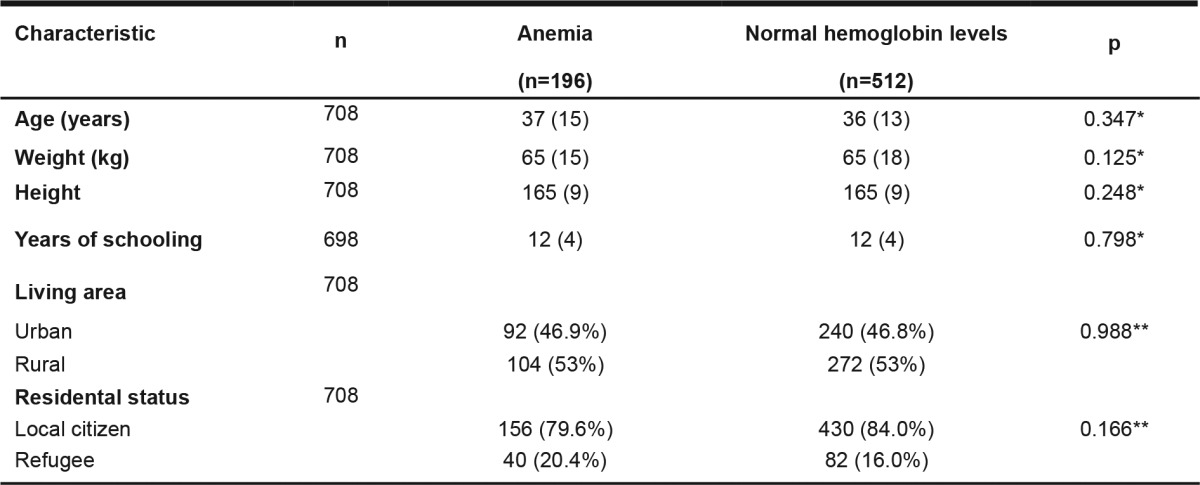
Values are expressed as median (IQR) or as proportions. * Mann-Whitney U-test, ** χ2-test
Table 2. Prevalence of anemia and univariate variable statistics for risk factors.
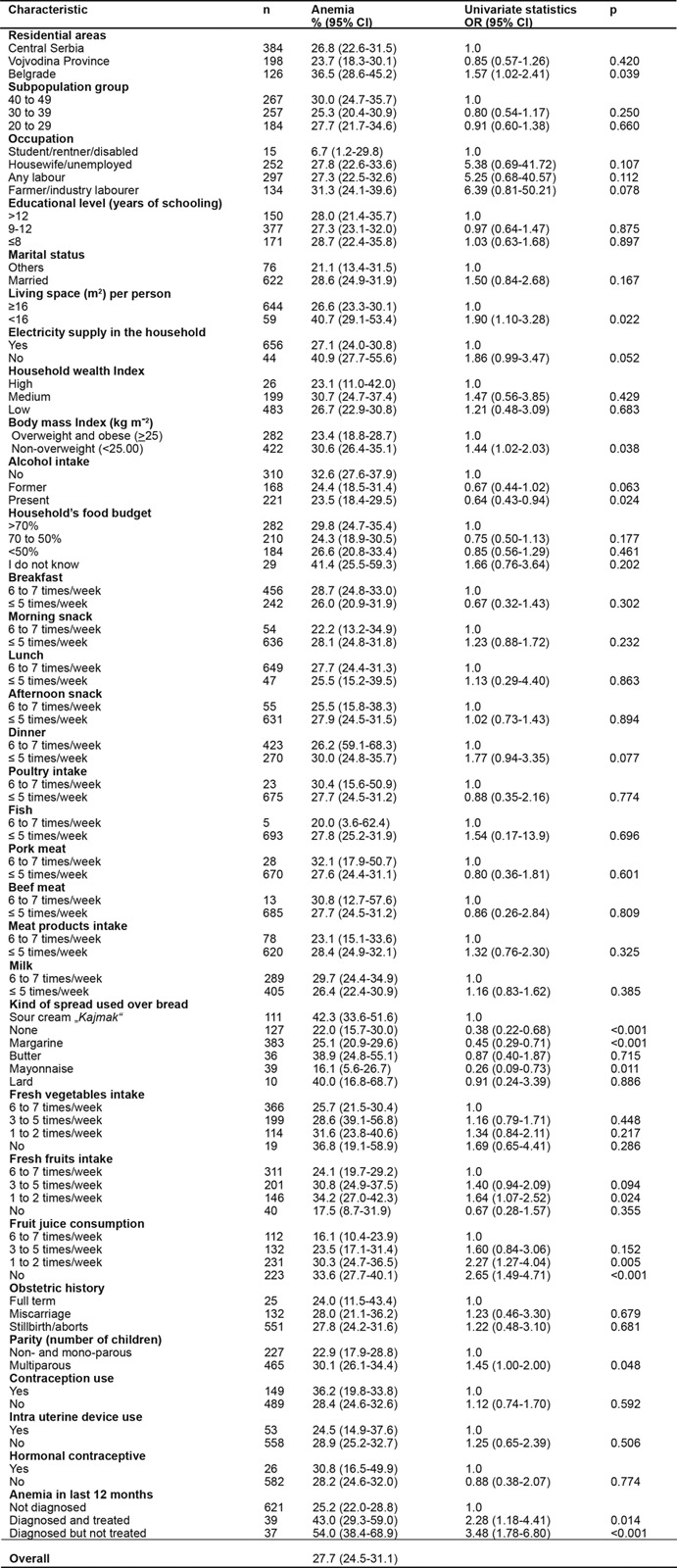
OR: Odds ratio, CI: Confidence interval, univariate binary logistic regression analysis was applied to test association of anemia with risk factors
Risk factors of anemia
Anemia was associated in the univariate analysis with eight risk factors. Those variables included, Belgrade residential area, deficit of living space per person (<16m2), BMI <25, alcohol consumption, kind of spread used over bread, inadequate fruit juice consumption (lack or 1-2 times a week), multiparous status (≥ 2 offsprings) and diagnosed, but not managed or managed anemia within last 12 months before study begin (Table 2).
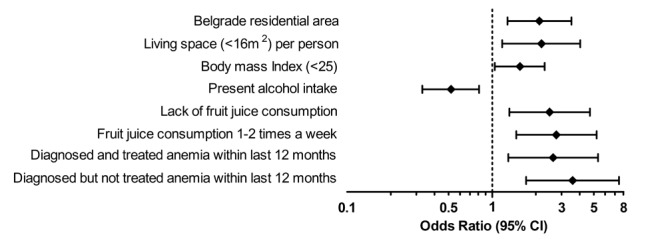
Multivariable logistic regression analysis revealed that Belgrade residential area [odds ratio 2.11 (95% CI 1.27, 3.50), p=0.004], living space per person less than 16m2 [2.18 (1.17, 4.03), p=0.014], BMI <25 [1.55 (1.04, 2.29), p=0.029], alcohol intake [0.52 (0.33, 0.81), p=0.004], lack of fruit juice consumption [2.48 (1.31, 4.70), p=0.005] or fruit juice drinking 1 to 2 times [2.76 (1.46, 5.23), p=0.002] a week and diagnosed but not managed [2.62 (1.29, 5.35), p=0.008] or managed [3.57 (1.71, 7.45), p<0.001] anemia within last 12 months were independent predictors of anemia (Figure 1).
Figure 1. Odds ratios for risk factors predicting anemia among Serbian non-pregnant women. Shown are the estimates (on a log10 scale) of the risk of possessing anemia. The diamonds represent point estimates confirmed by multivariable analysis. The horizontal lines indicate the 95% confidence intervals. Vertical dashed line on 1 designates no difference in haemoglobin values between participants with anemia and cases with normal hemoglobin levels.
Risk factors associated with anemia grades
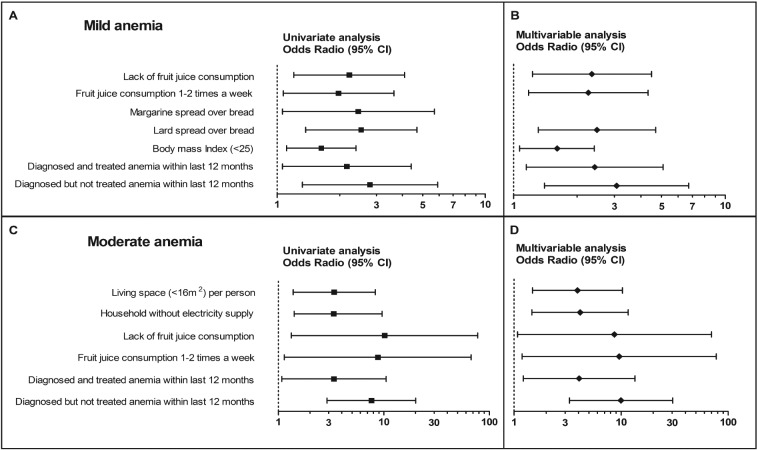
Relating to the anemic status among NPW, mild anemia in univariate analysis was associated with lack or fruit juice consumption 1 to 2 times a week, margarine and lard spread used over bread, BMI <25, previously diagnosed but treated or not treated anemia (Figure 2).
Figure 2. Odds ratios for risk factors predicting mild and moderate anemia among Serbian non-pregnant women. Shown are the estimates (on a log10 scale) of the risk of possessing mild (Panels, A and B) and moderate (Panels, C and D) anemia. The squares represent point estimates confirmed by univariate (Panels, A and C) and the diamonds by multivariable analyses (Panels, B and D). The horizontal lines indicate the 95% confidence intervals. Vertical dashed line on 1 designates no difference in haemoglobin values between participants with anemia and cases with normal hemoglobin levels.
Multivariable analysis showed that lack [2.35 (1.23-4.49), p=0.010] or fruit juice consumption 1 to 2 times [2.26 (1.18-4.33), p=0.014] a week, lard spread used over bread [2.48 (1.31-4.69), p=0.005], BMI <25 [1.61 (1.07-2.41), p=0.002] and confirmed diagnosis of anemia within last 12 months whether treated [2.42 (1.15-5.09), p=0.020] or not treated [3.07 (1.40-6.73), p=0.005] independently predicted mild anemia (Figure 2).
Living in households without electricity supply and without adequate living space, lack of fruit juice drinking or fruit juice consumption 1 to 2 times a week and presence of low Hb levels with or without therapy within last 12 months, increased likelihood of being moderately anemic in NPW (Figure 2).
After performing polynomial logistic regression analysis, (Figure 2) association was maintained for deficit of living space [3.92 (1.49-10.30), p=0.007] and electricity supply [4.15 (1.47-11.67), p=0.007], lack of [8.65 (1.08-69.94), p=0.042] or fruit juice consumption 1 to 2 times a week [9.62 (1.19-77.49), p=0.034] and diagnosed but not managed [9.99 (3.29-30.37), p<0.001] or managed [4.06 (1.22-13.51), p<0.001] anemia.
Although it was found in univariate analysis that living in households without electricity supply [12.25 (1.96-76.57), p=0.007] was related to severe anemia grade, that was further not confirmed by multivariable model.
Discussion
The overall prevalence of anemia among NHSS participants in our study was 27.7%. The most of NPW suffered of mild (21.9%) and moderate (5.1%) anemia, but few of them were affected by severe (0.7%) anemia. WHO1 suggests classification of the public significance of anemia according to the prevalence estimates of blood Hb levels and proposes four grades of significance for countries including “normal” (<5%), “mild” (50-19.9%), “moderate” (20.0-39.9%) and “severe” (≥40%) anemia. Accordingly, relating to the WHO classification our data clearly confirm that a public level of significance among Serbian woman is in third or “moderate” grade. Hence, the finding of high prevalence of anemia among NPW could be explained as continuation and worsening of anemia present in Serbian school aged children and throughout their adolescent age, that has been reported elsewhere40.
Our study has clearly shown that NPW living in Belgrade residential area were more than 2-times as likely to acquire anemia compared to females living in Vojvodina province and Central Serbia. This finding was further substantiated by study of Bentley and Griffiths21 who demonstrated that childbearing women from the urban, low standard of living group have almost 2-fold risk of anemia compared to the high urban, standard of living group. In Belgrade residential area, most developed area in Serbia, industrial production devastation, massive immigration and augmentation of unemployment in mid 1990s induced rapid decline of SES and poor health outcomes throughout vulnerable population groups including NPW.
Two additional risk factors of anemia confirmed by multivariable analysis, i.e. deficit of living space and electricity supply, deserve attention. We observed that shortage of living space per person (<16m2) increased more than twice the risk of anemia and almost 4-fold the risk of being moderately anemic among NPW, compared to their counterparts originating from households with sufficient space area. Furthermore, likelihood of being moderately anemic raised also by deficit of electricity supply in households in our study. Our finding is once again strong evidence in favor, that urban growth, overcrowding and pour housing are socioeconomic determinants of anemia.
SES associated with nutritional status and dietary intake, determines susceptibility to anemia in population group of NPW. Our study showed that NPW with BMI <25 had 61% higher odds of being anemic than females with a BMI ≥25. This was further substantiated by recent studies conducted in developing countries24. In cases with BMI ≥25 consumption of sufficient quantity energy reach food, even in the circumstances of its inadequate quality provides adequate iron supply and consequently decreases anemia risk. In addition, we confirmed that intake of lard as a spread over bread increases 2.5-times the risk of mild anemia. Since early 1990s, lard regarded as a “poverty food”41, and was often used in Serbia as a substitution for dairy fats and vegetable oils because of its high caloric content and low price.
Furthermore, beside high bioavailability rich foods, dietary fruits and vegetables were seldom consumed by NPW because of their expensiveness. We discovered that lack of or fruit juice consumption 1-2 times a week independently predicted anemia in study sample. Hence, absence of juice consumption increased 2- and almost 9-times the risk of mild and moderate anemia, respectively and likelihood of being anemic was also high, if juices have been drunk 1-2 times a week. This event is in agreement with results of a previous study in which multivariable statistic technique confirmed association of anemia with inadequate juice consumption18. Our results emphasize that, lack of dietary fruit consumption produces ascorbic acid deficiency and consequently poor non-hem iron absorption because of strong non-hem iron promoter absorption activity of ascorbic acid among NPW42. A recent study has reported protective activity of alcohol intake21 that is also confirmed in alcohol abusers in our study.
Additional risk factors for anemia such as presence of previous anemia diagnosis have been shown before27. However, the importance of assessing and monitoring Hb levels among NPW population at national level is emphasized by results in our study showing that previously diagnosed, but not treated or treated anemia within last 12 months increased odds of being anemic and raised the likelihood of mild and moderate anemia in our study.
This study suggests that anemia in NPW in Serbia could be reduced by improvement SES, with introduction of diagnostic and monitoring procedures, by improving health education about the importance of nutritive risk factors and by routinely screening throughout childbearing years. The present study also highlights the necessity of providing special attention on preventive and control measures of anemia toward introducing diagnostic procedures for iron-deficiency, as a prioritiy in the population group of NPW. Interventions directed to improve education are urgently required in Serbia to fill up the knowledge gaps in the vulnerable group of premenopausal women.
It is worth mentioning that we used measuring Hb concentration to evaluate prevalence of anemia as a proxy for iron deficiency anemia in the population which could be considered as the first limitation of our study. In addition, the second one, the type of study performed, because the cross sectional design we have conducted evaluates only “snapshot” of the existing frequency of anemia in the studied sample.
Despite such limitations, results of the present study elucidate our understanding of factors associated with anemia in the population group of NPW in Serbia. The findings of our study could raise the concern of decision makers and also assist health authorities’ implementation of continuous, national, science-based monitoring and control programs for anemia. However, further research is required to elucidate iron deficiency and other determinants of anemia among NPW.
It could be concluded that anemia remains a major public health problem among NPW in Serbia. Results of current cross-sectional study indicated that socioeconomic and nutritional factors, vitamin deficiency and presence of diagnosed but treated and not treated, anemia, are significant predictors of anemia. Such findings highlight the need for introduction of continuous monitoring and control programmes aimed to reduce high prevalence of anemia in Serbia.
Acknowledgements
We are particularly grateful to Professor Pekka Puska, from the National Public Health Institute (KTL) Finland, for his guidance in establishing the study and his supervision during the whole duration of the survey.
Conflicts of interest
None declared.
Funding
This study has been supported by grants-in-aids by the World Health Organization (2000); the United Nations International Children’s Fund (2000) and the European Community Humanitarian aid Office ECHO (2000).
References
- 1.Benoist B, McLean E, Cogswell M, Egli I, Wojdyla D. Worldwide prevalence of anemia 1993–2005. WHO Global Database on Anemia. World Health Organization, Geneva. 2008 [Google Scholar]
- 2.Shamah-Levy T, Villalpando S, Rivera JA, Mejía-Rodríguez F, Camacho-Cisneros M, Monterrubio EA. Anemia in Mexican women: a public health problem. Salud Publica Mex. 2003;45 Suppl 4:S499–S507. doi: 10.1590/s0036-36342003001000006. [DOI] [PubMed] [Google Scholar]
- 3.Ziauddin Hyder S, Persson LK, Chowdhury A, Ekström EC. Anaemia among non-pregnant women in rural Bangladesh. Public Health Nutr. 2001;4:79–83. doi: 10.1079/phn200055. [DOI] [PubMed] [Google Scholar]
- 4.Arnold DL, Williams MA, Miller RS, Qiu C, Sorensen TK. Iron deficiency anemia, cigarette smoking and risk of abruptio placentae. J Obstet Gynaecol Res. 2009;35:446–452. doi: 10.1111/j.1447-0756.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 5.Szerafin L, Jakó J. [Anemia in pregnancy: characteristics in Szabolcs-Szatmár-Bereg County, Hungary] Orv Hetil. 2010;151:1347–1352. doi: 10.1556/OH.2010.28887. [DOI] [PubMed] [Google Scholar]
- 6.Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. 1992;55:985–988. doi: 10.1093/ajcn/55.5.985. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen K. Is There a Causal Relationship between Iron Deficiency or Iron-Deficiency Anemia and Weight at Birth, Length of Gestation and Perinatal Mortality? J Nutr. 2001;131:590S–601S. doi: 10.1093/jn/131.2.590S. [DOI] [PubMed] [Google Scholar]
- 8.Lee HS, Kim MS, Kim MH, Kim YJ, Kim WY. Iron status and its association with pregnancy outcome in Korean pregnant women. Eur J Clin Nutr. 2006;60:1130–1135. doi: 10.1038/sj.ejcn.1602429. [DOI] [PubMed] [Google Scholar]
- 9.Mulayim B, Celik NY, Yanik FF. Helicobacter pylori infection detected by 14C-urea breath test is associated with iron deficiency anemia in pregnant women. J Obstet Gynaecol Res. 2008;34:980–985. doi: 10.1111/j.1447-0756.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–614S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 11.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Trans R Soc Trop Med Hyg. 2008;102:130–136. doi: 10.1016/j.trstmh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Scholz BD, Gross R, Schultink W, Sastroamidjojo S. Anaemia is associated with reduced productivity of women workers even in less-physically-strenuous tasks. Br J Nutr. 1997;77:47–57. doi: 10.1017/s0007114500002877. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization.United Nations Children’s Fund UNU. Iron deficiency anemia. Assessment, Prevention and Control. A guide for programme managers. World Health Organization, Geneva. 2001 [Google Scholar]
- 14.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 15.Chandyo RK, Strand TA, Ulvik RJ, Adhikari RK, Ulak M, Dixit H, et al. Prevalence of iron deficiency and anemia among healthy women of reproductive age in Bhaktapur, Nepal. Eur J Clin Nutr. 2007;61:262–269. doi: 10.1038/sj.ejcn.1602508. [DOI] [PubMed] [Google Scholar]
- 16.Zhu JH, Hu DJ, Hao L, Zhang BL, Cogswell ME, Bailey LB, et al. Iron, folate, and B(12) deficiencies and their associations with anemia among women of childbearing age in a rural area in Northern China. Int J Vitam Nutr Res. 2010;80:144–154. doi: 10.1024/0300-9831/a000014. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PH, Nguyen KC, Le Mai B, Nguyen TV, Ha KH, Bern C, et al. Risk factors for anemia in Vietnam. Southeast Asian J Trop Med Public Health. 2006;37:1213–1223. [PubMed] [Google Scholar]
- 18.Al-Quaiz JM. Iron deficiency anemia. A study of risk factors. Saudi Med J. 2001;22:490–496. [PubMed] [Google Scholar]
- 19.Singh MB, Fotedar R, Lakshminarayana J. Micronutrient deficiency status among women of desert areas of western Rajasthan, India. Public Health Nutr. 2009;12:624–629. doi: 10.1017/S1368980008002395. [DOI] [PubMed] [Google Scholar]
- 20.Morrone A, Nosotti L, Piombo L, Scardella P, Spada R, Pitidis A. Iron deficiency anaemia prevalence in a population of immigrated women in Italy. Eur J Public Health. 2012;22:256–262. doi: 10.1093/eurpub/ckq144. [DOI] [PubMed] [Google Scholar]
- 21.Bentley ME, Griffiths PL. The burden of anemia among women in India. Eur J Clin Nutr. 2003;57:52–60. doi: 10.1038/sj.ejcn.1601504. [DOI] [PubMed] [Google Scholar]
- 22.Shamah-Levy T, Villalpando-Hernández S, García-Guerra A, Mundo-Rosas V, Mejía-Rodríguez F, Domínguez-Islas CP. Anemia in Mexican women: results of two national probabilistic surveys. Salud Publica Mex. 2009;51 Suppl 4:515–522. doi: 10.1590/s0036-36342009001000006. [DOI] [PubMed] [Google Scholar]
- 23.Islam MZ, Lamberg-Allardt C, Bhuyan MA, Salamatullah Q. Iron status of premenopausal women in two regions of Bangladesh: prevalence of deficiency in high and low socio-economic groups. Eur J Clin Nutr. 2001;55:598–604. doi: 10.1038/sj.ejcn.1601190. [DOI] [PubMed] [Google Scholar]
- 24.Eckhardt CL, Torheim LE, Monterrubio E, Barquera S, Ruel MT. The overlap of overweight and anaemia among women in three countries undergoing the nutrition transition. Eur J Clin Nutr. 2008;62:238–246. doi: 10.1038/sj.ejcn.1602727. [DOI] [PubMed] [Google Scholar]
- 25.Bharati P, Som S, Chakrabarty S, Bharati S, Pal M. Prevalence of anemia and its determinants among nonpregnant and pregnant women in India. Asia Pac J Public Health. 2008;20:347–359. doi: 10.1177/1010539508322762. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Joshi S, Bhide P, Puranik B, Kanade A. Social dimensions related to anaemia among women of childbearing age from rural India. Public Health Nutr. 2011;14:365–372. doi: 10.1017/S1368980010002776. [DOI] [PubMed] [Google Scholar]
- 27.Pala K, Dundar N. Prevalence & risk factors of anaemia among women of reproductive age in Bursa, Turkey. Indian J Med Res. 2008;128:282–286. [PubMed] [Google Scholar]
- 28.Milman N, Clausen J, Byg KE. Iron status in 268 Danish women aged 18-30 years: influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol. 1998;77:13–19. doi: 10.1007/s002770050405. [DOI] [PubMed] [Google Scholar]
- 29.Dangour AD, Hill HL, Ismail SJ. Haemoglobin status of adult non-pregnant Kazakh women living in Kzyl-Orda region, Kazakhstan. Eur J Clin Nutr. 2001;55:1068–1075. doi: 10.1038/sj.ejcn.1601267. [DOI] [PubMed] [Google Scholar]
- 30.Tiong AC, Patel MS, Gardiner J, Ryan R, Linton KS, Walker KA, et al. Health issues in newly arrived African refugees attending general practice clinics in Melbourne. Med J Aust. 2006;185:602–606. doi: 10.5694/j.1326-5377.2006.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 31.United Nations Children’s Fund. Multiple Indicator Cluster Survey II. The Report for the Federal Republic of Yugoslavia. UNICEF, Belgrade. 2000 [Google Scholar]
- 32.United Nations Children’s Fund. Monitoring progress toward the goals of the world summit for children: a practical handbook for multiple-indicator surveys. UNICEF, New York. 1995 [Google Scholar]
- 33.Pravilnik o kvalitetu proizvoda od mleka i starter kultura. (Sl. glasnik RS, br.33/2010) [Google Scholar]
- 34.Statistical Office of the Republic of Serbia. Statistical Yearbook. Statistical Office of the Republic of Serbia, Belgrade. 2000 [Google Scholar]
- 35.Gordon CC, Chumlea WC, Roche AF. Stature, recumbent length, and weight. Lohman TG, Roche AF, Martorell R (Eds) Anthropometric standardization reference manual, Human Kinetics Books, Champaign, IL. 1988:3–8. [Google Scholar]
- 36.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854, WHO, Geneva. 1995 [PubMed] [Google Scholar]
- 37.Cohen PP, Short TG, Leung DH, Oh TE. A clinical evaluation of the Hemocue haemoglobinometer using capillary, venous and arterial samples. Anaesth Intensive Care. 1992;20:497–500. doi: 10.1177/0310057X9202000419. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. The prevalence of anemia among women: a tabulation of available information. WHO, Geneva. 1992 [Google Scholar]
- 39.Yip R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: special consideration of iron nutrition. Am J Clin Nutr. 2000;72:272S–279S. doi: 10.1093/ajcn/72.1.272S. [DOI] [PubMed] [Google Scholar]
- 40.Djokic D, Drakulovic MB, Radojicic Z, Crncevic Radovic L, Rakic L, Kocic S, et al. Risk factors associated with anemia among Serbian school-age children 7-14 years old: results of the first national health survey. Hippokratia. 2010;14:252–260. [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson A. The Penguin Companion to Food. Penguin Books, NY. 2002:530–531. [Google Scholar]
- 42.Backstrand JR, Allen LH, Black AK, de Mata M, Petto GH. Diet and iron status of nonpregnant women in rural Central Mexico. Am J Clin Nutr. 2002;76:156–164. doi: 10.1093/ajcn/76.1.156. [DOI] [PubMed] [Google Scholar]